Abstract
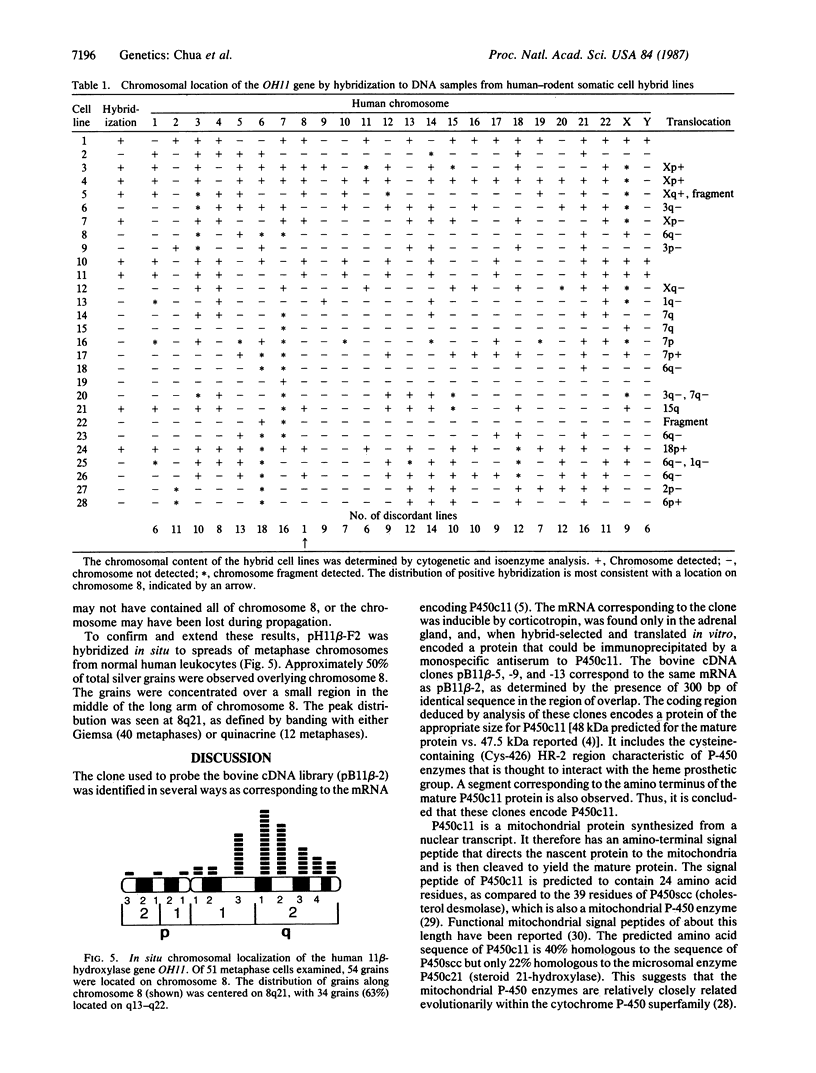
We have isolated bovine and human adrenal cDNA clones encoding the adrenal cytochrome P-450 specific for 11 beta-hydroxylation (P450c11). A bovine adrenal cDNA library constructed in the bacteriophage lambda vector gt10 was probed with a previously isolated cDNA clone corresponding to part of the 3' untranslated region of the 4.2-kilobase (kb) mRNA encoding P450c11. Several clones with 3.2-kb cDNA inserts were isolated. Sequence analysis showed that they overlapped the original probe by 300 base pairs (bp). Combined cDNA and RNA sequence data demonstrated a continuous open reading frame of 1509 bases. P450c11 is predicted to contain 479 amino acid residues in the mature protein in addition to a 24-residue amino-terminal mitochondrial signal sequence. A bovine clone was used to isolate a homologous clone with a 3.5-kb insert from a human adrenal cDNA library. A region of 1100 bp was 81% homologous to 769 bp of the coding sequence of the bovine cDNA except for a 400-bp segment presumed to be an unprocessed intron. Hybridization of the human cDNA to DNA from a panel of human-rodent somatic cell hybrid lines and in situ hybridization to metaphase spreads of human chromosomes localized the gene to the middle of the long arm of chromosome 8. These data should be useful in developing reagents for heterozygote detection and prenatal diagnosis of 11 beta-hydroxylase deficiency, the second most frequent cause of congenital adrenal hyperplasia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautbar C., Rösler A., Landau H., Cohen I., Nelken D., Cohen T., Levine C., Sack J., Benderli A., Moses S. No linkage between HLA and congenital adrenal hyperplasia due to 11-beta-hydroxylase deficiency. N Engl J Med. 1979 Jan 25;300(4):205–206. doi: 10.1056/nejm197901253000433. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Voutilainen R., Mohandas T. K., Miller W. L. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8962–8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Chrousos G. P., Mann D. W., Larsen J. W., Jr, Green I., McCluskey J., Loriaux D. L., Fletcher J. C., Koons G., Overpeck J. Pharmacologic suppression of the fetal adrenal gland in utero. Attempted prevention of abnormal external genital masculinization in suspected congenital adrenal hyperplasia. JAMA. 1985 Feb 15;253(7):1015–1020. [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard D. S., Kawasaki E. S., Bancroft F. C., Szabo P. Localization of a unique gene by direct hybridization in situ. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3755–3759. doi: 10.1073/pnas.78.6.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hellkuhl B., Grzeschik K. H. Assignment of a gene for arylsulfatase B to human chromosome 5 using human-mouse somatic cell hybrids. Cytogenet Cell Genet. 1978;22(1-6):203–206. doi: 10.1159/000130936. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M. E., John M. C., Simpson E. R., Waterman M. R. Regulation of cytochrome P-45011 beta gene expression by adrenocorticotropin. J Biol Chem. 1985 May 10;260(9):5760–5767. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Matteson K. J., Picado-Leonard J., Chung B. C., Mohandas T. K., Miller W. L. Assignment of the gene for adrenal P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase) to human chromosome 10. J Clin Endocrinol Metab. 1986 Sep;63(3):789–791. doi: 10.1210/jcem-63-3-789. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B., Levin W. The P450 gene superfamily: recommended nomenclature. DNA. 1987 Feb;6(1):1–11. doi: 10.1089/dna.1987.6.1. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkmeister J. W., New M. I., Dupont B., White P. C. Frequent deletion and duplication of the steroid 21-hydroxylase genes. Am J Hum Genet. 1986 Oct;39(4):461–469. [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yanagibashi K., Haniu M., Shively J. E., Shen W. H., Hall P. The synthesis of aldosterone by the adrenal cortex. Two zones (fasciculata and glomerulosa) possess one enzyme for 11 beta-, 18-hydroxylation, and aldehyde synthesis. J Biol Chem. 1986 Mar 15;261(8):3556–3562. [PubMed] [Google Scholar]
- Yoshioka H., Morohashi K., Sogawa K., Yamane M., Kominami S., Takemori S., Okada Y., Omura T., Fujii-Kuriyama Y. Structural analysis of cloned cDNA for mRNA of microsomal cytochrome P-450(C21) which catalyzes steroid 21-hydroxylation in bovine adrenal cortex. J Biol Chem. 1986 Mar 25;261(9):4106–4109. [PubMed] [Google Scholar]
- Zachmann M., Tassinari D., Prader A. Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients. J Clin Endocrinol Metab. 1983 Feb;56(2):222–229. doi: 10.1210/jcem-56-2-222. [DOI] [PubMed] [Google Scholar]





