Abstract
Loss of function mutations in the genes encoding either neurokinin B (NKB) or its receptor, NK3 (NK3R), result in hypogonadotropic hypogonadism, characterized by an absence of pubertal development and low circulating levels of LH and gonadal steroids. These studies implicate NKB and NK3R as essential elements of the human reproductive axis. Studies over the last two decades provide evidence that a group of neurons in the hypothalamic infundibular/arcuate nucleus form an important component of this regulatory circuit. These neurons are steroid-responsive and coexpress NKB, kisspeptin, dynorphin, NK3R and estrogen receptor α (ERα) in a variety of mammalian species. Compelling evidence in the human indicates these neurons function in the hypothalamic circuitry regulating estrogen negative feedback on gonadotropin-releasing hormone (GnRH) secretion. Moreover, in the rat, they form a bilateral, interconnected network that projects to NK3R-expressing GnRH terminals in the median eminence. This network provides an anatomical framework to explain how coordination among NKB/kisspeptin/dynorphin/NK3R/ERα neurons could mediate feedback information from the gonads to modulate pulsatile GnRH secretion. There is substantial (but indirect) evidence that this network may be part of the neural circuitry known as the “GnRH pulse generator”, with NK3R signaling as an important component. This theory provides a compelling explanation for the occurrence of hypogonadotropic hypogonadism in patients with inactivating mutations in the TAC3 orTACR3 genes. Future studies will be needed to determine whether NKB signaling plays a permissive role in the onset of puberty or is part of the driving force initiating the maturation of reproductive function.
1. Introduction
In 2009, Topaloglu et al. reported that loss of function mutations of the genes encoding either neurokinin B (NKB) or its cognate receptor, NK3 (NK3R) resulted in hypogonadotropic hypogonadism (Topaloglu et al., 2009). This seminal report implicates NKB signaling as an essential component for the onset of puberty and the control of gonadotropin secretion in the human. These findings were of great interest to us, based on our longstanding goal of understanding the physiological significance of the changes in NKB neurons in the hypothalamus of postmenopausal women (Rance and Young, 1991; Rance, 2009). The challenge now is to determine precisely how these neurons interface with the reproductive axis and whether these neurons are part of the signal for puberty. To provide a context for future studies, this review will summarize current knowledge of the role of NKB in the hypothalamic control of reproduction. For information regarding menopause and hypothalamic NKB/kisspeptin neurons, please refer to our previous article (Rance, 2009). A recent review on the relationship between NKB and placental physiology is also available (Page, 2010)
2. Molecular Biology of NKB: Nomenclature and Signaling
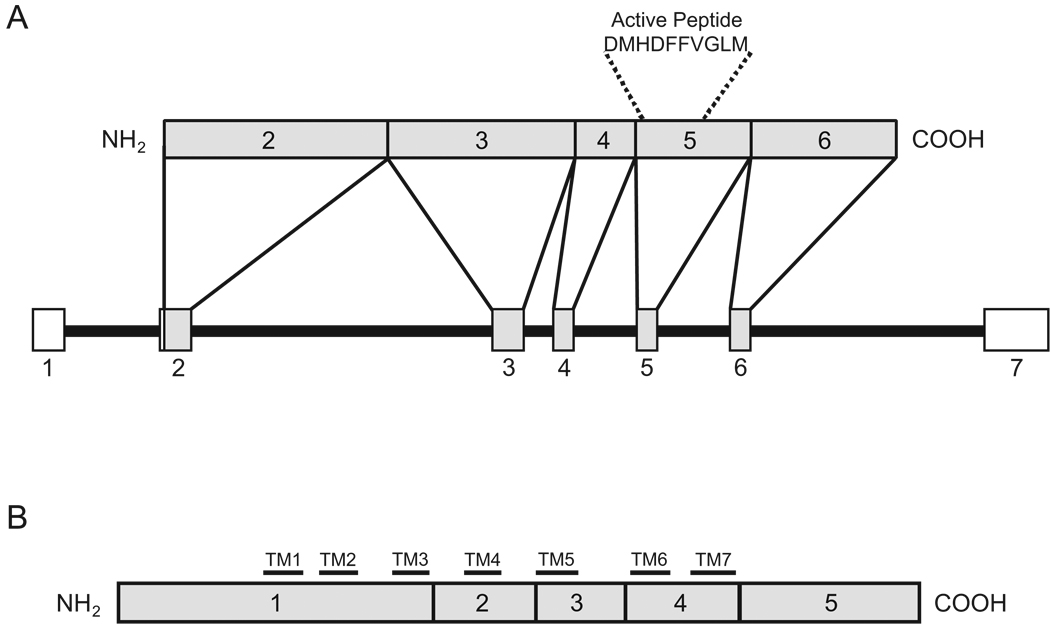
Neurokinin B is a member of the tachykinin family of peptides. Tachykinins are characterized by a common C-terminal amino-acid sequence (Phe-X-Gly-Leu-Met-NH2) and include substance P, neurokinin A and NKB, as well as neuropeptide K, neuropeptide γ, and hemokinin-1. NKB is the only tachykinin synthesized from the preprotachykinin-B gene (Almeida et al., 2004; Bonner et al., 1987; Helke et al., 1990; Kotani et al., 1986; Page et al., 2001) which is currently designated as TAC3 in humans, Tac3 in non-human primates, cattle and dogs and Tac2 in rodents. The TAC3 precursor mRNA contains 7 exons, 5 of which are translated to form the preprotachykinin B peptide (Figure 1). This prepropeptide undergoes enzymatic cleavage to form proneurokinin B, then NKB. The primary amino acid sequence of the final active peptide is encoded by exon 5 (Bonner et al., 1987; Kotani et al., 1986; Page et al., 2000). TAC3 precursor mRNA variants have been described, but the NKB peptide is widely conserved across vertebrates (Page et al., 2009). Because there are different names for the gene encoding NKB in different species (TAC3, Tac3 or Tac2), in this paper we will refer to mRNA products of this gene as NKB mRNA.
Figure 1.
A: Schematic diagram of human TAC3 gene and preprotachykinin B (connected by lines above the gene). The TAC3 gene contains 7 exons denoted by boxes 1–7 with introns represented by lines. Exons 2–6 (shaded boxes) are translated from mRNA to form preprotachykinin B. Exon 5 encodes the active NKB peptide. B: Schematic diagram of the NK3R protein. This G-protein coupled receptor is translated from five exons (shaded boxes) and the 7 transmembrane domains (TM1–7) are denoted by horizontal black bars (Takahashi et al., 1992). Figure A was modified from (Page et al., 2001) with permission.
NKB preferentially binds to NK3R, encoded by the TACR3 gene. Three tachykinin receptors have been identified, although the existence of additional receptors has been postulated (Grant et al., 2002; Pennefather et al., 2004). The three receptors (NK1R, NK2R, and NK3R) belong to the rhodopsin-like family of G-protein coupled receptors and share considerable structural homology (Almeida et al., 2004; Takahashi et al., 1992). Substance P, neurokinin A, and NKB exhibit strong preferential binding for NK1R, NK2R, and NK3R, respectively, but each can act as an agonist at the other receptors when present in sufficiently high concentrations (Linden et al., 2000; Pennefather et al., 2004; Regoli et al., 1994). NK3R species-differences have complicated the pharmacological study of the NKB-NK3R system. Most NK3R antagonists have similar activity on gerbil, guinea pig, dog, and human NK3Rs but substantially lower potency at rat and mouse NK3R (Leffler et al., 2009).
As a neuropeptide, NKB signaling may be associated with slow synaptic or non-synaptic communication (Salio et al., 2006). Neuropeptides are packaged in large dense core vesicles that may exocytose outside of the synaptic cleft at structurally nonspecialized sites (Huang and Neher, 1996; Salio et al., 2006; Zoli et al., 1999). Neuropeptides typically lack rapid reuptake mechanisms and are instead degraded by nonspecific enzymes such as endopeptidases and aminopeptidases (Hooper and Turner, 1985; Khawaja and Rogers, 1996). After NKB is bound to its receptor, NK3R activation increases intracellular Ca2+ concentration through inositol phospholipid hydrolysis. Alternatively, NK3R activation can increase intracellular cAMP levels through adenylate cyclase activation (Satake and Kawada, 2006). Activated NK3Rs are internalized and this feature has been used as an indirect measure of receptor activity (Haley and Flynn, 2006). A feature of G-protein related receptors, however, is that downstream cascades of the receptor can be either excitatory or inhibitory depending on which intracellular proteins are expressed (Kenakin, 1995). Other G-protein coupled receptors, such as the serotonin receptor, can associate with more than one intracellular cascade (Berg et al., 1998). Thus, the cellular responses arising from NK3R signaling are likely complex.
3. Changes in NKB neurons in the infundibular nucleus of postmenopausal women and aging rhesus monkeys
The first indication of a role for NKB in the reproductive axis came from studies comparing hypothalamic gene expression in premenopausal and postmenopausal women (Rance and Young, 1991). Menopause is characterized by degeneration of ovarian follicles and gonadotropin hypersecretion due to loss of steroid negative feedback (reviewed in (Rance, 2009)). In the hypothalamus of postmenopausal women, there is hypertrophy of a subgroup of neurons in the infundibular nucleus, the homologue of the arcuate nucleus in other species (Rance et al., 1990; Sheehan and Kovács, 1966). Neuronal hypertrophy also occurs in the infundibular nucleus of older men, but is less pronounced than that observed in older women (Rance et al., 1993). These findings correspond with the mild testicular dysfunction in older men (Rance et al., 1993), compared to the virtually complete loss of ovarian follicles in older women (Hansen et al., 2008).
Stereological studies indicate that postmenopausal neuronal hypertrophy is not secondary to cellular degeneration in the infundibular nucleus (Abel and Rance, 2000). The hypertrophied neurons express estrogen receptor α (ERα), NKB, substance P and kisspeptin mRNA, with marked increases in tachykinin and kisspeptin gene transcripts in postmenopausal women (Rance et al., 1990; Rance and Young, 1991; Rometo et al., 2007). Thus, the hypertrophied neurons express two mRNAs that are essential for reproduction, NKB and kisspeptin (de Roux et al., 2003; Oakley et al., 2009; Seminara et al., 2003). Dynorphin mRNA is also expressed in the hypertrophied neurons, but unlike the elevation of NKB and kisspeptin gene expression in postmenopausal women, the expression of the mRNA encoding dynorphin is reduced (Rometo and Rance, 2008).
Ovariectomy of young cynomolgus monkeys results in neuronal hypertrophy and increased NKB and kisspeptin gene expression similar to that seen in postmenopausal women (Rometo et al., 2007; Sandoval-Guzmán et al., 2004). These changes are reversed by estrogen replacement (Abel et al., 1999; Rometo et al., 2007), suggesting that the hypertrophy and increased NKB and kisspeptin gene expression in the infundibular nucleus of postmenopausal women is due to loss of ovarian estrogen. Numerous studies support the hypothesis that the hypertrophied infundibular neurons in postmenopausal women regulate estrogen negative feedback at a hypothalamic level (Rance, 2009).
Similar to the human, menopause in monkeys is characterized by ovarian failure and gonadotropin hypersecretion (Gore et al., 2004; Hodgen et al., 1977; Woller et al., 2002). Quantitative PCR has shown increased kisspeptin and kisspeptin receptor mRNA in the medial basal hypothalamus of postmenopausal monkeys and these changes are duplicated by ovariectomy of young rhesus monkeys (Kim et al., 2009). NKB gene expression is also increased in the arcuate nucleus of perimenopausal rhesus monkeys with low estrogen levels, and in monkeys after long-term ovariectomy (Eghlidi et al., 2010). Thus, as in women, NKB and kisspeptin gene expression is increased in the infundibular/arcuate nucleus of aging female rhesus monkeys, and these changes are likely due to the loss of ovarian steroids.
4. Hypogonadotropic hypogonadism due to mutations in the TAC3/TACR3 genes
Studies of individuals with TAC3 and TACR3 gene mutations provide unique insights into the role of NKB and NK3R signaling in the human reproductive axis. To date, approximately 40 patients with TAC3 and TACR3 mutations have been reported, with a worldwide distribution and a diverse racial mix (Gianetti et al., 2010; Guran et al., 2009; Topaloglu et al., 2009; Young et al., 2010). Like other cases of hypogonadotropic hypogonadism, individuals with inactivating mutations of TAC3 and TACR3 display an absence of pubertal development with low circulating levels of serum LH and low gonadal steroids. A high prevalence of microphallus is detected, indicating NKB/NK3R signaling is essential for the normal activation of the reproductive axis late in gestation (Gianetti et al., 2010; Guran et al., 2009; Topaloglu et al., 2009; Young et al., 2010).
Pulsatile GnRH administration to adults with TAC3 and TACR3 gene mutations restored serum LH and testosterone secretion in males and resulted in ovulation, pregnancy and a normal birth in a female (Young et al., 2010). These data suggest that the gonadotropin deficiency is due to disordered NK3R signaling at the level of the hypothalamus and not the anterior pituitary gland or gonads. A distinctive finding in patients with TAC3 and TACR3 gene mutations is the relative preservation of FSH secretion, compared to low levels of serum LH (Topaloglu et al., 2009; Young et al., 2010). This pattern of serum gonadotropins can be experimentally produced by slowing the frequency of GnRH pulses in the rhesus monkey (Wildt et al., 1981) or by administering GnRH in a continuous, non-pulsatile manner in the rat (Wise et al., 1979). Therefore, these data provide evidence of a change in the pattern of GnRH pulses in patients with deficits in NKB/NK3R signaling.
TAC3/TACR3 mutations were found in 5.5% of a large series of probands with normosmic hypogonadotropic hypogonadism (Gianetti et al., 2010). Interestingly, in this study, reproductive function was exhibited after discontinuation of steroid replacement therapy, including two female patients with frameshift mutations in the TAC3 gene who conceived spontaneously. Given the promiscuity of tachykinins and their receptors, activation of a redundant system was hypothesized to compensate for the absence of NKB in these two women (Gianetti et al., 2010). Based on our own data (Rance and Young, 1991), substance P signaling is a logical candidate for that redundant system. In any case, the distinctive reproductive phenotype of individuals with TAC3 or TACR3 mutations indicates unique functions for NKB signaling in the reproductive axis (Gianetti et al., 2010; Young et al., 2010).
5. Anatomic studies of NKB and NK3R in the hypothalamus and basal forebrain
5.1. Distribution of NKB neurons
The location of neurons expressing NKB mRNA has been mapped in detail in the human hypothalamus and basal forebrain (Chawla et al., 1997). NKB mRNA-expressing neurons are located predominantly in the infundibular nucleus and the anterior hypothalamic area. Magnocellular neurons in the septal region, diagonal band of Broca and nucleus basalis of Meynert also express NKB mRNA. The bed nucleus of the stria terminalis and the amygdala are also prominent sites of NKB mRNA-containing cell bodies and scattered neurons are identified in the adjacent neocortex (Chawla et al., 1997).
In the rat, the distribution of NKB neurons has been characterized using immunocytochemistry and in situ hybridization (Krajewski et al., 2010; Lucas et al., 1992; Marksteiner et al., 1992; Merchenthaler et al., 1992; Warden and Young, 1988). Immunocytochemical studies were facilitated by the use of an antibody directed against peptide 2, a sequence within the preprotachykinin B precursor that is not homologous to other preprotachykinins (Lucas et al., 1992; Marksteiner et al., 1992). A major population of NKB neurons resides in the arcuate nucleus of the rat. Small numbers of NKB neurons are also scattered throughout the anterior, lateral and dorsomedial hypothalamus and preoptic regions. In addition, NKB neurons are located in the amygdala, bed nucleus of the stria terminalis, septal nuclei, nucleus accumbens and diagonal band of Broca (Krajewski et al., 2010; Lucas et al., 1992; Marksteiner et al., 1992; Merchenthaler et al., 1992; Warden and Young, 1988). There have also been reports of proNKB-immunreactivity in magnocelluar paraventricular and supraoptic neurons (Hatae et al., 2001), but in situ hybridization studies have not confirmed the presence of NKB mRNA in these nuclei (Lucas et al., 1992; Marksteiner et al., 1992; Warden and Young, 1988). NKB neurons have also been described in the arcuate nucleus of the monkey (Abel et al., 1999; Ramaswamy et al., 2010; Sandoval-Guzmán et al., 2004), sheep (Foradori et al., 2006; Goodman et al., 2007; Goubillon et al., 2000; Pillon et al., 2003), goat (Wakabayashi et al., 2010) and mouse (Duarte et al., 2006; Navarro et al., 2009).
Projections of NKB neurons from the arcuate nucleus of the rat have been identified using dual-label immunofluorescence, anterograde tract-tracing and monosodium glutamate lesions (Burke et al., 2006; Krajewski et al., 2010) (Figure 2). A dense network of NKB/dynorphin fibers is found within the arcuate nucleus extending to the median eminence. NKB axons also form a dense periventricular pathway that projects rostrally from the arcuate nucleus to the magnocellular and parvicellular paraventricular nucleus, the anteroventral periventricular nucleus (AVPV), nuclei of the medial preoptic region, septal nuclei and the bed nucleus of the stria terminalis. The periventricular pathway projects dorsally to the dorsomedial nucleus and caudally to the ventral premammillary nucleus (Krajewski et al., 2010). Projections of arcuate NKB neurons also extend through a ventral hypothalamic tract to the lateral hypothalamus within the medial forebrain bundle.
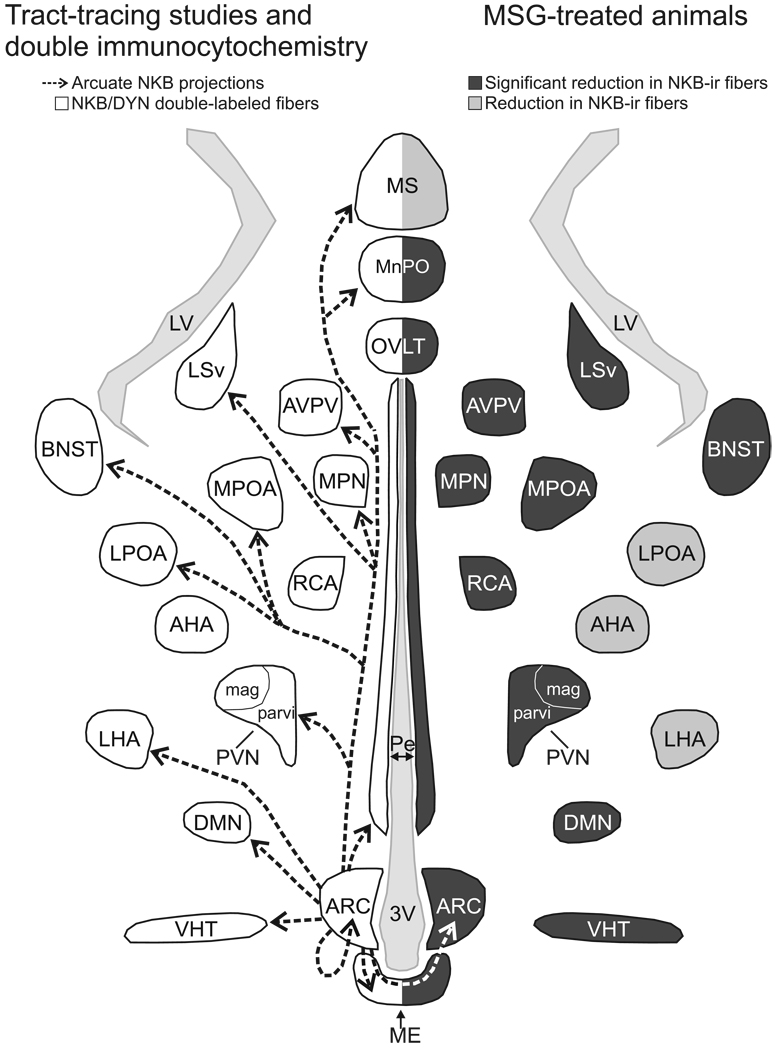
Figure 2.
Schematic diagram (horizontal plane) of projections from arcuate NKB/kisspeptin/dynorphin neurons in the rat revealed by anterograde tract-tracing and NKB/dynorphin immunofluorescence (left) and MSG-ablation of arcuate NKB neurons (right). Left: The arrows indicate the location of arcuate NKB projections labeled by a BDA injection into the arcuate nucleus (Krajewski et al., 2010). The outlined areas show where dual-labeled NKB/dynorphin fibers were identified (Burke et al., 2006). Right: The areas shaded in dark gray exhibited a significant reduction in NKB fibers in MSG-treated animals (Krajewski et al., 2010). The areas shaded in light gray exhibited a reduction of NKB-immunoreactivity in MSG-treated animals that was not significantly different from controls. The diverse projections of arcuate NKB neurons provide a mechanism to integrate the reproductive axis with multiple homeostatic and neuroendocrine circuits.
Abbreviations: 3V, third ventricle; AHA, anterior hypothalamic area; ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; DMN, dorsomedial nucleus; DYN, dynorphin; LHA, lateral hypothalamic area; LPOA, lateral preoptic area; LSv, ventral portion of the lateral septal nucleus; LV, lateral ventricle; ME, median eminence; MnPO, median preoptic nucleus; MSG, monosodium glutamate; MPN, medial preoptic nucleus; MPOA, medial preoptic area; MS, medial septal nucleus; OVLT,organum vasculosum of the lamina terminalis; Pe, periventricular hypothalamus; PVN mag, magnocellular division of the paraventricular nucleus; PVN parvi, parvicellular division of the paraventricular nucleus; RCA, retrochiasmatic area; VHT, ventral hypothalamic tract. Reproduced with permission from (Krajewski et al., 2010).
5.2. Distribution of neurons expressing NK3R
Many (but not all) of the regions receiving arcuate NKB projections in the rat also express NK3R (Ding et al., 1996; Krajewski et al., 2005; Shughrue et al., 1996). NK3R-immunoreactive neurons are identified in the arcuate nucleus (Burke et al., 2006; Krajewski et al., 2005). Moreover, NK3R neurons are found in the zona incerta and the lateral hypothalamic area and perifornical region. Magnocellular neurons of the paraventricular nucleus, supraoptic nucleus, and accessory magnocellular nucleus are intensely labeled with NK3R antibodies. There are also magnocellular NK3R neurons in the septal nuclei, diagonal band and nucleus basalis (Krajewski et al., 2005). Small numbers of neurons with low levels of NK3R-immunoreactivity are identified in the preoptic regions, anterior hypothalamic area, dorsomedial nucleus and mammillary nuclei (Ding et al., 1996; Krajewski et al., 2005).
The diverse projections of arcuate NKB neurons to multiple hypothalamic and limbic sites suggest that these sex-steroid responsive neurons participate in the regulation of numerous homeostatic, behavioral and neuroendocrine circuits (Krajewski et al., 2010). Thus, in addition to the essential function of NKB/NK3R signaling in the regulation of GnRH secretion, these neurons could relay sex-steroid information to optimize the internal milieu for reproduction. For example, we hypothesized that the hypertrophied NKB neurons in postmenopausal women could be involved with menopausal hot flushes (Rance and Young, 1991). This hypothesis is supported by the recent demonstration that focal microinfusion of picomolar amounts of the NK3R agonist, senktide, into the median preoptic nucleus/septal region produces a remarkable decrease in core temperature in the rat (Dacks et al., 2009). Projections of arcuate NKB neurons to the rostral hypothalamus and septal regions could constitute a pathway for NKB neurons to modulate the thermoregulatory axis (Krajewski et al., 2010).
6. An interconnected network of arcuate NKB/kisspeptin/dynorphin neurons
6.1. NKB neurons in the arcuate nucleus coexpress kisspeptin and dynorphin
A key feature of arcuate NKB neurons in multiple species is the coexpression of kisspeptin and dynorphin. First demonstrated in the ewe (Goodman et al., 2007), kisspeptin (or its mRNA) is coexpressed with NKB in the arcuate nucleus in the rat (Kirigiti et al., 2009), mouse (Navarro et al., 2009) and goat (Wakabayashi et al., 2010). There is also compelling evidence that NKB and kisspeptin mRNAs are coexpressed in the infundibular (arcuate) nucleus of the human (Rance and Young, 1991; Rometo et al., 2007) and monkey (Abel et al., 1999; Rometo et al., 2007; Sandoval-Guzmán et al., 2004). This conclusion is supported by the recent immunohistochemical demonstration of kisspeptin and NKB colocalization in the human (Hrabovszky et al., 2010) and monkey (Ramaswamy et al., 2010) infundibular nucleus.
Dynorphin is an endogenous opioid peptide involved with progesterone negative feedback on GnRH secretion in the luteal phase of the ewe (Goodman et al., 2004). Dynorphin is coexpressed with NKB in the arcuate nucleus of the rat (Burke et al., 2006; Ciofi et al., 2006), ewe (Foradori et al., 2006), mouse (Navarro et al., 2009) and goat (Wakabayashi et al., 2010). In postmenopausal women, dynorphin mRNA is decreased in the infundibular (arcuate) nucleus (Rometo and Rance, 2008), consistent with the decrease in dynorphin gene expression reported in ovariectomized ewes (Foradori et al., 2005). In the mouse, however, estradiol has been shown to suppress dynorphin gene expression in the arcuate nucleus (Gottsch et al., 2009; Navarro et al., 2009).
Coexistence of multiple peptides in a single neuron is a common finding in the central and peripheral nervous system (Salio et al., 2006). Typically, two or more peptides are stored together in large dense core vesicles and may be differentially released depending on their relative synthesis (Salio et al., 2006). Changing the balance among the relative amounts of peptides could modify the reproductive axis. For example, an increase in the gene transcription of excitatory peptides (NKB and kisspeptin) combined with a decrease in the transcription of an inhibitory peptide (dynorphin) could underlie the increased gonadotropin secretion in postmenopausal women (Rance and Young, 1991; Rance and Uswandi, 1996; Rance, 2009; Rometo et al., 2007; Rometo and Rance, 2008). Another characteristic of peptidergic neurons is the expression of a classical neurotransmitter in small clear synaptic vesicles, a different type of neurotransmitter packaging vesicle than that used to package neuropeptides (Salio et al., 2006). In the case of NKB/kisspeptin/dynorphin neurons, that neurotransmitter may be glutamate, based on the colocalization of vGLUT-2-immunoreactivity in the rat (Ciofi et al., 2006) and sheep (Lehman et al., 2010).
6.2. A bilateral network of NKB/kisspeptin/dynorphin neurons in the arcuate nucleus of the rat project to GnRH terminals in the median eminence
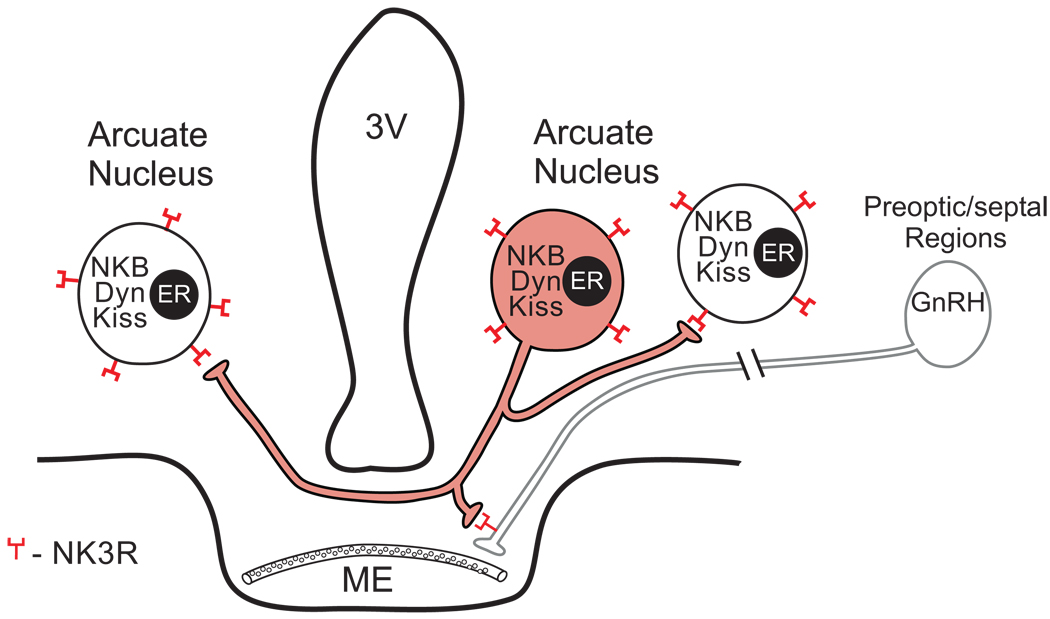
Through a series of studies, we have described a bilateral network of NKB neurons within the arcuate nucleus of the female rat that project to GnRH axons in the median eminence (Burke et al., 2006; Krajewski et al., 2005; Krajewski et al., 2010). The key features of this anatomic arrangement are illustrated in Figure 3. Arcuate NKB/kisspeptin/dynorphin neurons project extensively within the arcuate nucleus and extend across the median eminence to the contralateral arcuate nucleus (Krajewski et al., 2010). Within the arcuate nucleus there is a dense network of NKB/dynorphin axons and close apposition of these axons on NKB/dynorphin cell bodies and dendrites, indicative of communication between these neurons (Burke et al., 2006). Importantly, anterograde tract-tracing showed that NKB axons originating from the arcuate nucleus terminate on arcuate NKB neurons bilaterally (Krajewski et al., 2010).
Figure 3.
Schematic diagram of local projections of an arcuate NKB neuron in the rat (shaded). NKB neurons coexpress kisspeptin, dynorphin and ERα. Our tract-tracing studies showed that arcuate NKB neurons project to NKB neurons within the arcuate nucleus (bilaterally) and send projections to GnRH terminals within the median eminence. Branching of NKB axons within the arcuate nucleus and median eminence suggests that a single neuron could send collaterals to the median eminence, to the arcuate nucleus on both sides as well as other hypothalamic areas. Abbreviations: ERα, Estrogen Receptor α; DYN, Dynorphin; GnRH, gonadotropin releasing hormone; KISS, kisspeptin; ME, median eminence; NKB, neurokinin, 3V, third ventricle. This diagram is based on immunohistochemical and tract-tracing studies from several sources (Burke et al., 2006; Kirigiti et al., 2009; Krajewski et al., 2005; Krajewski et al., 2010).
A key feature of the arcuate nucleus network is the presence of NK3R on NKB/kisspeptin/dynorphin neurons (Burke et al., 2006). This feature has been shown in the rat (Burke et al., 2006), mouse (Navarro et al., 2009) and ewe (Amstalden et al., 2009). Approximately 20% of these neurons were reported to express kappa opioid receptor mRNA in the mouse (Navarro et al., 2009), but kisspeptin receptor mRNA has not been identified in arcuate NKB/kisspeptin/dynorphin neurons (Herbison et al., 2010). Bilateral connections among arcuate NKB/kisspeptin/dynorphin/ERα neurons via NK3R may provide an anatomic framework for coordinated activity of this important neuronal network.
From the arcuate nucleus, NKB fibers project to both the internal and external zones of the median eminence, including the lateral palisade zone, a site with dense GnRH terminals (Krajewski et al., 2005; Krajewski et al., 2010). Retrograde tract-tracing studies did not show uptake of injected fluorogold (a retrograde tracer) in arcuate NKB neurons after intraperitoneal injections (Krajewski et al., 2005). Therefore, it is unlikely that these neurons terminate on fenestrated capillaries of the hypophyseal portal system (Krajewski et al., 2005). However, within the median eminence, NKB fibers directly appose GnRH fibers at a location where punctate NK3R-immunoreactivity was identified on GnRH terminals (Krajewski et al., 2005). Tacr3 mRNA has been identified in GnRH neurons in the mouse, but the subcellular location of NK3R is not known in this species (Todman et al., 2005). Using electron microscopy, Ciofi et al. showed direct apposition between NKB fibers and GnRH axons in the median eminence without synaptic specializations (Ciofi et al., 2006). NKB would be expected to be released by large dense core vesicle exocytosis, a mode of neurotransmission that does not require the presence of classic synapses (Salio et al., 2006). There is also evidence of a similar network of arcuate NKB/kisspeptin/dynorphin neurons projecting to the median eminence of the monkey (Ramaswamy et al., 2008; Ramaswamy et al., 2010), mouse (Navarro et al., 2009), sheep (Foradori et al., 2006; Lehman et al., 2010) and goat (Wakabayashi et al., 2010).
7. Estrogen modulation of NKB gene expression
Classic studies revealed that neurons in the rat arcuate nucleus concentrate radioactive estradiol after intraperitoneal injections (Pfaff and Keiner, 1973). Subsequently, two isoforms of estrogen receptor were identified, ERα and ERβ. Studies using knockout mice indicate that ERα is critical for estrogen negative feedback on LH secretion (Dorling et al., 2003; Hewitt and Korach, 2002). Interestingly, GnRH neurons have only been shown to express ERβ, suggesting that estrogen negative feedback is exerted via a separate set of neurons (Hrabovszky et al., 2001). NKB and ERα coexpression has been documented in the arcuate nucleus of the rat (Burke et al., 2006), sheep (Goubillon et al., 2000) and human (Rance and Young, 1991), indicating that estrogens could act directly on arcuate NKB neurons. Progesterone receptors have also been demonstrated on these neurons in the ewe (Foradori et al., 2002). The transcription of NKB could be directly altered by estrogen receptors, as sequences corresponding to the estrogen response element (ERE) and the imperfect palindromic ERE have been reported upstream of the TAC3 gene transcription start site (Page et al., 2001). Studies are not yet available to verify if these are functional EREs.
Experiments in multiple species have documented sex-steroid modulation of the gene encoding NKB in the arcuate nucleus. NKB gene expression changes during the estrous cycle of the rat (Rance and Bruce, 1994). Ovariectomy increases NKB gene expression in arcuate nucleus of female monkeys (Eghlidi et al., 2010; Sandoval-Guzmán et al., 2004), rats (Rance and Bruce, 1994) and mice (Kauffman et al., 2009; Navarro et al., 2009), and orchidectomy increases NKB gene expression in the arcuate nucleus of male rats (Danzer et al., 1999) and mice (Kauffman et al., 2009). Furthermore, estrogen treatment of gonadectomized animals suppresses NKB gene expression in monkeys (Abel et al., 1999), mice (Dellovade and Merchenthaler, 2004; Navarro et al., 2009), sheep (Pillon et al., 2003) and rats of both genders (Danzer et al., 1999; Rance and Bruce, 1994). The suppression of NKB gene expression by estradiol does not occur in ERα knockout mice, implicating ERα as an essential element (Dellovade and Merchenthaler, 2004).
8. Sexual dimorphism of NKB neurons
Although gonadectomy and steroid replacement modify NKB gene expression in both sexes (Danzer et al., 1999; Rance and Bruce, 1994), gender differences exist in the number and morphology of NKB neurons. Increased numbers of NKB neurons have been identified in the arcuate nucleus of ewes compared to rams (Cheng et al., 2010; Goubillon et al., 2000). Prenatal testosterone treatment of ewes results in a male-type pattern, indicating an organizational effect of androgens early in development (Cheng et al., 2010; Goubillon et al., 2000). In the rat, there are dense projections of NKB axons around blood vessels in the median eminence in the male, compared to a more diffuse distribution in the female, and this morphology is altered by treatment with gonadal steroids even after the postnatal period (Ciofi et al., 2006; Ciofi et al., 2007). Finally, the response of NKB neurons to gonadectomy before puberty differs by gender. Prepubertal female mice exhibit increased NKB and kisspeptin gene expression in the arcuate nucleus in response to gonadectomy, while this response is delayed until after puberty in male mice (Kauffman et al., 2009).
9. Pharmacological studies of the effects of NK3R agonists on LH secretion
Pharmacological administration of NKB or NK3R agonists has resulted in disparate effects on LH secretion, depending on the animal model and gonadal status. Initial studies showed that intraventricular injections of senktide, a potent and selective NK3R agonist, significantly decreased serum LH in ovariectomized rats treated with very low levels of estradiol (Sandoval-Guzmán and Rance, 2004). Inhibitory effects on LH secretion have also been observed after intraventricular injection of sentktide in ovariectomized mice (Navarro et al., 2009) and direct injection of NKB into the arcuate nucleus of ovariectomized goats (Wakabayashi et al., 2010). On the other hand, senktide stimulated LH secretion in the ewe during the follicular phase but not during the luteal phase, showing differing effects depending on gonadal status (Billings et al., 2010). More recently, stimulation of LH secretion was observed in prepubertal rhesus monkeys after intravenous infusion of either NKB or senktide (Ramaswamy et al., 2010). NKB also modulates the effects of kisspeptin on LH secretion. For example, intraventricular co-administration of NKB and kisspeptin amplified kisspeptin’s stimulatory effects in male rodents while co-administration of NKB and kisspeptin to mouse hypothalamic explants inhibited kisspeptin’s positive effect on GnRH release (Corander et al., 2010). Thus, the effects of pharmacological administration of NKB or senktide are complex and contradictory. This complexity contrasts with kisspeptin, which consistently stimulates LH secretion in a wide variety of mammalian species and experimental settings (Dhillo et al., 2006; Han et al., 2005; Plant, 2006; Thompson et al., 2004).
While administration of NK3R agonists clearly alters LH secretion, the interpretation of these studies is complicated by many factors. First, senktide is a potent and selective NK3R agonist, but NKB binds to other tachykinin receptors (Laufer et al., 1986; Pennefather et al., 2004). Second, there may be species differences in the efficacy of pharmacological agents (Leffler et al., 2009) and in the location of NK3R (Amstalden et al., 2009; Krajewski et al., 2005). Third, the steroid environment could alter the basal activity of the neurons, relative levels of gene expression (see section 7) and the number or responsiveness of receptors (Kelly et al., 2003). Finally, endogenous neuropeptides are released in specific spatial and temporal patterns, while intraventricular infusion of a pharmacological agent could simultaneously interact with NK3R at numerous sites at nonphysiologic concentrations. For NK3R agonists in particular, there could be direct effects on GnRH neurons, as well as indirect effects via the arcuate nucleus that could modulate the pattern of GnRH secretion and thereby alter the responsiveness of the anterior pituitary gland (see section 10 below).
10. Putative role of arcuate NKB neurons in the “GnRH pulse generator”
10.1. GnRH neurons and the pulsatile secretion of LH
GnRH is released in a pulsatile manner and this pattern is necessary for pulsatile release of LH (Clarke and Cummins, 1982). In turn, pulsatile LH secretion is a prerequisite for normal ovarian function (Knobil, 1990). Electrophysiological recordings from the arcuate nucleus in monkeys (Knobil, 1981), rats (Kimura et al., 1991; Kinsey-Jones et al., 2008) and goats (Maeda et al., 1995; Wakabayashi et al., 2010) reveal dramatic, transient increases in neuronal firing rates (of approximately 2000 spikes/minute) that occur every 20–120 minutes depending upon species, steroid status or other factors. These “multiunit volleys” are timed with pulses in serum LH levels, which peak minutes after the end of each volley. Based on this strong temporal correlation, it has been hypothesized that the multiunit volleys come from a group of neurons termed the “GnRH pulse generator,” which signals GnRH neurons to fire (Knobil, 1981). It should be noted, however, that this concept is highly controversial, with a wide divergence of opinion regarding the relationship between the multiunit volleys and the control of GnRH release.
While the anatomical/cellular substrate of the multiunit activity is unknown, GnRH neurons themselves have been proposed to be the source. In the monkey, GnRH neurons are close to the site of electrical activity (Silverman et al., 1986) and in the rat, unmyelinated GnRH axons passing through the adjacent median eminence are also in close proximity to the multiunit activity (Kimura et al., 1991). Indeed, GnRH neurons are probably capable of generating oscillations autonomously (Herbison, 2006; Moenter et al., 2003). Cultures of GnRH-secreting GT1 cells, primary hypothalamic neuronal cultures, and nasal placode explants all exhibit spontaneous oscillations and pulsatile release of GnRH (Charles and Hales, 1995; Krsmanović et al., 1992; Martínez de la Escalera et al., 1992; Terasawa et al., 1999; Wetsel et al., 1992). Despite the fact that GnRH neurons are not confined to a single location or nucleus, they are extensively interconnected providing an anatomical framework for synchronization (Campbell et al., 2009). Because isolated multi-unit recordings of GnRH neurons in vivo are not possible due to their scattered location, the contribution of GnRH neurons to the multiunit volleys remains to be been established.
10.2. Evidence for NKB/kisspeptin/dynorphin neurons in the arcuate nucleus for the modulation of pulsatile GnRH secretion
Even if GnRH neurons generate pulsatile activity, this would not exclude the possibility of an external oscillator in the arcuate nucleus that relays information from sex-steroids to influence pulsatile GnRH secretion (a GnRH pulse modulator). Modulation of GnRH neurons via external sources might also need to be pulsatile, or at least coordinated, to ensure synchronized GnRH secretion. There are currently several lines of evidence that arcuate NKB/kisspeptin/dynorphin neurons could modulate pulsatile GnRH secretion, and be the source of the multiunit volleys of electrical activity that are timed with pulses of serum LH.
NKB/kisspeptin/dynorphin neurons are located in the arcuate nucleus where the multiunit volleys of activity are present in monkeys (Knobil, 1981), rats (Kimura et al., 1991; Kinsey-Jones et al., 2008) and goats (Maeda et al., 1995; Ohkura et al., 2009; Wakabayashi et al., 2010).
Destruction of the arcuate nucleus in the rhesus monkey (with relative preservation of more lateral GnRH neurons) abolishes spontaneous and estrogen-induced LH secretion (Plant et al., 1978; Plant and Ramaswamy, 2009).
NKB/kisspeptin/dynorphin neurons in the arcuate nucleus of the rat form a bilateral interconnected network that could provide an anatomical framework for the coordination and synchronization of activity (Burke et al., 2006; Krajewski et al., 2010).
NKB/kisspeptin/dynorphin neurons in the arcuate nucleus project to GnRH terminals in the median eminence in the rat (Krajewski et al., 2005; Krajewski et al., 2010) monkey (Ramaswamy et al., 2008), and sheep (Lehman et al., 2010), an ideal location for final modulation of GnRH output (Moenter et al., 2003)
The expression of NKB and kisspeptin mRNAs in arcuate neurons (discussed in section 7) and the frequency of multiunit activity and LH pulses (Mori et al., 1991; Nishihara et al., 1999; Wakabayashi et al., 2010) are modulated in a similar direction by ovariectomy and steroid replacement.
There is strong evidence that progesterone inhibition of LH pulse frequency in the luteal phase of the ewe is mediated by NKB/kisspeptin/dynorphin neurons in the arcuate nucleus (Goodman et al., 2004; Lehman et al., 2010).
In monkeys, the duration of each multiunit volley increases over a 4–6 week period after ovariectomy (O'Byrne et al., 1993) consistent with a time-course of cellular remodeling in the form of neuronal hypertrophy (Rance et al., 1990; Rance and Young, 1991). On the other hand, estradiol treatment of ovariectomized monkeys reduces the duration of multiunit activity within hours (O'Byrne et al., 1993), consistent with the time course that estradiol supresses arcuate NKB mRNA in the ewe (Pillon et al., 2003).
Kisspeptin is released in a pulsatile manner into the stalk-median eminence of female rhesus monkeys and these pulses occur with the majority of GnRH pulses (Keen et al., 2008). Based on morphological descriptions, the kisspeptin released in the stalk-median eminence of monkeys is likely secreted from NKB/kisspeptin-coexpressing neurons in the arcuate nucleus (Ramaswamy et al., 2008; Ramaswamy et al., 2010; Rometo et al., 2007; Sandoval-Guzmán et al., 2004) and could have direct effects on GnRH axons at the level of the median eminence (d'Anglemont de Tassigny et al., 2008).
The frequency of multiunit volleys and pulsatile LH secretion are altered by infusions of NKB or dynorphin into the arcuate nucleus of the goat (Wakabayashi et al., 2010), consistent with the identification of NK3R (Amstalden et al., 2009; Burke et al., 2006; Navarro et al., 2009) and kappa opioid receptor mRNA (Navarro et al., 2009) on arcuate NKB/kisspeptin/dynorphin neurons.
Patients with hygonadotropic hypogonadism due to TAC3 and TACR3 mutations exhibit low levels of serum LH but normal, or nearly normal, levels of FSH (Topaloglu et al., 2009; Young et al., 2010). This profile of gonadotropin secretion can be simulated by changing the pattern of pulsatile GnRH secretion (Wildt et al., 1981; Wise et al., 1979; Young et al., 2010).
In contrast to the normal frequency and low amplitude LH pulses detected in patients with kisspeptin receptor gene mutations (Tenenbaum-Rakover et al., 2007), no LH pulses were reported in untreated patients with TAC3 and TACR3 mutations (Gianetti et al., 2010; Young et al., 2010). After gonadal steroid treatment of patients with TAC3 and TACR3 mutations, despite some recovery of function, LH pulse frequency is slow or irregular (Gianetti et al., 2010). We hypothesize that loss of functional NK3R signaling on arcuate NKB/kisspeptin/dynorphin neurons leads to alterations in the frequency of LH pulses due to dysfunctional coordination of the arcuate nucleus network.
In summary, there is strong evidence for proposing that arcuate (infundibular) NKB/kisspeptin/dynorphin neurons are part of the neural network influencing the pulsatile secretion of GnRH and contribute to the multiunit activity known as the GnRH pulse generator. This hypothesis does not exclude GnRH neurons in generating pulses or a role of other neurons within the arcuate nucleus or elsewhere. It must be emphasized that there is currently no definitive demonstration of the source of multiunit activity and thus, these concepts are speculative. It is exciting, however, to have substantial clues on a cellular identity of the multiunit activity, and these data are currently being used to develop models of how GnRH pulses are generated (Lehman et al., 2010; Navarro et al., 2009; Okamura et al., 2010; Wakabayashi et al., 2010). Of note, if this theory is correct, then NK3R signaling on arcuate NKB/kisspeptin/dynorphin neurons may represent an integral part of network coordination (Amstalden et al., 2009; Burke et al., 2006; Navarro et al., 2009; Wakabayashi et al., 2010). Because pulsatile GnRH secretion is essential for normal reproductive function, dysfunctional coordination of the NKB/kisspeptin/dynorphin network may explain the etiology of hypogonadotropic hypogonadism in individuals with TAC3/TACR3 mutations.
Conclusions
Although the structure of the GnRH decapeptide was elucidated in 1971, debate continues on the relative importance of neuronal circuits controlling GnRH neuronal secretion. This is due to the complexity of a system regulated by multiple inputs (sex-steroids, seasons, stress, circadian rhythms, and energy balance) with cell bodies that are widely scattered in the basal forebrain. A new focus has arisen from studying the phenotype of individuals with TAC3 or TACR3 gene mutations which clearly show the essential nature of NKB/NK3R signaling in the human reproductive axis. Based on a wide variety of studies, it is likely that this critical function is carried out by an interconnected network of sex-steroid sensitive neurons in the infundibular/arcuate nucleus that coexpress NKB/kisspeptin/dynorphin and project to GnRH axons in the median eminence. Connections among the NKB/kisspeptin/dynorphin neurons within the arcuate nucleus provide an anatomic framework to explain how these neurons could be coordinated bilaterally to relay feedback information from the ovaries to modulate pulsatile GnRH secretion. NK3R may function within the arcuate nucleus network to coordinate activity and may also be part of the input signal to GnRH axons in the median eminence. On the whole, these studies provide a compelling explanation for the occurrence of hypothalamic hypogonadotropic hypogonadism in patients with inactivating mutations in the TAC3 or TACR3 genes.
Future studies are necessary to determine if the lack of puberty in individuals with TAC3 or TACR3 gene mutations is due to dysfunction in hypothalamic control mechanisms or loss of a specific signal that drives GnRH neurons to initiate sexual maturation. Virtually no information is available on the participation of NKB neurons in the onset of puberty in experimental animals. Although Tacr3 knockout mice have been generated (Kung et al., 2004; Nordquist et al., 2008; Siuciak et al., 2007), detailed studies of the reproductive axis of these mice have not been published. It will also be very important to determine the role of NKB signaling in the early activation of the reproductive axis in the neonatal period. We eagerly anticipate the results of future studies on this system.
Acknowledgements
The authors are grateful for the expert advice and continuing support of Dr. Nathaniel T. McMullen, who also provided comments on this manuscript. We would also like to acknowledge the Graduate Interdisciplinary Programs in Neuroscience and Physiology at the University of Arizona.
Grant Support: This work was supported by National Institutes of Health (NIH) National Institute on Aging Grants R01 AG09214 and AG032315. M.A.S. and P.A.D. were supported by the NIH Predoctoral Training Program in Neuroscience (5T32-AG007434). P.A.D. was also supported by NIH National Institute on Aging Pre-doctoral Training Fellowship (1F31-AG030881) and the ARCS Foundation. M.C. was supported by a scholarship from the Science Foundation Arizona.
References
- Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J. Clin. Endocrinol. Metab. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J. Comp. Neurol. 2000;424:679–688. doi: 10.1002/1096-9861(20000904)424:4<679::aid-cne9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr. Med. Chem. 2004;11:2045–2081. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalization in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2009;22:1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner TI, Affolter H-U, Young AC, Young WS., III A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Mol. Brain Res. 1987;2:243–249. doi: 10.1016/0169-328x(87)90031-3. [DOI] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J. Comp. Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Gaidamaka G, Han S-K, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Hales TG. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1–7) neurons. J. Neurophysiol. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Gutierrez GM, Young WS, III, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J. Comp. Neurol. 1997;384:429–442. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Ciofi P, Lapirot OC, Tramu G. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience. 2007;146:630–642. doi: 10.1016/j.neuroscience.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteininzing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GSH, O’Rahilly S, Dhillo WS, Semple RK, Coll AP. The effects of neurokinin B upon gonadotrophin release in male rodents. J. Neuroendocrinol. 2010;22:181–187. doi: 10.1111/j.1365-2826.2009.01951.x. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MBL, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Brown J, Rance NE. NK3 receptor activation in the median preoptic nucleus reduces core temperature in the rat. Soc. Neurosci. Abstr. 2009 Program No. 274.3. [Google Scholar]
- Danzer SC, Price RO, McMullen NT, Rance NE. Sex steroid modulation of neurokinin B gene expression in the arcuate nucleus of adult male rats. Mol. Brain Res. 1999;66:200–204. doi: 10.1016/s0169-328x(99)00024-8. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145:736–742. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Ding Y-Q, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat J. Comp. Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- Duarte CR, Schütz B, Zimmer A. Incongruent pattern of neurokinin B expression in rat and mouse brains. Cell Tissue Res. 2006;323:43–51. doi: 10.1007/s00441-005-0027-x. [DOI] [PubMed] [Google Scholar]
- Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J. Neuroendocrinol. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LFG, Costa EMF, Bilharinho de Mendonca B, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J. Clin. Endocrinol. Metab. 2010;95:2857–2867. doi: 10.1210/jc.2009-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J. Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubillon M-L, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- Grant AD, Akhtar R, Gerard NP, Brain SD. Neurokinin B induces oedema formation in mouse lung via tachykinin receptor-independent mechanisms. J. Physiol. 2002;543:1007–1014. doi: 10.1113/jphysiol.2002.018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J. Clin. Endocrinol. Metab. 2009;94:3633–3639. doi: 10.1210/jc.2009-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1242–R1250. doi: 10.1152/ajpregu.00773.2005. [DOI] [PubMed] [Google Scholar]
- Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Hatae T, Kawano H, Karpitskiy V, Krause JE, Masuko S. Arginine-vasopressin neurons in the rat hypothalamus produce neurokinin B and co-express the tachykinin NK-3 receptor and angiotensin II type 1 receptor. Arch. Histol. Cytol. 2001;64:37–44. doi: 10.1679/aohc.64.37. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Krause JE, Mantyh PW, Couture R, Bannon MJ. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990;4:1606–1615. [PubMed] [Google Scholar]
- Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, Plant TM, Pfaff DW, Challis JG, de Kretser DM, Richards JS, Wassarman PM, editors. In Knobil and Neill's Physiology of Reproduction. Vol. II. Elsevier; 2006. pp. 1415–1482. ed.^eds. [Google Scholar]
- Herbison AE, d'Anglemont de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev. Endocr. Metab. Disord. 2002;3:193–200. doi: 10.1023/a:1020068224909. [DOI] [PubMed] [Google Scholar]
- Hodgen GD, Goodman AL, O'Connor A, Johnson DK. Menopause in rhesus monkeys: model for study of disorders in the human climacteric. Am. J. Obstet. Gynecol. 1977;127:581–584. doi: 10.1016/0002-9378(77)90352-0. [DOI] [PubMed] [Google Scholar]
- Hooper NM, Turner AJ. Neurokinin B is hydrolysed by synaptic membranes and by endopeptidase-24.11 ('enkephalinase') but not by angiotensin converting enzyme. FEBS Lett. 1985;190:133–136. doi: 10.1016/0014-5793(85)80443-9. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur. J. Neurosci. 2010:31. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Huang LYM, Neher E. Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am. J. Physiol. Endocrinol. Metab. 2009;297:E1212–E1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann. N. Y. Acad. Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II: agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int. J. Biochem. Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Nishihara M, Hiruma H, Funabashi T. Naloxone increases the frequency of the electrical activity of luteinizing hormone-releasing hormone pulse generator in long-term ovariectomized rats. Neuroendocrinology. 1991;53:97–102. doi: 10.1159/000125704. [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149:1004–1008. doi: 10.1210/en.2007-1505. [DOI] [PubMed] [Google Scholar]
- Kirigiti MA, True C, Ciofi P, Grove KL, Smith MS. Kisspeptin and NKB fiber distribution in the adult female rat: relationship to GnRH cell bodies and fibers/terminals in the median eminence. Endocrine Soc. Abstr. 2009:P3–P223. [Google Scholar]
- Knobil E. Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol. Reprod. 1981;24:44–49. doi: 10.1095/biolreprod24.1.44. [DOI] [PubMed] [Google Scholar]
- Knobil E. The GnRH pulse generator. Am. J. Obstet. Gynecol. 1990;163:1721–1727. doi: 10.1016/0002-9378(90)91435-f. [DOI] [PubMed] [Google Scholar]
- Kotani H, Hoshimaru M, Nawa H, Nakanishi S. Structure and gene organization of bovine neuromedin K precursor. Proc.Natl.Acad.Sci.USA. 1986;83:7074–7078. doi: 10.1073/pnas.83.18.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence J. Comp. Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanović LZ, Stojilković SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol. Res. 2004;50:611–615. doi: 10.1016/j.phrs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Laufer R, Gilon C, Chorev M, Selinger Z. Characterization of a neurokinin B receptor site in rat brain using a highly selective radioligand. J. Biol. Chem. 1986;261:10257–10263. [PubMed] [Google Scholar]
- Leffler A, Ahlstedt I, Engberg S, Svensson A, Billger M, Öberg L, Bjursell MK, Lindström E, von Mentzer B. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochem. Pharmacol. 2009;77:1522–1530. doi: 10.1016/j.bcp.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arucate nucleus: a central node in the control of GnRH secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Reutter MA, McCarson KE, Seybold VS. Time-dependent changes in neurokinin3 receptors and tachykinins during adjuvant-induced peripheral inflammation in the rat. Neuroscience. 2000;98:801–811. doi: 10.1016/s0306-4522(00)00160-3. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience. 1992;51:317–345. doi: 10.1016/0306-4522(92)90318-v. [DOI] [PubMed] [Google Scholar]
- Maeda K-I, Tsukamura H, Ohkura S, Kawakami S, Nagabukuro H, Yokoyama A. The LHRH pulse generator: a mediobasal hypothalamic location. Neurosci. Biobehav. Rev. 1995;19:427–437. doi: 10.1016/0149-7634(94)00069-d. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. J. Comp. Neurol. 1992;317:341–356. doi: 10.1002/cne.903170403. [DOI] [PubMed] [Google Scholar]
- Martínez de la Escalera G, Choi ALH, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Maderdrut JL, O'Harte F, Conlon JM. Localization of neurokinin B in the central nervous system of the rat. Peptides. 1992;13:815–829. doi: 10.1016/0196-9781(92)90192-6. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front. Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology. 1991;53:392–395. doi: 10.1159/000125746. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara M, Takeuchi Y, Tanaka T, Mori Y. Electrophysiological correlates of pulsatile and surge gonadotrophin secretion. Rev. Reprod. 1999;4:110–116. doi: 10.1530/ror.0.0040110. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Savignac H, Pauly-Evers M, Walker G, Knoflach F, Borroni E, Glaentzlin P, Bohrmann B, Messer J, Ozmen L, Albientz A, Spooren W. Characterization of behavioral response to amphetamine, tyrosine hydroxylase levels, and dopamine receptor levels in neurokinin 3 receptor knockout mice. Behav. Pharmacol. 2008;19:518–529. doi: 10.1097/FBP.0b013e32830cd7f5. [DOI] [PubMed] [Google Scholar]
- O'Byrne KT, Chen M-D, Nishihara M, Williams CL, Thalabard J-C, Hotchkiss J, Knobil E. Ovarian control of gonadotropin hormone-releasing hormone pulse generator activity in the rhesus monkey: duration of the associated hypothalamic signal. Neuroendocrinology. 1993;57:588–592. doi: 10.1159/000126411. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr. Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J. Neuroendocrinol. 2009;21:813–821. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- Okamura H, Murata K, Sakamoto K, Wakabayashi Y, Ohkura S, Takeuchi Y, Mori Y. Male effect pheromone tickles the gonadotrophin-releasing hormone pulse generator. J. Neuroendocrinol. 2010;22:825–832. doi: 10.1111/j.1365-2826.2010.02037.x. [DOI] [PubMed] [Google Scholar]
- Page NM, Woods RJ, Gardiner SM, Lomthaisong K, Gladwell RT, Butlin DJ, Manyonda IT, Lowry PJ. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature. 2000;405:797–800. doi: 10.1038/35015579. [DOI] [PubMed] [Google Scholar]
- Page NM, Woods RJ, Lowry PJ. A regulatory role for neurokinin B in placental physiology and pre-eclampsia. Regul. Pept. 2001;98:97–104. doi: 10.1016/s0167-0115(00)00239-1. [DOI] [PubMed] [Google Scholar]
- Page NM, Morrish DW, Weston-Bell NJ. Differential mRNA splicing and precursor processing of neurokinin B in neuroendocrine tissues. Peptides. 2009;30:1508–1513. doi: 10.1016/j.peptides.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Page NM. Neurokinin B and pre-eclampsia: a decade of discovery. Reprod Biol Endocrinol. 2010;8:4. doi: 10.1186/1477-7827-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J. Neuroendocrinol. 2003;15:749–753. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur. J. Endocrinol. 2006;155:S11–S16. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta) Peptides. 2009;30:67–75. doi: 10.1016/j.peptides.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010 doi: 10.1210/en.2010-0223. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., III Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J. Clin. Endocrinol. Metab. 1990;71:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- Rance NE, Young WS., III Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- Rance NE, Uswandi SV, McMullen NT. Neuronal hypertrophy in the hypothalamus of older men. Neurobiol.Aging. 1993;14:337–342. doi: 10.1016/0197-4580(93)90119-v. [DOI] [PubMed] [Google Scholar]
- Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J. Clin. Endocrinol. Metab. 1996;81:3540–3546. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D, Boudon A, Fauchére J-L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J. Neuroendocrinol. 2008;20:1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J. Neuroendocrinol. 2004;16:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr. Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N.Engl.J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sheehan HL, Kovács K. The subventricular nucleus of the human hypothalamus. Brain. 1966;89:589–614. doi: 10.1093/brain/89.3.589. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J. Comp. Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Wilson R, Kesner JS, Knobil E. Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology. 1986;44:168–171. doi: 10.1159/000124641. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Martin AN, Chapin DS, Stock J, Nadeau DM, Kantesaria S, Bryce-Pritt D, McLean S. Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology (Berl) 2007;194:185–195. doi: 10.1007/s00213-007-0828-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanaka A, Hara M, Nakanishi S. The primary structure and gene organization of human substance P and neuromedin K receptors. Eur. J. Biochem. 1992;204:1025–1033. doi: 10.1111/j.1432-1033.1992.tb16724.x. [DOI] [PubMed] [Google Scholar]
- Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J. Clin. Endocrinol. Metab. 2007;92:1137–1144. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140:1432–1441. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Todman MG, Han S-K, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat. Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MK, Young WS., III Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. J. Comp. Neurol. 1988;272:90–113. doi: 10.1002/cne.902720107. [DOI] [PubMed] [Google Scholar]
- Wetsel WC, Valenca MM, Merchenthaler I, Liposits Z, López FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4149–4153. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- Wise PM, Rance N, Barr GD, Barraclough CA. Further evidence that luteinizing hormone-releasing hormone also is follicle-stimulating hormone-releasing hormone. Endocrinology. 1979;104:940–947. doi: 10.1210/endo-104-4-940. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J. Clin. Endocrinol. Metab. 2002;87:5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- Young J, Bouligand J, Francou B, Raffin-Sanson M-L, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J. Clin. Endocrinol. Metab. 2010;95:2287–2295. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Syková E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]