Abstract
Objective
To test the hypothesis that perioperative statin use reduces acute kidney injury (AKI) following cardiac surgery
Design
Retrospective analysis of prospectively collected data from an ongoing clinical trial
Setting
Quaternary-care university hospital
Participants
Three hundred twenty-four elective adult cardiac surgery patients
Interventions
None
Measurements and Main Results
We assessed the association of preoperative statin use, early postoperative statin use, and acute statin withdrawal with the incidence of AKI. Early postoperative statin use was defined as statin treatment within the first postoperative day. Statin withdrawal was defined as discontinuation of preoperative statin treatment prior to surgery until at least postoperative day 2. Logistic regression and propensity score modeling were used to control for AKI risk factors. Sixty-eight of 324 patients (21.0%) developed AKI. AKI patients stayed in the hospital longer (P=0.03) and were more likely to develop pneumonia (P=0.002) or die (P=0.001). Higher body mass index (P=0.003), higher central venous pressure (P=0.03), and statin withdrawal (27.4 vs. 14.7%, P=0.046) were associated with a higher incidence of AKI, while early postoperative statin use was protective (12.5 vs. 23.8%, P=0.03). Preoperative statin use did not affect risk of AKI. In multivariate logistic regression, age (P=0.03), male gender (P=0.02), body mass index (P<0.001), and early postoperative statin use (OR 0.32, 95% CI 0.14–0.72, P=0.006) independently predicted AKI. Propensity score-adjusted risk assessment confirmed the association between early postoperative statin use and reduced AKI (OR 0.30, 95% CI 0.13–0.70, P=0.005).
Conclusions
Early postoperative statin use is associated with a lower incidence of AKI among both chronic statin users and statin-naïve cardiac surgery patients.
Keywords: acute kidney injury, acute renal failure, cardiac surgery, statin, oxidative stress, obesity
Introduction
Half a million people undergo cardiac surgery each year in the United States (1). Renal dysfunction complicates 8–24% percent of these surgeries and leads to dialysis in 1–5% (2–5). Acute Kidney Injury (AKI) associated with cardiac surgery increases cardiac and infectious morbidity, increases utilization of healthcare resources, extends intensive care unit length of stay, and independently predicts death (4–6). Although advancements have led to less invasive surgical techniques, off-pump coronary artery bypass, better myocardial preservation, and reduced mortality, the incidence of renal dysfunction persists (7).
Renal ischemia-reperfusion injury (8), impaired vasodilatation (9), neurohormonal activation (10), oxidative stress (11), inflammation (12), and atheroembolism (13) promote AKI following cardiac surgery and cardiopulmonary bypass. In contrast, statins limit oxidant production (14), improve endothelial function (15), and reduce inflammation (16). Long-term statin use preserves renal function in patients with coronary artery disease (17), and short-term statin use reduces myocardial damage in patients undergoing cardiac catheterization (18, 19). Conversely, statin withdrawal is associated with myocardial injury in vascular surgery patients (20) and limits nitric oxide bioavailability and increases reactive oxygen species (ROS) generation in rodents (21). In experimental models of ischemia-reperfusion, short-term statin treatment reduces renal dysfunction and markers of inflammation (22). The aim of this study was to test the hypothesis that perioperative statin therapy affects the risk of AKI following cardiac surgery.
Methods
Data were obtained from participants in the ongoing Renin-Angiotensin, Inflammation, and Post-op Atrial Fibrillation (RAP) study (NCT00141778). The RAP study has been approved by the Vanderbilt University Institutional Review Board for Research on Human Subjects and is conducted according to the Declaration of Helsinki. All patients provided written informed consent. This analysis of RAP study data was approved by the data and safety monitoring board of the RAP study. The RAP study tests the hypothesis that interruption of the renin-angiotensin-aldosterone system by either angiotensin-converting enzyme (ACE) inhibition (ramipril) or aldosterone receptor antagonism (spironolactone) decreases the incidence of atrial fibrillation after elective cardiac surgery. Exclusion criteria for the RAP study include chronic atrial fibrillation, serum creatinine > 1.6mg/dl, a left ventricle ejection fraction < 30%, evidence of coagulopathy, emergency surgery, and serum potassium > 5.0 mEq/L. One week to 4 days prior to surgery, patients are randomized to treatment with placebo, spironolactone (25 mg/d), or ramipril (1.25 mg the first two days, followed by 2.5 mg/d). Randomization is stratified by preoperative statin use, as well as by age and prior ACE inhibitor, angiotensin receptor blocker, or mineralocorticoid receptor antagonist use. The primary end point of the RAP study is the occurrence of atrial fibrillation from surgery until hospital discharge. Secondary end points include intraoperative mean arterial pressure, intraoperative and postoperative vasopressor requirements, death, length of hospital stay, and serum potassium and creatinine concentrations.
A research nurse determines preoperative medication use by inspection of the subject’s medical record and direct questioning of the subject and postoperative medication use by inspection of the inpatient medication administration record. Statins are defined as hydroxymethylglutaryl-coenzyme A reductase inhibitors. Early postoperative statin use is defined as statin treatment within the first postoperative day.
Study Population
Data were analyzed for all patients who had enrolled in the RAP study from April 18, 2005 through November 18, 2008. Four hundred fifty-six prescreened subjects gave consent and were screened for the RAP study. One hundred thirty-two subjects were excluded or withdrew (Figure 1). Three hundred twenty-four subjects had completed the RAP study at the time of this analysis and comprised this dataset.
Figure 1.
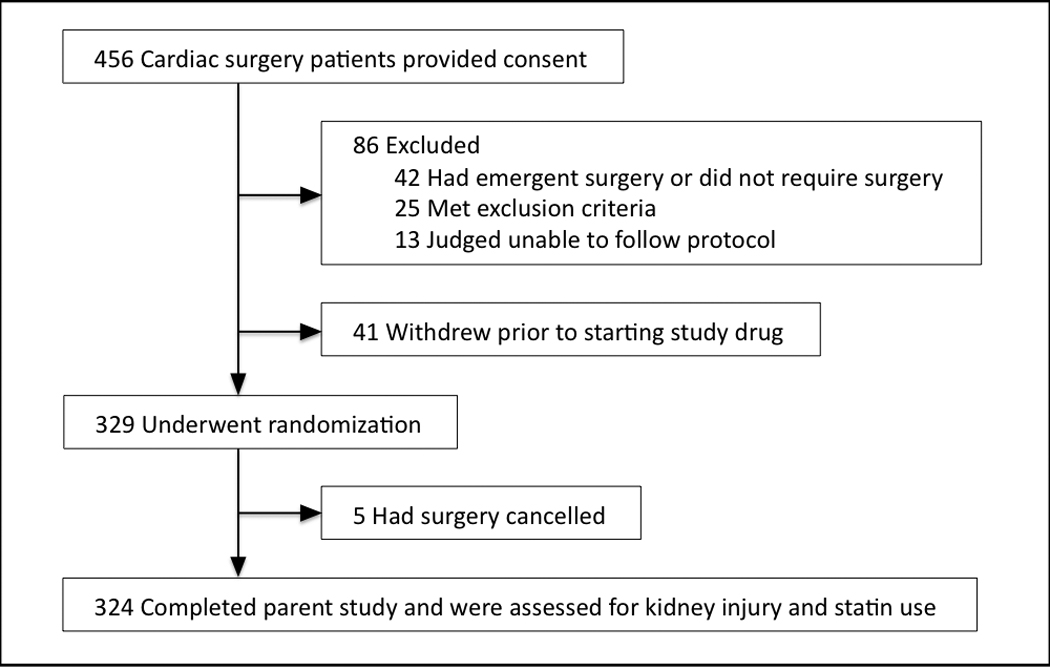
Subject enrollment and study completion.
Between those RAP study subjects who consented and were not studied and the 324 subjects studied, there were no differences in subject characteristics, medical history, medication use, or proposed surgery, except those not studied were more likely to be taking spironolactone (p<0.001) and had a lower left ventricle ejection fraction (p=0.02), reflecting the exclusion criteria of the study.
Patient Treatment
Anesthetic management, cardiopulmonary bypass (CPB), surgical management, and transfusion and resuscitation management are conducted according to institutional protocols as previously described (23). Patients received general endotracheal anesthesia, consisting of induction with a combination of propofol, midazolam, fentanyl, or etomidate and maintenance with isoflurane, pancuronium, and fentanyl. Monitoring included standard modalities (EKG, temperature, invasive blood pressure, pulse oximetry, and gas monitoring), central venous pressure or pulmonary artery catheter monitoring, and transesophageal echocardiography (TEE).
Anticoagulation for CPB consisted of 400 U/kg unfractionated porcine heparin and maintenance of activated coagulation time > 400 seconds. Temperature management involves cooling to 28° C to 30° C, temperature-uncorrected blood gas management (α stat), and cold antegrade and retrograde cardioplegia techniques. At the conclusion of CPB, anticoagulation was reversed with 250 mg protamine, with an additional 50 mg administered in the next 10 minutes in the presence of ongoing microvascular bleeding and an activated coagulation time 10% greater than baseline.
Vasopressors and inotropes were used for separation from CPB for the following criteria: left ventricular ejection fraction <40%, CPB time >120 minutes, a cardiac index <2 L / min / m2, or evidence of new-onset ventricular dysfunction by TEE. Patients were transfused with packed red blood cells to a hemoglobin concentration of 8 or 10 g/dL in the setting of persistent hemorrhage. Platelets were administered if hemorrhage persisted following protamine reversal of heparin, poor clot quality existed, and the absolute platelet count was <80,000, or if CPB time exceeded 120 minutes. Likewise, fresh frozen plasma was administered if hemorrhage persisted following protamine reversal of heparin, poor clot quality exists, and the INR exceeded 1.5. Six % hetastarch and isotonic saline were administered if the patient was hypotensive, cardiac filling pressures were low, and the left ventricle reflected hypovolemia by TEE.
Aprotinin was administered to 18 repeat sternotomy or multiple valve surgery subjects, and ε- aminocaproic acid was given to the remaining 265 subjects undergoing CPB. No subjects received tranexamic acid. Twenty coronary artery bypass-grafting (CABG) patients received intraoperative cardiac catheterization and iodinated radiocontrast to assess grafts or place stents in non-left anterior descending coronary artery lesions.
Patients were transported to the intensive care unit (ICU) intubated, mechanically ventilated, and sedated on propofol infusion. Postoperative care was at the discretion of the intensivist with consultation from the patient’s surgeon. If patients were normothermic and hemodynamically stable, and chest tube drainage was <100 ml/h, propofol was discontinued, and patients were assessed for extubation. Postoperative medication use and fluid managment, including the administration of statins, was at the discretion of the intensive care physicians. Some intensivists administered a statin in the immediate postoperative period, at times via an intubated patient’s oral gastric tube, and some intensivists did not initiate postoperative statin treatment until subjects resumed their outpatient medications several days postoperatively. Isotonic saline was administered if a patient was hypotensive (mean arterial pressure < 60 mmHg), cardiac filling pressures were low (central venous pressure < 10 mmHg and pulmonary artery occlusion pressure < 18 mmHg), and cardiac index was less than 2.0 l/min/m2. Serum creatinine and blood urea nitrogen concentrations were determined daily until hospital discharge.
AKI was defined according to Acute Kidney Injury Network (AKIN) criteria (24). Specifically, AKI was defined as an increase in a patient’s serum creatinine concentration of 50% or 0.3 mg/dl within 48 hours of surgery. The AKIN urine output criteria for AKI diagnosis were not used due to confounding by intravascular hypovolemia and diuretic use, both of which are common among cardiac surgery patients. Baseline creatinine was determined during the preoperative anesthesiology assessment clinic visit for outpatients and on the morning of surgery for inpatients. Baseline glomerular filtration rate was estimated using the modification of diet in renal disease formula (25). Postoperative atrial fibrillation was defined as any atrial fibrillation from postoperative ICU admission until hospital discharge. A research nurse collected each subject’s continuous telemetry tracing and daily electrocardiogram, and a blinded cardiologist evaluated all tracings. Myocardial infarction was defined as postoperative ST segment elevation with increased serum troponin concentrations, temporally separate from the increased troponin concentrations associated with cardiac surgery. Pneumonia was defined as pulmonary infiltrate with systemic signs of infection (temperature > 39° C or leukocytosis > 10,700/ul).
Statistical Analysis
Univariate analysis was performed to characterize subjects who did and did not develop AKI, as well as subjects who did and did not use statins preoperatively and early postoperatively. The Mann-Whitney U test was used for continuous variables and the chi-square test or likelihood ratio for between-group comparisons of categorical variables. Data are presented as mean ±SD or number and percent. Risk for developing AKI was then evaluated using logistic regression. Established risk factors for AKI were considered for logistic regression modeling with preoperative and early postoperative statin use. The number of variables included in the model was based on the criteria of one variable per 10 events (26), which allowed seven variables in the final model. A priori determined risk factors for cardiac surgery-associated AKI include age, baseline creatinine, history of diabetes, female gender, left ventricular ejection fraction <35%, anemia, diuretic use, length of cardiopulmonary bypass, length of aortic cross-clamp time, intraoperative radiocontrast administration, red blood cell transfusion, and surgical re-exploration (2, 27, 28). The randomization of RAP study drug was stratified on statin use, age, and ACE inhibitor, angiotensin receptor blocker, or aldosterone receptor antagonist use. Consequently, the distribution of RAP study drug assignment, and therefore RAP study drug effect, was similar among statin users and non-users. As a result, we did not unblind study drug in the parent trial or include study drug in regression modeling. Potentially significant confounding variables between early postoperative statin use and AKI, including preoperative statin therapy, coronary artery bypass surgery, and time to extubation, were also considered for logistic regression modeling. Limiting the total number of included factors to seven, we created a regression model that included the strongest known predictors of AKI and any possible confounders between early postoperative statin use and AKI. This final regression model consisted of age, gender, preoperative statin use, baseline creatinine, body mass index (BMI), cardiopulmonary bypass time, and early postoperative statin use. Data are reported as the estimated odds ratios (ORs) and 95% confidence intervals (CIs) (with a value of P<0.05 considered statistically significant).
Because of potential inequities between statin users and non-users, the numerous known risk factors for AKI, and potential confounding between early postoperative statin use and AKI, we also conducted propensity score analyses using logistic regression on preoperative statin therapy, baseline creatinine, age, gender, history of diabetes, history of hypertensive disease, BMI, baseline left ventricular ejection fraction, preoperative diuretic use, preoperative ACE inhibitor use, valvular surgery, use of cardiopulmonary bypass, cardiopulmonary bypass time, aortic cross-clamp time, intraoperative inotrope support, intraoperative coronary catheterization, postoperative surgical re-exploration, postoperative diuretic use, and time to extubation. All data were analyzed using SPSS (Version 17.0, SPSS, Chicago, IL) or using the statistical package SAS for Windows (Version 9, Cary, NC).
Results
Preoperative Statin Use
Table 1 provides baseline and intraoperative characteristics of those patients taking a statin prior to surgery and those who were not. Patients taking a statin preoperatively were older, more likely to be male, have diabetes or hypertension, and more likely to be taking a diuretic, ACE inhibitor, or an angiotensin receptor blocker. Preoperative statin users were also more likely to undergo coronary bypass grafting vs. valvular heart surgery and less likely to undergo CPB.
Table 1.
Characteristics According to Preoperative and Early Postoperative Statin Use
| Characteristic | No Preop Statin (n=150) |
Preop Statin (n=174) |
P | No Early Postop Statin (n=244) |
Early Postop Statin (n=80) |
P |
|---|---|---|---|---|---|---|
| Age, years | 54 .7±13.0 | 63.3±10.0 | <0.001 | 57.9±12.7 | 63.6±9.3 | <0.001 |
| Men, n (%) | 87 (58) | 128 (73) | 0.003 | 149 (61) | 66 (83) | <0.001 |
| Black, n (%) | 11 (7.3) | 8 (4.6) | 0.30 | 14 (5.7) | 5 (6.3) | 0.87 |
| Mean arterial pressure, mmHg | 94±12 | 92±12 | 0.22 | 93±12 | 94±12 | 0.66 |
| Heart rate, bpm | 67±14 | 64±14 | 0.04 | 66±14 | 63±13 | 0.19 |
| LVEF, % | 57±9 | 57±10 | 0.91 | 58±9 | 56±10 | 0.22 |
| Body Mass Index, kg/m2 | 29.2±7.3 | 29.4±5.6 | 0.24 | 29.3±6.7 | 29.3±5.5 | 0.64 |
| Medical history, n (%) | ||||||
| Diabetes | 21 (14) | 53 (31) | <0.001 | 52 (21) | 22 (26) | 0.25 |
| Hypertension | 68 (45) | 136 (78) | <0.001 | 146 (60) | 58 (73) | 0.04 |
| Smoking | 27 (18) | 39 (22) | 0.33 | 50 (21) | 16 (20) | 0.92 |
| Medications, preop, n (%) | ||||||
| Statin | 0 (0) | 174 (100) | 0 | 106 (43) | 68 (85) | <0.001 |
| Diuretic | 38 (25) | 68 (39) | 0.009 | 81 (33) | 25 (31) | 0.75 |
| ACE inhibitor* | 38 (25) | 72 (41) | 0.002 | 70 (29) | 40 (51) | <0.001 |
| Angiotensin blocker* | 19 (13) | 37 (21) | 0.04 | 44 (18) | 12 (15) | 0.53 |
| Treatment Group A:B:C, n (%) † |
45(30.2): 52(34.9): 52(34.9) |
56(32.2): 62(35.6): 56(32.2) |
0.87 | 75(30.9): 85(35.0): 83(34.2) |
26(32.5): 29(36.3): 25(31.3) |
0.89 |
| Creatinine, mg/dl | 1.0±0.2 | 1.0±0.2 | 0.30 | 1.0±0.2 | 1.0±0.2 | 0.95 |
| eGFR, ml/min/1.73m2 | 81±22 | 78±19 | 0.35 | 79±21 | 80±19 | 0.41 |
| CABG surgery, n (%) | 31 (21) | 105 (60) | <0.001 | 83 (34) | 53 (67) | <0.001 |
| On-pump surgery, n (%) | 141 (94) | 142 (82) | 0.001 | 224 (92) | 59 (74) | <0.001 |
| CPB time, min‡ | 130±72 | 124±49 | 0.88 | 131±66 | 110±40 | 0.03 |
| Cross clamp time, min§ | 100±48 | 91±40 | 0.20 | 101±45 | 77±33 | 0.003 |
| Inotrope use, n (%) | 61 (41) | 64 (37) | 0.47 | 102 (42) | 23 (29) | 0.04 |
| Transfusion, intraop, unit | ||||||
| Packed red blood cells | 1.0±1.8 | 1.3±2.2 | 0.13 | 1.1±2.6 | 1.1±2.2 | 0.12 |
| Fresh frozen plasma | 0.6±2.3 | 0.6±1.6 | 0.33 | 0.7±2.1 | 0.5±1.3 | 0.43 |
| Platelets (5-pack) | 0.1±0.5 | 0.2±0.5 | 0.19 | 0.2±0.5 | 0.2±0.6 | 0.65 |
ACE inhibitor and angiotensin receptor blocker use was discontinued 4–7 days preoperatively.
blinded ramipril, aldactone, or placebo treatment group.
among CPB patients.
among subjects undergoing aortic cross-clamp.
ACE, angiotensin converting enzyme; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass.
The incidence of AKI was similar in those patients who had taken a statin prior to surgery and those who had not (22.4% vs. 19.3%, P = 0.50), despite the higher prevalence of AKI risk factors among preoperative statin users. Other postoperative outcomes, including atrial fibrillation, time to extubation, length of stay, and mortality were also similar between the two groups. Postoperative myocardial infarction tended to be more common among preoperative statin users compared to non-users (3.4% vs. 0.7%, P = 0.07).
Early Postoperative Statin Use
Table 1 also shows baseline and intraoperative characteristics of those patients treated with a statin within one day following surgery (early postoperative use) and those who were not. Patients receiving early postoperative statin therapy were older, more likely to be male, more likely to have taken a statin prior to surgery, more likely to have hypertension, and more likely to undergo CABG, while being less likely to undergo CPB than patients who did not receive a statin in the early postoperative period. Patients receiving early postoperative statin therapy also experienced shorter aortic cross-clamp and cardiopulmonary bypass times than patients not receiving early postoperative statin therapy and less intraoperative inotropic support.
Table 2 shows outcome data for subjects who were given a statin within one day of surgery and those who were not. Subjects who received a statin within the first postoperative day were less likely to develop AKI. In addition, they tended to have a shorter length of hospital stay and tended to be less likely to develop postoperative atrial fibrillation. Early postoperative statin users had increased chest tube output within the first 24h of ICU admission but experienced shorter time to extubation. The incidence of postoperative myocardial infarction, surgical re-exploration, blood product transfusion, pneumonia, or death was not different in patients who received early versus late postoperative statin therapy.
Table 2.
Outcomes according to Early Postoperative Statin Use
| Characteristic | No Early Postop Statin (n=244) |
Early Postop Statin (n=80) |
P |
|---|---|---|---|
| AKI, n (%) | 58 (23.8) | 10 (12.5) | 0.03 |
| Chest tube output first 24h ICU, ml | 649±550 | 694±437 | 0.03 |
| PRBC transfusion postop, units | 1.1±2.6 | 1.0±2.8 | 0.23 |
| Surgical Reexploration, n (%) | 10 (4.1) | 2 (2.5) | 0.51 |
| Time to extubation, hours | 16.8±41.0 | 9.0±8.7 | 0.02 |
| Atrial Fibrillation, n (%) | 77 (31.6) | 17 (21.3) | 0.08 |
| Myocardial infarction, n (%) | 5 (2.0) | 2 (2.5) | 0.80 |
| Pneumonia, n (%) | 6 (2.5) | 4 (5.0) | 0.25 |
| Length of stay, days | 6.5±6.9 | 5.2±2.0 | 0.08 |
| Death, n (%) | 4 (1.6) | 1 (1.3) | 0.81 |
ICU, intensive care unit; PRBC, packed red blood cell.
Statin Withdrawal
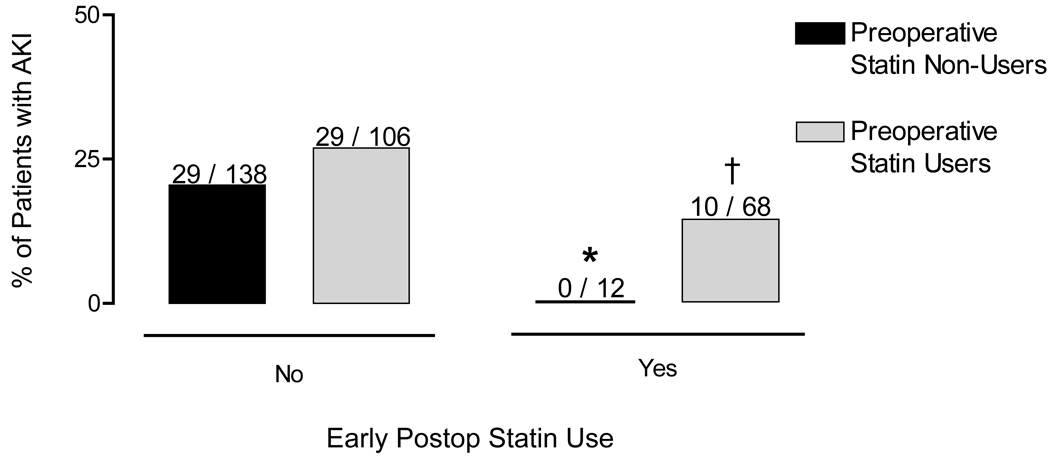
One hundred six of the preoperative statin users were withdrawn from statin use on the day of surgery and postoperative day 1, and 68 were not. The incidence of AKI was 27.4% among subjects withdrawn from statin therapy and 14.7% among those not (P=0.046). Figure 2 shows AKI incidence rates of subjects separated by statin withdrawal and preoperative and early postoperative statin use.
Figure 2.
Incidence of Acute Kidney Injury (AKI) among subjects, separated by statin exposure. Early postoperative statin use was associated with a reduced incidence of AKI among statin-naïve subjects (0% vs. 21%, *, P=0.02) and among preoperative statin-using subjects (14.7 vs. 27.4%, †, P=0.046).
Acute Kidney Injury
Sixty-eight patients (21.0%) developed acute kidney injury within 48 hours following cardiac surgery. Patients who developed AKI stayed in the hospital longer (8.7±11.9 days vs. 5.5±2.6 days; P=0.001), were more likely to develop pneumonia (8.8% vs. 1.6%; P=0.002), and had an increased risk of death (5.9% vs. 0.4%; P=0.001), compared to patients who did not develop AKI. No subjects received dialysis or died prior to measurement of AKI.
There were no differences in age, gender, baseline renal function, past medical history, vital signs, or preoperative medication use between patients who developed AKI and those who did not (Table 3). Patients who developed AKI had a significantly higher BMI and higher preoperative central venous pressure compared to those who did not.
Table 3.
Preoperative Subject Characteristics according to Development of AKI
| Characteristic | No AKI (n=256) | AKI (n=68) | P |
|---|---|---|---|
| Age, years | 58.9±12.5 | 61.0±11.1 | 0.19 |
| Men, n (%) | 165 (64.7) | 50 (73.5) | 0.17 |
| Black, n (%) | 16 (6.3) | 3 (4.4) | 0.57 |
| Mean arterial pressure, mmHg | 93±12 | 94±11 | 0.31 |
| Heart rate, bpm | 65±14 | 66±14 | 0.68 |
| CVP, mmHg | 12.1±5 | 13.7±5 | 0.03 |
| LVEF, % | 57±10 | 56±10 | 0.49 |
| Body Mass Index, kg/m2 | 28.8±6.0 | 31.5±7.6 | 0.003 |
| Medical history, n (%) | |||
| Diabetes mellitus | 57 (22.3) | 17 (25.0) | 0.63 |
| Hypertension | 158 (61.7) | 46 (67.6) | 0.37 |
| Smoking | 51 (19.9) | 15 (22.1) | 0.70 |
| Medication use preoperative, n (%) | |||
| Statin | 135 (52.7) | 39 (57.4) | 0.50 |
| Diuretic | 78 (30.5) | 28 (41.2) | 0.09 |
| ACE inhibitor* | 90 (35.2) | 20 (29.4) | 0.37 |
| Angiotensin receptor blocker* | 42 (16.4) | 14 (20.6) | 0.42 |
| Creatinine, mg/dl | 1.0±0.2 | 1.0±0.2 | 0.93 |
| eGFR, ml/min/1.73m2 | 78±19 | 82±25 | 0.78 |
ACE inhibitor and angiotensin receptor blocker use was discontinued 4–7 days preoperatively.
ACE, angiotensin converting enzyme; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; CVP, central venous pressure.
CABG, valvular heart surgery, intraoperative arterial pressure, intraoperative inotrope use, intraoperative norepinephine use, intraoperative coronary catheterization, aprotinin use, use of CPB, duration of CPB, or aortic cross-clamp time did not predict the development of AKI in univariate analysis (Table 4). Administration of intraoperative blood products, postoperative chest tube drainage, and intake-output fluid balances on day of surgery and postoperative day one were similar between patients who developed AKI and patients who did not. In contrast, subjects with lower arterial pressure, higher central venous pressure, who received norepinephrine use, or who received more packed red blood cells in the postoperative period were more likely to develop AKI. In addition, patients who required surgical re-exploration (trend) were more likely to develop AKI.
Table 4.
Intra and Postoperative Characteristics according to Development of AKI
| Characteristic | No AKI (n=256) | AKI (n=68) | P |
|---|---|---|---|
| CABG surgery, n (%) | 105 (41.0) | 31 (45.6) | 0.50 |
| On-pump surgery, n (%) | 224 (87.5) | 59 (86.8) | 0.87 |
| CPB time, min (among CPB patients) | 123±51 | 142±90 | 0.33 |
| Cross-clamp time, min (among subjects undergoing aortic cross-clamp) | 93±40 | 102±53 | 0.54 |
| Aprotinin use, n (%) | 14 (5.5) | 4 (6.0) | 0.87 |
| Intraoperative coronary catheterization, n (%) | 15 (5.9) | 5 (7.5) | 0.63 |
| Inotropic use, n (%) | 101 (39.5) | 24 (35.3) | 0.53 |
| Norepinephrine use, intraoperative, n (%) | 208 (81.1) | 54 (79.1) | 0.71 |
| Mean blood pressure, intraoperative, mmHg* | 74±14 | 71±13 | 0.10 |
| Time to extubation, hours | 13.9±37.4 | 18.3±30.3 | 0.37 |
| Transfusion, intraoperative, units | |||
| Packed red blood cells | 1.2±1.9 | 1.2±2.4 | 0.58 |
| Fresh frozen plasma | 0.5±1.3 | 1.1±3.3 | 0.28 |
| Pooled Platelets (5-pack) | 0.14±0.45 | 0.26±0.68 | 0.15 |
| Surgical Re-exploration, n (%) | 7 (2.7) | 5 (7.4) | 0.07 |
| Packed red blood cell transfusion, postop, units | 0.9±2.1 | 1.7±4.1 | 0.03 |
| Diuretic use, day of surgery or postop day 1, n (%) | 33 (12.9) | 12 (17.6) | 0.31 |
| Fluid balance, day of surgery, liters | +0.5±1.1 | +0.6±1.0 | 0.50 |
| Fluid balance, postop day 1, liters | −0.6±1.3 | −0.5+1.3 | 0.51 |
| Norepinephrine use, postop day 1, n (%) | 94 (36.6) | 39 (57.1) | 0.003 |
| Mean blood pressure, postop day 1, mmHg | 75±10 | 70±10 | 0.002 |
| CVP, postop day 1, mmHg | 10±5 | 12±5 | 0.01 |
following protamine administration.
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; CVP, central venous pressure; postop, postoperative.
Logistic Regression Models
In a multivariate logistic regression model that included preoperative statin use, early postoperative statin use, age, gender, baseline creatinine, BMI, and cardiopulmonary bypass time as variables, early postoperative statin use, age, gender, and BMI were significantly associated with the development of AKI (Table 5). The Hosmer and Lemeshow test for goodness of fit demonstrated no lack of fit for the model (P=0.54).
Table 5.
Multivariate Logistic Regression Model for Acute Kidney Injury
| Variable | Odds Ratio | P value | 95% CI | |
|---|---|---|---|---|
| Preoperative Statin Use | 1.24 | 0.50 | 0.66 | 2.32 |
| Early Postop Statin Use | 0.32 | 0.006 | 0.14 | 0.72 |
| Age, years | 1.03 | 0.03 | 1.004 | 1.06 |
| Gender, Male vs. Female | 2.41 | 0.02 | 1.19 | 4.89 |
| Baseline creatinine, mg/dl | 0.35 | 0.16 | 0.08 | 1.49 |
| Body mass index, kg/m2 | 1.08 | <0.001 | 1.04 | 1.13 |
| CPB time, minutes | 1.002 | 0.20 | 1.00 | 1.01 |
CI, confidence interval; BMI, body mass index; CPB, cardiopulmonary bypass.
Because preoperative statin use, hypertension, CABG, aortic cross-clamp time, intraoperative inotrope use, and time to extubation were associated with early postoperative statin use, we evaluated the possibility of confounding by these variables in the logistic regression, by sequentially including them one variable at a time to the main model (Table 5). None of these variables altered the association between postoperative statin use and AKI within the model, changed the significance of the other factors within the model, or proved to be confounders.
For example, when time to extubation, a variable which may reflect illness severity and could influence early postoperative statin use, was added to the model, early postoperative statin use (OR 0.32, 95% CI 0.14–0.73, P=0.006), gender (OR 2.40 male versus female, 95% CI 1.18–4.88, P=0.02), age (OR 1.03, 95% CI 1.00–1.06, P=0.03), and baseline BMI (OR 1.08, 95% CI 1.04–1.13, P<0.001) remained associated with risk of AKI. Moreover, time to extubation, although predictive of early postoperative statin use, had no association with the development of AKI in the model (OR 1.00, P=0.60). When intraoperative inotrope use was added to the model, the effect of postoperative statin use on AKI remained highly significant (OR 0.34, 95% CI 0.15–0.75, P=0.007), as did the effect when history of hypertension was added to the model (OR 0.34, 95% CI 0.16–0.75, P=0.008).
Because lower arterial blood pressure, norepinephrine use, and higher central venous pressure on postoperative day 1 were associated with a higher incidence of AKI, we also evaluated the possibility of confounding by these variables using logistic regression. None of these variables altered the association between early postoperative statin use or any of the other variables in our model and the development of AKI. For example, when norepinephrine use on postoperative day 1 was added to the model, early postoperative statin use (OR 0.36, 95% CI 0.15–0.85, P=0.02), gender (OR 2.80 male versus female, 95% CI 1.32–5.94, P=0.007), age (OR 1.03, 95% CI 1.00–1.06, P=0.04), and baseline BMI (OR 1.10, 95% CI 1.05–1.15, P<0.001) remained associated with risk of AKI. Norepinephrine use on postoperative day 1 was also associated with risk of AKI (OR 2.18, 95% CI 1.20–3.99, P=0.01).
In order to account for further imbalances between early postoperative statin users and non-users, we also conducted a sensitivity analysis using a propensity score (constructed using logistic regression described in the Statistical Methods section). After adjusting for the propensity score, the effect of early postoperative statin use on AKI remained highly significant (Table 6). The balance of the distribution of all the covariates used in constructing the propensity score between early postoperative statin users and non-users was satisfactory within the lower and upper half of the propensity score subgroups except for the use of CPB and CPB time. Thus, we examined the relationship between postoperative statin use and AKI using an additional logistic regression that included early postoperative statin use, the propensity score, the use of CPB, and CPB time. The relationship of early postoperative statin use to AKI (OR 0.30, 95% CI 0.13–0.69, P=0.005) remained consistent with the multivariate logistic regression.
Table 6.
Propensity Score Adjusted Regression Model for Acute Kidney Injury
| Variable | Odds Ratio | P value | 95% CI | |
|---|---|---|---|---|
| Early Postop Statin Use | 0.30 | 0.005 | 0.13 | 0.70 |
| Propensity Score | 3.81 | 0.057 | 0.96 | 15.15 |
CI, confidence interval.
Discussion
We examined the relationship between pre- and postoperative statin use and AKI in patients undergoing cardiac surgery. We report for the first time that early postoperative statin use is associated with a lower incidence of AKI. Conversely withdrawal of statin therapy in the preoperative period increases risk of AKI. This finding is consistent with data from recent studies suggesting that statin administration decreases AKI following suprarenal aortic cross-clamping (29) and hastens recovery from AKI following lower extremity or abdominal aneurysm vascular surgery (30).
Early postoperative statin treatment was associated with reduced AKI but preoperative statin use was not. This finding is consistent with recent studies in coronary catheterization patients showing that statin bolus enhances the myocardial protective effect of chronic statin use (31). Likewise, short-term perioperative statin treatment protects statin-naïve coronary catheterization patients (19). AKI following cardiac surgery results from injury sustained during the continuum of surgery and the early postoperative period. Consequently, statin intervention within the first postoperative day may have a greater effect on the development of AKI than preoperative statin use. Experimental findings support this observation. Within 6–18 hours, statin treatment improves endothelial function and reduces the generation of ROS (32, 33). Subsequently, statin use reduces endothelium-leukocyte interactions, platelet reactivity, and systemic concentrations of interleukin-6, interleukin-8, tumor necrosis factor-α, and circulating leukocyte number and activity in coronary surgery patients (34, 35). Since early postoperative statin use was associated with less AKI, but preoperative statin use was not, statin effects on ROS generation and renal endothelium may affect AKI more than statin alteration of inflammation.
This analysis identified BMI as an independent risk factor for AKI following cardiac surgery. After adjusting for known AKI risk factors and the effect of early postoperative statin use on AKI, every 1 kg/m2 increase in BMI resulted in an 8.2 % increase in the odds of AKI. Prior studies have established BMI as a risk factor for wound infections and atrial fibrillation following cardiac surgery, but effects on the risk of AKI have been inconsistent (36, 37). On the other hand, obesity is associated with endothelial dysfunction (38), promotes inflammation (39), and predicts the progression of chronic kidney disease (40).
This observational study has several limitations. Patients with moderate and severe baseline renal insufficiency (creatinine > 1.6 mg/dl) are excluded from the RAP study, and this exclusion likely accounts for the lack of association between baseline creatinine and AKI within this analysis. Preexisting renal dysfunction is a significant predictor of postoperative AKI, and the association between statin use and AKI needs to be studied in this patient population. Likewise, because we studied only patients undergoing non-emergency surgery, we avoided studying patients who had undergone recent cardiac catheterization with intravenous radiocontrast exposure or intra-aortic balloon pump counterpulsation, which are both risk factors for AKI. Among the small number of patients who received intraoperative coronary catheterization, no difference in AKI was observed. These exclusion criteria for the RAP study may limit the generalizability of our findings.
Patients were not randomized to statin use, and this may have resulted in a selection bias for lower-risk patients among those treated with a statin within the first postoperative day. We addressed this potential for selection bias by including all known risk factors for AKI in our propensity score-adjusted regression model, including preoperative predictors of AKI, intraoperative indicators of severity of surgical intervention, and postoperative markers of prolonged recovery. In this manner, we analyzed the relationship between early postoperative statin use and AKI after adjusting for differences in AKI risk factors between early statin users and non-users. Factors such as surgical re-exploration and time to extubation, which may have influenced the timing of the initiation of oral statin treatment, were not associated with AKI. We sought to control for all confounders between statin use and AKI. However, unknown factors may exist.
Likewise, we may have missed a renal protective effect of preoperative statin use due to confounding by indication. Despite preoperative statin users increased risk of atherosclerotic disease and inflammation, AKI incidence remained similar to the AKI incidence among non-preoperative statin users. Prior studies of preoperative statin use and AKI have yielded mixed results. One observational trial reported a 46% odds reduction in AKI (41), while a large but heterogeneous meta-analysis of observational studies reported no reduction (42).
In summary, we found that early postoperative statin use is associated with reduced AKI following cardiac surgery in a non-randomized, retrospective study, even after correcting for differences between statin users and non-users. Prospective, randomized, placebo-controlled trials are needed, however, to evaluate the effect of both preoperative and early postoperative statin use on the risk of AKI following cardiac surgery. If AKI is limited by early postoperative statin use, thousands of elective and urgent cardiac surgery patients could benefit from early postoperative statin treatment.
Acknowledgments
We would like to thank Carol Meisch, RN, Carol Bowling, RN, and Anthony DeMatteo for their nursing assistance and for database management.
This work was supported by the National Institutes of Health [R01HL65193, R01HL77389, R01HL085740, and UL1RR024975] and institutional departmental funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frederic T. Billings, IV, Department of Anesthesiology, Division of Critical Care, Vanderbilt University.
Mias Pretorius, Department of Anesthesiology, Cardiothoracic Division, Vanderbilt University.
Edward D. Siew, Department of Internal Medicine, Division of Nephrology, Vanderbilt University.
Chang Yu, Department of Biostatistics, Vanderbilt University.
Nancy J. Brown, Department of Internal Medicine, Division of Clinical Pharmacology, Vanderbilt University.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Mangano CM, Diamondstone LS, Ramsay JG, et al. The Multicenter Study of Perioperative Ischemia Research Group. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med. 1998;128(3):194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Del Duca D, Iqbal S, Rahme E, et al. Renal failure after cardiac surgery: timing of cardiac catheterization and other perioperative risk factors. Ann Thorac Surg. 2007;84(4):1264–1271. doi: 10.1016/j.athoracsur.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14(5):1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 6.Ryckwaert F, Boccara G, Frappier JM, et al. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med. 2002;30(7):1495–1498. doi: 10.1097/00003246-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Swaminathan M, Shaw AD, Phillips-Bute BG, et al. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med. 2007;35(10):2286–2291. doi: 10.1097/01.ccm.0000282079.05994.57. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GJ, Angelini GD. Side effects of cardiopulmonary bypass: what is the reality? J Card Surg. 2004;19(6):481–488. doi: 10.1111/j.0886-0440.2004.04101.x. [DOI] [PubMed] [Google Scholar]
- 9.Andersson LG, Bratteby LE, Ekroth R, et al. Renal function during cardiopulmonary bypass: influence of pump flow and systemic blood pressure. Eur J Cardiothorac Surg. 1994;8(11):597–602. doi: 10.1016/1010-7940(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 10.McFarlane SI, Winer N, Sowers JR. Role of the natriuretic peptide system in cardiorenal protection. Arch Intern Med. 2003;163(22):2696–2704. doi: 10.1001/archinte.163.22.2696. [DOI] [PubMed] [Google Scholar]
- 11.Perrella MA, Edell ES, Krowka MJ, et al. Endothelium-derived relaxing factor in pulmonary and renal circulations during hypoxia. Am J Physiol. 1992;263(1 Pt 2):R45–R50. doi: 10.1152/ajpregu.1992.263.1.R45. [DOI] [PubMed] [Google Scholar]
- 12.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112(3):676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 13.Scolari F, Tardanico R, Zani R, et al. Cholesterol crystal embolism: A recognizable cause of renal disease. Am J Kidney Dis. 2000;36(6):1089–1109. doi: 10.1053/ajkd.2000.19809. [DOI] [PubMed] [Google Scholar]
- 14.Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154(1):87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 15.O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95(5):1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 16.Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108(4):426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 17.Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. J Clin Pathol. 2004;57(7):728–734. doi: 10.1136/jcp.2003.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasceri V, Patti G, Nusca A, et al. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110(6):674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 19.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49(12):1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104(6):1326–1333. doi: 10.1213/01.ane.0000263029.72643.10. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Endres M, Laufs U. Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35(11) Suppl 1:2708–2711. doi: 10.1161/01.STR.0000143319.73503.38. [DOI] [PubMed] [Google Scholar]
- 22.Sharyo S, Yokota-Ikeda N, Mori M, et al. Pravastatin improves renal ischemia-reperfusion injury by inhibiting the mevalonate pathway. Kidney Int. 2008;74(5):577–584. doi: 10.1038/ki.2008.210. [DOI] [PubMed] [Google Scholar]
- 23.Fleming GA, Murray KT, Yu C, et al. Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery. Circulation. 2008;118(16):1619–1625. doi: 10.1161/CIRCULATIONAHA.108.790162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 27.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 28.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119(4):495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 29.Schouten O, Kok NF, Boersma E, et al. Effects of statins on renal function after aortic cross clamping during major vascular surgery. Am J Cardiol. 2006;97(9):1383–1385. doi: 10.1016/j.amjcard.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 30.Welten GM, Chonchol M, Schouten O, et al. Statin use is associated with early recovery of kidney injury after vascular surgery and improved long-term outcome. Nephrol Dial Transplant. 2008;23(12):3867–3873. doi: 10.1093/ndt/gfn381. [DOI] [PubMed] [Google Scholar]
- 31.Di Sciascio G, Patti G, Pasceri V, et al. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54(6):558–565. doi: 10.1016/j.jacc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Jones SP, Gibson MF, Rimmer DM, 3rd, et al. Direct vascular and cardioprotective effects of rosuvastatin, a new HMG-CoA reductase inhibitor. J Am Coll Cardiol. 2002;40(6):1172–1178. doi: 10.1016/s0735-1097(02)02115-0. [DOI] [PubMed] [Google Scholar]
- 33.Wagner AH, Kohler T, Ruckschloss U, et al. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20(1):61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 34.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96(5A):24F–33F. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chello M, Patti G, Candura D, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34(3):660–667. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]
- 36.Pan W, Hindler K, Lee VV, et al. Obesity in diabetic patients undergoing coronary artery bypass graft surgery is associated with increased postoperative morbidity. Anesthesiology. 2006;104(3):441–447. doi: 10.1097/00000542-200603000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Moulton MJ, Creswell LL, Mackey ME, et al. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996;94(9 Suppl):II87–II92. [PubMed] [Google Scholar]
- 38.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112(1):32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 39.Festa A, D'Agostino R, Jr, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25(10):1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 40.Ramos LF, Shintani A, Ikizler TA, et al. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19(3):593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabata M, Khalpey Z, Pirundini PA, et al. Renoprotective effect of preoperative statins in coronary artery bypass grafting. Am J Cardiol. 2007;100(3):442–444. doi: 10.1016/j.amjcard.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 42.Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J. 2008;29(12):1548–1559. doi: 10.1093/eurheartj/ehn198. [DOI] [PubMed] [Google Scholar]