Abstract
Residential isolation segregation (a measure of residential inter-racial exposure) has been associated with rates of preterm birth (<37 weeks gestation) experienced by black women. Epidemiologic differences between very preterm (<32 weeks gestation) and moderately preterm births (32–36 weeks) raise questions about whether this association is similar across gestational ages, and through what pathways it might be mediated. Hierarchical Bayesian models were fit to answer three questions: is the isolation-prematurity association similar for very and moderately preterm birth; is this association mediated by maternal chronic disease, socioeconomic status, or metropolitan area crime and poverty rates; and how much of the geographic variation in black-white very preterm birth disparities is explained by isolation segregation? Singleton births to black and white women in 231 U.S. metropolitan statistical areas in 2000–2002 were analyzed and isolation segregation was calculated for each. We found that among black women, isolation is associated with very preterm birth and moderately preterm birth. The association may be partially mediated by individual level socioeconomic characteristics and metropolitan level violent crime rates. There is no association between segregation and prematurity among white women. Isolation segregation explains 28% of the geographic variation in black-white very preterm birth disparities. Our findings highlight the importance of isolation segregation for the high-burden outcome of very preterm birth, but unexplained excess risk for prematurity among black women is substantial.
Keywords: Preterm birth, Racial disparity, Residential segregation, Bayesian modeling, Social determinants, USA
Introduction
Preterm birth (<37 weeks gestation) is the leading cause of infant mortality in the US (Callaghan, MacDorman, Rasmussen, Qin, & Lackritz, 2006). However a small subset— approximately 2% of all US births (Martin, Hamilton, Sutton, Ventura, Menacker et al., 2009)— who are born less than 32 weeks (very preterm birth) account for 95% of preterm-associated infant mortality. Very preterm birth is also a leading risk factor for pediatric morbidities such as cerebral palsy and mental retardation (Yeargin-Allsopp, Van Naarden Braun, Doernberg, Benedict, Kirby et al., 2008), and substantial economic costs for neonatal care, rehabilitative and educational services through a child's life (Behrman & Butler, 2007). The stubborn persistence of racial disparities in prematurity further contributes to the urgency of this public health problem. Black women in the US have 43% greater risk than white women for delivering moderately preterm at 32–36 weeks gestation, and 2–3 times the risk of delivering at less than 32 weeks gestation (Martin, Kung, Mathews, Hoyert, Strobino et al., 2008).
It is common in epidemiologic studies to combine all births less than 37 weeks into a single group, but these births are not only heterogeneous with regards to the magnitude of the racial disparity; there may also be etiological differences related to gestational age. Infection and inflammation are leading causes of very preterm birth, particularly for black women, but less important in near-term births (Goldenberg, Hauth, & Andrews, 2000). On the other hand, assisted reproductive technology, increases in medical intervention in pregnancy, and societal changes in maternal age at conception may contribute to the recent increase in moderately preterm births (Branum & Schoendorf, 2002; Raju, 2006).
Further efforts to disentangle the causes of these profound racial disparities and minimize the burdens that result, requires greater attention to the heterogeneity within common epidemiologic definitions of disease.
Pathways to prematurity
Preterm birth is a complex health problem with social, environmental, behavioral and genetic determinants of individuals' risk (Kramer & Hogue, 2009a). While numerous risk factors for preterm birth have been reported, their failure to explain a significant portion of the racial disparity has lead investigators to look further upstream in the causal chain to place-based contextual effects and structural inequities in access to resources. For instance, controlling for individual-level risk factors, neighborhood violent crime and poverty rates have been associated with increased risk of preterm birth (Kaufman, Dole, Savitz, & Herring, 2003; Messer, Kaufman, Dole, Savitz, & Laraia, 2006; O'Campo, Burke, Culhane, Elo, Eyster et al., 2008).
The association between preterm birth and neighborhood context could be mediated by a combination of material and psychosocial pathways related to access to safe living conditions, maternal perceptions of neighborhood quality, and exposure to discrimination or stressful life events (Collins, David, Handler, Wall, & Andes, 2004; Collins, David, Symons, Handler, Wall et al., 1998). These acute or chronic stressors may interact with maternal neuroendocrine and immune function resulting in preterm birth, or could be mediated by the prevalence of poorly controlled chronic hypertension, or individual behavioral responses (e.g. smoking) to stressful environments, each of which could differentially pattern risk (Kramer & Hogue, 2009a).
While local residential neighborhoods have been useful constructs for conceptualizing contextual rather than individual determinants of health disparities, some exposures may operate differently at different geographic scales. Health-relevant policies and social context may be patterned at the scale of counties, urbanized areas or states (Bird, 1995; Centers for Disease Control and Prevention, 2002; Sims, Sims, & Bruce, 2007). The risk of very preterm birth varies substantially more among metropolitan statistical areas (MSA's) for black women than it does for white women (Kramer & Hogue, 2008). The neighborhoods within these MSA's may still be the proximally relevant exposure, but the propensity for some MSA's to have more unhealthy neighborhoods than others suggests additional patterning forces.
Residential segregation and prematurity
The spatial segregation of blacks and whites across residential neighborhoods may indicate the extent of regional structural inequality and institutionalized racism which could explain MSA-level disparities in infant mortality and low birthweight (Ellen, 2000; Laveist, 1989; Polednak, 1996). More recently, segregation has been associated with other indicators of perinatal health including preterm birth (Bell, Zimmerman, Almgren, Mayer, & Huebner, 2006; Osypuk & Acevedo-Garcia, 2008), teen pregnancy (Sucoff & Upchurch, 1998), and smoking during pregnancy (Bell, Zimmerman, Mayer, Almgren, & Huebner, 2007). While studies of small area (e.g. neighborhood) segregation find poor health outcomes for both black and white women as segregation increases (Grady & Ramirez, 2008; Mason, Messer, Laraia, & Mendola, 2009), studies focusing on MSA's suggest a cross-level interaction where segregation is associated with increased risk for black but not white women (Ellen, 2000; Kramer & Hogue, 2008). This is likely due to the fact that very few white women live in predominantly black neighborhoods in highly segregated MSA's.
Residential segregation is often distinguished along several `dimensions' describing unique spatial patterns of residential settlement in urban and suburban areas (Massey & Denton, 1988). Three dimensions commonly employed in research on segregation and health outcomes are isolation, evenness, and clustering. Isolation is the probability of interaction between blacks and whites within neighborhoods; evenness describes the racial distribution within neighborhoods to the overall MSA composition; clustering is the aggregation of racially homogenous neighborhoods in sub-regions of a metropolitan area.
Although correlated, these patterns may capture distinct aspects of health-relevant exposures (Kramer & Hogue, 2009b). For example Bell, et al (2006) report a reduced risk for preterm birth when clustering of predominantly black neighborhoods is present, but a elevated risk of prematurity in the presence of isolation segregation. The authors suggest that conditional on the degree of isolation, clustering enhances social support and networks for black women and families, which may reduce risk by buffering against psychosocial stressors, and facilitating health protective behaviors. Isolation on the other hand may capture economic disenfranchisement and poverty concentration, and is associated with elevated violent crime rates, diminished access to healthy food options, and reduced access to preventive healthcare (Collins, 1999; Morland & Filomena, 2007; Shihadeh & Flynn, 1996). These factors may result in racial differences in risk factors for preterm birth including exposure to psychosocial stress, and prevalence of chronic diseases such as diabetes and hypertension.
Study Questions
Thus three observations motivate this study. First, prematurity may best be understood as a heterogeneous outcome, resulting from complex health processes, with at least one domain of difference being very preterm versus moderately preterm births. Second, the magnitude of black-white racial disparities is not constant but varies geographically and at different geographic scales. This variation in relative risk may offer clues as to some causes of the disparity. Finally, residential segregation has been hypothesized to be a distal determinant of racial disparities in prematurity, but this relationship has never been looked at for very preterm birth, and is incompletely understood in terms of mediating pathways.
From these three observations we pose three questions.
Is isolation segregation associated similarly with very versus moderate preterm birth?
Which individual or metropolitan characteristics mediate the association between isolation and preterm birth?
Does isolation segregation explain the geographic variation in black-white disparities of very preterm birth?
Methods
Data sources
Individual level variables
At the individual level, each singleton live births born in 2000–2002 to non-Hispanic white or black mothers living in US metropolitan statistical areas was abstracted from National Center for Health Statistics natality files. Individuals are geo-located within MSA's but not within specific neighborhoods. Births were categorized as very preterm (from 20 to less than 32 weeks), moderately preterm (from 32 to less than 37 weeks), or term (37 to 44 weeks).
Variables including maternal education, marital status, smoking, and chronic disease status were also abstracted from birth records. These variables are hypothesized to be mediators of a segregation effect on prematurity. Residential segregation may influence family social structure, area school quality and adult educational attainment (Card & Rothstein, 2007; Howell-Moroney, 2005); maternal education and marital status are each associated with preterm birth, and thus to the degree that segregation influences metropolitan-level patterns they could mediate a health effect. Pre-conceptional chronic diseases such as hypertension and diabetes are also associated with preterm birth (Ehrenthal, Jurkovitz, Hoffman, Kroelinger, & Weintraub, 2007), and there is some evidence that prevalent chronic disease mediates some of the segregation association with low birthweight for black women (Grady & Ramirez, 2008). Smoking during pregnancy is also a risk factor for preterm birth, and the rate of smoking may vary by degree of segregation (Bell et al., 2007). Because smoking is not measured on California birth records, this variable is coded with a level to indicate missing values to retain California births in all models including smoking.
Other variables captured for individual births include maternal age, parity, and history of a prior preterm birth.
Metropolitan level variables
Metropolitan statistical areas were chosen as the contextual unit of analysis. MSA's represent contiguous counties surrounding a core city which are deemed by the federal government to be economically and socially integrated (OMB, 2000). The geographic scale of MSA's are particularly well suited for this study because residential segregation is conceptualized as a process of sorting individuals into living environments on the basis of race and class. This process happens across regional residential housing markets; therefore simultaneously recognizing the housing choices of economically and socially linked urban and suburban communities is beneficial. We analyzed 231 MSA's which had a population of at least 100,000 and had a non-Hispanic black population of at least 5,000 in the 2000 Census.
Metropolitan population size (categorized as <500,000, 500,000–1 million, or >1 million), and Census region (Northeast, Southeast, Midwest, West) were obtained from the 2000 Census. Because there may be variation in both segregation and very preterm birth risk across these variables, we control for them in all models to better describe the independent association of segregation.
Potential mediators between isolation and prematurity at the metropolitan level were chosen based on evidence from neighborhood-level contextual determinants reviewed above, and include violent crime and poverty rates. The mechanisms for an effect of these variables on pregnancy health could result from chronic exposure to psychosocial stressors and subsequent `weathering' or premature aging of maternal neuroendocrine and vascular function (Geronimus, 1996). Alternatively effects could be material in nature, related to access to health promoting resources including health and dental care (Haas, Phillips, Sonneborn, McCulloch, Baker et al., 2004; Kushel, Gupta, Gee, & Haas, 2006).
The murder rate per 100,000 persons in each metropolitan area was obtained from federal statistics for 2000 (Federal Bureau of Investigation, 2000). The black poverty rate as well as the ratio of black to white poverty rates in each MSA were calculated from Census 2000 data to represent indicators of area-based racial inequity (US Census Bureau, 2000). We used measures of the exposure of poor children to high poverty neighborhoods as calculated by Acevedo-Garcia, et al (2007) to approximate spatial poverty concentration. This variable is the proportion in each MSA of black children under 18 years of age whose families are below the poverty line and live in census tracts with median household incomes less than 80% of the median household income for the MSA as a whole in 2000.
Measuring residential segregation
The primary segregation dimension of interest is isolation, although unevenness/clustering was also measured (Massey & Denton, 1988). We use explicitly spatial adaptations of common census-tract derived segregation indices to measure these dimensions(Reardon & O'Sullivan, 2004). The strength of these spatial measures is that they minimize measurement bias introduced by reliance on the arbitrary size and shapes of census tracts by applying a consistent definition of residential neighborhoods within MSAs in the calculation segregation indices (Kramer, Cooper, Drews-Botsch, Waller, & Hogue, 2010). The spatial segregation indices also allow a flexible definition the size or scale of neighborhoods, recognizing that the granularity of segregation may be an attribute of the segregation pattern in and of itself (Lee, Reardon, Firebaugh, Farrell, Matthews et al., 2008). The spatial isolation index is calculated using the formula:
| Eq. 1 Spatial isolation index |
τp is the total population density for each point p in region R (the MSA), and T is the total MSA population. is the proportion black in the spatial area or residential environment of point p. We defined the residential environment as a 500-meter radius circle around each point p in the MSA. This definition of neighborhood is based on exploratory analysis of best model fit at different spatial scales (Kramer et al., 2010).
Reardon & O'Sullivan argue that the clustering dimension of segregation is simply an uneven distribution of predominantly black neighborhoods, and thus an index of evenness such as the dissimilarity index would measure clustering if the spatial scale of the local area were sufficiently broad. We operationalized clustering (or sub-regional unevenness) using a spatial adaptation of the dissimilarity index:
| Eq. 2. Spatial dissimilarity index |
Again, τp is the total population density at point p, while π and πp are the proportion black in the MSA overall and at point p respectively. denotes the proportion black in the spatial area around point p which for clustering is set as a 4000-meter radius circle. More detail on the calculation method and correlation between isolation and clustering is provided in the electronic technical appendix available with the online version of the paper [SUPPLEMENTARY FILE]. The resulting indices each range from 0 to 1; for isolation 0 represents highest inter-racial exposure and 1 is highest isolation. For the dissimilarity index 0 suggests least clustering or most even distribution across large sub-regions, while 1 suggests a high degree of clustering. Each segregation index was standardized to a mean of 0, standard deviation of 1, so that model interpretations are made in terms of a 1-standard deviation change in the relevant index.
Analysis
All analyses were conducted using hierarchical Bayesian logistic regression models (Gelman & Hill, 2007). The setup for each model follows this template:
| Eq. 3. Hierarchical Bayesian logistic model |
In the first level yi is the binary pregnancy outcome for the ith woman, αj is a random intercept for the jth MSA, β is a vector of parameters for individual variables, and X is a matrix of individual level covariates. Each parameter is assigned a prior probability distribution. Relatively uninformative priors are assigned to the β-parameters, while the α-intercept has an informative prior, in the form of the second level of the model. The random intercepts (alphas) are assumed to come from a normal distribution with a variance of . The mean of the distribution is the sum of a global intercept, γ0, and the vector of γ- parameters corresponding to the MSA-level covariates in matrix U, including segregation. All models reported control for census region and metropolitan size as MSA-level confounders and except for crude models adjust for maternal age, parity and history of prior preterm birth at the individual level.
For question one, concerning the association of segregation with very and moderately preterm births, separate models were fit for white and black women, and for very preterm and moderately preterm birth as the dependent variable.
Question two concerns possible mediation of a segregation-prematurity association by hypothesized mechanisms. Models of the crude association of individual covariates and segregation indices with preterm birth are adjusted only for MSA region and size. Baseline models controlling for possible individual and metropolitan level confounders are denoted M1. Mediation was evaluated by comparing the magnitude of the segregation-preterm birth association with and without the candidate mediator(s), with meaningful attenuation of the association suggesting the variable is a mediator in the pathway, or alternately a non-causal confounder of the crude association. Potential mediation by socioeconomic status is evaluated by adding variables for maternal education and marital status (model M2); likewise maternal health status is considered as a mediator by entering chronic hypertension, diabetes and smoking variables (model M3). Model M4 includes all of the above covariates. The primary segregation pattern of interest is spatial isolation; to consider whether spatial clustering segregation contributes to the model, a final model with both segregation indices is fit (M5).
Question three, concerning the geographic variation in the racial disparity of preterm birth attributable to segregation includes births to white and black mothers. The model structure is similar to the previous questions with the addition of a binary variable for race, and an associated random slope for this variable:
| Eq. 4. Model for black-white disparity |
The random intercept, αj, and the priors remain the same as the previously described model (equation 3). δj is the MSA-specific relative black-white disparity accounting for individual and area covariates. γrace0 is then the average excess risk across all MSA's for black as compared with white women, and γrace1 is the vector of second-level parameters corresponding to the matrix, U, of MSA-level covariates including isolation segregation. σ2race is the variation in the disparity across MSA's.
Bayesian models were fit with WinBUGS 1.4 (Lunn, Thomas, Best, & Spiegelhalter, 2000) using R 2.7 (R Development Core Team, 2008) and the R2WinBUGS package (Sturtz, Ligges, & Gelman, 2005). All models were run with three chains, each for 50,000 iterations with the first half discarded; convergence was evaluated by visual inspection of the trace plots of the posterior parameter estimates from each chain, as well as an R-hat statistic of 1.1 or lower for each parameter (Gelman & Hill, 2007). Relative improvements in model fit were assessed using the deviance information criterion (DIC) (Spiegelhalter, Best, Carlin, & van der Linde, 2002).
Results
Of 6,180,544 eligible births during the study period, 23.5% were born to black mothers (Table 1). In this sample black women experienced three times the risk as white women for very preterm birth, and 60% greater risk for moderately preterm birth. Racial differences in the importance of individual risk factors result in variation in the racial disparity across covariates. For instance, higher maternal education and being married are more strongly protective for white women than for black women, so that the relative racial disparity is smaller among women without a high school degree, or among unmarried women.
Table 1.
Distribution of singleton live births, 231 metropolitan statistical areas, 2000–2002
| Non-Hispanic black mothers |
Non-Hispanic white mothers |
B/W Risk Ratio |
||||||
|---|---|---|---|---|---|---|---|---|
| N births | %VPT | %MPT | N births | %VPT | %MPT | VPT | MPT | |
|
|
|
|
||||||
| TOTAL | 1,452,943 | 3.45 | 12.38 | 4,727,601 | 1.09 | 7.62 | 3.17 | 1.62 |
| Maternal age | ||||||||
| <15 | 8,036 | 6.25 | 17.26 | 2,875 | 3.90 | 14.50 | 1.60 | 1.19 |
| 15–19 | 259,919 | 3.71 | 13.30 | 331,585 | 1.88 | 9.36 | 1.97 | 1.42 |
| 20–24 | 462,992 | 3.05 | 11.89 | 931,881 | 1.26 | 8.06 | 2.42 | 1.48 |
| 25–29 | 335,450 | 3.16 | 11.41 | 1,260,933 | 0.94 | 7.28 | 3.36 | 1.57 |
| 30–34 | 235,487 | 3.69 | 12.18 | 1,377,871 | 0.89 | 7.01 | 4.15 | 1.74 |
| 35–39 | 121,912 | 4.17 | 14.15 | 681,430 | 1.08 | 7.66 | 3.86 | 1.85 |
| 40+ | 29,147 | 4.85 | 16.04 | 141,026 | 1.36 | 9.34 | 3.57 | 1.72 |
| Maternal educationa | ||||||||
| <12 years | 346,198 | 3.92 | 14.30 | 485,564 | 1.83 | 9.99 | 2.14 | 1.43 |
| 12 years | 555,945 | 3.44 | 12.53 | 1,322,573 | 1.31 | 8.30 | 2.63 | 1.51 |
| >12 years | 525,107 | 3.01 | 10.93 | 2,879,627 | 0.84 | 6.91 | 3.58 | 1.58 |
| Marital status | ||||||||
| Married | 467,200 | 2.83 | 10.94 | 3,736,839 | 0.89 | 7.16 | 3.18 | 1.53 |
| Unmarried | 985,743 | 3.74 | 13.06 | 990,762 | 1.85 | 9.37 | 2.02 | 1.39 |
| Parity | ||||||||
| Primiparous | 432,811 | 3.42 | 11.49 | 1,609,656 | 1.24 | 7.80 | 2.76 | 1.47 |
| Multiparous | 1,020,132 | 3.46 | 12.76 | 3,117,945 | 1.01 | 7.53 | 3.43 | 1.69 |
| Prior preterm or SGA birtha | ||||||||
| Yes | 20,637 | 11.18 | 26.25 | 54,813 | 3.84 | 22.22 | 2.91 | 1.18 |
| No | 1,420,965 | 3.32 | 12.18 | 4,618,748 | 1.05 | 7.44 | 3.16 | 1.64 |
| Chronic hypertensiona | ||||||||
| Yes | 20,519 | 8.54 | 21.34 | 35,256 | 3.46 | 16.31 | 2.47 | 1.31 |
| No | 1,421,083 | 3.36 | 12.25 | 4,628,305 | 1.07 | 7.55 | 3.14 | 1.62 |
| Diabetesa | ||||||||
| Yes | 41,956 | 3.42 | 16.92 | 135,907 | 1.17 | 11.94 | 2.92 | 1.42 |
| No | 1,399,646 | 3.44 | 12.25 | 4,537,654 | 1.08 | 7.49 | 3.19 | 1.64 |
| Tobacco usea | ||||||||
| Yes | 122,275 | 4.94 | 15.85 | 576,214 | 1.84 | 10.02 | 2.68 | 1.58 |
| No | 1,237,533 | 3.33 | 12.15 | 3,707,273 | 0.99 | 7.38 | 3.36 | 1.65 |
| Region | ||||||||
| Northeast | 275,059 | 3.36 | 11.42 | 1,017,475 | 1.01 | 6.68 | 3.33 | 1.71 |
| Southeast | 741,877 | 3.52 | 12.84 | 1,639,918 | 1.19 | 8.48 | 2.96 | 1.51 |
| Midwest | 316,917 | 3.60 | 12.68 | 1,213,167 | 1.13 | 7.65 | 3.19 | 1.66 |
| West | 119,090 | 2.77 | 10.94 | 857,041 | 0.94 | 7.07 | 2.95 | 1.55 |
|
|
||||||||
Source: National Center for Health Statistics Natality Files, 2000–2002
Abbreviations: VPT: Very preterm birth (greater than 20 but less than 32 weeks gestation); MPT: Moderately preterm birth (greater than or equal to 32 weeks but less than 37 weeks gestation)
Demographic factor does not sum to total due to missing values in natality files
For both black and white women, risk for preterm birth varied regionally, with the Western metropolitan areas having substantially lower risk than other areas for black women; for white women risk is higher in the Northeast and Midwest but lower in the West and Southeast.
Across the 231 metropolitan areas analyzed, isolation segregation ranged from 0.06 (Salt Lake City, Utah) to 0.86 (Gary, Indiana), with a median value of 0.51 and an interquartile range of 0.36– 0.64. The measure of spatial clustering ranged from 0.22 (Lawton, Oklahoma) to 0.79 (also in Gary, Indian) with a median value of 0.51 and an interquartile range of 0.43–0.59. The Spearman rank correlation of MSA isolation and clustering was 0.47 (p<.001). After standardizing the indices, MSA's ranged from approximately -2 to +2 standard deviations for each index.
Table 2 reports odds ratios for very preterm birth among black women for models with isolation, individual covariates, and control for region and population size. In the crude model, each standard deviation increase in isolation is associated with an 11% increased odds of very preterm birth for black women (95% CI 1.08, 1.14). Models M1–M4 consider this association under different specifications of covariates. History of prior preterm birth, chronic hypertension, or tobacco use is each an important predictor of very preterm birth risk; however the independent association of isolation with very preterm birth remains relatively unchanged with adjustment for such factors. A modest reduction in the association is seen in model M2 controlling for socioeconomic characteristics (OR 1.10, 95% CI 1.07, 1.14) compared with the baseline adjusted M1 model (OR 1.12, 95% CI 1.08, 1.15).
Table 2.
Parameter estimates for very preterm birth among black women
| Crudea |
M1 -- Age, parity and prior preterm birth |
M2 -- Socioeconomic condition pathway |
M3 -- Maternal health pathway |
M4 -- All covariate model |
M5 -- Dual Index model |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||||
|
|
||||||||||||||||||
| Individual level | ||||||||||||||||||
| Maternal age | ||||||||||||||||||
| <15 | 2.12 | 1.93 | 2.34 | 2.23 | 2.03 | 2.45 | 1.66 | 1.51 | 1.83 | 2.29 | 2.08 | 2.50 | 1.80 | 1.62 | 1.97 | 1.80 | 1.64 | 1.99 |
| 15–19 | 1.18 | 1.15 | 1.21 | 1.22 | 1.18 | 1.25 | 0.97 | 0.94 | 1.00 | 1.23 | 1.19 | 1.27 | 1.02 | 0.98 | 1.05 | 1.02 | 0.98 | 1.05 |
| 20–24 | 0.96 | 0.93 | 0.98 | 0.96 | 0.94 | 0.99 | 0.86 | 0.84 | 0.89 | 0.97 | 0.94 | 0.99 | 0.88 | 0.85 | 0.90 | 0.88 | 0.86 | 0.90 |
| 25–29 | ref | ref | ref | ref | ref | ref | ||||||||||||
| 30–34 | 1.21 | 1.17 | 1.24 | 1.21 | 1.17 | 1.24 | 1.30 | 1.26 | 1.34 | 1.19 | 1.16 | 1.23 | 1.26 | 1.23 | 1.30 | 1.27 | 1.23 | 1.31 |
| 35–39 | 1.42 | 1.37 | 1.47 | 1.41 | 1.37 | 1.46 | 1.54 | 1.49 | 1.60 | 1.35 | 1.31 | 1.40 | 1.46 | 1.41 | 1.51 | 1.46 | 1.41 | 1.51 |
| 40+ | 1.71 | 1.62 | 1.82 | 1.71 | 1.61 | 1.81 | 1.85 | 1.74 | 1.96 | 1.58 | 1.49 | 1.68 | 1.70 | 1.60 | 1.80 | 1.70 | 1.61 | 1.80 |
| Parity | ||||||||||||||||||
| Primiparous | ref | ref | ref | ref | ref | ref | ||||||||||||
| Multiparous | 1.07 | 1.05 | 1.09 | 1.00 | 0.97 | 1.02 | 0.97 | 0.95 | 0.99 | 0.97 | 0.95 | 1.00 | 0.96 | 0.94 | 0.98 | 0.96 | 0.94 | 0.98 |
| Prior preterm or SGA birth | ||||||||||||||||||
| Yes | 4.75 | 4.54 | 4.98 | 4.77 | 4.57 | 5.00 | 4.69 | 4.47 | 4.91 | 4.56 | 4.34 | 4.79 | 4.53 | 4.31 | 4.74 | 4.54 | 4.34 | 4.75 |
| No | ref | ref | ref | ref | ref | ref | ||||||||||||
| Maternal education | ||||||||||||||||||
| <12 years | 1.27 | 1.25 | 1.30 | 1.21 | 1.18 | 1.24 | 1.15 | 1.11 | 1.18 | 1.15 | 1.12 | 1.18 | ||||||
| 12 years | 1.10 | 1.08 | 1.12 | 1.08 | 1.06 | 1.11 | 1.06 | 1.04 | 1.09 | 1.06 | 1.04 | 1.09 | ||||||
| >12 years | ref | ref | ref | ref | ||||||||||||||
| Marital status | ||||||||||||||||||
| Married | ref | ref | ref | ref | ||||||||||||||
| Unmarried | 1.36 | 1.33 | 1.39 | 1.47 | 1.44 | 1.51 | 1.43 | 1.40 | 1.47 | 1.43 | 1.40 | 1.47 | ||||||
| Chronic hypertension | ||||||||||||||||||
| Yes vs no | 3.03 | 2.87 | 3.18 | 2.77 | 2.64 | 2.93 | 2.75 | 2.61 | 2.90 | 2.75 | 2.61 | 2.90 | ||||||
| Diabetes | ||||||||||||||||||
| Yes vs. no | 0.95 | 0.90 | 1.00 | 0.90 | 0.85 | 0.95 | 0.91 | 0.87 | 0.97 | 0.91 | 0.87 | 0.96 | ||||||
| Tobacco usec | ||||||||||||||||||
| Yes vs no | 1.58 | 1.54 | 1.63 | 1.53 | 1.48 | 1.57 | 1.38 | 1.34 | 1.42 | 1.38 | 1.34 | 1.42 | ||||||
| MSA-level | ||||||||||||||||||
| Isolationb | 1.11 | 1.08 | 1.14 | 1.12 | 1.08 | 1.15 | 1.10 | 1.07 | 1.14 | 1.13 | 1.10 | 1.17 | 1.11 | 1.08 | 1.15 | 1.15 | 1.10 | 1.19 |
| Clusteringb | 1.05 | 1.02 | 1.08 | 0.95 | 0.92 | 0.99 | ||||||||||||
| Population size | ||||||||||||||||||
| <500,000 | 1.09 | 1.04 | 1.16 | 1.10 | 1.04 | 1.16 | 1.08 | 1.02 | 1.14 | 1.08 | 1.03 | 1.14 | 1.07 | 1.01 | 1.13 | 1.05 | 0.99 | 1.11 |
| 500k – 1 million | 1.08 | 1.01 | 1.15 | 1.09 | 1.02 | 1.16 | 1.09 | 1.01 | 1.16 | 1.08 | 1.01 | 1.15 | 1.07 | 1.00 | 1.14 | 1.07 | 1.00 | 1.14 |
| 1 million + | ref | ref | ref | ref | ref | ref | ||||||||||||
| Region | ||||||||||||||||||
| Northeast | ref | ref | ref | ref | ref | ref | ||||||||||||
| Southeast | 0.98 | 0.92 | 1.05 | 1.04 | 0.96 | 1.12 | 1.07 | 0.99 | 1.15 | 1.07 | 1.00 | 1.15 | 1.09 | 1.02 | 1.17 | 1.04 | 0.97 | 1.13 |
| Midwest | 0.98 | 0.91 | 1.06 | 1.01 | 0.93 | 1.09 | 1.00 | 0.92 | 1.08 | 1.01 | 0.93 | 1.09 | 1.00 | 0.93 | 1.09 | 1.01 | 0.94 | 1.09 |
| West | 0.84 | 0.76 | 0.92 | 0.86 | 0.78 | 0.95 | 0.88 | 0.80 | 0.98 | 0.93 | 0.84 | 1.03 | 0.94 | 0.85 | 1.03 | 0.91 | 0.83 | 1.01 |
| σ 2 α | 0.134 | 0.135 | 0.126 | 0.129 | 0.125 | |||||||||||||
| DIC | 416696 | 415134 | 414758 | 413557 | 413559 | |||||||||||||
|
| ||||||||||||||||||
Abbreviations: MSA: metropolitan statistical area
Crude models for individual covariates all include random intercepts for MSA, and adjustment for region and population size
The odds ratios for segregation indices refer to the change in risk of very preterm birth for a 1-SD change in segregation index
California does not collect smoking on birth certificates so smoking parameter estimates exclude births in California. The variable was coded such that California births are not omitted from the model so all other parameters are inclusive.
The independent association of clustering segregation (measured with spatial dissimilarity index) with very preterm birth in black women seen in the crude model results (OR 1.05, 95% CI 1.02–1.08) was similar across models M1 to M4 (data not shown). However in model M5, with both isolation and clustering segregation, the isolation-very preterm birth association strengthened (OR 1.15, 95% CI 1.10, 1.19), while the independent association of clustering appears to be modestly protective (OR 0.95, 95% CI 0.92, 0.99). Put another way, among MSA's with average clustering (e.g. standardized index=0), the odds ratio for very preterm birth in black women living in the MSA's with 1 standard deviation (SD) above the mean isolation level to those living in MSA's 1 SD below the mean isolation (2 SD change) was 1.32 (95% CI 1.21–1.42). This 2-SD change represents the range of observed isolation values conditional on clustering (online electronic technical appendix).
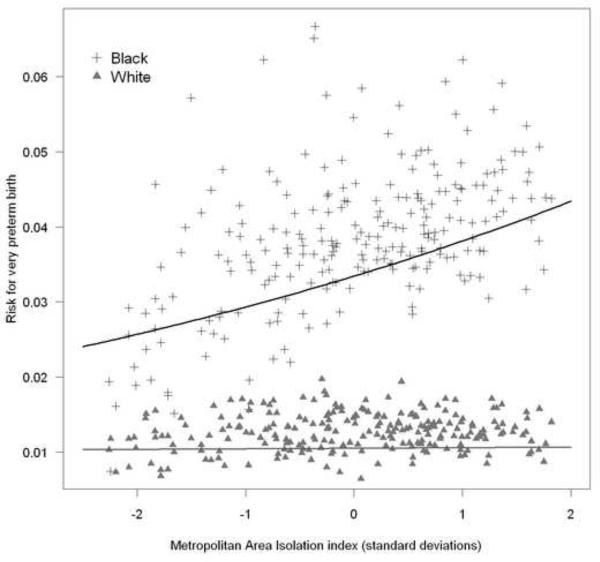
There was no association between clustering or isolation segregation and very preterm birth in any of the preceding models for white women (data not shown). Figure 1 plots the observed and model predicted risks using model M5 for black and white women. As MSA isolation increases the average very preterm birth risk for black women increases, while for white women there is no similar association.
Figure 1. Metropolitan area very preterm birth risk by isolation segregation for black and white women.
Symbols (cross and triangles) represent observed risk for VPT birth in each of 231 MSA's for black and white women respectively; lines represent model predicted risk for VPT birth from Table 2, Model M5.
Table 3 reports parallel model results with moderately preterm birth as the outcome. The point estimate for the association of isolation with moderately preterm birth is half as strong as it is with very preterm birth. While a one standard deviation increase in isolation conditional on clustering and all individual covariates increased very preterm birth odds 15% among black women, it increased moderately preterm birth odds only 8%. There was no association between segregation and moderately preterm birth in white women (data not shown).
Table 3.
Parameter estimates for moderately preterm birth among black women
| Crudea |
M1 -- Age, parity and prior preterm birth |
M2 -- Socioeconomic condition pathway |
M3 -- Maternal health pathway |
M4 -- All covariate model |
M5 -- Dual Index model |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||||
|
|
||||||||||||||||||
| MSA-level | ||||||||||||||||||
| Isolationb | 1.06 | 1.04 | 1.09 | 1.07 | 1.04 | 1.10 | 1.06 | 1.03 | 1.09 | 1.08 | 1.05 | 1.11 | 1.07 | 1.04 | 1.09 | 1.08 | 1.04 | 1.11 |
| Clusteringb | 1.04 | 1.02 | 1.07 | 0.99 | 0.96 | 1.02 | ||||||||||||
| σ 2 α | 0.134 | 0.134 | 0.131 | 0.132 | 0.132 | |||||||||||||
| DIC | 1065390 | 1062570 | 1062380 | 1060170 | 1060180 | |||||||||||||
|
| ||||||||||||||||||
Abbreviations: MSA: metropolitan statistical area
Crude models for individual covariates all include random intercepts for MSA, and adjustment for region and population size
The odds ratios for segregation indices refer to the change in risk of very preterm birth for a 1-SD change in segregation index
Table 4 builds on model M5, by further considering metropolitan characteristics which could mediate the association with segregation. Statistical control for MSA murder rate modestly attenuates the isolation-very preterm birth association from 1.15 to 1.12 (95% CI 1.07, 1.17). There is also a substantial reduction in the DIC (suggesting improved model fit) in the models including murder rates or poverty concentration as compared to model M5.
Table 4.
Candidate metropolitan level mediating variables for association between segregation and very preterm birth among black women
| Murder |
Poverty |
Poverty rate ratio |
Poverty concentration |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||
|
|
||||||||||||
| Isolationa | 1.12 | 1.07 | 1.17 | 1.15 | 1.10 | 1.20 | 1.15 | 1.10 | 1.20 | 1.16 | 1.11 | 1.21 |
| Clusteringa | 0.95 | 0.91 | 0.98 | 0.95 | 0.91 | 0.99 | 0.96 | 0.92 | 0.99 | 0.95 | 0.91 | 1.00 |
| Murder rateb | 1.12 | 1.03 | 1.21 | |||||||||
| Black poverty ratec | 1.03 | 0.98 | 1.07 | |||||||||
| Black-white poverty rate ratio | 1.00 | 0.97 | 1.03 | |||||||||
| Black poverty concentrationd | 0.98 | 0.96 | 1.01 | |||||||||
| σ 2 α | 0.120 | 0.125 | 0.127 | 0.122 | ||||||||
| DIC | 410126 | 413558 | 413558 | 406547 | ||||||||
NOTE: All models are also adjusted for maternal age, education, parity, marital status, smoking, history of prior preterm birth, chronic hypertension or diabetes, MSA region and MSA population size.
Odds ratios for isolation and clustering indices correspond to the change in the outcome for a 1-SD increase in segregation
Murder rate is scaled so that a 1-unit change in murder rate is equivalent to 10 murders/100,000 persons
Black poverty rate is scaled so that a 1-unit change is equivalent to 10% change in poverty rate
Black poverty concentration is the proportion of children in poor families who also live in high poverty neighborhoods. It is scaled so that a 1-unit change is equivalent to 10% change in this proportion.
In Table 5, models with both black and white women assess the change in the adjusted racial disparity for very preterm birth under various model specifications. Change in the disparity is calculated as a percentage excess risk explained (Lynch, Kaplan, Cohen, Tuomilehto, & Salonen, 1996): (ORcrude-ORadjusted)/(ORcrude−1).
Table 5.
Black-white disparities in very preterm birth under different model specifications
| OR | 95%CI | % disparity explained | σ 2race | DIC | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Crude | 3.24 | 3.20 | 3.28 | |||
| All individual variables | 2.68 | 2.37 | 3.02 | 25.0 | 0.107 | 960703 |
| Individual + isolation | 2.49 | 2.25 | 2.77 | 33.4 | 0.077 | 960681 |
| Individual + isolation + clustering | 2.51 | 2.25 | 2.80 | 32.5 | 0.075 | 960677 |
Inclusion of all individual covariates reduced the crude black-white odds ratio by approximately 25%. The addition of isolation segregation reduced the disparity an additional 8%, and there was a concomitant 28% decrease in the inter-MSA variation in the disparity (σ2race) from a variance of 0.107 to 0.077 with control for isolation. Addition of clustering added little to the model in terms of model fit or explanation of the disparity.
Valid measurement of gestational age is notoriously hard to capture even in prospective clinical studies and differential misclassification by race and class are particular concerns when using birth certificate data. Very low birthweight (VLBW) is highly correlated with very preterm birth and more reliably measured. To assess sensitivity of our findings to misclassification of gestational age, models were fit with VLBW as an alternate outcome. Parameter magnitude and direction were similar in these models, suggesting misclassification of gestational age is not a significant source of bias.
To assess the sensitivity of our findings to the choice of spatial segregation measures, models were repeated using traditional census-tract-derived isolation, dissimilarity, and spatial proximity indices (Massey & Denton, 1988). Odds ratios for the association of these indices with preterm birth were similar in direction, but smaller in magnitude than with spatial indices. The difference is magnitude of association may be due to our use of a highly granular and consistent neighborhood definition as compared with the coarser and more variably sized pattern identified with census tracts (Kramer et al., 2010).
In models which included segregation categorized into quintiles, there was no evidence of non-linearities in the segregation-preterm birth association.
Discussion
The black-white racial disparity in preterm birth in the US is a stubborn problem which defies simple explanations. Residential segregation has been proposed as a fundamental cause of racial disparities in health because of the manner in which segregation may constrain some individuals' economic attainment, health, and welfare (Williams & Collins, 2001). Consistent with prior work (Bell et al., 2006; Osypuk & Acevedo-Garcia, 2008) this study finds evidence that for black women, independent of measured individual and area level risk factors, living in a metropolitan area characterized by high isolation segregation significantly increases risk for preterm birth. We further demonstrate that the association is nearly twice as strong for the outcome of very preterm birth as compared to near-term births, an important finding because of the substantial public health burden and increased racial gap for very preterm birth risk.
The different associations by gestational age may also hint at mechanisms by which segregation affects individual health. For example bacterial vaginosis is much more strongly associated with very preterm birth as compared to moderately preterm birth (Goldenberg, Iams, Mercer, Meis, Moawad et al., 1998). The higher prevalence of bacterial vaginosis among black women may be due to experiences of stress, discrimination or poor social support (Culhane, Rauh, & Goldenberg, 2006; Paul, Boutain, Manhart, & Hitti, 2008). Women living in neighborhoods characterized by social stressors such as high violent crime rates and economic disadvantage have both increased overall risk for poor pregnancy outcomes, as well as accelerated age-associated risk for poor outcomes consistent with Geronimus' weathering hypothesis (Cerda, Buka, & Rich-Edwards, 2008; Geronimus, 1996; Masi, Hawkley, Piotrowski, & Pickett, 2007). However living in MSA's characterized by high racial isolation means different things for white and black women. Poor whites tend to live in mixed-income neighborhoods, while blacks—and particularly poor blacks—living in highly segregated cities tend to live in neighborhoods with high poverty rates and lower economic opportunity (Osypuk, Galea, McArdle, & Acevedo-Garcia, 2009). High MSA isolation segregation may therefore be a risk marker of racial differences in exposures to chronic stress, lack of social support, and discrimination thereby disproportionately affecting black women's immune status and infection prevalence (Hogue & Bremner, 2005). There was a modest attenuation of the segregation-very preterm birth association with inclusion of MSA murder rate, a finding consistent with prior research (Masi et al., 2007; Messer et al., 2006). Violent crime could be a direct source of chronic stress, or it could be a marker of another process such as poor social support, low economic opportunity, or infrastructure decay.
We found, as have other investigators (Bell et al., 2006), that isolation and clustering segregation patterns have different independent associations with prematurity. Clustering and isolation are modestly correlated variables and each separately is associated with increased risk for prematurity in black women. However in models considering the joint relationship it appears that the isolation construct best captures the negative effect of segregation, while clustering is conditionally protective (very preterm birth) to null (moderately preterm birth). MSA's which are divergent on these two dimensions tend to be smaller in size and have smaller black populations (electronic technical appendix and Kramer et al., 2010). Possible explanations for a different association of clustering with prematurity conditional on isolation include buffering aspects of social networks and black political empowerment (Laveist, 1993).
Our findings can also be viewed in the context of research on neighborhood-level ethnic density and pregnancy outcomes. One study in Chicago neighborhoods reported lower risk for low birthweight among black women living in predominantly black neighborhoods after controlling for area deprivation and individual socioeconomic status (Roberts, 1997), while studies in New York City and North Carolina found increased risk for low birth weight and preterm birth for black women in predominantly black neighborhoods (Grady & McLafferty, 2007; Mason et al., 2009). Ethnic enclave effects may also differ by maternal nativity (foreign born versus US born) (Grady & McLafferty, 2007), and by ethnicity (Hispanic versus non-Hispanic) (Osypuk, Bates, & Acevedo-Garcia, 2010). Such conflicting or complex associations may be attributable to regionally specific forces which affect individual selection into neighborhoods (Oakes, 2004) or makeup the broader regional social context. In a study which simultaneously measured neighborhood racial composition and city-level segregation, it was in fact city-level segregation which was found to have the independent (and deleterious) association with pregnancy health of black women, with no remaining significant association of neighborhood racial composition (Reichman, Teitler, & Hamilton, 2009).
Residential segregation has a statistically significant association with excess risk of preterm birth in black women, yet it independently accounts for only 8% of the black-white racial disparity, and in fact adjustment for all measured covariates leaves two-thirds of the disparity unexplained! Modeled inter-MSA variance decreases by about 28% when isolation is added to the models, suggesting that segregation accounts for a portion of this geographic variation. The persisting disparity results from unmeasured risk factors which may include racial differences in lifecourse health behaviors (Lu & Halfon, 2003), residual confounding by socioeconomic status (Kaufman & Cooper, 2008) , pervasive exposures to chronic stress, or genetic and epigenetic interactions with any of the above (Hogue & Bremner, 2005).
Because of the history of slavery and racial inequality following the Civil War and into the current century, the patterns and consequences of black-white segregation we describe may be uniquely American. While our results may not be fully generalizable to other countries, ethnic and economic residential segregation likely occurs in all urbanized areas, and comparable tools can be applied to understanding the consequences of such segregation, although relationships may be similarly complex (Pickett, Shaw, Atkin, Kiernan, & Wilkinson, 2009).
Limitations
This cross-sectional, vital records-based observational study is limited by lack of information on important variables. Such variables include detailed individual biological and social exposures, and measures of neighborhoods in addition to metropolitan areas. Because birth records are not nationally available with neighborhood-level geocodes it is not possible to distinguish whether the contextual effect of segregation or poverty is primarily exerted at the neighborhood or the metropolitan level.
Conclusions
Isolation segregation, previously associated with risk for low birthweight and preterm birth, is most strongly associated with very preterm birth, an outcome where the racial gap is also largest and the burden of mortality and morbidity is most severe. The association between racial isolation segregation and preterm birth persists under numerous model specifications, and is only attenuated modestly with control for individual socioeconomic variables and metropolitan murder rate. While important, this association remains small in magnitude and, like other known risk factors, explains only a fraction of the racial disparity. Future work should continue to explore the manner in which structural processes in urban areas influence pregnancy outcomes. These efforts will likely be most effective if combined with improved measurement at multiple scales of study from individual clinical and biological information, to neighborhood environment, and metropolitan characteristics (Kramer & Hogue, 2009a).
Supplementary Material
Acknowledgments
Author Comments: This work was supported in part by a Health Resource and Service Administration Maternal and Child Health training grant (T03MC07651 to M. K.) and a National Institute of Health Reproductive, Perinatal, and Pediatric Health Training grant (T32 HD052460 to M. K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo-Garcia D, McArdle N, Osypuk TL, Lefkowitz B, Krimgold BK. Children left behind: How metropolitan areas are failing America's children. Harvard School of Public Health; 2007. diversitydata.org. [Google Scholar]
- Behrman RE, Butler AS, editors. Preterm birth: Causes, consequences, and prevention. National Academy Press, Institute of Medicine; Washington, D.C.: 2007. [PubMed] [Google Scholar]
- Bell JF, Zimmerman FJ, Almgren GR, Mayer JD, Huebner CE. Birth outcomes among urban African-American women: a multilevel analysis of the role of racial residential segregation. Soc Sci Med. 2006;63(12):3030–3045. doi: 10.1016/j.socscimed.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Bell JF, Zimmerman FJ, Mayer JD, Almgren GR, Huebner CE. Associations between residential segregation and smoking during pregnancy among urban African-American women. J Urban Health. 2007;84(3):372–388. doi: 10.1007/s11524-006-9152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird ST. Separate black and white infant mortality models: differences in the importance of structural variables. Soc Sci Med. 1995;41(11):1507–1512. doi: 10.1016/0277-9536(95)00029-7. [DOI] [PubMed] [Google Scholar]
- Branum AM, Schoendorf KC. Changing patterns of low birthweight and preterm birth in the United States, 1981–98. Paediatr Perinat Epidemiol. 2002;16(1):8–15. doi: 10.1046/j.1365-3016.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- Card D, Rothstein J. Racial segregation and the black-white test score gap. Journal of Public Economics. 2007;91(11–12):2158–2184. [Google Scholar]
- Centers for Disease Control and Prevention Racial and ethnic disparities in infant mortality rates--60 largest U.S. cities, 1995–1998. MMWR Morb Mortal Wkly Rep. 2002;51(15):329–332. 343. [PubMed] [Google Scholar]
- Cerda M, Buka SL, Rich-Edwards JW. Neighborhood influences on the association between maternal age and birthweight: a multilevel investigation of age-related disparities in health. Soc Sci Med. 2008;66(9):2048–2060. doi: 10.1016/j.socscimed.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA. Racism and health: segregation and causes of death amenable to medical intervention in major U.S. cities. Ann N Y Acad Sci. 1999;896:396–398. doi: 10.1111/j.1749-6632.1999.tb08152.x. [DOI] [PubMed] [Google Scholar]
- Collins JW, David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. 2004;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW, Jr., David RJ, Symons R, Handler A, Wall S, Andes S. African-American mothers' perception of their residential environment, stressful life events, and very low birthweight. Epidemiology. 1998;9(3):286–289. [PubMed] [Google Scholar]
- Culhane JF, Rauh VA, Goldenberg RL. Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep. 2006;8(6):459–464. doi: 10.1007/s11908-006-0020-x. [DOI] [PubMed] [Google Scholar]
- Ehrenthal DB, Jurkovitz C, Hoffman M, Kroelinger C, Weintraub W. A population study of the contribution of medical comorbidity to the risk of prematurity in blacks. Am J Obstet Gynecol. 2007;197(4):409. doi: 10.1016/j.ajog.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Ellen IG. Is segregation bad for your health? The case of low birth weight. Brookings-Wharton Papers on Urban Affairs. 2000;2000:203–229. [Google Scholar]
- Federal Bureau of Investigation Uniform Crime Reports: Index of crime by metropolitan statistical area. 2000 [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge University press; New York: 2007. [Google Scholar]
- Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996;42(4):589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health. 1998;88(2):233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SC, McLafferty S. Segregation, nativity, and health: Reproductive health inequalities for immigrant and native-born black women in new york city. Urban Geography. 2007;28(4):377–397. [Google Scholar]
- Grady SC, Ramirez IJ. Mediating medical risk factors in the residential segregation and low birthweight relationship by race in New York City. Health Place. 2008;14(4):661–677. doi: 10.1016/j.healthplace.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Haas JS, Phillips KA, Sonneborn D, McCulloch CE, Baker LC, Kaplan CP, et al. Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual's county of residence. Med Care. 2004;42(7):707–714. doi: 10.1097/01.mlr.0000129906.95881.83. [DOI] [PubMed] [Google Scholar]
- Hogue CJ, Bremner JD. Stress model for research into preterm delivery among black women. Am J Obstet Gynecol. 2005;192(5 Suppl):S47–55. doi: 10.1016/j.ajog.2005.01.073. [DOI] [PubMed] [Google Scholar]
- Howell-Moroney M. The geography of opportunity and unemployment: An integrated model of residential segregation and spatial mismatch. Journal of Urban Affairs. 2005;27(4):353–377. [Google Scholar]
- Kaufman JS, Cooper RS. Race in epidemiology: new tools, old problems. Ann Epidemiol. 2008;18(2):119–123. doi: 10.1016/j.annepidem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kaufman JS, Dole N, Savitz DA, Herring AH. Modeling community-level effects on preterm birth. Ann Epidemiol. 2003;13(5):377–384. doi: 10.1016/s1047-2797(02)00480-5. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Cooper H, Drews-Botsch C, Waller L, Hogue C. Do measures matter? Comparing surface-density-derived and census-tract-derived measures of racial residential segregation. International Journal of Health Geographics. 2010 doi: 10.1186/1476-072X-9-29. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MR, Hogue CR. Place matters: variation in the black/white very preterm birth rate across U.S. metropolitan areas, 2002–2004. Public Health Rep. 2008;123(5):576–585. doi: 10.1177/003335490812300507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MR, Hogue CR. What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiologic Reviews. 2009a;31:84–98. doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MR, Hogue CR. Is segregation bad for your health? Epidemiologic Reviews. 2009b;31:178–194. doi: 10.1093/epirev/mxp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71–77. doi: 10.1111/j.1525-1497.2005.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laveist TA. Linking Residential Segregation to the Infant-Mortality Race Disparity in United-States Cities. Sociology and Social Research. 1989;73(2):90–94. [Google Scholar]
- Laveist TA. Segregation, poverty, and empowerment: health consequences for African Americans. Milbank Q. 1993;71(1):41–64. [PubMed] [Google Scholar]
- Lee BA, Reardon SF, Firebaugh G, Farrell CR, Matthews SA, O'Sullivan D. Beyond the Census Tract: Patterns and Determinants of Racial Segregation at Multiple Geographic Scales. Am Sociol Rev. 2008;73(5):489–514. doi: 10.1177/000312240807300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7(1):13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS--a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing. 2000;10:235–337. [Google Scholar]
- Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? Am J Epidemiol. 1996;144(10):934–942. doi: 10.1093/oxfordjournals.aje.a008863. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2006. National Vital Statistics Report. 2009;57(7) [PubMed] [Google Scholar]
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121(4):788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Piotrowski ZH, Pickett KE. Neighborhood economic disadvantage, violent crime, group density, and pregnancy outcomes in a diverse, urban population. Soc Sci Med. 2007;65(12):2440–2457. doi: 10.1016/j.socscimed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Mason SM, Messer LC, Laraia BA, Mendola P. Segregation and preterm birth: the effects of neighborhood racial composition in North Carolina. Health Place. 2009;15(1):1–9. doi: 10.1016/j.healthplace.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey DS, Denton NA. The Dimensions of Residential Segregation. Social Forces. 1988;67(2):281–315. [Google Scholar]
- Messer LC, Kaufman JS, Dole N, Savitz DA, Laraia BA. Neighborhood crime, deprivation, and preterm birth. Ann Epidemiol. 2006;16(6):455–462. doi: 10.1016/j.annepidem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Morland K, Filomena S. Disparities in the availability of fruits and vegetables between racially segregated urban neighbourhoods. Public Health Nutrition. 2007;10(12):1481–1489. doi: 10.1017/S1368980007000079. [DOI] [PubMed] [Google Scholar]
- O'Campo P, Burke JG, Culhane J, Elo IT, Eyster J, Holzman C, et al. Neighborhood deprivation and preterm birth among non-Hispanic Black and White women in eight geographic areas in the United States. Am J Epidemiol. 2008;167(2):155–163. doi: 10.1093/aje/kwm277. [DOI] [PubMed] [Google Scholar]
- Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Soc Sci Med. 2004;58(10):1929–1952. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- OMB . Final Report and Recommendations From the Metropolitan Area Standards Review Committee to the Office of Management and Budget Concerning Changes to the Standards for Defining Metropolitan Areas. Washington DC: 2000. [Google Scholar]
- Osypuk TL, Acevedo-Garcia D. Are Racial Disparities in Preterm Birth Larger in Hypersegregated Areas? Am J Epidemiol. 2008;167(11):1295–1304. doi: 10.1093/aje/kwn043. [DOI] [PubMed] [Google Scholar]
- Osypuk TL, Bates LM, Acevedo-Garcia D. Another Mexican birthweight paradox? The role of residential enclaves and neighborhood poverty in the birthweight of Mexican-origin infants. Soc Sci Med. 2010;70(4):550–560. doi: 10.1016/j.socscimed.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osypuk TL, Galea S, McArdle N, Acevedo-Garcia D. Quantifying Separate and Unequal: Racial-Ethnic Distributions of Neighborhood Poverty in Metropolitan America. Urban Aff Rev Thousand Oaks Calif. 2009;45(1):25–65. doi: 10.1177/1078087408331119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Boutain D, Manhart L, Hitti J. Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc Sci Med. 2008;67(5):824–833. doi: 10.1016/j.socscimed.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Shaw RJ, Atkin K, Kiernan KE, Wilkinson RG. Ethnic density effects on maternal and infant health in the Millennium Cohort Study. Soc Sci Med. 2009;69(10):1476–1483. doi: 10.1016/j.socscimed.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Polednak AP. Trends in US urban black infant mortality, by degree of residential segregation. Am J Public Health. 1996;86(5):723–726. doi: 10.2105/ajph.86.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Raju TN. Epidemiology of late preterm (near-term) births. Clin Perinatol. 2006;33(4):751–763. doi: 10.1016/j.clp.2006.09.009. abstract vii. [DOI] [PubMed] [Google Scholar]
- Reardon SF, O'Sullivan D. Measures of spatial segregation. Sociological Methodology. 2004;34:121–162. [Google Scholar]
- Reichman NE, Teitler JO, Hamilton ER. Effects of neighborhood racial composition on birthweight. Health & Place. 2009;15(3):784–791. doi: 10.1016/j.healthplace.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM. Neighborhood social environments and the distribution of low birthweight in Chicago. Am J Public Health. 1997;87(4):597–603. doi: 10.2105/ajph.87.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh ES, Flynn N. Segregation and crime: The effect of black social isolation on the rates of black urban violence. Social Forces. 1996;74(4):1325–1352. [Google Scholar]
- Sims M, Sims TL, Bruce MA. Urban poverty and infant mortality rate disparities. J Natl Med Assoc. 2007;99(4):349–356. [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter D, Best N, Carlin B, van der Linde A. Bayesian measure of model complexity and fit. Jounral of the Royal Statistical Society. 2002;64(4):583–639. [Google Scholar]
- Sturtz S, Ligges U, Gelman A. R2WinBUGS: A package for running WinBUGS from R. Journal of Statistical Software. 2005;12(3):1–16. [Google Scholar]
- Sucoff CA, Upchurch DM. Neighborhood context and the risk of childbearing among metropolitan-area black adolescents. American Sociological Review. 1998;63(4):571–585. [Google Scholar]
- US Census Bureau 2000 Decennial Census. 2000 Summary File 3. [Google Scholar]
- Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121(3):547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.