Abstract
Over the past decade, the existence of transgenic mouse models in which reporter genes are expressed under control of the gonadotropin-releasing hormone (GnRH) promoter has made possible the electrophysiological study of these cells. Here we review the intrinsic and synaptic properties of these cells that have been revealed by these approaches, with a particular regard to burst generation. Advances in our understanding of neuromodulation of GnRH neurons and synchronization of this network are also discussed.
Introduction
The gonadotropin-releasing hormone (GnRH) neuronal system is the final common pathway for central regulation of fertility. Through its regulation of the synthesis and secretion of the pituitary gonadotropins luteinizing hormone (LH, often used as a surrogate marker for GnRH release) and follicle-stimulating hormone (FSH), GnRH exerts control over the hypothalamo-pituitary gonadal axis. Multiple inputs from the internal environment (e.g., steroid hormones, energy balance, stress) and external environment (e.g., photoperiod, pheromones, endocrine disruptors) can alter fertility through central actions that ultimately alter the release of GnRH. Even before the sequencing of the GnRH decapeptide [7], both the innate function of this system and its response to various stimuli were intensely studied. During much of this time, the ability to tease out the neurobiological mechanisms underlying observations of altered GnRH or LH release was limited by the technical approaches available and the scattered distribution of GnRH neurons. It was possible, for example, to determine if a factor acted centrally by intracerebroventricular application or treatment of hypothalamic explants or cultures, and to speculate about upstream afferents through the localization of steroid receptors or expression of the immediate early gene cFos [57,77,127], knife cut studies [10] or localized administration of steroids [87]. But understanding the intrinsic, synaptic and network properties of these neurons was very limited in mammalian systems. In contrast, electrophysiological work in the teleost Dwarf Gourami provided considerable insight into pacemaker activities of the terminal nerve GnRH neurons, which can be easily identified by eye [105,106].
Despite the limitations largely precluding direct study of mammalian GnRH neurons, a great deal of knowledge about the reproductive neuroendocrine system was acquired and has served as the basis for designing and interpreting experiments that are now taking advantage of new model systems. The pioneering work of Ernst Knobil’s group describing circhoral oscillations of LH in the circulation [34], the importance of episodic GnRH stimulation for pituitary function [8,156] and central electrical correlates with LH release [157] still provides the fundamental description of this system as a pulse generator, and drives many current research questions. Several groups independently developed methods to measure directly GnRH release [13,28,78,79]. Both immortalized GnRH neuron cell lines [88,118] and primary embryonic cultures derived from the migratory pathway of GnRH neurons first in the primate [144] and later in the mouse [41], also provided insight into the function of this system. None of these approaches, however, allowed examination of native adult GnRH neurons in the context of at least a portion of their normal network using the powerful electrophysiological methods that were being applied to other central neuronal cell types that could be identified by anatomical location.
Martin Kelly’s group was the first to study individual GnRH neurons using electrophysiological recordings in acutely prepared brain slices, demonstrating that GnRH neurons were acutely hyperpolarized by estradiol in the guinea pig model [66]. This and subsequent work in this model [73,150] identified the first intrinsic properties of mammalian GnRH neurons. The effort needed to accumulate GnRH neurons, however, was heroic; in the first study, which utilized procion yellow labeling during blind sharp electrode recordings followed by post hoc identification, only five of 102 cells studied were GnRH positive on post hoc examination.
About a decade ago, promoter-driven reporter genes were applied to the identification of GnRH neurons. The cell-specifically and strongly expressed GnRH promoter is well suited to this approach and was used to drive expression of modified jellyfish Aequorea victoria green fluorescent protein (GFP), beta galactosidease or the calcium indicator pericam [49,61,62,65,129,132,139,155] in GnRH neurons from mouse, rat and medaka. Primarily using the GFP models, considerable progress has been made over the past decade in understanding of the neurobiology of GnRH neurons. These studies will form the basis of this review. The reader is also pointed to a recent excellent review on intracellular calcium dynamics in GnRH neurons [60], and previous reviews on the physiology of the GnRH neuronal system [53,92].
An overview of electrophysiological approaches used in the GnRH system
The purpose of this review is not to provide a complete primer on electrophysiological approaches, which are available elsewhere [55,125]. What follows is a brief overview of the different approaches that have been used on GnRH neurons, their plusses and minuses and the types of data that can be acquired with each.
Targeted extracellular
In this type of recording, a pipette filled with a solution mimicking the extracellular milieu is placed in contact with a cell under visual control [99]. Although no high-resistance seal is formed, the signal obtained is essentially limited to the contacted cell. This approach records the action currents that underlie action potential firing. A main advantage is the pattern of activity of a neuron can be observed for hours without altering its intracellular milieu. The main disadvantage is that the type of information is limited to firing pattern and underlying mechanisms cannot be probed except at a rudimentary level such as determination if a particular type of input is required for a response [112].
Patch-clamp
In this type of recording, a high-resistance (“gigaohm”) seal is formed between the pipette and the target cell’s membrane; the tight seal greatly reduces electrical noise. There are several varieties of this approach.
On-cell-recordings are made after a gigaohm seal has been obtained without subsequently penetrating the membrane. This maintains intracellular milieu while allowing recording of channel activity within the membrane circumscribed by the pipette or of action current activity. Disadvantages include lack of access to channel elements beyond the pipette, and that the recordings can spontaneous rupture to the whole-cell configuration.
Whole-cell
This is the most versatile of recording modes. From the on-cell configuration the membrane within the pipette is broken while maintaining the gigaohm seal. This provides electrical access to the interior of the cell and allows the pipette solution to dialyze the cell. The advantage of this dialysis is that one can manipulate ionic or other composition to favor isolation and detection of conductances via specific ion channels. The disadvantage is that cellular constituents that are important for ion channel and other function can become diluted.
Perforated-patch
Perforated-patch is a modification of the whole-cell approach in which electrical access is gained not by physically breaking the membrane within the pipette but rather by inclusion in the pipette of a pore-forming antibiotic such as nystatin, amphotericin or gramicidin. This minimizes dialysis of important cellular components. Gramicidin is particularly useful in studies of the intrinsic ion channel of the GABAA receptor as the current through this channel is primarily carried by the chloride ion and gramicidin pores are not permeable to chloride [72]. The disadvantages of these recordings are reduced stability (recordings must be monitored to ensure they do not spontaneously rupture to the whole-cell configuration), increased noise due to pore disruption of the gigaohm seal and, importantly, reduced access to the cell, which can negatively impact recording quality.
Both voltage-clamp and current-clamp can be made in whole-cell or perforated-patch configurations. In voltage-clamp, the cell’s membrane potential is clamped at specific values via feedback circuits in the amplifier. The current needed to maintain the commanded potentials are recorded. Specific channel blockers and membrane potential changes can be used to isolate and record individual currents. In current-clamp, membrane potential is monitored. Clamp is a misnomer as all that is “clamped” is the current input to the cell. The cell membrane is able to respond to not only any clamped current input but also any intrinsic or synaptic changes that subsequently occur; membrane potential changes are not regulated by amplifier feedback circuitry as they are in voltage-clamp.
It is important to monitor quality of whole-cell and perforated-patch recordings throughout a study. Series resistance (Rs, also called access resistance, Ra) is a function of the resistance of the recording pipette (Rs is minimally twice this value) and the quality of the recording. As Rs increases, the ability to accurately record signals is reduced. Changes in Rs can alter measured currents independent of treatment; as Rs increases, current amplitude decreases and duration increases. In our experience, a change of >10% makes a recording unacceptable.
Intrinsic properties of GnRH neurons
Observations of the episodic activity of multiunit electrical activity and hormone release at the whole animal level [90,157] coupled with the electrophysiology magnocellular neuroendocrine neurons [5,38,39,96,151,152] showing that action potential firing, particular in bursts, was needed for hormone release, generated several hypotheses about the electrophysiological properties of GnRH neurons that were testable with the development of GnRH-reporter gene mice. That GnRH neurons would fire action potentials was almost beyond question. The mechanisms by which firing was generated and the pattern of firing were, and remain, important questions. There are two main mechanisms by which a neuron’s membrane potential can be depolarized to the threshold for action potential firing: intrinsic changes in ion conductances and synaptic conductances. The evidence presented below support the working hypothesis that GnRH neurons can intrinsically generate patterned firing activity but that they may require external or network interactions to generate fully functional pulsatile hormone release.
Studies using both whole-cell and extracellular recordings found a majority of GnRH neurons in acutely prepared brain slices, as well as those mechanically isolated from brain slices or in cultures of embryonic mouse and primate neurons, fired spontaneous action potentials, or spikes [3,70,71,101,128,132,140]. Spontaneous here means that the cells fired without input from the experimenter, but does not differentiate between synaptically generated vs. intrinsically generated spikes. Quiescent cells were also observed, as were periodic transitions from firing to quiescence. Quiescence is likely an important contributor in this system to the generation of interpulse intervals of hormone release.
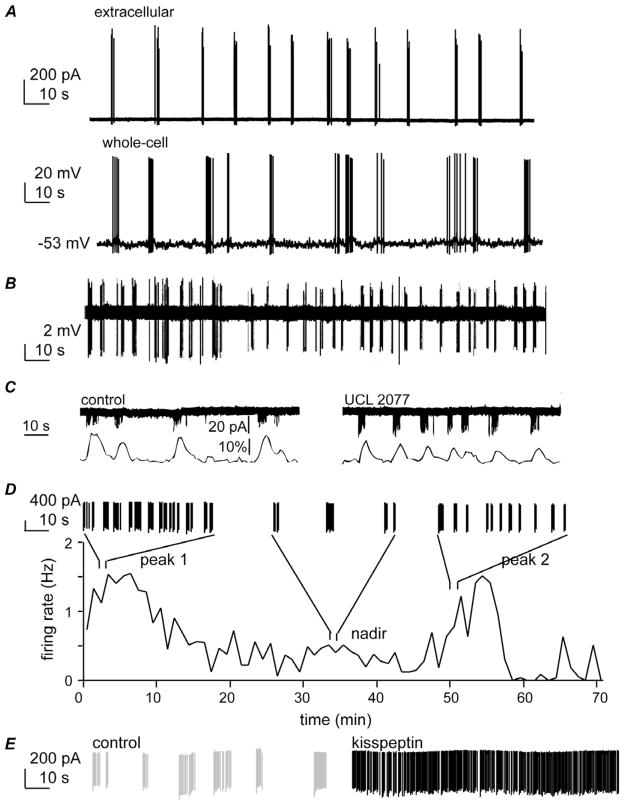
Importantly, in various preparations, the majority of spontaneously firing neurons exhibited bursts of action potentials. Bursts were comprised of two to several action potential spikes and intraburst frequency varied from ~2–25 Hz. Interburst period varied from a few to many seconds both within and among cells [3,70,75,102,140]. Of interest, bursts recorded in the whole-cell and extracellular configurations have similar characteristics (Figure 1A), indicating fundamental function of GnRH neurons is preserved in whole-cell recordings (e.g., [23,101]). Several lines of evidence indicated that spontaneous bursting activity is intrinsic, arising at least in part from GnRH neurons themselves. First, pure cultures of immortalized GT1 GnRH neurons exhibit burst firing [15]. Second, burst and action potential firing persist in GnRH neurons within brain slices after blockade of ionotropic GABA and glutamate receptors, which receive fast synaptic transmission inputs to these cells [101]. Third, and perhaps the most convincing demonstration that synaptic input is not needed for GnRH neurons to fire spontaneously, isolated GnRH neurons continue to fire in a burst pattern (Figure 1B, [70]). Moreover, the bursting pattern of these isolated neurons varied over time suggesting longer-term changes in GnRH neuron activity may also be intrinsic to these cells (Figure 1D). Interestingly, irregular burst firing is also a characteristic of hypophysiotropic fish GnRH neurons, in contrast to the strict pacemaker activity of type 2 and type 3 GnRH neurons in fish [62].
Figure 1.
Importance of burst firing to GnRH neuron physiology. A. Burst firing examples in extracellular (top) and whole-cell (bottom) recordings (Chu and Moenter, unpublished). B. Isolated GnRH neurons exhibit burst firing, indicating synaptic input is not needed for this activity (with permission from [70]). C. Blocking slow calcium-activated potassium channels reduces interburst interval (top) and associated changes in intracellular calcium levels (bottom, with permission from [75]. D. GnRH neurons exhibit endogenous shifts in interburst interval (with permission from [102]). E. Burst firing of an extracellularly recorded GnRH neuron under control conditions (grey) and 8 minutes after exposure to 1 nM kisspeptin, illustrating that neuromodulators can alter GnRH neuron burst properties (Pielecka-Fortuna and Moenter, unpublished). Together these data suggest bursting is an intrinsic characteristic of GnRH neurons that spontaneously changes over time perhaps due to changes in neuromodulation in the network.
The ionic conductances intrinsic to GnRH neurons that underlie this firing pattern have been an area of intense interest. A cell’s resting, or interspike, membrane potential is important to burst firing as it helps determine the types of ion channels that are active; the ion channels present and the distribution of ions that flow through these channels are in turn critical determinants of the cell’s interspike potential. The interspike potential of the majority of GnRH neurons is between −65 and −50 mV recorded in either whole-cell or perforated-patch configuration [32,70,71,75,132,139,140], with embryonic and isolated GnRH neurons tending to be more towards the depolarized end of that range. Measurements made using a non-invasive method [148] suggested a more hyperpolarized membrane potential of ~−75 mV [31]. The latter is of interest for potential activation of channels requiring hyperpolarization, discussed below.
Another important characteristic of mammalian GnRH neurons is that they have a high input resistance (Rin). Rin measures cell membrane conductivity. Under physiological conditions, GnRH neuron Rin is typically near one gigaohm [32,70,75,139,140,161]. This indicates these cells have relatively few channels open near their resting or interspike potential. Lower Rin has been reported in GnRH neurons with longer dendrites, perhaps reflecting more active channels in this segment of membrane [119]. Because Rin is high, small currents can have a large effect on membrane potential. This is important, as many of the conductances that contribute to intrinsic firing activity are relatively small.
Thus far, several promising candidates have been identified that may be involved in aspects of burst firing in GnRH neurons: T-type calcium currents (IT), calcium-activated potassium currents of the afterhyperpolarization (IKCa), persistent sodium currents and sodium currents of the afterdepolarization (IsADP) and hyperpolarization-activated non-specific cation currents (Ih).
T-type (low-voltage activated) calcium conductances
T-type calcium currents are intriguing with regard to burst initiation. T-type channels are an example of voltage-gated inactivating channels, a group that also includes the sodium channels underlying action potential generation and some varieties of potassium channels and calcium channels [55]. These channels have two voltage-sensitive molecular gates that control ion flux, and both must be open for current to flow. The activation gate opens with depolarization allowing current to flow, but this flow is transient as the inactivation gate closes with depolarization, typically soon after opening of the activation gate. Hyperpolarization, such as after an action potential, in response to an inhibitory input or due to an inherently hyperpolarized interspike potential, closes the activation gate but opens the inactivation gate allowing for subsequent depolarization to activate the channel.
GnRH neurons from rats and mice express CaV3 subunits, which code for the conducting alpha subunit of T-type channels [63,164]. T-type channels can be activated at hyperpolarized membrane potentials, such as the interspike potential [109]. The inward current through these channels can serve as a driving force for depolarization to action potential threshold and thus spike or burst initiation. In mouse GnRH neurons, T-type channels activate beginning about −70 mV and are one contributor to rebound action potential firing after hyperpolarization in GnRH neurons [100,136,164]. In the rat, activation of T-type channels is only observed at more depolarized potentials [63,65], thus whether they are involved in burst initiation in this species remains to be determined.
Calcium-activated potassium currents
Once an action potential is initiated, the depolarization during its spike activates other channels that contribute to the characteristic repetitive firing of the burst as well as to its eventual termination. In GnRH neurons, the spike of the action potential is followed first by a very characteristic afterhyperpolarization (AHP), during which the membrane is more hyperpolarized that before spike initiation, and then by a slow afterdepolarization (sADP), during which the membrane is more depolarized than before spike initiation. The AHP in many neurons, including GnRH neurons, is at least in part shaped by activation of calcium-activated potassium channels (IKCa). As the name implies, these channels have increased probability of opening in the presence of elevated intracellular calcium. Messenger RNA for both large (BK) and small (SK) conductance calcium-activated potassium channels are expressed and functional in GnRH neurons from mouse, rat and guinea pig [11,23,26,56,64,75,81,130,131]. BK channels have very rapid kinetics and contribute, along with A-type, D-type and delayed rectifier channels [32,108,124], to the repolarization of the membrane during the downstroke of the action potential spike. BK channels can also participate in early spike frequency adaptation [45]. SK channels have slower kinetics, decaying over a few hundred milliseconds, and help shape the AHP and contribute to later aspects of spike frequency adaptation in some neurons [44] but not in others [40].
Spike frequency adaptation is the gradual slowing and sometimes cessation of action potentials upon prolonged (100s of milliseconds) depolarization. The extent of spike frequency adaptation exhibited by GnRH neurons varies with reports. In some, there is a small reduction in spike frequency but no cessation of firing during a depolarizing current injection [11,23,26,32,132] whereas in others spikes attenuate more quickly and completely [64,75,81]; in the latter, blocking SK channels with apamin ameliorates the cessation of firing. The reasons for these differences in spike frequency adaptation are not clear. The reports of minimal adaptation used the whole-cell or sharp electrode recordings, whereas those in which strong adaptation was observed utilized perforated-patch. It is possible that the caveats of either approach contribute to the results obtained. Reduced access or seal leak with perforated-patch could contribute to adaptation to depolarizing current; likewise, a critical component for adaptation could be dialyzed from the cell in the whole-cell mode. Arguing against the latter, the burst duration in GnRH neurons in whole-cell and in extracellular recordings is similar, suggesting burst termination is still possible in the whole-cell configuration. Upon prolonged induced or spontaneous action potential firing in GnRH neurons, AHP amplitude is reduced as the spike train proceeds; this is more severe with greater spike frequency [81]. The reduced AHP is accompanied by mild [23,81,132] to severe [64,128] attenuation of spike amplitude. These are likely related phenomena; both may arise as a result of reduced recovery from inactivation of the fast sodium and potassium channels underlying the upstroke and repolarization of the spike, respectively, as a spike train proceeds. In this regard, reduction of AHP amplitude by blocking BK channels in magnocellular neurons causes increased spike failure [69].
A recent paper suggested involvement of a third type of calcium-activated potassium current in burst firing of GnRH neurons [75]. This is a very slow current (referred to as sIAHP-UCL based on pharmacology) that can take seconds to decay and is conducted via molecularly uncharacterized channels [108,149]. There was a very nice correlation between burst firing and transient elevations in intracellular calcium in GnRH neurons, which lasted ~10 seconds [75], suggesting an interaction between these phenomena. Consistent with this, Ca transients were dependent upon burst firing, and both intra and extracellular calcium, the later of which appears to enter via L-type channels that would be activated during an action potential spike [65,136]. Elevated intracellular calcium provides a mechanism for activation of all three types of IKCa during a burst.
Interestingly, about 40% of GnRH neurons exhibited an interburst interval of ~20 seconds. This is similar to the period of large oscillations in membrane potential observed in ~5% of GnRH neurons [24] and some other reports of burst firing (Figure 1). What might generate this apparently characteristic interval? Induction of calcium-activated potassium channels by a prolonged (600 ms) depolarizing (−60 mV to 60 mV) step revealed currents that could be blocked by a combination of apamin, which inhibits some SK-mediated currents, and UCL2007, which blocks the slow uncharacterized conductance sIAHP-UCL. The latter conductance peaked ~20 seconds after the depolarizing step and was proposed to regulate interburst interval. In extracellular recordings of burst firing, blocking this conductance increased the number of bursts/unit time, consistent with this hypothesis (Figure 1C). It is important to point out, however, that the extent to which the current is activated during a burst of firing in GnRH neurons will require further investigation. A protocol resembling a single action potential spike (2 ms depolarization from −60 mV to +20 mV) generated a current that was similar in amplitude and apamin sensitivity, but had dissipated within ~10 ms [23], suggesting current during a normal burst may not achieve the same duration as following a prolonged depolarization, although it would likely persist longer than observed after a single spike. Nonetheless, this observation is of interest as the channels conducting sIAHP-UCL are a downstream target for a number of neurotransmitters and neuromodulators [149], thus might be a point of modulation of GnRH neuron intrinsic properties by afferent inputs. Interesting questions for future studies are whether modulation of this conductance underlies intrinsic changes in interburst intervals within the same GnRH neuron, which have been observed in longer recordings [102], and whether neuromodulators induce changes in this conductance or an intrinsic cycle in the GnRH neuron (Figure 1D, E). Of interest, GnRH iteslf alters pacemaking of teleost terminal nerve GnRH neurons in part via altering calcium-activated potassium and calcium conductances [2].
Hyperpolarization-activated non-specific cation currents (Ih)
Ih can contribute to rhythmic activity in both neuronal and non-neuronal systems [67,84–86]. GnRH neurons exhibit Ih [27,141,163]. Ih is a slowly activating and noninactivating conductance that tends to stabilize membrane potential [123]. Blocking this current in GnRH neurons leads to inhibition of action potential firing and also alters the properties of bursts. Ih increases the rate of membrane repolarization during the AHP, suggesting it interacts with calcium-activated potassium channels in regulating spike frequency during bursts [27]. Blocking Ih also reduces the number of spikes per burst, perhaps because of interactions with another conductance, IsADP, which is active for ~1s after action potential spikes.
IsADP
Following the AHP, GnRH neurons undergo a prolonged period of depolarization relative to the prespike potential called the slow afterdepolarization (sADP). This phenomenon is prolonged in GnRH neurons and other neuroendocrine neurons in comparison to that observed in hippocampal pyramidal neurons [80,89]. Dendritic structure may affect the amplitude of the sADP [119]. GnRH neurons exhibit both induced and spontaneous sADPs following AHPs, implying an intimate interconnection between these events [26]. The main current underlying the sADP following induced spikes is a TTX-sensitive current. Blocking calcium entry with the non-specific calcium channel blocker cadmium, which would subsequently reduce activation of calcium-activated potassium channels, alters the timing and amplitude of the sADP implying these various conductances interact in sculpting the final interspike waveform. Mouse GnRH neurons also exhibit a persistent sodium current that may contribute to depolarization to threshold and burst initiation in these cells as it does in Dwarf Gourami terminal nerve GnRH neurons [104,105], however mammalian GnRH neurons appear to lack resurgent sodium currents that contribute to repetitive firing in other cell types [68,154]. Ih may also be involved in sADPs following spontaneously generated spikes in GnRH neurons, but Ih does not appear to be activated following induction of single spikes; of note the AHP following induced spikes is of reduced amplitude, suggesting the hyperpolarization during the AHP is important for a fast component of Ih activation and any contribution of this current to the sADP. Aging reduces the sADP as well as spontaneous activity in GnRH neurons [153], implying this membrane phenomenon is important for generating GnRH neuron activity.
Other ionic conductances in GnRH neurons
In addition to the conductances discussed above that likely shape burst firing, GnRH neurons express other voltage-gated conductances. These cells exhibit a prominent transient A-type potassium current [32,163]. This current activates at potentials near the interspike potential and can act to delay the initiation of action potential firing in response to depolarization [32]. Current through A-type channels may oppose the action of IT or a persistent sodium current to initiate bursting. A-type as well as delayed rectifier potassium channels, which require the depolarization of the action potential to be activated, play an important role in spike repolarization and setting the amplitude of the AHP allowing other contuctances such as Ih to be activated. GnRH neurons also express ATP-sensitive potassium channels (KATP) that are involved in regulating response to metabolic cues in other neurons and pancreatic islets [163]. GnRH neurons in mice and rats also exhibit all major subclasses of high-voltage activated calcium currents (L, N, P/Q and predominantly R) [56,65,100,130,136]. Their activation during the spike can contribute to short-term changes in membrane current, calcium-induced calcium release signaling to the nucleus to alter gene transcription, and activation of Ca-dependent channels.
In sum, a number of intrinsic characteristics of GnRH neurons have now been described and an emerging picture is that alterations in burst firing pattern due to regulation of the above and potentially other ion channels will be critical aspects of GnRH neuron electrophysiology. Figure 1 illustrates a striking similarity in burst firing in studies across several labs. How the electrophysiological findings in single GnRH neurons at the millisecond to second timescale relate or translate to the episodic release of GnRH into the portal blood, which occurs with a period from several minutes to several hours, and to the surge mode of release, occurring once per cycle, have not been determined and are important topics for future research.
Steroid modulation of GnRH neuron firing pattern and underlying conductances
A critical aspect of the GnRH neuronal system is its feedback regulation by steroid hormones. GnRH neurons express the beta isoform of the estradiol receptor [54,58,59], but most other steroid receptors have only rarely, if at all, been detected in GnRH neurons. This has lead to an overall view in the field that steroid regulation of GnRH neurons engages steroid-sensitive afferent neurons [158]. Direct electrophysiological studies of GnRH neurons have supported and extended this concept in several ways.
Estradiol has long been of particular interest in the regulation of GnRH neurons because of its involvement in both negative homeostatic feedback in males and during much of the cycle in females, and positive feedback generation of the preovulatory GnRH surge in females [20,35,52,91]. Not surprisingly it is the best-studied steroid in terms of its electrophysiological effects. Much of this has been recently reviewed with regard to surge generation [20] and only the highlights will be addressed here. Ovariectomy with replacement with estradiol capsules producing physiological levels generates daily afternoon LH surges in mice for ~ 5 days [18], similar to rats and hamsters [76,97]. The daily switch between negative feedback in the AM and positive feedback in the PM is mediated by the alpha isoform of the estradiol receptor [17], and provides a model to examine these different feedback modes without a change in estradiol level. During negative feedback, GnRH neuron activity is suppressed and quiescence increased. Mechanistically, this may be attributed to both synaptic (reduced GABAergic and glutamatergic transmission) and intrinsic (reduced whole-cell calcium current) changes [19,22,136]. In contrast, during positive feedback, GnRH neuron activity, GABAergic transmission and whole-cell calcium current are elevated. Although burst pattern has not been formally analyzed in this model, some speculation is possible. First, synaptic initiation of bursting would be reduced during negative and increased during positive feedback. Second, the reduction of whole-cell calcium current during negative feedback might reduce activation of IKCa leading to bursts with fewer spikes, further reducing activity. The opposite would be predicted for negative feedback.
Longer times post ovariectomy plus estradiol can be used to examine negative feedback [102]. Long-term extracellular recordings of GnRH neurons reveal peaks and troughs in firing rate that occur with an interval similar to episodic hormone release in vivo [101,110,111]. Fast-Fourier transform examination of repetitive activity in these longer recordings reveals that interburst interval is reduced during peaks in firing rate. Estradiol negative feedback did not alter burst characteristics in this model, but did alter longer term patterning of the bursts. Specifically, estradiol reduced the rate at which bursts became closer together (peak in firing) and further apart (trough in firing) [102].
Progesterone and androgens also influence overall GnRH neuron firing rate. Although detailed studies of burst dynamics have not yet been performed, it will be interesting to determine if similar changes in burst properties are revealed, and to identify the conductances being altered to effect these changes, and the mechanisms (neuromodulation?) by which these changes are brought about. Both synaptic [134] and intrinsic mechanisms are likely involved. With regard to the latter, calcium currents have recently been shown to be modulated by progesterone and DHT, suggesting they and downstream calcium-sensitive mechanisms may be a central point for burst regulation [137].
Estradiol, as well as other steroids, can also have rapid actions on cells [51,66,73]. In mouse GnRH neurons, acute application of estradiol has differential effects that depend on dose and engage different mechanisms [23]. Low physiological levels present during most of the cycle inhibited GnRH neurons in an ERα-dependent manner that involved reductions in GABAergic transmission. In contrast, high physiological estradiol levels excited GnRH neurons directly via ERβ, inducing changes in the currents underlying the AHP and sADP, inhibiting the former and increasing the latter. These changes were both mimicked and occluded by blockers of BK and SK channels. The temporal relationship among the underlying currents is key to producing the membrane response; the combination of BK and SK blockers does not affect IsADP amplitude, but does reduce the latency to the peak of this current, suggesting IAHP, which flows in the opposite direction to IsADP, may delay onset of IsADP [26]. Primate embryonic GnRH neurons were also acutely excited by high physiological estradiol [3] via classical receptors and potentially the membrane estradiol receptor GPR30 [94,95,143]. Of interest, estradiol rapidly altered the burst firing pattern in the primate neurons, which raises the interesting question of whether rapid alterations in IKCa produced the increase in burst duration and intraburst frequency [3]. The acute effects of estradiol have not been carefully examined in this regard in the mouse, merely the effects on overall firing frequency [23]. Acute application of estradiol did increase L and R type calcium currents via ERβ and GPR30 dependent mechanisms, respectively, in the mouse [136] making it likely that burst dynamics are altered.
Neurotransmitters, neuromodulators and GnRH neuron function
When discussing either acute or chronic effects of steroids on GnRH neurons, the likelihood of intermediate neurons being involved necessitates an examination of these factors. The primary conveyors of fast synaptic transmission (mediated by ionotropic vs. metabotropic receptors) are GABA and glutamate. GnRH neurons receive spontaneous glutamatergic transmission via both AMPA/KA and NMDA receptors [16,22,132,138], but the frequency of these currents is low and not all GnRH neurons exhibit evidence of spontaneous innervation. This may be an artifact of recordings made at the cell soma as small currents generated in the distal dendrites might not be detected but might still be important for cell function. In this regard, AMPA-mediated currents in the proximal dendrite of modeled GnRH neurons can contribute to action potential initiation [120] whereas those received in distal segments of the dendrite cannot. In this particular model, the assumption was made that GnRH neuron dendrites are passive and cannot generate action potentials. Recent work has demonstrated expression of voltage-gated sodium channels in GnRH neuron dendrites and also shown that perhaps as many as 2/3 of GnRH neuron action potentials are initiated in dendrites [121]. This is an intriguing finding as it suggests that the distal inputs may be able to contribute to GnRH neuron firing by generating action potentials that can propagate through the thin fiber of the dendrite. An interesting question for future study will be whether the proportion of action potentials generated in different cell regions is altered by reproductive state; for example a steroid milieu that suppresses sodium channel trafficking to dendrites would be expected to make GnRH neurons less responsive to distal inputs.
Essentially all GnRH neurons receive GABAergic transmission. The consequence of GABAA receptor activation has been controversial in the GnRH field but is a topic of another recent review (Herbison and Moenter, in preparation) and will not be discussed here. Studies of how various factors that regulate GnRH neurons alter GABAergic transmission demonstrate good agreement that factors that increase GnRH neuron activity (DHT, estradiol during positive feedback, kisspeptin) increase GABAergic transmission, whereas factors that reduce GnRH neuron activity (fasting, progesterone, estradiol negative feedback) reduce GABAergic transmission [16,19,25,113,133–135]. The interaction between GABAergic and glutamatergic inputs has not been addressed except in one modeling study, which suggests that a GABA-mediated event correctly timed before a sub-threshold AMPA-mediated event can lead to summation needed for action potential initiation [122]. This result mandates further studies of the patterning of these two inputs in different physiological milieu.
A plethora of neuromodulators has been proposed to regulate GnRH neurons. With the advent of direct electrophysiological studies, we have begun to understand which of these act directly, which act indirectly and what the mechanisms are. RT-PCR amplification of mRNA from identified GnRH neurons has shown they express receptors for a number of neuroactive substances [146]. Here we will briefly review those that have been directly studied using electrophysiology.
Kisspeptin is a neuromodulator that was identified through genetic screens of patients with hypothalamic infertility [30,126]. Its excitatory actions on GnRH release have been extensively studied and reviewed [4,29,103] and here only the electrophysiological aspects will be touched upon. The ability of kisspeptin to increase GnRH neuron activity increases during postnatal life through adulthood [46]; a loss in responsiveness of kisspeptin neurons may contribute to age-related changes in reproduction [74,93]. The kisspeptin receptor, GPR54, is expressed on GnRH neurons and can act directly via a number of mechanisms including activation of non-specific cation channels and closure of potassium channels, including inhibiting induction of inwardly rectified potassium currents by GABAB receptor agonists [82,112,165,166]. Kisspeptin also excites other central neurons [6,112]. Estradiol potentiates the excitatory actions of kisspeptin on GnRH neurons by increasing GABAergic and glutamatergic transmission to these cells [113]. All of these mechanisms would increase activity of GnRH neurons.
Kisspeptin has been heralded as essential for GnRH neuron function; it is important to point out, however, that there is evidence both in vivo and in vitro for GnRH neuron activity in the absence of kisspeptin [14,70]. Kisspeptin activated a majority of GnRH neurons in OVX mice treated or not with estradiol [112], but a smaller percentage in diestrous females ([37,46] Pielecka-Fortuna and Moenter unpublished observations). Of interest, GnRH neurons that express vGluT2 are sensitive to kisspeptin but not to agonists of group I metabotropic glutamate receptors [37], whereas GnRH neurons that are sensitive to mGluR agonists are not activated by kisspeptin. Thus there is considerable evidence for GnRH neurons that are not excited by kisspeptin, and thus might drive fertility in the absence of this peptide.
Kisspeptin is an RFamide peptide. Another RFamide that is emerging as a regulator of GnRH neurons is RFRP-3, which is the mammalian analogue of gonadotropin-inhibitory hormone (GnIH) originally identified in birds to inhibit pituitary gonadotropin release [147]. RFRP-3 inhibits GnRH neurons in brain slices from mice; the inhibition persists in the absence of fast synaptic transmission and appears to activate a potassium current suggesting it is a direct effect on GnRH neurons [36,160]. Interestingly this peptide could prevent or reverse activation of GnRH neurons by kisspeptin suggesting intracellular interactions, such as mentioned above for GABAB, determine the net effect up the GnRH neuron [160].
Several other modulators have been examined for effects on GnRH neuron activity. These include direct inhibition of GnRH neurons by norepinephrine via alpha 1 or beta adrenergic receptors [47], by somatostatin via somatostatin receptor 2 [9], and by melanin-concentrating hormone via MCHR1 through activation of a potassium channel [159]. Finally, vasoactive intestinal polypeptide (VIP) activates GnRH neurons in an estradiol and time-of-day dependent manner, that may be direct and/or transsynaptic [21].
Coordination of GnRH neurons
The episodic nature of GnRH release mandates some level of coordination among these cells. Studies in GT1 cells suggested that not every cell participates in every burst of activity [98]. Similarly, oscillations in intracellular calcium levels indicate coordination among a part of the population [1]. Unfortunately, relatively little experimental evidence can positively support a mechanism for how the GnRH neuronal network produces pulses, however some findings that point to future experiments are worthy of note.
Work in the primate embryonic GnRH culture system suggests a possible role for non-neuronal cells [142,145] in synchronizing calcium oscillations. In this regard, GnRH neurons appear to form local feedback circuits to regulate their upstream GABAergic neurons and preliminary evidence supports a potential role for the gliotransmitter prostaglandin in this process [25,43]. Glial-GnRH neuron interactions have also been proposed to be important for pubertal maturation of the GnRH neurosecretory system [83,115,117], and recent work has shown functional interactions between GnRH neuron terminals and tanacytes are very plastic and related to neurosecretion [114,116].
A very interesting recent report examined the dendritic structure of GnRH neurons [12]. This study found considerable bundling and appositions among GnRH neuronal dendrites particularly in the vertical orientation. Occasional “horizontal” GnRH neuron orientations were observed that crossed several vertically organized bundles. No vesicles nor gap junctions were identified at points of GnRH dendrite to GnRH dendrite contact within the GnRH neurons, suggesting neither traditional synaptic or gap junction communication mechanisms are utilized, unlike in teleost terminal nerve GnRH neurons [50]. It is still possible that this anatomical organization participates in the communication among GnRH neurons. Shared synaptic inputs to GnRH neurons were also observed, suggesting input from outside the GnRH network may be involved in synchronization. Coupled with recent reports that GnRH neuron dendrites can exhibit active properties [121], this is an interesting observation that will require further study.
GnRH itself has been proposed as a regulator of GnRH neurons. Although expression of GnRH peptide is not needed for burst firing, other aspects of GnRH neuronal physiology might be affected [42]. In vivo studies indicate GnRH inhibits GnRH release [33,107]. In brain slice electrophysiology, GnRH had a dose dependent effect on firing rate in male mice, with low levels (20 nM) inhibiting firing and higher levels (2 μM) increasing firing [162]. GnRH also exerts a biphasic effect (transient decrease followed by acceleration) on terminal nerve GnRH neuron pacemaking activity in teleosts [2]. In slices from mouse brain, GnRH activates an M-type potassium current [161], suggesting the inhibitory effect is at least in part direct and that excitatory effects may be transsynaptic. In this regard, GnRH altered GABAergic transmission in a dose-dependent manner with 20 nM suppressing GABAergic transmission and 2 μM having no effect [16]; the effect of 2 μM GnRH on GABAergic transmission became stimulatory when signaling via Gi was blocked with pertussis toxin, suggesting a possible interaction between GnRH and other neuromodulators at upstream GABA neurons. A recent report found a different response to GnRH with most neurons being excited regardless of dose except in proestrous female mice [48]. At present no explanation exists; it is curious, however, that inhibition by GnRH is observed at the time when GnRH levels are likely to be the highest and the activity of these cells most prolonged.
Summary
The past decade has seen a major increase in our knowledge of the intrinsic and synaptic properties of GnRH neurons. The expanding repertoire of intrinsic and synaptic properties can continue to be incorporated into sophisticated models that generate new hypotheses to be tested about the network. Questions we are now poised to address include: What are the roles of non-GnRH neuron and non-neuronal elements in coordinating the network? How do the various conductances that contribute to burst firing, and have largely been studied individually, interact to sculpt the membrane response? Do functional subpopulations of GnRH neurons serve different roles, for example local circuit regulation, control of distal central elements vs. neuroendocrine function? What pattern of GnRH activity is optimal for hormone release? Where is GnRH release besides the median eminence and what is its function? And finally, how do steroid milieu, circadian signals, metabolic cues and other factors influencing reproduction, such as stress, alter the answers to these questions?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1 neurons. Endocrinology. 2008;149:5325–5327. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe H, Oka Y. Mechanisms of the modulation of pacemaker activity by GnRH peptides in the terminal nerve-GnRH neurons. Zoological Science. 2002;19:111–128. doi: 10.2108/zsj.19.111. [DOI] [PubMed] [Google Scholar]
- 3.Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akazome Y, Kanda S, Okubo K, Oka Y. Functional and evolutionary insights into vertebrate kisspeptin systems from studies of fish brain. J Fish Biol. 2010;76:161–182. doi: 10.1111/j.1095-8649.2009.02496.x. [DOI] [PubMed] [Google Scholar]
- 5.Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- 6.Arai AC. The role of kisspeptin and GPR54 in the hippocampus. Peptides. 2009;30:16–25. doi: 10.1016/j.peptides.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Baba Y, Arimura A, Schally AV. On the tryptophan residue in porcine LH and FSH-releasing hormone. Biochem Biophys Res Comm. 1971;45:483–487. doi: 10.1016/0006-291x(71)90844-8. [DOI] [PubMed] [Google Scholar]
- 8.Belchetz P, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 9.Bhattarai JP, Kaszas A, Park SA, Yin H, Park SJ, Herbison AE, Han S-K, Abraham IM. Somatostatin Inhibition of Gonadotropin-Releasing Hormone Neurons in Female and Male Mice. Endocrinology. 2010 doi: 10.1210/en.2010-0148. in press. [DOI] [PubMed] [Google Scholar]
- 10.Blake CA, Sawyer CH. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974;94:730–736. doi: 10.1210/endo-94-3-730. [DOI] [PubMed] [Google Scholar]
- 11.Bosch MA, Kelly MJ, Rønnekleiv OK. Distribution neuronal colocalization, and 17β-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in Guinea pig brain. Endocrinology. 2002;143 doi: 10.1210/endo.143.3.8708. [DOI] [PubMed] [Google Scholar]
- 12.Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gondadotropin-releasing hormone neurons. Proc Natl Acad Sci, USA. 2009;106:10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraty A, Locatelli A. Effect of time after castration on secretion of LHRH and LH in the ram. Journal of Reproduction & Fertility. 1988;82:263–269. doi: 10.1530/jrf.0.0820263. [DOI] [PubMed] [Google Scholar]
- 14.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023. doi: 10.1111/j.1365-2826.2009.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles AC, Hales TG. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. Journal of Neurophysiology. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Moenter SM. GABAergic Transmission to Gonadotropin-Releasing Hormone (GnRH) Neurons Is Regulated by GnRH in a Concentration-Dependent Manner Engaging Multiple Signaling Pathways. J Neurosci. 2009;29:9809–9818. doi: 10.1523/JNEUROSCI.2509-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian CA, Moenter SM. The Neurobiology of Preovulatory and Estradiol-Induced Gonadotropin-Releasing Hormone Surges. Endocr Rev. 2010 doi: 10.1210/er.2009-0023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian CA, Moenter SM. Vasoactive Intestinal Polypeptide Can Excite Gonadotropin-Releasing Hormone Neurons in a Manner Dependent on Estradiol and Gated by Time of Day. Endocrinology. 2008;149:3130–3136. doi: 10.1210/en.2007-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol Suppresses Glutamatergic Transmission to Gonadotropin-Releasing Hormone Neurons in a Model of Negative Feedback in Mice. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.075077. biolreprod.108.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential Regulation of Gonadotropin-Releasing Hormone Neuron Activity and Membrane Properties by Acutely Applied Estradiol: Dependence on Dose and Estrogen Receptor Subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Z, Moenter SM. Acute estradiol alters gonadotropin-releasing hormone (GnRH) neuron excitability. Soc Neurosci Abstract Viewer. 2007 [Google Scholar]
- 25.Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci. 2005;25:5740–5749. doi: 10.1523/JNEUROSCI.0913-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26:11961–11973. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. doi: 10.1523/JNEUROSCI.1687-10.2010. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson J, Han S-K, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Molecular and Cellular Endocrinology. 2010 doi: 10.1016/j.mce.2010.01.026. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 30.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the Kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-Type {gamma}-Aminobutyric Acid Receptors Excites Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 32.DeFazio RA, Moenter SM. Estradiol Feedback Alters Potassium Currents and Firing Properties of Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol. 2002;16:2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 33.DePaolo LV, King RA, Carrillo AJ. In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology. 1987;120:272–279. doi: 10.1210/endo-120-1-272. [DOI] [PubMed] [Google Scholar]
- 34.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–853. doi: 10.1210/endo-87-5-850. [DOI] [PubMed] [Google Scholar]
- 35.Docke F, Dorner G. The mechanism of the induction of ovulation by oestrogens. J Endocrinol. 1965;33:491–499. doi: 10.1677/joe.0.0330491. [DOI] [PubMed] [Google Scholar]
- 36.Ducret E, Anderson GM, Herbison AE. RFamide-Related Peptide-3, a Mammalian Gonadotropin-Inhibitory Hormone Ortholog, Regulates Gonadotropin-Releasing Hormone Neuron Firing in the Mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 37.Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory Effects of the Puberty-Initiating Peptide Kisspeptin and Group I Metabotropic Glutamate Receptor Agonists Differentiate Two Distinct Subpopulations of Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. Journal of Physiology. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutton A, Dyball RE, Poulain DA, Wakerley JB. The importance of short interpulse intervals in determining vasopressin release from the isolated neurohypophysis [proceedings] Journal of Physiology. 1978;280:23P. [PubMed] [Google Scholar]
- 40.Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Developmental Biology. 1994;166:331–348. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- 42.Gill JC, Wadas B, Chen P, Portillo W, Reyna A, Jorgensen E, Mani S, Schwarting GA, Moenter SM, Tobet SA, Kaiser UB. The gonadotropin-releasing hormone (GnRH) neuronal population is normal in size and distribution in GnRH-deficient and GnRH receptor-mutant hypogonadal mice. Endocrinology. 2008;149:4596–4604. doi: 10.1210/en.2008-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glanowska KM, Moenter SM. Are astrocytes components of a local circuit modulating GABAergic transmission to GnRH neurons? Soc Neurosci Abstract Viewer. 2008 [Google Scholar]
- 44.Greffrath W, Mageri W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J Neuroendocrinol. 2004;16:577–588. doi: 10.1111/j.1365-2826.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- 45.Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han SK, Herbison AE. Norepinephrine Suppresses Gonadotropin-Releasing Hormone Neuron Excitability in the Adult Mouse. Endocrinology. 2008;149:1129–1135. doi: 10.1210/en.2007-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han SK, Lee K, Bhattarai JP, Herbison AE. Gonadotrophin-releasing hormone (GnRH) exerts stimulatory effects on GnRH neurones in intact adult male and female mice. J Neuroendocrinol. 2010;22:188–195. doi: 10.1111/j.1365-2826.2009.01950.x. [DOI] [PubMed] [Google Scholar]
- 49.Han SK, Todman MG, Herbison AE. Endogenous GABA Release Inhibits the Firing of Adult Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- 50.Haneda K, Oka Y. Coordinated Synchronization in the Electrically Coupled Network of Terminal Nerve Gonadotropin-Releasing Hormone Neurons as Demonstrated by Double Patch-Clamp Study. Endocrinology. 2008;149:3540–3548. doi: 10.1210/en.2008-0299. [DOI] [PubMed] [Google Scholar]
- 51.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets? Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 52.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 53.Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. 3. Raven Press; New York: 2006. pp. 1415–1482. [Google Scholar]
- 54.Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- 55.Hille B. Ionic Channels of Excitable Membranes. 3. Sinauer Associates, Inc; Sunderland: 2001. [Google Scholar]
- 56.Hiraizumi Y, Nishimura I, Ishii H, Tanaka N, Takeshita T, Sakuma Y, Kato M. Rat GnRH neurons exhibit large conductance voltage- and Ca2+-Activated K + (BK) currents and express BK channel mRNAs. J Physiol Sci. 2008;58:21–29. doi: 10.2170/physiolsci.RP013207. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman GE, Lee WS, Attardi B, Yann V, Fitzsimmons MD. Luteinizing hormone-releasing hormone neurons express c-fos antigen after steroid activation. Endocrinology. 1990;126:1736–1741. doi: 10.1210/endo-126-3-1736. [DOI] [PubMed] [Google Scholar]
- 58.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- 59.Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- 60.Jasoni CL, Romano N, Constantin S, Lee K, Herbison AE. Calcium dynamics in gonadotropin-releasing hormone neurons. Front neuroendocrinol. 2010 doi: 10.1016/j.yfrne.2010.05.005. in press. [DOI] [PubMed] [Google Scholar]
- 61.Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell Type-Specific Expression of a Genetically Encoded Calcium Indicator Reveals Intrinsic Calcium Oscillations in Adult Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2007;27:860–867. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanda S, Nishikawa K, Karigo T, Okubo K, Isomae S, Abe H, Kobayashi D, Oka Y. Regular pacemaker activity characterizes gonadotropin-releasing hormone 2 neurons recorded from green fluorescent protein-transgenic medaka. Endocrinology. 2010;151:695–701. doi: 10.1210/en.2009-0842. [DOI] [PubMed] [Google Scholar]
- 63.Kato M, Tanaka N, Ishii H, Yin C, Sakuma Y. Ca2+ Channels and Ca2+-Activated K+< Channels in Adult Rat Gonadotrophin-Releasing Hormone Neurones. Journal of Neuroendocrinology. 2009;21:312–315. doi: 10.1111/j.1365-2826.2009.01849.x. [DOI] [PubMed] [Google Scholar]
- 64.Kato M, Tanaka N, Usui S, Sakuma Y. The SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones. The Journal of Physiology. 2006;574:431–442. doi: 10.1113/jphysiol.2006.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of Voltage-Gated Calcium Currents in Gonadotropin-Releasing Hormone Neurons Tagged with Green Fluorescent Protein in Rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 66.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 67.Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends in Endocrinology & Metabolism. 2002;13:409–410. doi: 10.1016/s1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- 68.Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. Journal of Neuroscience. 2003;23:4899–4912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klyachko VA, Ahern GP, Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron. 2001;31:1015–1025. doi: 10.1016/s0896-6273(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 70.Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. Journal of Neuroscience. 2002;22:2313–2322. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3918–3922. doi: 10.1073/pnas.92.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. Journal of Neuroscience Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 73.Lagrange AH, Ronnekleiv OK, Kelly MJ. Estradiol-17 beta and mu-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 74.Lederman M, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–320. doi: 10.1016/j.neuropharm.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee K, Duan W, Sneyd J, Herbison AE. Two Slow Calcium-Activated Afterhyperpolarization Currents Control Burst Firing Dynamics in Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2010;30:6214–6224. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- 77.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 78.Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117:711–721. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- 79.Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107:1782–1790. doi: 10.1210/endo-107-6-1782. [DOI] [PubMed] [Google Scholar]
- 80.Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. Journal of Physiology. 1997;505:95–106. doi: 10.1111/j.1469-7793.1997.095bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X, Herbison AE. Small-Conductance Calcium-Activated Potassium Channels Control Excitability and Firing Dynamics in Gonadotropin-Releasing Hormone (GnRH) Neurons. Endocrinology. 2008;149:3598–3604. doi: 10.1210/en.2007-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Lee K, Herbison AE. Kisspeptin Excites Gonadotropin-Releasing Hormone Neurons through a Phospholipase C/Calcium-Dependent Pathway Regulating Multiple Ion Channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lomniczi A, Cornea A, Costa ME, Ojeda SR. Hypothalamic Tumor Necrosis Factor-{alpha} Converting Enzyme Mediates Excitatory Amino Acid-Dependent Neuron-to-Glia Signaling in the Neuroendocrine Brain. J Neurosci. 2006;26:51–62. doi: 10.1523/JNEUROSCI.2939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luthi A, Bal T, McCormick DA. Periodicity of thalamic spindle waves is abolished by ZD7288,a blocker of Ih. Journal of Neurophysiology. 1998;79:3284–3289. doi: 10.1152/jn.1998.79.6.3284. [DOI] [PubMed] [Google Scholar]
- 85.Luthi A, McCormick DA. Modulation of a pacemaker current through Ca(2+)-induced stimulation of cAMP production. Nature Neuroscience. 1999;2:634–641. doi: 10.1038/10189. [DOI] [PubMed] [Google Scholar]
- 86.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurons. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McManus CJ, Goodman RL, Llanza NV, Miroslav V, Dobbins AB, Connors JM, Hileman SM. Inhibition of luteinizing hormone secretion by localized administration of estrogen, but not dihydrotestosterone, is enhanced in the ventromedial hypothalamus during feed restriction in the young wether. Biol Reprod. 2005;73:781–789. doi: 10.1095/biolreprod.105.042275. [DOI] [PubMed] [Google Scholar]
- 88.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 89.Metz AE, Jarsky T, Martina M, Spruston N. R-Type Calcium Channels Contribute to Afterdepolarization and Bursting in Hippocampal CA1 Pyramidal Neurons. J Neurosci. 2005;25:5763–5773. doi: 10.1523/JNEUROSCI.0624-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130:503–510. doi: 10.1210/endo.130.1.1727719. [DOI] [PubMed] [Google Scholar]
- 91.Moenter SM, Chu Z, Christian CA. Neurobiological Mechanisms Underlying Oestradiol Negative and Positive Feedback Regulation of Gonadotrophin-Releasing Hormone Neurones. Journal of Neuroendocrinology. 2009;21:327–333. doi: 10.1111/j.1365-2826.2009.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 93.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–3708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G Protein-Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noel SD, Keen KL, Filardo EJ, Terasawa E. Possible role of G-protein-coupled receptor GPR30 in estrogen action in primate LHRH neurons, Abstract viewer/itinerary planner. Society for Neuroscience 2005 program no. 725.6. 2005 online. [Google Scholar]
- 96.Nordmann JJ, Stuenkel EL. Electrical properties of axons and neurohypophysial nerve terminals and their relationship to secretion in the rat. Journal of Physiology. 1986;380:521–539. doi: 10.1113/jphysiol.1986.sp016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norman RL, Spies HG. Neural control of the estrogen-dependent twenty-four-hour periodicity of LH release in the golden hamster. Endocrinology. 1974;95:1367–1372. doi: 10.1210/endo-95-5-1367. [DOI] [PubMed] [Google Scholar]
- 98.Nunemaker CS, DeFazio RA, Geusz ME, Herzog ED, Pitts GR, Moenter SM. Long-term recordings of networks of immortalized GnRH neurons reveal episodic patterns of electrical activity. Journal of Neurophysiology. 2001;86:86–93. doi: 10.1152/jn.2001.86.1.86. [DOI] [PubMed] [Google Scholar]
- 99.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biological Procedures Online. 2003;5:53–62. doi: 10.1251/bpo46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nunemaker CS, DeFazio RA, Moenter SM. Calcium current subtypes in GnRH neurons. Biology of Reproduction. 2003;69:1914–1922. doi: 10.1095/biolreprod.103.019265. [DOI] [PubMed] [Google Scholar]
- 101.Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–2292. doi: 10.1210/endo.143.6.8869. [DOI] [PubMed] [Google Scholar]
- 102.Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144:823–831. doi: 10.1210/en.2002-220585. [DOI] [PubMed] [Google Scholar]
- 103.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oka Y. Characterization of TTX-resistant persistent Na+ current underlying pacemaker potentials of fish gonadotropin-releasing hormone (GnRH) neurons. Journal of Neurophysiology. 1996;75:2397–2404. doi: 10.1152/jn.1996.75.6.2397. [DOI] [PubMed] [Google Scholar]
- 105.Oka Y. Tetrodotoxin-resistant persistent Na+ current underlying pacemaker potentials of fish gonadotrophin-releasing hormone neurones. Journal of Physiology. 1995;482:1–6. doi: 10.1113/jphysiol.1995.sp020494. [erratum appears in J Physiol (Lond) 1995 Mar 15;483(Pt 3):811] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oka Y, Matsushima T. Gonadotropin-releasing hormone (GnRH)-immunoreactive terminal nerve cells have intrinsic rhythmicity and project widely in the brain. Journal of Neuroscience. 1993;13:2161–2176. doi: 10.1523/JNEUROSCI.13-05-02161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology. 1995;62:248–258. doi: 10.1159/000127011. [DOI] [PubMed] [Google Scholar]
- 108.Pedarzani P, Stocker M. Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 110.Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74:931–937. doi: 10.1095/biolreprod.105.049619. [DOI] [PubMed] [Google Scholar]
- 111.Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147:1474–1479. doi: 10.1210/en.2005-1029. [DOI] [PubMed] [Google Scholar]
- 112.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin Acts Directly and Indirectly to Increase Gonadotropin-Releasing Hormone Neuron Activity and Its Effects Are Modulated by Estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pielecka-Fortuna J, Moenter SM. Kisspeptin Increases {gamma}-Aminobutyric Acidergic and Glutamatergic Transmission Directly to Gonadotropin-Releasing Hormone Neurons in an Estradiol-Dependent Manner. Endocrinology. 2010;151:291–300. doi: 10.1210/en.2009-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prevot V, Bellefontaine N, Baroncini M, Sharif A, Hanchate NK, Parkash J, Campagne C, Seranno Sd. Gonadotrophin-Releasing Hormone Nerve Terminals, Tanycytes and Neurohaemal Junction Remodelling in the Adult Median Eminence: Functional Consequences for Reproduction and Dynamic Role of Vascular Endothelial Cells. Journal of Neuroendocrinology. 2010;22:639–649. doi: 10.1111/j.1365-2826.2010.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity. Journal of Neuroscience. 2003;23:10622–10632. doi: 10.1523/JNEUROSCI.23-33-10622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prevot V, Dehouck B, Poulain P, Beauvillain JC, Buee-Scherrer V, Bouret S. Neuronal-glial-endothelial interactions and cell plasticity in the postnatal hypothalamus: implications for the neuroendocrine control of reproduction. Psychoneuroendocrinology. 2007:32S1. doi: 10.1016/j.psyneuen.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 117.Prevot V, Lomniczi A, Corfas G, Ojeda SR. erbB-1 and erbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology. 2005;146:1465–1472. doi: 10.1210/en.2004-1146. [DOI] [PubMed] [Google Scholar]
- 118.Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler GB, Jr, Wondisford FE. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3402–3406. doi: 10.1073/pnas.88.8.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roberts C, O’Boyle M, Suter K. Dendrites determine the contribution of after depolarization potentials (ADPs) to generation of repetitive action potentials in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Journal of Computational Neuroscience. 2009;26:39–53. doi: 10.1007/s10827-008-0095-5. [DOI] [PubMed] [Google Scholar]
- 120.Roberts CB, Best JA, Suter KJ. Dendritic Processing of Excitatory Synaptic Input in Hypothalamic Gonadotropin Releasing-Hormone Neurons. Endocrinology. 2006;147:1545–1555. doi: 10.1210/en.2005-1350. [DOI] [PubMed] [Google Scholar]
- 121.Roberts CB, Campbell RE, Herbison AE, Suter KJ. Dendritic Action Potential Initiation in Hypothalamic Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2008;149:3355–3360. doi: 10.1210/en.2008-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts CB, Hemond P, Suter KJ. Synaptic integration in hypothalamic gonadotropin releasing hormone (GnRH) neurons. Neuroscience. 2008;154:1337–1351. doi: 10.1016/j.neuroscience.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Ann Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 124.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurbiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 125.Sakman B, Neher E. Single-Channel Recording. 2. Plenum Press; New York, NY: 1995. [Google Scholar]
- 126.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. New England Journal of Medicine. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [see comment] [DOI] [PubMed] [Google Scholar]
- 127.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 128.Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. Journal of Neuroscience. 2001;21:1067–1075. doi: 10.1523/JNEUROSCI.21-03-01067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. Journal of Neuroscience. 1999;19:5955–5966. doi: 10.1523/JNEUROSCI.19-14-05955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spergel DJ. Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during and after puberty. Endocrinology. 2007;148:2383–2390. doi: 10.1210/en.2006-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spergel DJ, Catt KJ, Rojas E. Immortalized GnRH neurons express large-conductance calcium-activated potassium channels. Neuroendocrinology. 1996;63:101–111. doi: 10.1159/000126946. [DOI] [PubMed] [Google Scholar]
- 132.Spergel DJ, Ulrich D, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neuroscience. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sullivan SD, DeFazio RA, Moenter SM. Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. Journal of Neuroscience. 2003;23:8578–8585. doi: 10.1523/JNEUROSCI.23-24-08578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod. 2005;72:33–41. doi: 10.1095/biolreprod.104.033126. [DOI] [PubMed] [Google Scholar]
- 135.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun J, Chu Z, Moenter SM. Diurnal In Vivo and Rapid In Vitro Effects of Estradiol on Voltage-Gated Calcium Channels in Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun J, Moenter SM. Progesterone inhibits and androgen potentiates voltage-gated calcium currents in gonadotropin-releasing hormone (GnRH) neurons. doi: 10.1210/en.2010-0385. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suter KJ. Control of firing by small (S)-[alpha]-amino-3-hydroxy-5-methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Neuroscience. 2004;128:443–450. doi: 10.1016/j.neuroscience.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 139.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- 140.Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM. Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology. 2000;141:3731–3736. doi: 10.1210/endo.141.10.7690. [DOI] [PubMed] [Google Scholar]
- 141.Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons. Soc Neurosci Abstr. 2003;33 doi: 10.1523/JNEUROSCI.1687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Terasawa E, Keen KL, Grendell RL, Golos TG. Possible Role of 5′-Adenosine Triphosphate in Synchronization of Ca2+ Oscillations in Primate Luteinizing Hormone-Releasing Hormone Neurons. Mol Endocrinol. 2005;19:2736–2747. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- 143.Terasawa E, Noel SD, Keen KL. Rapid Action of Oestrogen in Luteinising Hormone-Releasing Hormone Neurones: The Role of GPR30. Journal of Neuroendocrinology. 2009;21:316–321. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133:2379–2390. doi: 10.1210/endo.133.5.8404690. [DOI] [PubMed] [Google Scholar]
- 145.Terasawa E, Richter TA, Keen KL. A role for non-neuronal cells in synchronization of intracellular calcium oscillations in primate LHRH neurons. Progress in Brain Research. 2002;141:283–291. doi: 10.1016/S0079-6123(02)41099-0. [DOI] [PubMed] [Google Scholar]
- 146.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 147.Tsutsui K, Bentley GB, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010 doi: 10.1016/j.yfrne.2010.03.001. in press. [DOI] [PubMed] [Google Scholar]
- 148.Verheugen JA, Fricker D, Miles R. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J Neuorsci. 1999;19:2546–2555. doi: 10.1523/JNEUROSCI.19-07-02546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci. 2003;18:3155–3166. doi: 10.1111/j.1460-9568.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 150.Wagner EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- 151.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Research. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- 152.Wakerley JB, Poulain DA, Dyball RE, Cross BA. Activity of phasic neurosecretory cells during haemorrhage. Nature. 1975;258:82–84. doi: 10.1038/258082a0. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Garro M, Dantzler HA, Taylor JA, Kline DD, Kuehl-Kovarik MC. Age Affects Spontaneous Activity and Depolarizing Afterpotentials in Isolated Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2008;149:4938–4947. doi: 10.1210/en.2008-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Y, Garro M, Kuehl-Kovarik MC. Estradiol attenuates multiple tetrodotoxin-sensitive sodium currents in isolated gonadotropin-releasing hormone neurons. Br Res. 2010 doi: 10.1016/j.brainres.2010.05.031. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wayne NL, Kuwahara K, Aida K, Nagahama Y, Okubo K. Whole-Cell Electrophysiology of Gonadotropin-Releasing Hormone Neurons that Express Green Fluorescent Protein in the Terminal Nerve of Transgenic Medaka (Oryzias latipes) Biology of Reproduction. 2005;73:1228–1234. doi: 10.1095/biolreprod.105.042721. [DOI] [PubMed] [Google Scholar]
- 156.Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- 157.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39:256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 158.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proceedings of the National Academy of Sciences. 2009;106:17217–17222. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. The Journal of Physiology. 2009;587:1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu C, Roepke TA, Zhang C, Ronnekleiv OK, Kelly MJ. Gonadotropin-Releasing Hormone (GnRH) Activates the M-Current in GnRH Neurons: An Autoregulatory Negative Feedback Mechanism? Endocrinology. 2008;149:2459–2466. doi: 10.1210/en.2007-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology. 2004;145:728–735. doi: 10.1210/en.2003-0562. [DOI] [PubMed] [Google Scholar]
- 163.Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang C, Bosch MA, Rick EA, Kelly MJ, Ronnekleiv OK. 17{beta}-Estradiol Regulation of T-Type Calcium Channels in Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang C, Bosch MA, Ronnekleiv OK, Kelly MJ. {gamma}-Aminobutyric Acid B Receptor Mediated Inhibition of Gonadotropin-Releasing Hormone Neurons Is Suppressed by Kisspeptin-G Protein-Coupled Receptor 54 Signaling. Endocrinology. 2009;150:2388–2394. doi: 10.1210/en.2008-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin Depolarizes Gonadotropin-Releasing Hormone Neurons through Activation of TRPC-Like Cationic Channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]