Abstract
Objective
To evaluate the effects and mechanisms of action of Vitamin D on human uterine leiomyoma (HuLM) cell proliferation in vitro.
Design
Laboratory study.
Setting
University hospitals.
Patients(s)
Not applicable.
Interventions(s)
Not applicable.
Main Outcome Measure(s)
HuLM cells were treated with 1, 25-dihydroxyvitamin D3 (Vitamin D) and cell proliferation was assayed by the MTT technique. PCNA, BCL-2, BCL-w, CDK-1 and COMT protein levels were analyzed by Western blotting. COMT mRNA and enzyme activity were assayed by quantitative RT-PCR and HPLC analysis, respectively. The role of COMT was evaluated in stable HuLM cells by silencing COMT expression.
Result(s)
Vitamin D inhibited the growth of HuLM cells by 47% ± 0.03 at 1 µM and by 38% ± 0.02 at 0.1 µM compared to control cells at 120 hours of treatment (P < 0.05). Vitamin D inhibited ERK activation and downregulated the expression of BCL-2, BCL-w, CDK1 and PCNA. Western blot, RT-PCR and enzyme assay of COMT demonstrated inhibitory effects of Vitamin D on COMT expression and enzyme activity. Silencing endogenous COMT expression abolished Vitamin D-mediated inhibition of HuLM cell proliferation.
Conclusion(s)
Vitamin D inhibits growth of HuLM cells through the down-regulation of PCNA, CDK1 and BCL-2, and suppresses COMT expression and activity in HuLM cells. Thus, hypovitaminosis D appears to be a risk factor for uterine fibroids.
Keywords: Vitamin D, uterine leiomyoma, proliferation, hypovitaminosis D, Catechol-O-methyltransferase, COMT
INTRODUCTION
Uterine leiomyomas (fibroids) are the most common benign tumors in uterus, occurring in 20–25% of premenopausal women (1–3). Myomectomy and hysterectomy are the only available surgical methods for treatment of uterine fibroids (4, 5). Uterine leiomyomas are 3–4 times higher in black women than in white women (6). The exact reason for such ethnic disparity is not clear. We have recently reported a differential ethnic distribution of specific functional genetic variants in estrogen receptor alpha and COMT, and their association with uterine fibroid susceptibility (7–9).
COMT is a ubiquitous enzyme that catalyzes the S-adenosyl-L-methyleonine-dependent methyl conjugate of the hydroxyl group of catechol estrogens. Regulation of COMT activity may modulate the biologic effects of estrogen and play an etiological role in uterine fibroid formation (10). We have recently reported increased COMT mRNA and protein expression in uterine leiomyoma in comparison to adjacent normal myometrium (10).
Solar ultraviolet B-Photons change 7-dehydrocholesteryl in the skin to pre-vitamin D3 which finally converts into Vitamin D3 (11). Vitamin D deficiency is associated with metabolic bone disease as well as cancers, Type-1 diabetes, and rheumatoid arthritis and schizophrenia (12). Vitamin D deficiency is more prevalent among African Americans (40–45%) compared to Caucasians (4%) (13). This high occurrence of Vitamin D deficiency in African Americans is due to the melanin levels in the skin, as well as an increased incidence of lactase deficiency affecting dairy product intake (13).
In this study, we demonstrate the growth inhibitory effect of Vitamin D on immortalized human uterine leiomyoma (HuLM) cells and that the inhibitory effects are exerted by decreasing the expression of PCNA, the cell cycle regulatory protein CDK1 and anti-apoptotic proteins BCL-2 and BCL-w. In addition, silencing the endogenous expression of COMT by COMT specific shRNA reduces the inhibitory effects of Vitamin D on HuLM cell proliferation. Our results indicate an important role for Vitamin D in regulating HuLM cell proliferation and thus the growth of uterine fibroids.
MATERIALS AND METHODS
Reagents and Antibodies
1, 25-dihydroxyvitamin D3 (Vitamin D), estradiol and beta-actin antibodies were purchased from Sigma-Aldrich (St. Louis, MO). PCNA, BCL-2, BCL-w, BCL-xL, CDK1, total ERK and the horseradish peroxidase conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-ERK antibody was purchased from Cell Signaling (Beverly, MA) and the COMT antibody was from Chemicon (Temecula, CA).
Cell Culture
The HuLM cell line was a kind gift from Dr. Darlene Dixon as described previously (14). HuLM cell line and shRNA-COMT cell lines were cultured and maintained in SmBm medium containing 5% FBS, 0.1% insulin, 0.2% hFGF-B, 0.1% GA-100 and 0.1% hEGF (Lonza, Walkersville, MD). Primary LM298 cells were established as described previously (15,16) according to the policies of the Institutional Review Board of the University of Texas Medical Branch, Galveston, TX and the Meharry Medical College, Nashville, TN (IRB #01-111). These cells were passaged three times and they express both estrogen and progesterone receptors, and maintained similar characteristics to HuLM cells. Once these cells were about 70% confluent in culture medium, the media was replaced by DMEM-12 (phenol free) supplemented with 10% charcoal stripped FBS and 10 nM estradiol, and then treated with Vitamin D (Sigma-Aldrich).
Construction of Stable shRNA-COMT Human Leiomyoma Cells
To test whether the cellular effects of Vitamin D could be mediated via endogenous COMT, we proceeded to silence the expression of endogenous COMT by short hairpin RNA (shRNA) technology. The MISSION TRC pLKO.1-5 plasmid DNAs, which contains 5 distinct human COMT (17) specific shRNA sequences, were purchased from Sigma-Aldrich. These plasmid DNA constructs were stably transfected into HuLM cells and colonies were selected with puromycin (Sigma, 2.5 µg/ml). Stable clones were verified for COMT expression by western blot analyses using anti-COMT antibody. Clones that expressed lowest levels of sCOMT (soluble) and mCOMT (membrane-bound) were selected for further evaluation.
Cell Proliferation Assay
2×103 cells/well from each pool of HuLM, shRNA-COMT and primary LM298 cells were seeded in 96 well plates and allowed to grow for 24 hours at 37°C. Cells were then treated with various concentrations of Vitamin D and the relative cell numbers were assayed at 24 h, 72 h and 120 h using MTT assay according to the manufacturer's recommendation (Sigma). The mean ± standard error (SE) was calculated for each triplicate measurement.
Real Time PCR
Total RNA was prepared from HuLM cells treated with Vitamin D using an RNA extraction kit (Qiagen, Valencia, CA). One microgram of total RNA from each sample was reverse-transcribed and an equal amount of cDNA (40 ng) was used for amplification of either GAPDH or COMT (Applied Biosystems) and detected real-time by Bio-Rad IQ5.
Western Blot Analyses
HuLM cells were treated with Vitamin D for 48 h and cell lysates were analyzed by western blots as described previously (18). Western blot analyses were performed using primary antibodies against PCNA (1:500), BCL-2 (1:200), BCL-xL (1:250), BCL-w (1:500), CDK1 (1:1,000), phospho-ERK (1:1,000), total ERK (1:1,000) and COMT (1:100,000). The intensities of specific protein bands were quantified and the values of β-actin, detected by anti-β-actin antibody (1:5,000), were used to normalize the level of corresponding target protein bands.
High Performance Liquid Chromatography (HPLC)
The COMT activity, in the control and Vitamin D treated HuLM cells, was assayed using established HPLC-based methods (19). The COMT enzyme activity was expressed in units per milligram of total protein.
Statistical Analyses
All data were expressed as the mean ± standard error (SE) of all values obtained from three replicates. Statistical significance was determined using Student’s t-test. P less than 0.05 (P<0.05) was considered to be statistically significant.
RESULTS
Vitamin D Inhibits Growth of HuLM Cells
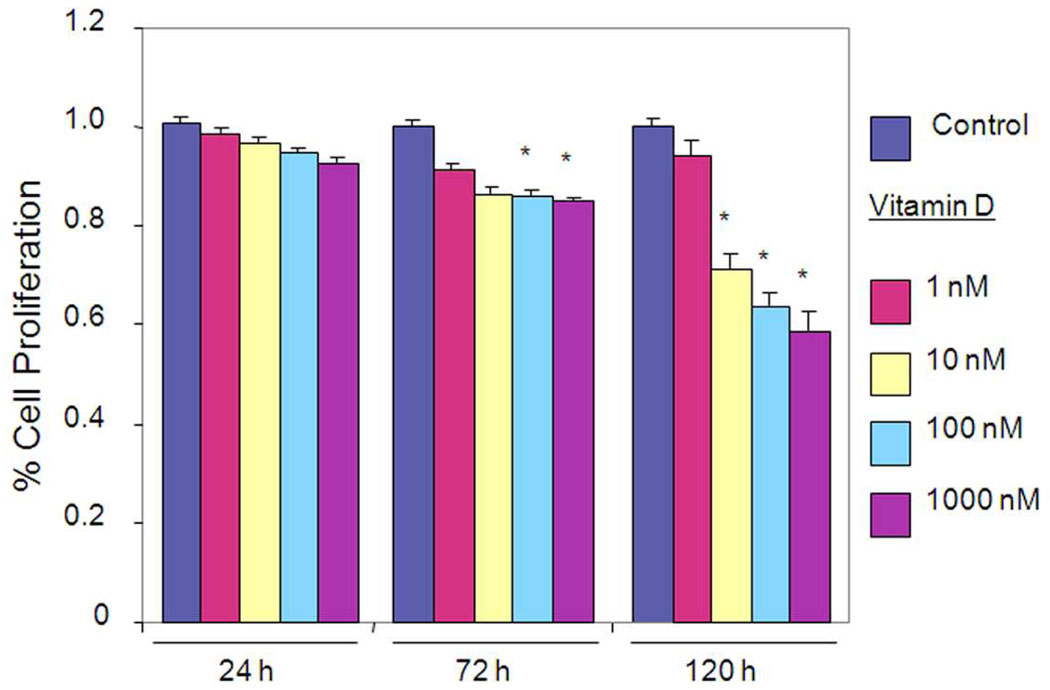
To evaluate the effect of Vitamin D on cell growth, HuLM cells were treated with various concentrations of Vitamin D for up to 120 hours, and cell growth and proliferation were determined using MTT assay (Fig. 1A). Vitamin D decreased growth of HuLM cells when compared with untreated HuLM cells (Fig. 1A). At 120 hours treatment, Vitamin D significantly inhibited proliferation of leiomyoma cells by 47% ±0.03 at 1 µM concentration, and 38% ±0.02 at 0.1 µM concentration when compared with untreated control (P < 0.05). These results suggest that Vitamin D inhibits proliferation of HuLM cells.
Figure 1. Effect of Vitamin D on proliferation of HuLM cells.
2×103 HuLM cells were seeded in 96-well plates and treated with 1, 25-dihydroxyvitamin D3 (Vitamin D). Ethanol vehicle was added to the control cells. Relative cell numbers were assessed at each time point using MTT assay. Individual data points are the mean ± SE of triplicate determinations. *P < 0.05 compared with corresponding control.
Vitamin D Reduces PCNA, CDK1 Expression and Cellular ERK Activity in HuLM Cells
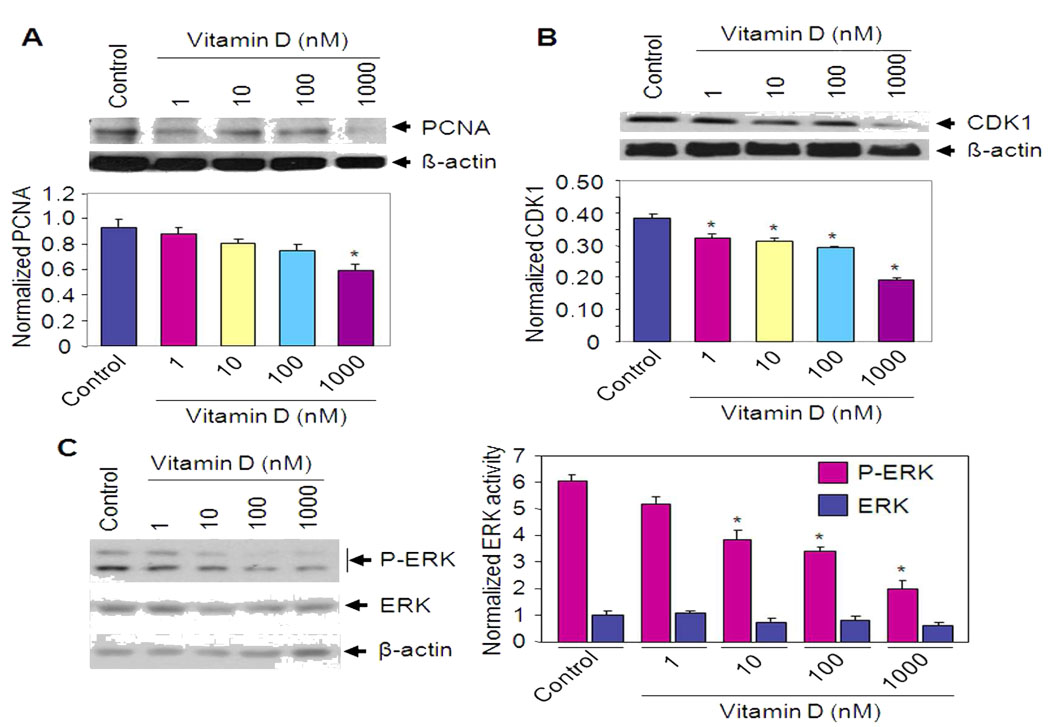
To test whether Vitamin D affects the expression of PCNA, we performed western blot analysis using lysates from Vitamin D treated HuLM cells. At 1 µM concentration, Vitamin D decreased PCNA protein expression by approximately 40% when compared with untreated control (P < 0.05) (Fig. 2A). The cyclin-dependent kinase 1 (CDK1) is required for G2-M transition during the cell cycle (20). Vitamin D treatment reduced CDK1 expression in HuLM cells when compared with untreated control cells (Fig. 2B). At 1 µM concentration of Vitamin D, we observed a 2 fold reduction (P < 0.05) of CDK1 protein expression. Western blot analyses indicate that Vitamin D decreased phosphorylation of ERK in HuLM cells when compared with untreated control cells. However, the total levels of ERK were not affected by Vitamin D (Fig. 2C), suggesting a role for Vitamin D in the regulation of cellular growth by modulating ERK MAP kinase activation in leiomyoma cells.
Figure 2. Effect of Vitamin D on PCNA and CDK1 expression as well as anti-apoptotic BCL-2 and BCL-w in HuLM cells.
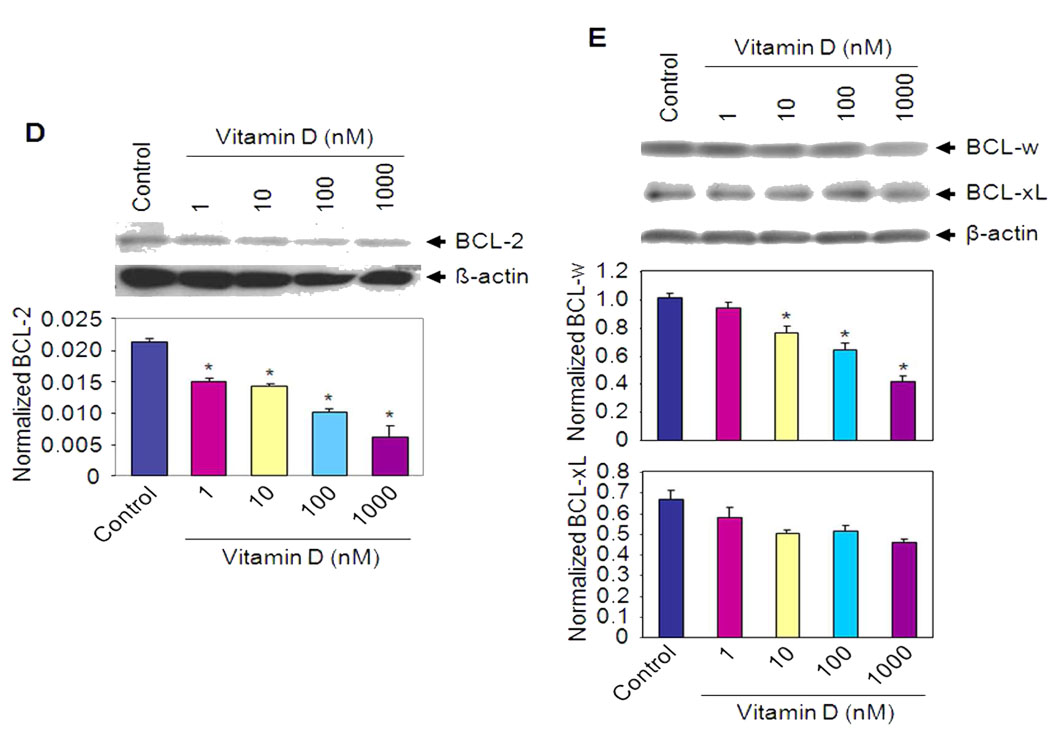
HuLM cells were treated with different doses of Vitamin D for 48 h as indicated. Lysates prepared from both treated and untreated control cells were analyzed by western blotting with anti-PCNA (A) and anti-CDK1 (B) antibodies. The intensity of each protein signal was quantified and normalized with corresponding β-actin. *P < 0.05 compared to untreated control. (C) HuLM cells were serum starved and treated with Vitamin D for 8 h as indicated. Cell lysates were prepared and were analyzed by western blotting using anti-phospho-ERK and anti-ERK antibodies. β-actin was used as loading control (left panel). The signal intensity of phospho-ERK (P-ERK) and total ERK (ERK) were quantified and normalized as above. *P < 0.05 compared to untreated control (right panel). (D and E) HuLM cells were treated with Vitamin D for 48 h as indicated. Lysates from both Vitamin D-treated and untreated control cells were analyzed by western blotting with anti-BCL-2, anti-BCL-xL and anti-BCL-w antibodies. The intensity of each band was normalized with corresponding β-actin as above (bottom panel). *P < 0.05 compared to untreated control.
Vitamin D Reduces Anti-apoptotic BCL-2 and BCL-w Protein Expression in HuLM Cells
To verify whether Vitamin D affects the expression of anti-apoptotic genes, we performed western blot analyses. HuLM cells were treated with different doses of Vitamin D for 48 h and cell lysates were analyzed for BCL-2, BCL-xL and BCL-w protein expression. BCL-2 and BCL-w protein levels were significantly down-regulated by Vitamin D treatment when compared with untreated control cells (Fig. 2D and E, P < 0.05). However, we did not observe any significant changes in BCL-xL protein expression by Vitamin D in HuLM cells.
Vitamin D Reduces COMT mRNA and Protein Expression and Inhibits COMT Enzyme Activity in HuLM Cells
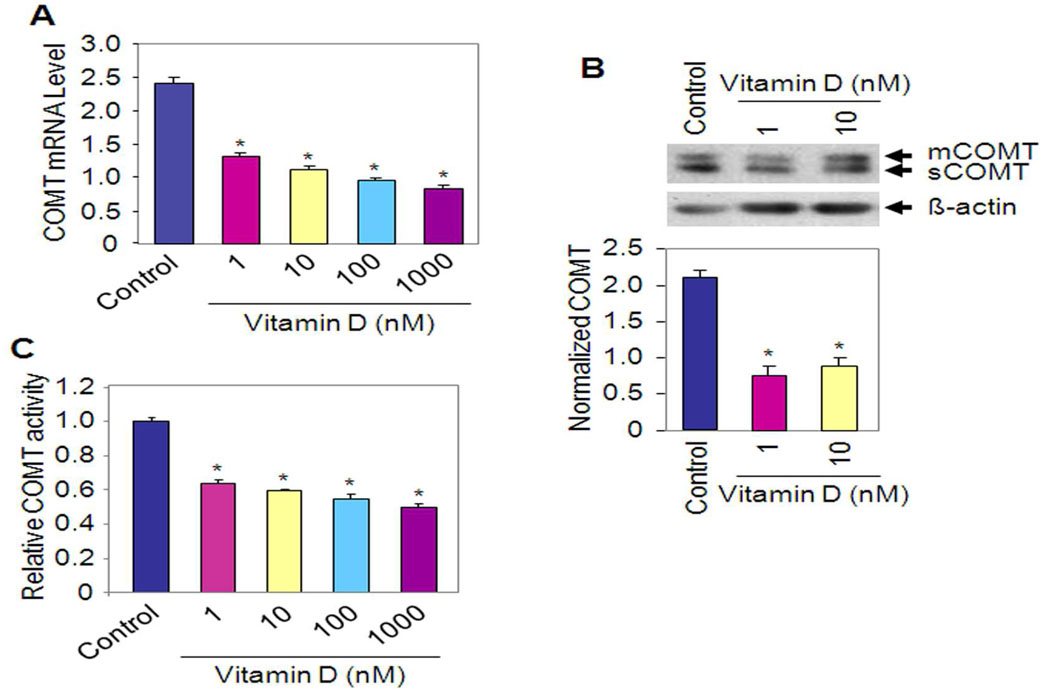
COMT plays an important role in the pathophysiology of many human disorders, including estrogen related cancers (21). COMT is upregulated in human leiomyoma compared to adjacent normal myometrium (9, 10, 22). We observed that Vitamin D inhibits COMT mRNA expression at 100 nM concentration in HuLM cells when compared with untreated control (P < 0.05) as determined by quantitative real time PCR (Fig. 3A). However, further inhibition by 1 µM concentration of Vitamin D was not observed (Fig. 3A). Using western blot analyses we found that Vitamin D reduces COMT protein expression in HuLM cells when compared with untreated control (Fig. 3B). In addition, Vitamin D reduced COMT enzyme activity (50%, P < 0.05) in HuLM cells at 1 µM concentration when compared with untreated control (Fig. 3C). These results suggest that Vitamin D is a potent COMT inhibitor in HuLM cells, both at the expression and enzyme activity levels.
Figure 3. Effect of Vitamin D on COMT mRNA and protein expression, and enzyme activity.
(A) Quantitative analysis of COMT mRNA expression levels with real time RT-PCR after treatment of HuLM cells with different doses of Vitamin D (1 nM to 1 µM) for 48 h. Expression levels of COMT mRNA are shown as the mean ratio of target-to-reference gene ± SE relative to the vehicle-treated control. Results are expressed as mean ± SE of three independent experiments. *P < 0.05 compared to untreated control. (B) HuLM cells were treated with Vitamin D for 48 h and cell lysates were prepared and analyzed by western blotting with anti-COMT antibody. The intensity of each protein signal was quantified and normalized with corresponding β-actin. *P < 0.05 compared to untreated control. (C) COMT enzyme activity was assayed after the treatment of HuLM cells with different doses of Vitamin D as indicated. COMT enzyme activity was expressed in units per milligram of total protein. *P < 0.05 compared to untreated control.
Vitamin D Reduces Proliferation of HuLM Cells via Cooperation with Endogenous COMT
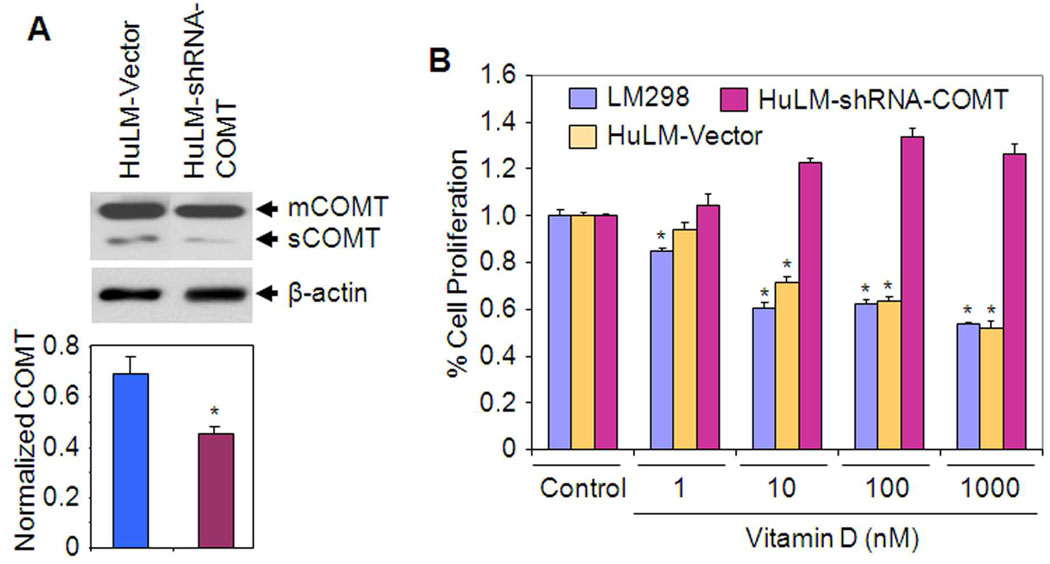
Since Vitamin D significantly reduced COMT mRNA expression, protein levels and enzyme activity in HuLM cells, we hypothesized that Vitamin D-mediated growth inhibitory effect may be mediated via endogenous COMT in HuLM cells. To test this hypothesis, we have generated stable COMT-deficient HuLM cell lines by stable transfection with COMT-specific shRNA. The shRNA-COMT clone expressed markedly reduced levels of endogenous COMT for both mCOMT and sCOMT as compared to the vector control HuLM cells (Fig. 4A). This shRNA-COMT clone, the vector control HuLM cells as well as primary LM298 cells were analyzed for cell growth using MTT-proliferation assay. We observed that Vitamin D inhibited growth of both vector control HuLM and LM298 cells when compared with untreated control cells (Fig. 4B). Interestingly, silencing endogenous COMT expression by shRNA in HuLM cells (shRNA-COMT) abolished the Vitamin D-induced growth inhibition (Fig. 4B). Cumulatively, these results suggest that Vitamin D reduces growth of HuLM cells, at least in part, through cooperation with endogenous COMT.
Figure 4. Effect of endogenous COMT silencing on Vitamin D-mediated inhibition of HuLM cell proliferation.
(A) The vector control HuLM cells (HuLM-Vector) and COMT-deficient HuLM cell lines (shRNA-COMT) were cultured and cell lysates were prepared. Equal amounts of each cell lysate from representative stably transfected colonies were analyzed for endogenous COMT expression by western blotting using anti-COMT antibody. Endogenous levels of mCOMT (membrane bound) and sCOMT (soluble) were determined in wild type HuLM cells, a vector control clone and a shRNA-COMT clone that expresses significantly lower levels of COMT using western blot analyses with anti-COMT antibody (top panel). The intensity of COMT signals (mCOMT and sCOMT) was quantified and normalized with corresponding β-actin as above. *P < 0.05 compared to untreated control (bottom panel). (B) 2×103 cells from each pool of the above vector control HuLM cells and the shRNA-COMT clone and in addition primary LM298 cells were seeded in 96-well plates and treated with Vitamin D for MTT-proliferation assay as described previously (43). Relative cell numbers were assessed at 120 h and individual data points are the mean ± SE of triplicate determinations. *P < 0.05 when compared with corresponding control.
DISCUSSION
In this study, we have observed that both HuLM and LM298 cells are highly sensitive to the growth inhibitory effect of Vitamin D. Our observation that Vitamin D significantly reduces the growth of HuLM cells, suggests a potential role of Vitamin D in myometrial homeostasis and tumor prevention. Vitamin D deficiency might conceivably be a risk factor for the initiation and progression of uterine fibroids. Considering that Vitamin D deficiency is more prevalent among African Americans (40–45%) compared to Whites (4%), (12) one may hypothesize that the markedly higher prevalence of uterine fibroids in African American women compared to other ethnic groups (7, 8, 23, 24) might be attributed, at least partially, to the ethnically differential prevalence of Vitamin D deficiency.
Vitamin D is a potent regulator of cellular growth in both normal and cancer cells (12). Vitamin D suppresses the proliferation of malignant tumor cells as well as induces differentiation and apoptosis (25). Additionally, Vitamin D analogue has been shown to potentiate anti-tumor activity in a murine squamous cell carcinoma model system (26). No prior reports have explored the mechanism of action of Vitamin D on human uterine leiomyoma cells. Therefore, the aim of our study was to determine whether Vitamin D affects HuLM cell proliferation, and subsequently begin to understand the molecular mechanisms of how Vitamin D affects the growth of uterine leiomyoma cells in vitro.
PCNA is a cell proliferation marker that is involved in cell growth and proliferation as well as tumorigenicity. Our results indicate that Vitamin D reduces the expression of PCNA in leiomyoma cells. Therefore, reduction in the expression of PCNA by Vitamin D treatment suggests a potential role for Vitamin D in the regulation of growth of HuLM cells. The cyclin-dependent kinase 1 (CDK1) is known as a mitosis promoting factor, and it is required for G2-M transition during the cell cycle (19). Inhibition of CDK1 by the inhibitor roscovitine reduces proliferation of several tumor cells (27). Our results showed that Vitamin D significantly suppresses the expression of CDK1 in HuLM cells. This important observation demonstrates a potential role of Vitamin D in the regulation of cell cycle regulatory CDK1. Vitamin D is a potent growth inhibitor of breast cancer cells and it inhibits serum induced MAP kinase activation in breast cancer cells (28). Consistent with the above results we also observed that Vitamin D reduces MAPK ERK activation in HuLM cells. Therefore, uterine leiomyoma could potentially be a good target for Vitamin D treatment. Such work is currently underway in our laboratory using various uterine fibroid preclinical animal models (29).
Vitamin D induces apoptosis in the MCF-7 breast cancer cell line (30, 31). Vitamin D may control members of the BCL-2 family, which have been shown to play a major role in the signaling mechanism of apoptosis. This protein family consists of both anti-apoptotic proteins such as BCL-2, BCL-xL and BCL-w, and pro-apoptotic proteins including Bax, Bak and Bad (32). Our results show repression of BCL-2 and BCL-w gene expression in HuLM cells upon treatment with Vitamin D. These results indicate that the growth inhibitory role of Vitamin D is also mediated by blocking the expression of anti-apoptotic BCL-2 and BCL-w.
The COMT enzyme catalyzes the transfer of a methyl group from S adenosyl-methionine to one of the phenolic hydroxyl groups of a variety of catechols including catecholestrogens and catecholamine neurotransmitters (33, 34). COMT is known to convert the catecholestrogens, 2- and 4-hydroxyestrogen, to 2- and 4-methoxyestrogen respectively (34). We and others have shown that 2-hydroxyestrogen can act as estrogen antagonists in a variety of cells including human myometrial and leiomyoma cells (7; 8, 22, 35, 36) while their methylated counterpart, 2- methoxyestrogen, can act as estrogen agonists in multiple biologic assays (37, 38, 39, 40). Such hormonal activity of these estrogen metabolites suggests that modulation of COMT expression and activity would affect the balance between 2- hydroxyestrogen versus 2-methoxyestrogens and have a considerable effect on cellular estrogen bioactivity (7, 8, 22). We have recently reported on wide-spread expression of the COMT protein in many human reproductive tissues, including placenta, decidua vera, myometrium as well as endometrium (7, 8, 22, 41, 42). However, the possibility of interaction between Vitamin D and COMT expression in HuLM is yet to be explored. We have previously reported the upregulation of COMT in human leiomyomas compared to adjacent normal myometrium, detected by immunohistochemical analyses, quantitative RT-PCR and microarray (7, 8). Here we demonstrate that COMT mRNA, protein and enzyme activity are significantly reduced in HuLM cells upon Vitamin D treatment. Additionally, cell growth assays using shRNA-COMT cells, where COMT expression has been considerably silenced, were non-responsive to Vitamin D mediated growth inhibition. These findings collectively suggest the necessity of COMT for a Vitamin D-induced anti-proliferative effect on HuLM cells. Thus, Vitamin D deficiency might explain the higher expression of COMT in uterine leiomyoma and might constitute a modifiable risk factor for uterine fibroids.
In conclusion, Vitamin D inhibits growth of HuLM cells by reducing the expression of PCNA and CDK1 as well as suppression of anti-apoptotic BCL-2 and BCL-w. In addition, we report for the first time that Vitamin D reduces COMT mRNA and protein expression as well as the enzyme activity in HuLM cells. Silencing the endogenous COMT expression in HuLM cells by COMT specific shRNA reduces the growth inhibitory effect of Vitamin D. Our findings suggest that Vitamin D deficiency may be an important risk factor in growth and progression of uterine fibroids.
Acknowledgement
We appreciate Dr. Veera Rajaratnam, Director of Scientific Publications at the Center for Women’s Health Research, Meharry Medical College, for the excellent scientific editing and expertise rendered for graphics of this manuscript.
Financial Support: NIH/NICHD 1 R01 HD046228-01 to AA and RCMI grant G12 RR03032
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Healy DL, Lawson SR, Abbott M, Baird DT, Fraser HM. Toward removing uterine fibroids without surgery: subcutaneous infusion of a luteinizing hormone-releasing hormone agonist commencing in the luteal phase. J Clinic Endocrinol & Metabol. 1986;63:619–625. doi: 10.1210/jcem-63-3-619. [DOI] [PubMed] [Google Scholar]
- 2.Walker CL, Cesen-Cummings K, Houle C, Baird D, Barrett JC, Davis B. Protective effect of pregnancy for development of uterine leiomyoma. Carcinogenesis. 2001;22:2049–2052. doi: 10.1093/carcin/22.12.2049. [DOI] [PubMed] [Google Scholar]
- 3.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 4.Elder-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70:687–691. doi: 10.1016/s0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- 5.Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyoma in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril. 2001;75:405–410. doi: 10.1016/s0015-0282(00)01714-3. [DOI] [PubMed] [Google Scholar]
- 6.Day Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Highly cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-α polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006a;86:3686–3693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Invest. 2006b;13:136–144. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Othman E, Al-Hendy A. Molecular genetics and racial disparities of uterine leiomyomas. Best Prac Res Clin Obstet Gynecol. 2008;22:589–601. doi: 10.1016/j.bpobgyn.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salama SA, Shu-Leong Ho, Hui-Qun W, Tenhunen J, Tilgmann C, Al-Hendy A. Hormonal regulation of catechol-O-methyl transferase activity in women with uterine leiomyomas. Fertil and Steril. 2006;86:259–262. doi: 10.1016/j.fertnstert.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Hollick MF, Vitamin D. In: Modern nutrition in health and disease. 9th ed. Shils ME, Olson JA, Shike M, Ross CA, editors. Baltimore: Williams and Williams; 1999. [Google Scholar]
- 12.Hollick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clinical Nutrition. 2004;80:1678s–1688s. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 13.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 14.Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moor AB, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82:719–728. doi: 10.1097/01.lab.0000017499.51216.3e. [DOI] [PubMed] [Google Scholar]
- 15.Rauk PN, Surti U, Roberts JM, Michalopoulos G. Mitogenic effect of basic fibroblast growth factor and estradiol on cultured human myometrial and leiomyoma cells. Am J Obstet Gynecol. 1995;173:571–577. doi: 10.1016/0002-9378(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hendy A, Lee EJ, Wang HQ, Copland A. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–1631. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom K, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Ulmanen I. Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta. 1995;1251:1–10. doi: 10.1016/0167-4838(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Al-Hendy M, Richard-Davis G, Montgomery-Rice V, Rajaratnam V, Al-Hendy A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil and Steril. 2009 doi: 10.1016/j.fertnstert.2009.08.065. (In Press) PMID: 19819432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiaoyu S, Namrata P, Arthur W. A refined HPLC method to measure catecholamine-o methyltransferase activity in selected brain regions. J Neuroscience Methods. 2005;144:137–142. doi: 10.1016/j.jneumeth.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 21.Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm HW, Malherbe P. Human catechol-O-methyltransferase: cloning and expression of the membrane associated form. Proc. Natl Acad Sci USA. 1991;88:1416–1420. doi: 10.1073/pnas.88.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wentz MJ, Jamaluddin M, Garfield R, Al-Hendy A. Regulation of catechol-O-methyltransferase expression in human myometrial cells. Obstet Gynecol. 2006;108:1439–1447. doi: 10.1097/01.AOG.0000243775.73788.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeCherney AH. Current Obstetric&Gynecologic Diagnosis&Treatment. 9th Ed. 2003. p. 693. [Google Scholar]
- 24.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 25.Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, et al. Antiproliferative action of Vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 26.Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res. 1997;57:3759–3764. [PubMed] [Google Scholar]
- 27.Goke R, Barth P, Schmidt A, Samans B, Lankat-Buttgereit B. Programmed cell death protein 4 suppresses CDK-1/cdc 2 via induction of p21Waf1/Cip1. Am J Physiol Cell Physiol. 2004;287:C1541–C1546. doi: 10.1152/ajpcell.00025.2004. [DOI] [PubMed] [Google Scholar]
- 28.Capiati DA, Rossi AM, Picotto G, Benassati S, Boland RL. Inhibition of Serum-Stimulated Mitogen Activated Protein Kinase by 1a,25(OH)2-Vitamin D3 in MCF-7 Breast Cancer Cells. J Cell Biochem. 2004;93:384–397. doi: 10.1002/jcb.20165. [DOI] [PubMed] [Google Scholar]
- 29.Hassan MH, Salama SA, Zhang D, Arafa HMM, Hamada FMA, Fouad H, et al. Gene therapy targeting leiomyoma: adenovirusmediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil Steril. 2010;93:239–250. doi: 10.1016/j.fertnstert.2008.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James SY, Mackay AG, Colston KW. Effects of 1, 25-dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J. Steroid Biochem Mol Biol. 1996;58:395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- 31.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1, 25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 32.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 33.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Bio Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- 34.Creveling CR. The role of catechol-O-methyltransferase in the inactivation of catecholestrogen. Cellular Mol Neurobiol. 2003;23:289–291. doi: 10.1023/A:1023680302975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandewalle B, Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cellular Endocrinol. 1989;61:239–246. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 36.Bradlow LH. 2-Hydroxyesterone: the ‘good’ estrogen. J Endocrinol. 1996;150:S259–S265. [PubMed] [Google Scholar]
- 37.Banerjee SN, Sengupta K, Banerjee S, Saxena NK, Banerjee SK. 2-Methoxyestradiol exhibits a biphasic effect on VEGF-A in tumor cells and upregulation is mediated through ER-a: a possible signaling pathway associated with the impact of 2-ME2 on proliferative cells. Neoplasia. 2003;5:417–426. doi: 10.1016/s1476-5586(03)80044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippert C, Seeger H, Mueck AO. The effect of endogenous estradiol metabolites on the proliferation of human breast cancer cells. Life Sciences. 2003;72:877–883. doi: 10.1016/s0024-3205(02)02305-6. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z-J, Zhu BT. Concentration-dependent mitogenic and antiproliferative actions of 2 methoxyestradiol in estrogen receptorpositive human breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:265–275. doi: 10.1016/j.jsbmb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland TE, Schuliga M, Harris T, Eckhardt BL, Anderson RL, Quan L, et al. 2-methoxyestradiol is an estrogen receptor agonist that supports tumor growth in murine xenograft models of breast cancer. Clinical Cancer Res. 2005;11:1722–1732. doi: 10.1158/1078-0432.CCR-04-1789. [DOI] [PubMed] [Google Scholar]
- 41.Salih SM, Salama MD, Salama A, Fadl AA, Nagamani M, Al-Hendy A. Expression and cyclic variations of catechol-O-methyltransferase in human endometrial stroma. Fertil Steril. 2008;90:789–797. doi: 10.1016/j.fertnstert.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harirah H, Thota C, Wentz MJ, Zaman W, Al-Hendy A. Elevated expression of catechol O-methyltransferase is associated with active labor and increased PGE2 production by human fetal membranes. Am J Obstet and Gynecol. 2009;201:496. doi: 10.1016/j.ajog.2009.05.041. e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salama SA, Kamel M, Awad M, Nasser AH, Al-Hendy A, Botting S, et al. Catecholestrogens induce oxidative stress and malignant transformation in human endometrial glandular cells: Protective effect of catechol-O-methyltransferase. Int J Cancer. 2008;123:1246–1254. doi: 10.1002/ijc.23653. [DOI] [PubMed] [Google Scholar]