Abstract
The initiation of mammalian puberty requires a sustained increase in pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus. This increase is brought about by coordinated changes in transsynaptic and glial-neuronal communication, consisting of an increase in neuronal and glial stimulatory inputs to the GnRH neuronal network and the loss of transsynaptic inhibitory influences. GnRH secretion is stimulated by transsynaptic inputs provided by excitatory amino acids (glutamate) and at least one peptide (kisspeptin), and by glial inputs provided by growth factors and small bioactive molecules. The inhibitory input to GnRH neurons is mostly transsynaptic and provided by GABAergic and opiatergic neurons; however, GABA has also been shown to directly excite GnRH neurons. There are many genes involved in the control of these cellular networks, and hence in the control of the pubertal process as a whole. Our laboratory has proposed the concept that these genes are arranged in overlapping networks internally organized in a hierarchical fashion. According to this concept, the highest level of intra-network control is provided by transcriptional regulators that, by directing expression of key subordinate genes, impose genetic coordination to the neuronal and glial subsets involved in initiating the pubertal process. More recently, we have begun to explore the concept that a more dynamic and encompassing level of integrative coordination is provided by epigenetic mechanisms.
Keywords: hypothalamus, GnRH neurons, epigenetics, glial-neuronal communication, transcriptional regulation, timing of puberty
1. Introduction
There is little doubt that different regulatory systems contribute to controlling the pubertal process in mammals. The key, decisive, event required for puberty to occur is an increase in pulsatile gonadotrophin-releasing hormone (GnRH) release from GnRH neurons. While in primates these neurosecretory cells are mostly located in the medial basal hypothalamus, they reside exclusively in the preoptic area of rodents (Ojeda and Skinner, 2006;Plant and Witchel, 2006). It is also clear that the pubertal increase in GnRH secretion is initiated and maintained by changes in transsynaptic (Kordon et al., 1994;Ojeda and Terasawa, 2002) and glial (Ojeda et al., 2003;Ojeda and Terasawa, 2002) inputs to the GnRH neuronal network. The transsynaptic changes consist of a coordinated increase in excitatory inputs and a reduction in inhibitory influences (Ojeda and Terasawa, 2002;Plant and Witchel, 2006;Terasawa and Fernandez, 2001); the glial component of the system is mostly facilitatory, and provided by growth factors and small diffusible molecules that directly or indirectly stimulate GnRH secretion (Ojeda et al., 2003;Ojeda and Skinner, 2006).
In recent years, new components of each of these regulatory systems have been identified. Thus, in addition to glutamatergic neurons (Brann, 1995;Ojeda and Skinner, 2006;Plant and Witchel, 2006), which are widely distributed throughout the basal forebrain, the hypothalamus contains two discrete subsets of neurons that produce the peptide kisspeptin (also termed metastin), a powerful stimulator of GnRH release (Kauffman et al., 2007;Oakley et al., 2009;Shahab et al., 2005). In rodents, one group of kisspeptin neurons is located in the periventricular region of the anteroventral periventricular nucleus (AVPV), and the other in the periventricular region of the arcuate nucleus (ARC) (Clarkson et al., 2009). Kisspeptin neurons of the ARC produce two additional peptides: neurokinin B (TAC3) (Navarro et al., 2009), which is encoded by a gene recently shown to be required for puberty to occur (Topaloglu et al., 2008), and dynorphin, an opioid peptide that inhibits GnRH secretion (Kinoshita et al., 1982;Navarro et al., 2009;Schulz et al., 1981). Altogether, these observations indicate that the excitatory transsynaptic regulation of GnRH secretion is provided by neurons that use glutamate, kisspeptin (Dungan et al., 2006;Ojeda and Skinner, 2006), and perhaps neurokinin B (Topaloglu et al., 2008), for transsynaptic communication.
With regard to the inhibitory transsynaptic circuitry controlling GnRH release, it appears clear that GABAergic and opiatergic neurons are central players [reviewed in (Terasawa and Fernandez, 2001)]. However, a rapidly accumulating body of evidence is beginning to strengthen the concept that a product of the RFamide-related peptide gene (RFRP), which is the mammalian ortholog of the peptide gonadotrophin-inhibiting hormone (GnIH) in birds (Ebling and Luckman, 2008), is a physiological inhibitor of GnRH neurons in mammals (Ducret et al., 2009;Gibson et al., 2008;Tsutsui et al., 2010). These observations suggest that RFRP-containing neurons provide an additional, and perhaps important, transsynaptic mode of inhibitory control to GnRH neurons during reproductive maturation. Of these three inhibitory systems, the RFamide-component seems to be the simplest, because it is composed of one or two peptides (RFRP1 and RFRP3) and a single receptor termed GPR147 (Hinuma et al., 2000;Tsutsui et al., 2010), which appears to be expressed in GnRH neurons (Ducret et al., 2009). The GABA controlling system is endowed with a higher level of complexity, because GABA can inhibit GnRH secretion indirectly, via effects exerted on neurons connected to the GnRH neuronal network (Ojeda and Skinner, 2006;Terasawa and Fernandez, 2001), but can also stimulate GnRH neurons directly through activation of GABAA receptors (DeFazio et al., 2002;Moenter and DeFazio, 2005). The opioid inhibitory system is even more complex, because opiatergic neurons employ different peptides and several receptors to inhibit GnRH release (Kordon et al., 1994); as in the case of GABAergic inputs, opiatergic inhibition may be exerted directly on GnRH neurons (Dudas and Merchenthaler, 2006) or indirectly on neurons involved in the stimulatory control of the GnRH neuronal network, such as kisspeptin neurons (Navarro et al., 2009).
Other studies have shown that, in addition to transsynaptic inputs, the pubertal activation of GnRH secretion also requires information from glial cells (Ojeda et al., 2000;Ojeda and Terasawa, 2002). Both astrocytes and ependymoglial cells lining the ventral surface of the third ventricle (called tanycytes) produce cell-cell signalling molecules that stimulate GnRH release, and that are necessary for the timing of puberty [reviewed in (Lomniczi and Ojeda, 2009)]. Glial cells contribute to the pubertal activation of GnRH secretion via two complementary mechanisms. One of them involves growth factors of at least four different families. Transforming growth factor-beta (TGFβ), of the TGFβ superfamily, is recognized by cell-membrane receptors endowed with serine-threonine kinase activity and that are located on GnRH neurons (Prevot et al., 2000). Upon binding, TGFβ enhances GnRH gene expression and GnRH secretion (reviewed in (Mahesh et al., 2006;Prevot, 2002)). Growth factors of the other three families, including the epidermal growth factor (EGF) family, basic fibroblast growth factor (bFGF), and insulin-like growth factor 1 (IGF-I), are recognized by receptors with tyrosine kinase activity. Some of these receptors (FGFR, IGF-1R) are expressed in GnRH neurons, but erbB receptors (which recognize EGF and EGF-like peptides) are mostly expressed on glial cells themselves. Genetic disruption of erbB receptors delays female sexual development due, at least in part, to impaired erbB ligand-induced glial prostaglandin E2 (PGE2) release (Lomniczi and Ojeda, 2009). While growth factors of glial origin set in motion glia-to-neuron signaling pathways, at least one neuron-to-glia regulatory pathway initiated by glutamatergic neurons, has been shown to facilitate astrocytic signaling mediated by erbB receptors (Dziedzic et al., 2003).
The second mechanism of glia-to-GnRH neuron communication involves plastic rearrangement in cell adhesiveness. The adhesion of glial cells to GnRH neurons appears to require at least three different cell-cell communications systems. One is thought to be provided by the sialylated form of the neural cell adhesion molecule NCAM (PSA-NCAM) (Parkash and Kaur, 2005;Perera et al., 1993). PSA-NCAM is abundant in brain regions endowed with a high degree of postnatal plasticity (Gascon et al., 2007), such as the medial basal hypothalamus-median eminence (ME) region (Perera et al., 1993). PSA-NCAM is especially abundant in GnRH nerve terminals and glial cells of the ME (Parkash and Kaur, 2005), suggesting an involvement in glia-GnRH nerve terminal adhesiveness. Another adhesive system employs Synaptic Cell Adhesion Molecule 1 (SynCAM1) (Ojeda et al., 2008), and the other is based on the interaction of neuronal contactin with glial Receptor-like Protein Tyrosine Phosphatase-β (RPTPβ) (Parent et al., 2007). Because in all three cases the participating proteins contain intracellular domains with signaling capabilities, it is likely that the interaction of glial cells with GnRH neurons may not only involve secreted bioactive molecules, but also the activation of cell-cell signaling mechanisms set in motion by these and, likely other, adhesive molecules (reviewed in (Lomniczi and Ojeda, 2009).
2. The neuroendocrine control of puberty involves many genes with different functions
Several studies have suggested that no isolated pathway or cellular subset is solely responsible for the neuroendocrine control of puberty (Eaves et al., 2004;Gajdos et al., 2008;Krewson et al., 2004;Ojeda et al., 2006;Seminara and Crowley, Jr., 2001). Instead, the initiation of puberty may be controlled by regulatory gene networks composed of multiple functional modules (Ojeda et al., 2006). For instance, mutations affecting different genes, including GNRHR (Bedecarrats and Kaiser, 2007), GPR54 (de Roux et al., 2003;Seminara et al., 2003), KiSS1 (Lapatto et al., 2007), TAC3 and TACR3 (Topaloglu et al., 2008), result in pubertal failure. Recent genome-wide association studies demonstrated an association of a sequence variation in LIN28B (a gene encoding an RNA-binding protein) with the age at menarche (He et al., 2009;Ong et al., 2009;Perry et al., 2009;Sulem et al., 2009), and showed that variants of at least 10 other genes are also associated with early menarche (Perry et al., 2009;Sulem et al., 2009). Very recently, it was reported that – complementing the observations in humans - mice overexpressing Lin28a have delayed puberty (Zhu et al., 2010). Notwithstanding the importance of this finding, it is currently unknown if Lin28a overexpression delays puberty due to a general effect on body metabolism or via a mechanism involving an alteration in GnRH secretion. Studies from our laboratory identified and functionally characterized several other components of the regulatory system controlling the onset of female puberty, including novel molecules required for glutamate release (Choi et al., 2008;Ha et al., 2008), homeostatic maintenance of GnRH neuron excitability (Garcia-Rudaz et al., 2008), unidirectional glia-to-GnRH neuron signaling (Lomniczi et al., 2006), and glia-GnRH neuron adhesive communication (Parent et al., 2007;Sandau et al., 2009). We also identified transcriptional regulators of the pubertal process, including the POU-domain gene Oct2 (Ojeda et al., 1999), the homeodomain gene Ttf1/Nkx2.1 (Mastronardi et al., 2006), and a novel gene provisionally termed chromosome 14 open reading frame 4 (C14ORF4) (Rampazzo et al., 2000), which we termed Eap1 (Enhanced At Puberty1) (Heger et al., 2007). Based on a variety of experimental approaches, including the use of antisense oligodeoxynucleotides (Ojeda et al., 1999), Cre-loxP-mediated, neuron-specific conditional gene deletion (Mastronardi et al., 2006) and siRNA-mediated region-specific knock-down of gene expression (Heger et al., 2007) we proposed that these genes occupy a central position in the hierarchical arrangement of networks controlling the pubertal process (Ojeda et al., 2006). Although in most of these studies the endpoints were somatic manifestations of puberty, such as vaginal opening and age at first ovulation, the inference was that GnRH secretion had been affected by manipulating the expression of these genes selectively in the hypothalamus.
3. Genes controlling puberty are organized in functional networks
From the above considerations it may be concluded that there are genes required for the acquisition of reproductive function, such as GPR54, KiSS1 and TAC3, and genes that contribute to defining the correct timing of the pubertal process, such as those encoding molecules involved in the flow of glia-neuron communication, transsynaptic information, glia-GnRH neuron adhesive behavior, and intracellular signaling. Others, such as OCT2, TTF1/NKX2.1, EAP1, and LIN28B appear to be involved in the transcriptional control of puberty. As such, they are expected to control the expression of subordinate genes that are necessary for the neuron-to neuron and glia-to neuron regulation of GnRH secretion at puberty [reviewed in (Ojeda et al., 2006;Ojeda et al., 2010)].
Using a high-throughput approach (DNA arrays) we provided evidence for the existence of an additional gene network that, according to our results, contributes to the hypothalamic to control of puberty (Roth et al., 2007). Although the genes composing this network have diverse cellular functions, they share the common feature of being first identified as involved in tumor suppression/tumor formation (Roth et al., 2007). Because of this, we termed the network “TSG” (for Tumor Supressor Gene) network. Quantitative PCR studies verified the array results (Parent et al., 2008;Roth et al., 2007) and in silico analysis of transcription factor recognition sites present in gene promoters indicated that Kiss1, Gpr54, and Syncam1, are subordinate genes of the network. Interestingly, before the role of kisspeptin in the control of puberty was discovered, the KiSS1 gene was known as a suppressor of tumor metastases (Ohtaki et al., 2001;Steeg et al., 2003).
Cis-regulatory analysis of shared TSG binding sites predicted the existence of five central hubs (Cdp/Cutl1, Maf, p53, Yy1, and Usf2) controlling the TSG network at the transcriptional level (Roth et al., 2007). This analysis involves the computer-assisted inspection of the 5′flanking region of the genes of interest for predicted transcription factor binding sites using databases such as TransFac (Matys et al., 2003;Wingender et al., 1996) and P-MATCH (http://www.gene-regulation.com/pub/programs.html), the establishment of directional links among genes encoding transcription factors to all genes containing a binding site for those transcription factors, and the visualization of these interactions using the on-line tool CytoScape (http://www.expasy.org). Several hubs were identified and found to be connected to both subordinate genes encoding proteins required for intracellular signaling and cell-cell communication, and to other presumptive non-TSG upper-echelon genes (Oct2, Ttf1, and Eap1) involved in the transcriptional regulation of the pubertal process. Immunohistochemistry and in situ hybridization analysis demonstrated that these three genes, as well as other subordinate nodes of the network, are expressed in neuronal and/or glial subsets involved in the control of GnRH secretion, including GnRH neurons themselves (Heger et al., 2007;Mastronardi et al., 2006;Ojeda et al., 2008;Roth et al., 2007). As it now stands, the current model of this TSG network is imperfect and needs to be fully validated by functional studies. Such studies are necessary to not only verify in silico predictions, but also to provide a more accurate and comprehensive architecture of the network. Also in need of definition is the identity of the neuronal and glial subsets expressing each putative central hub, and the possibility that individual central hubs have different, cell-specific, puberty-related developmental patterns of expression. Following this line of reasoning, we have now obtained evidence that the Kiss1 gene is not only controlled by TTF1 and Eap1, two non-TSG transcriptional regulators (Heger et al., 2007;Mastronardi et al., 2006), but also by CUTL1 and YY1 (Mueller, Dietzel, Tefs, Kiess, Danne, Loche, Lomniczi, Ojeda and Heger, in preparation), two putative central hubs of the TSG network.
It is likely that the TSG network is just one of several sub-networks involved in the control of puberty. As indicated above, Oct2, Ttf1, and Eap1 may form part of another, functionally connected network, because they do not behave as TSGs. This notion is supported by the absence of a discernible pattern of EAP1 under-expression in a panel of human tumors (Heger and Ojeda, unpublished). Lin28b, on the other hand, may be a hub of the TSG network, because of its role in puberty and its well-established contribution to cancer biology (Viswanathan et al., 2009). Based on both in silico models and experimental data, we envision a controlling system in which transcriptional regulators are shared by different neuronal and glial subsets, with sets of subordinate genes specifically expressed in particular cellular subsets.
It is tempting to speculate that these transcriptional control systems extend to genes encoding enzymes involved in processing precursors of puberty-related peptides, such as kisspeptin, dynorphin, RFRP1/3, etc. Likewise, little is known about the transcriptional machinery operating in hypothalamic astrocytes to control the production of growth factors (e.g. TGFα, neuregulins), and small molecules (e.g., prostaglandin E2, glutamate, ATP) involved in stimulating GnRH release. Of all of these factors, only TGFα expression has been shown to be controlled by a gene (Oct2) involved in the transcriptional regulation of puberty.
A strategy that can be used to assess the involvement of transcription factors in the control of the pubertal process is the use of gain-of-function or loss-of-function approaches (Davidson et al., 2002;Ideker et al., 2001). We have employed gain-of-function approaches to define the involvement of astroglial cells (Ma et al., 1994;Prevot et al., 2003;Rage et al., 1997;Sandau et al., 2009) and specific neuronal subsets (Bilger et al., 2001;Heger et al., 2003) in the hypothalamic control of puberty, and loss-of-function strategies to identify three upstream transcriptional regulators of the pubertal process (Heger et al., 2007;Mastronardi et al., 2006;Ojeda et al., 1999), and four subordinate genes involved in neuron-neuron communication (Choi et al., 2008;Garcia-Rudaz et al., 2008;Ha et al., 2008;Sandau et al., 2009). Because lentiviruses (Tiscornia et al., 2003) can efficiently deliver small interfering (si)RNAs to tissues or cells of interest (Garcia-Rudaz et al., 2007;Heger et al., 2007), we have used this delivery system to determine if decreasing the production of EAP1 would alter the onset of female puberty and adult reproductive cyclicity (Heger et al., 2007). We observed that Eap1 siRNA-producing lentiviral particles injected bilaterally into the preoptic area (POA) of juvenile 23-day-old rats results in delayed puberty, disrupted estrous cyclicity, reduced plasma LH, FSH and estradiol levels, and delayed growth of ovarian follicles. We conducted additional experiments to determine if EAP1 is also important for the hypothalamic control of menstrual cyclicity in nonhuman primates (Dissen et al., 2009), and found that knocking down EAP1 expression in the arcuate nucleus (ARC) of the hypothalamus abolished menstrual cyclicity (Dissen, Lomniczi, Heger, and Ojeda, in preparation). These results indicate that EAP1 is crucial for the ARC to maintain menstrual cyclicity in higher primates. Because EAP1 has an intrinsic repressive activity (Heger et al., 2007), EAP1 knock-down may delay puberty and prevent reproductive cyclicity by relieving an EAP1 repressive influence on inhibitory systems controlling GnRH output. These inhibitory systems may be inherent to the transsynaptic control of GnRH neurons (e.g., opioid peptides, GABA, GnIH) or – as detailed below – involve more encompassing regulatory systems, able to impose a broader level of transcriptional repression to the gene networks controlling the pubertal process.
4. The transcriptional repression of puberty
Though not yet proven, emerging evidence is beginning to surface suggesting that puberty may not occur earlier because it is held in check by a developmental program involving transcriptional/posttranscriptional repression of genes that are stimulatory to the pubertal process. For instance, nucleotide polymorphisms near the LIN28B gene in chromosome 6(q21) are associated with earlier puberty and shorter stature in girls (Ong et al., 2009;Perry et al., 2009;Sulem et al., 2009). LIN28B and its homolog LIN28 (located on a different chromosome) encode RNA-binding proteins that control gene expression via posttranscriptional regulation (Moss et al., 1997). However, only sequence variation in LIN28B is associated with early menarche (Ong et al., 2009). Increasing Lin28b expression In C. Elegans (Moss et al., 1997) and Lin28a in mice (Zhu et al., 2010) leads to developmental delay. Lin28b exerts this effect by preventing the early expression of genes, which should normally be activated at a subsequent phase of development (Ambros and Horvitz, 1984;Moss et al., 1997). Mammalian LIN28A and B control cellular function by blocking the maturation of let-7 miRNA precursors into mature miRNAs. An excess of LIN28B has been shown to reduce production of this miRNA family leading to derepression of let-7 miRNA target genes (Viswanathan et al., 2009). Because some of these targets are oncogenic (for instance K-Ras and c-Myc), let-7 miRNAs are considered to be TSGs (Viswanathan et al., 2009). Considering that Lin28a overexpression in mice results in delayed puberty (Zhu et al., 2010), both Lin28 and let-7 miRNAs can be considered as repressive components of the TSG network controlling the onset of puberty. As such, Lin28a and/or Lin28b expression would be expected to decline in the hypothalamus at the time of puberty, resulting in derepression of let-7 miRNA maturation. A reduction in Lin28b mRNA abundance does, in fact, occur both in the agonadal monkey hypothalamus at the expected time of puberty (Matagne, Ojeda and Plant, unpublished), and in ovary-intact female rats during prepubertal development (Tena-Sempene, Ojeda et al, unpublished observations). In this latter study, we also observed that let-7 miRNA levels increase in the hypothalamus as Lin28b mRNA abundance decreases.
It is likely that there are other potential mechanisms of transcriptional/posttranscriptional repression that may be involved in controlling the timing of puberty. For instance, we recently observed that expression of a family of genes encoding Zinc-finger (ZNF)-containing proteins changes in the hypothalamus of castrated male nonhuman primate at the expected time of puberty (Matagne et al., 2009). The expression profiles of these genes was inversely correlated to the prepubertal changes in LH output, i.e., it was elevated during the late juvenile period when GnRH secretion is turned off, and low as GnRH neuronal activity increases at the initiation of puberty. ZNF genes encode proteins that function as transcriptional repressors (Urrutia, 2003;Vogel et al., 2006). In other ongoing high-throughput studies, we identified two families of transcriptional repressors expressed in the pre and peripubertal female rat hypothalamus (Lomniczi et al., 2010). Intriguingly, expression of genes belonging to one of these families decreases at puberty, while that of members from the other family increases. Family 1 consists of genes of the POZ-ZF (poxvirus and Zinc finger) family of transcriptional regulators, also known as BTB (broad complex, Tramtrack, bric-à-brac) (Kelly and Daniel, 2006). POZ-ZF genes are transcriptional repressors implicated in the control of a variety of developmental processes; because alterations of their function result in tumorigenesis and developmental disorders, many of them are considered to be TSGs (Kelly and Daniel, 2006). We observed that expression of a subset of these genes, known as the POK (POZ and Krüppel) subfamily, increases in the hypothalamus of female rats at puberty, suggesting that POK proteins may be repressing downstream repressors of the pubertal process. Family 2 consists of genes of the Polycomb group (PcG). The PcG silencing complex is considered as a master regulator of genomic programs, because it acts at different stages of development to define which sets of genes are active and which ones are quiescent (Kohler and Villar, 2008;Schwartz and Pirrotta, 2007;Simon and Kingston, 2009). We found that expression of key members of this complex decreases in the hypothalamus at puberty, suggesting that PcG proteins might provide a repressive influence on the initiation of puberty by inhibiting the expression of downstream genes whose activation is ultimately required for puberty to occur. Like POK genes, genes of the PcG silencing complex can be considered as TSGs, because they have tumor suppressor activity (Classen et al., 2009;Kelly and Daniel, 2006;Martinez et al., 2009). Based on these considerations, the concept of a TSG network controlling puberty can be refined by postulating that the network's core is composed of both trans-activational and repressive nodes. While the former move the process along by facilitating the sequential activation of key stimulatory events, the latter may impose a repressive, and likely encompassing, tone to the system, so that premature reproductive maturation is prevented. Obviously, these preliminary observations will need to be thoroughly tested before they can be employed to support the idea that transcriptional repression is an integral component of the developmental program controlling mammalian puberty at a hypothalamic level.
5. The epigenetic control of puberty
While advancing our knowledge, the aforementioned findings were made only within the context of a genetic framework. As such, they do not explain how inherited, permanent changes in DNA sequence can regulate gene expression dynamically, while also imposing an encompassing level of coordination and transcriptional plasticity to gene sets, such as those controlling female reproductive development. It appears intuitively obvious that alternative mechanisms of control must exist. We believe that a powerful biological regulatory system that meets these requirements is epigenetics – i.e., those heritable changes in gene expression that occur without changing the primary nucleotide sequence of a gene (Herman and Baylin, 2003;Wolffe and Matzke, 1999). Chemical modification of DNA or chromatin-associated proteins, particularly histones, has a major influence on chromatin structure and gene expression. DNA can be modified by methylation of cytosine residues in CpG dinucleotides (Bjornsson et al., 2004;Jaenisch and Bird, 2003), and the N-terminal tails of histone proteins are subject to a wide range of different modifications, including acetylation, methylation, phosphorylation and ubiquitylation (Jenuwein and Allis, 2001;Kouzarides, 2007). Epigenetic mechanisms can not only provide gene-specific gate keeper functions (Garcia-Bassets et al., 2007), but are also endowed with an unsuspected degree of plasticity able to transiently change gene expression within hours (Miller and Sweatt, 2007), and even minutes (Kangaspeska et al., 2008;Metivier et al., 2008). Even more remarkable, epigenetic regulation of certain genes (Metivier et al., 2008), including the gene encoding ERα (Kangaspeska et al., 2008), is unexpectedly cyclic, exhibiting a periodicity that results in a rapid, tight and dynamic control of gene expression. It is now clear that epigenetic information is also essential for a variety of neural functions, including memory formation (Miller and Sweatt, 2007), recovery of learning and memory (Fischer et al., 2007), dendritic development (Wu et al., 2007), neuronal and behavioral plasticity (Kumar et al., 2005), estrogen-induced gene expression (Perillo et al., 2008;Subramanian et al., 2008), glial-neuronal interactions (Shen et al., 2008), circadian rhythms (Nakahata et al., 2008), and sexual differentiation of the brain (McCarthy et al., 2009).
The potential contribution of epigenetics to the pubertal process has never been formally addressed. We have now obtained evidence (Lomniczi et al., 2010) suggesting that an epigenetic mechanism of transcriptional repression operating in the hypothalamus plays a significant role in timing the initiation of female puberty. We obtained results suggesting that the PcG group of transcriptional silencers is a major contributor to this repressive mechanism. We observed that hypothalamic expression of core components of the PcG complex decreases at puberty, and that this change is associated with acquisition of epigenetic silencing marks (DNA methylation, repressive histones) and loss of activating histone marks from their promoter regions. In additional studies, we explored the concept that PcG genes target for repression downstream genes directly involved in the stimulatory control of GnRH secretion at puberty. Using the Kiss1 gene as a prototype, we found that PcG proteins interact with the 5′ flanking region of this gene, and that the pubertal increase in Kiss1 expression is accompanied by the acquisition of epigenetic modifications associated with gene activation, i.e. DNA demethylation, recruitment of activating histones and loss of repressive histones. These results support the notion that epigenetic mechanisms are integral components of the neuroendocrine process controlling female puberty.
6. Conclusions and perspectives
The observations discussed in this article support the notion that the onset of puberty depends on the contribution of more than one gene. There are many genes and many pathways that contribute to the process, but as seen in embryonic developmental processes, puberty also appears to be controlled at the transcriptional/post-transcriptional level by discrete groups of genes. “Activators” might move the process along by promoting key developmental events; “repressors” might prevent the untimely activation of activating genes (Fig. 1). We speculate that both inhibitory and stimulatory pathways of transcriptional/post-transcriptional regulation operate within neuronal and glial populations involved in controlling the onset of puberty, and that an encompassing level of regulation is provided by two layers of gene repression, acting in concert with epigenetic mechanisms. One of these layers, formed by transcriptional/posttranscriptional “repressors” (e.g. PcG genes, Lin28b, etc) may function to prevent the premature activation of puberty-inducing genes (such as Kiss1, Tac3, Ttf1, Eap1, Oct2, etc); the other layer, located more centrally in the network (e.g., POK, ZNF genes, and possibly others), may modulate in a temporally dynamic manner the expression of repressors (Fig. 2). As such, these more centrally located genes can be considered as “repressors of repressors”. Both layers may also directly control key subordinate genes of the network (such as those operating within kisspeptin, GABAergic, glutamatergic, opioid, and RFRP neurons, in addition to genes expressed in glial cells). The model also predicts that an encompassing level of coordination and transcriptional plasticity to these gene networks is provided, not by variations in DNA sequence, but by changes in epigenetic information (Lomniczi et al., 2010;Ojeda and Lomniczi, 2010), i.e. heritable changes in gene expression that occur without changing the primary nucleotide sequence of a gene. Much research is obviously needed to verify or challenge the validity of these concepts.
Figure 1. The hypothetical transcriptional control of mammalian puberty by “activators” and “repressors” operating in the female neuroendocrine brain.
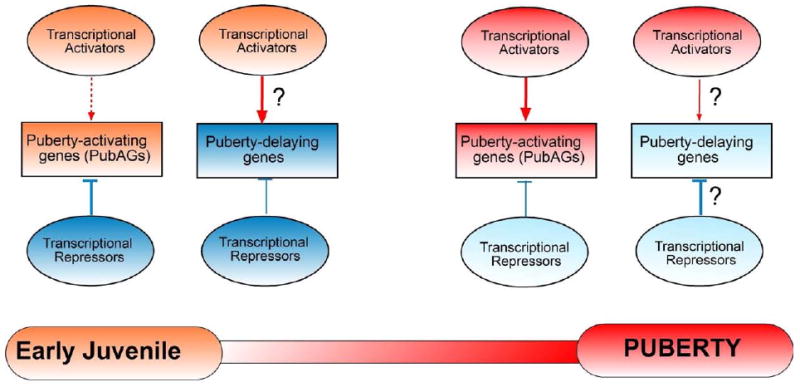
According to this model, during early juvenile days there are sets of genes repressing downstream genes involved in advancing the pubertal process (for instance, the effectors Kiss1 and Tac3 and the transcriptional regulators Ttf1, Oct2 and Eap1- collectively referred to as puberty-activating genes, PubAGs). In contrast, transcriptional repression of cellular systems inhibitory to puberty (e.g., dynorphin and RFRP neurons, and/or genes encoding prepropeptide processing enzymes in different inhibitory neuronal systems) would be negligible. At this time of development, trans-activation of PubAGs genes would be minimal, whereas trans-activation of puberty-delaying genes may be enhanced. The model predicts that the transcriptional inhibition of PubAGs is lifted at or before puberty, and replaced by increased trans-activation of gene expression. Simultaneously, the repression of puberty-delaying genes is expected to be enhanced.← = stimulation; ⊥ = inhibition. Whether a similar, but “reversed”, mechanism operates in primates at the time of the infantile-juvenile transition, when GnRH secretion is turned off is a possibility that requires experimental consideration.
Figure 2. General organization of a hypothetical transcriptional complex controlling the activational arm of female puberty.
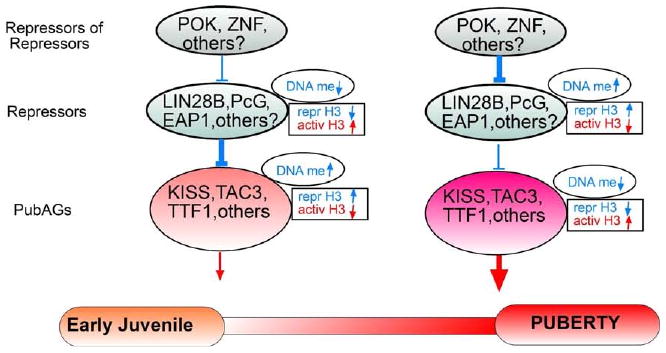
According to this model the highest level of control is exerted by repressors of repressors (e.g., POK, ZNF genes); because these genes repress other repressors (e.g. PcG genes), their influence would be expected to be low during juvenile development, increasing at puberty. In contrast, the repressive influence of a second, less “central” level of control formed by “repressors” (e.g. PcG genes) would be higher during juvenile development than at puberty, because these genes function to prevent the premature activation of puberty activating genes (PubAGs). As a result of these changes in repressive tone, PubAG expression (e.g, Kiss1, Tac3, TTF1), would increase at and/or preceding the onset of puberty. Because Eap1 predominantly represses gene expression, it may be considered as a second tier repressor, but more information is needed before an accurate assignment can be made. An additional level of regulation is provided by epigenetic mechanisms. According to existing information derived from studies performed in our laboratory, these mechanisms involve opposite changes in DNA methylation (DNA me) and association of modified histones to PcG and PubAG promoters (repr H3 = histones associated with gene repression; activ H3 = histones associated with gene activation). Not considered in this model is the possibility that repressors of repressors also modulate the activity of neuronal populations involved in the inhibitory control of puberty; should this be the case, an increased repressive influence of these factors at puberty would remove transsynaptic inhibitory influences on GnRH neurons, allowing stimulatory inputs to operate at full force. Lastly, this model does not consider “further upstream” mechanisms of control that may govern the expression of repressors of repressors, and that are likely to impose an even more encompassing regulation of the transcriptional cascade controlling puberty onset.
Acknowledgments
This work was supported in part by the National Institutes of Health grants HD25123, MH65438, HD050798, RR000163, and through cooperative agreement U-54 HD18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Abbreviations
- GnRH
Gonadotrophin-Releasing Hormone
- AVPV
anteroventral periventricular nucleus
- ARC
arcuate nucleus
- RFRP
RFamide-related peptide gene
- GnIH
gonadotrophin-inhibiting hormone
- TGFβ
Transforming growth factor-beta
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- IGF-I
insulin-like growth factor 1
- PGE2
prostaglandin E2
- NCAM
neural cell adhesion molecule
- PSA-NCAM
polysialylated NCAM
- ME
median eminence
- SynCAM1
Synaptic Cell Adhesion Molecule 1
- RPTPβ
Receptor-like Protein Tyrosine Phosphatase-β
- Eap1
Enhanced At Puberty1
- TSG
Tumor Supressor Gene
- POA
preoptic area
- ARC
arcuate nucleus
- ZNF
Zinc-finger
- POZ-ZF
poxvirus and Zinc finger
- BTB
broad complex - Tramtrack, bric-à-brac
- PcG
Polycomb group
- GABA
gamma amino butyric acid
- GNRHR
gonadotrophin releasing hormone receptor
- TAC3
G-protein coupled receptor 54, tachykinin 3
- TAC3
tachykinin 3 receptor
- POU
Pit1-Oct2-Unc86
- TGFα
transforming growth factor alpha
- LH
luteinizing hormone
- FSH
follicle stimulating hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Bedecarrats GY, Kaiser UB. Mutations in the human gonadotropin-releasing hormone receptor: insights into receptor biology and function. Semin Reprod Med. 2007;25:368–378. doi: 10.1055/s-2007-984743. [DOI] [PubMed] [Google Scholar]
- Bilger M, Heger S, Brann DW, Paredes A, Ojeda SR. A conditional, tetracycline-regulated increase in gamma amino butyric acid production near LHRH nerve terminals disrupts estrous cyclicity in the rat. Endocrinology. 2001;142:2102–2114. doi: 10.1210/endo.142.5.8166. [DOI] [PubMed] [Google Scholar]
- Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20:350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Brann DW. Glutamate: A major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology. 1995;61:213–225. doi: 10.1159/000126843. [DOI] [PubMed] [Google Scholar]
- Choi J, Ha CM, Choi EJ, Jeong CS, Park JW, Baik JH, Park JY, Costa ME, Ojeda SR, Lee BJ. Kinesin superfamily-associated protein 3 is preferentially expressed in glutamatergic neurons and contributes to the excitatory control of female puberty. Endocrinology. 2008;149:6146–6156. doi: 10.1210/en.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont dTX, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D. A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK-STAT signaling. Nat Genet. 2009;41:1150–1155. doi: 10.1038/ng.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, Yuh CH, Minokawa T, Amore G, Hinman V, Arenas-mena C, Otim O, Brown CT, Livi CB, Lee PY, Revilla R, Rust AG, Pan ZJ, Schilstra MJ, Clarke PJC, Arnone MI, Rowen L, Cameron RA, McClay DR, Hood L, Bolouri H. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Lomniczi A, Neff TL, Hobbs TR, Kohama SG, Kroenke CD, Galimi F, Ojeda SR. In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods. 2009;49:70–77. doi: 10.1016/j.ymeth.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- Dudas B, Merchenthaler I. Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol. 2006;18:79–95. doi: 10.1111/j.1365-2826.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Dziedzic B, Prevot V, Lomniczi A, Jung H, Cornea A, Ojeda SR. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates erbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci. 2003;23:915–926. doi: 10.1523/JNEUROSCI.23-03-00915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves L, Silberg J, Foley D, Bulik C, Maes H, Erkanli A, Angold A, Costello EJ, Worthman C. Genetic and environmental influences on the relative timing of pubertal change. Twin Res. 2004;7:471–481. doi: 10.1375/1369052042335278. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Luckman SM. RFAmide-related peptide: another sexy peptide? Endocrinology. 2008;149:899–901. doi: 10.1210/en.2007-1765. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Gajdos ZK, Butler JL, Henderson KD, He C, Supelak PJ, Egyud M, Price A, Reich D, Clayton PE, Le ML, Hunter DJ, Henderson BE, Palmert MR, Hirschhorn JN. Association studies of common variants in 10 hypogonadotropic hypogonadism genes with age at menarche. J Clin Endocrinol Metab. 2008;93:4290–4298. doi: 10.1210/jc.2008-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu XD, Rosenfeld MG. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rudaz C, Deng V, Matagne V, Ronnekleiv O, Bosch M, Han V, Percy AK, Ojeda SR. FXYD1, a modulator of Na(+), K(+)-ATPase activity, facilitates female sexual development by maintaining GnRH neuronal excitability. J Neuroendocrinol. 2008;21:108–122. doi: 10.1111/j.1365-2826.2008.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rudaz C, Luna F, Tapia V, Kerr B, Colgin L, Galimi F, Dissen GA, Rawlings ND, Ojeda SR. Fxna, a novel gene differentially expressed in the rat ovary at the time of folliculogenesis, is required for normal ovarian histogenesis. Development. 2007;134:945–957. doi: 10.1242/dev.02795. [DOI] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res Rev. 2007;56:101–118. doi: 10.1016/j.brainresrev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, III, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CM, Choi J, Choi EJ, Costa ME, Lee BJ, Ojeda SR. NELL2, a neuron-specific EGF-like protein, is selectively expressed in glutamatergic neurons and contributes to the glutamatergic control of GnRH neurons at puberty. Neuroendocrinology. 2008;88:199–211. doi: 10.1159/000139579. [DOI] [PubMed] [Google Scholar]
- He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009 doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger S, Mastronardi C, Dissen GA, Lomniczi A, Cabrera R, Roth CL, Jung H, Galimi F, Sippell W, Ojeda SR. Enhanced at puberty 1 (EAP1) is a new transcriptional regulator of the female neuroendocrine reproductive axis. J Clin Invest. 2007;117:2145–2154. doi: 10.1172/JCI31752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger S, Seney M, Bless E, Schwarting GA, Bilger M, Mungenast A, Ojeda SR, Tobet SA. Overexpression of glutamic acid decarboxylase-67 (GAD-67) in GnRH neurons disrupts migratory fate and female reproductive function in mice. Endocrinology. 2003;144:2566–2579. doi: 10.1210/en.2002-221107. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, Goodlett DR, Aebersold R, Hood L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–115. doi: 10.1038/nature06640. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kelly KF, Daniel JM. POZ for effect--POZ-ZF transcription factors in cancer and development. Trends Cell Biol. 2006;16:578–587. doi: 10.1016/j.tcb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Kinoshita F, Nakai Y, Katakami H, Imura H. Suppressive effect of dynorphin-(1-13) on luteinizing hormone release in conscious castrated rats. Life Sci. 1982;30:1915–1919. doi: 10.1016/0024-3205(82)90472-6. [DOI] [PubMed] [Google Scholar]
- Kohler C, Villar CB. Programming of gene expression by Polycomb group proteins. Trends Cell Biol. 2008;18:236–243. doi: 10.1016/j.tcb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Kordon C, Drouva SV, Martínez de la Escalera G, Weiner RI. Role of classic and peptide neuromediators in the neuroendocrine regulation of luteinizing hormone and prolactin. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2nd. Vol. 1. Raven Press; New York: 1994. pp. 1621–1681. [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krewson TD, Supelak PJ, Hill AE, Singer JB, Lander ES, Nadeau JH, Palmert MR. Chromosomes 6 and 13 harbor genes that regulate pubertal timing in mouse chromosome substitution strains. Endocrinology. 2004;145:4447–4451. doi: 10.1210/en.2004-0543. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Lomniczi A, Cornea A, Costa ME, Ojeda SR. Hypothalamic tumor necrosis factor-α converting enzyme (TACE) mediates excitatory amino acid-dependent neuron-to-glia signaling in the neuroendocrine brain. J Neurosci. 2006;26:51–62. doi: 10.1523/JNEUROSCI.2939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi A, Loche A, Ojeda SR. Epigenetic regulation of female puberty. 40th Ann Mtg Society for Neuroscience; San Diego, CA. November 13-17, 2010. [Google Scholar]
- Lomniczi A, Ojeda SR. A role for glial cells of the neuroendocrine brain in the central control of female sexual development. In: Parpura V, Haydon P, editors. Astrocytes in (Patho)Physiology of the Nervous System. Springer; NY: 2009. pp. 487–511. [Google Scholar]
- Ma YJ, Dissen GA, Merlino G, Coquelin A, Ojeda SR. Overexpression of a human transforming growth factor alpha (TGFα) transgene reveals a dual antagonistic role of TGFα in female sexual development. Endocrinology. 1994;135:1392–1400. doi: 10.1210/endo.135.4.7925101. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Schuettengruber B, Sakr S, Janic A, Gonzalez C, Cavalli G. Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling. Nat Genet. 2009;41:1076–1082. doi: 10.1038/ng.414. [DOI] [PubMed] [Google Scholar]
- Mastronardi C, Smiley GG, Raber J, Kusakabe T, Kawaguchi A, Matagne V, Dietzel A, Heger S, Mungenast AE, Cabrera R, Kimura S, Ojeda SR. Deletion of the Ttf1 gene in differentiated neurons disrupts female reproduction without impairing basal ganglia function. J Neurosci. 2006;26:13167–13179. doi: 10.1523/JNEUROSCI.4238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne V, Ramaswamy S, Lomniczi A, Plant TM, Ojeda SR. Program No 703.9, 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Hypothalamic expression of a gene cluster encoding transcriptional repressors and mapping to chromosome 19 is developmentally regulated and linked to sexual maturation in the rhesus monkey. Online. [Google Scholar]
- Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Munch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metivier R, Gallais R, Tiffoche C, Le PC, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Dubay C, Lomniczi A, Kaidar G, Matagne V, Sandau US, Dissen GA. Gene networks and the neuroendocrine regulation of puberty. Mol Cell Endocrinol. 2010;324:3–11. doi: 10.1016/j.mce.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Hill J, Hill DF, Costa ME, Tapia V, Cornea A, Ma YJ. The Oct-2 POU-domain gene in the neuroendocrine brain: A transcriptional regulator of mammalian puberty. Endocrinology. 1999;140:3774–3789. doi: 10.1210/endo.140.8.6941. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A. The Epigenetics of Mammalian Puberty. 14th International Congress of Endocrinology; Kyoto, Japan. March 26-30, 2010. [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Ma YJ, Dziedzic B, Prevot V. Astrocyte-neuron signaling and the onset of female puberty. In: Bourguignon JP, Plant TM, editors. The Onset of Puberty in Perspective. Elsevier Science B.V; Amsterdam: 2000. pp. 41–57. [Google Scholar]
- Ojeda SR, Prevot V, Heger S, Lomniczi A, Dziedzic B, Mungenast A. Glia-to neuron signaling and the neuroendocrine control of female puberty. Ann Med. 2003;35:244–255. doi: 10.1080/07853890310005164. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. The Physiology of Reproduction. 3rd. Academic Press/Elsevier; San Diego: 2006. pp. 2061–2126. [Google Scholar]
- Ojeda SR, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier; New York: 2002. pp. 589–659. [Google Scholar]
- Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, Bingham SA, Brage S, Smith GD, Ekelund U, Gillson CJ, Glaser B, Golding J, Hardy R, Khaw KT, Kuh D, Luben R, Marcus M, McGeehin MA, Ness AR, Northstone K, Ring SM, Rubin C, Sims MA, Song K, Strachan DP, Vollenweider P, Waeber G, Waterworth DM, Wong A, Deloukas P, Barroso I, Mooser V, Loos RJ, Wareham NJ. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009;41:729–733. doi: 10.1038/ng.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AS, Matagne V, Westphal M, Heger S, Ojeda S, Jung H. Gene expression profiling of hypothalamic hamartomas: a search for genes associated with central precocious puberty. Horm Res. 2008;69:114–123. doi: 10.1159/000111815. [DOI] [PubMed] [Google Scholar]
- Parent AS, Mungenast AE, Lomniczi A, Sandau US, Peles E, Bosch MA, Ronnekleiv OK, Ojeda SR. A contactin-receptor-like protein tyrosine phosphatase beta complex mediates adhesive communication between astroglial cells and gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2007;19:847–859. doi: 10.1111/j.1365-2826.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- Parkash J, Kaur G. Neuronal-glial plasticity in gonadotropin-releasing hormone release in adult female rats: role of the polysialylated form of the neural cell adhesion molecule. J Endocrinol. 2005;186:397–409. doi: 10.1677/joe.1.06156. [DOI] [PubMed] [Google Scholar]
- Perera AD, Lagenaur CF, Plant TM. Postnatal expression of polysialic acid-neural cell adhesion molecule in the hypothalamus of the male rhesus monkey (Macaca mulatta) Endocrinology. 1993;133:2729–2735. doi: 10.1210/endo.133.6.7694845. [DOI] [PubMed] [Google Scholar]
- Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C, Avvedimento EV. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science. 2008;319:202–206. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- Perry JR, Stolk L, Franceschini N, Lunetta KL, Zhai G, McArdle PF, Smith AV, Aspelund T, Bandinelli S, Boerwinkle E, Cherkas L, Eiriksdottir G, Estrada K, Ferrucci L, Folsom AR, Garcia M, Gudnason V, Hofman A, Karasik D, Kiel DP, Launer LJ, van MJ, Nalls MA, Rivadeneira F, Shuldiner AR, Singleton A, Soranzo N, Tanaka T, Visser JA, Weedon MN, Wilson SG, Zhuang V, Streeten EA, Harris TB, Murray A, Spector TD, Demerath EW, Uitterlinden AG, Murabito JM. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009;41:648–650. doi: 10.1038/ng.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. The Physiology of Reproduction. 3rd. Academic Press/Elsevier; San Diego: 2006. pp. 2177–2230. [Google Scholar]
- Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–255. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- Prevot V, Bouret S, Croix D, Takumi T, Jennes L, Mitchell V, Beauvillain JC. Evidence that members of the TGFβ Superfamily play a role in regulation of the GnRH neuroendocrine axis: expression of a type 1 serine-threonine kinase receptor for TGFβ and activin in GnRH neurones and hypothalamic areas of the female rat. J Neuroendocrinol. 2000;12:665–670. doi: 10.1046/j.1365-2826.2000.00508.x. [DOI] [PubMed] [Google Scholar]
- Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rage F, Hill DF, Sena-Esteves M, Breakefield XO, Coffey RJ, Costa ME, McCann SM, Ojeda SR. Targeting transforming growth factor α expression to discrete loci of the neuroendocrine brain induces female sexual precocity. Proc Natl Acad Sci USA. 1997;94:2735–2740. doi: 10.1073/pnas.94.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampazzo A, Pivotto F, Occhi G, Tiso N, Bortoluzzi S, Rowen L, Hood L, Nava A, Danieli GA. Characterization of C14orf4, a novel intronless human gene containing a polyglutamine repeat, mapped to the ARVD1 critical region. Biochem Biophys Res Commun. 2000;278:766–774. doi: 10.1006/bbrc.2000.3883. [DOI] [PubMed] [Google Scholar]
- Roth CL, Mastronardi C, Lomniczi A, Wright H, Cabrera R, Mungenast AE, Heger S, Jung H, Dubay C, Ojeda SR. Expression of a tumor-related gene network increases in the mammalian hypothalamus at the time of female puberty. Endocrinology. 2007;148:5147–5161. doi: 10.1210/en.2007-0634. [DOI] [PubMed] [Google Scholar]
- Sandau US, Mungenast AE, Lomniczi A, Alderman Z, Sardi SP, Fogel AI, Taylor B, Parent AS, Biederer T, Corfas G, Ojeda SR. SynCAM 1, a synaptic adhesion molecule, is expressed in astrocytes and contributes to the neuroendocrine control of Female Sexual Development via a cis Interaction with Glial erbB4 Receptors. 2009 [Google Scholar]
- Schulz R, Wilhelm A, Pirke KM, Gramsch C, Herz A. Beta-endorphin and dynorphin control serum luteinizing hormone level in immature female rats. Nature. 1981;294:757–759. doi: 10.1038/294757a0. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Crowley WF., Jr Perspective: the importance of genetic defects in humans in elucidating the complexities of the hypothalamic-pituitary-gonadal axis. Endocrinology. 2001;142:2173–2177. doi: 10.1210/endo.142.6.8261. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJM, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nature Neuroscience. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Steeg PS, Ouatas T, Halverson D, Palmieri D, Salerno M. Metastasis suppressor genes: Basic biology and potential clinical use. Clin Breast Cancer. 2003;4:51–62. doi: 10.3816/cbc.2003.n.012. [DOI] [PubMed] [Google Scholar]
- Subramanian K, Jia D, Kapoor-Vazirani P, Powell DR, Collins RE, Sharma D, Peng J, Cheng X, Vertino PM. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Mol Cell. 2008;30:336–347. doi: 10.1016/j.molcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Rafnar T, Holm H, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Alexandersen P, Feenstra B, Boyd HA, Aben KK, Verbeek AL, Roeleveld N, Jonasdottir A, Styrkarsdottir U, Steinthorsdottir V, Karason A, Stacey SN, Gudmundsson J, Jakobsdottir M, Thorleifsson G, Hardarson G, Gulcher J, Kong A, Kiemeney LA, Melbye M, Christiansen C, Tryggvadottir L, Thorsteinsdottir U, Stefansson K. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet. 2009;41:734–738. doi: 10.1038/ng.383. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Ilawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2008;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010 doi: 10.1016/j.yfrne.2010.03.001. Article in Press. [DOI] [PubMed] [Google Scholar]
- Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003;4:231. doi: 10.1186/gb-2003-4-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, Mermel CH, Beroukhim R, Azam M, Teixeira J, Meyerson M, Hughes TP, Llovet JM, Radich J, Mullighan CG, Golub TR, Sorensen PH, Daley GQ. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel MJ, Guelen L, de WE, Peric-Hupkes D, Loden M, Talhout W, Feenstra M, Abbas B, Classen AK, van SB. Human heterochromatin proteins form large domains containing KRAB-ZNF genes. Genome Res. 2006;16:1493–1504. doi: 10.1101/gr.5391806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E, Dietze P, Karas H, Knuppel R. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res. 1996;24:238–241. doi: 10.1093/nar/24.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010 doi: 10.1038/ng.593. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


