Abstract
Kisspeptin has been recognized as a key regulator of GnRH secretion during puberty and adulthood, conveying the feedback influence of endogenous gonadal steroids onto the GnRH system. Understanding the functional roles of this peptide depends on knowledge of the anatomical framework in which it acts, including the location of kisspeptin-expressing cells in the brain and their connections. In this paper, we review current data on the anatomy of the kisspeptin neuronal network, including its colocalization with gonadal steroid hormone receptors, anatomical sites of interaction with the GnRH system, and recent evidence of neurochemical heterogeneity among different kisspeptin neuronal populations. Evidence to date suggests that kisspeptin cells in mammals comprise an interconnected network, with reciprocal connections both within and between separate cell populations, and with GnRH neurons. At the same time, there is more functional and anatomical heterogeneity in this system than originally thought, and many unanswered questions remain concerning anatomical relationships of kisspeptin neurons with other neuroendocrine and neural systems in the brain.
Keywords: kisspeptin, hypothalamus, neuroanatomy, GnRH, steroid receptor
1. Introduction
Kisspeptin is one of a family of RFamide-related peptides (RFRP) that is now recognized as an essential endogenous regulator of the GnRH neuroendocrine system (Oakley et al., 2009). Kisspeptin and its related peptides are ligands for the orphan G protein-coupled receptor 54 (GPR54, now called Kiss1 receptor), mutations of which produce hypogonadotropic hypogonadism and a failure to enter puberty in humans (de Roux et al., 2003; Seminara et al., 2003) and mice (Seminara et al., 2003). Kisspeptin was subsequently shown to be an extraordinarily potent stimulator of GnRH/LH secretion in a variety of species (Irwig et al., 2004; Jayasena et al., 2009; Messager et al., 2005; Shahab et al., 2005), and because of the presence of Kiss1 receptors in GnRH neurons (Han et al., 2005; Irwig et al., 2004) and the ability of GnRH antagonists to block the effects of kisspeptin (Shahab et al., 2005), early work quickly suggested that this influence was conveyed directly upon the GnRH neuroendocrine system.
Because of its key role in reproduction, there has been avid interest in identifying the location of kisspeptin neurons, and characterizing the neural circuitry by which kisspeptin acts to stimulate GnRH release and regulate reproductive neuroendocrine function (Oakley et al., 2009). An understanding of the functional role of kisspeptin signalling in the brain depends on the anatomical framework within which kisspeptin acts, i.e., knowing the location of neuronal cell bodies that synthesize the peptide, and their afferent/efferent connections. The primary aim of this review is to update our current understanding of the anatomical organization of the kisspeptin network; in this context, we would note that there has been one previous, excellent review of the neuroanatomy of the kisspeptin system (Mikkelsen and Simonneaux, 2009). However, in light of the recent addition of information from a wider variety of mammalian species, we viewed it as timely and worthwhile to re-evaluate the range of data reported to see where consistent patterns might emerge concerning the organization of the kisspeptin neural network. In addition, we review anatomical evidence of steroid receptor colocalization in kisspeptin neurons, findings supporting the existence of direct connections between kisspeptin and GnRH neurons, and recent evidence of phenotypic heterogeneity among subsets of kisspeptin cells which may contribute to their physiological functions. Finally, we end with a consideration of current gaps in this knowledge and some suggestions of future studies to fill those gaps.
2. Distribution of Kisspeptin Cells and Fibers in the Mammalian Brain
The location of kisspeptin cell bodies in the mammalian brain has been examined by two primary techniques: in situ hybridization (ISH) to detect cells expressing Kiss1 mRNA transcripts, and immunocytochemistry (ICC), using either fluorescent or histochemical detection methods, to visualize kisspeptin peptide (Table 1). Initially, the use of ICC to detect kisspeptin-positive cell populations and fibers was confounded by the use of antibodies that cross-reacted with other members of the RFRP peptide family (Brailoiu et al., 2005). More recently, an antibody generated by Caraty and colleagues targeted against the C-terminal end of kisspeptin has been shown to be specific in a number of species both by careful preabsorption controls (Clarkson et al., 2009; Franceschini et al., 2006; Goodman et al., 2007) and the use of Kiss1 knockout mice as negative controls (Clarkson et al., 2009). Studies using other kisspeptin antibodies have performed similar controls (Greives et al., 2007; Ohkura et al., 2009; Ramaswamy et al., 2008). Thus in our analysis of the location of kisspeptin cells and fibers (Tables 1 and 2) we have omitted ICC studies that utilized antibodies which have been shown to cross-react with other RFRP peptides (e.g., from Phoenix Pharmaceuticals) and where appropriate controls for such cross-reactivity are lacking.
Table 1.
Distribution of Kisspeptin/Kiss1 Cells in the Mammalian Nervous System
+++, large (50–150); ++, moderate (15–50); +, few (<15 or numbers not reported);
Includes cells in PeN;
Caraty anti-Kp10 used for ICC detection
Table 2.
Distribution of Kisspeptin fibers in the Mammalian Nervous System
| Species | ARC | POA | AVPVa | Internal ME | External ME | PVN | DMH | SON | BNST | Lateral Septum | Other Regions | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | ++ | ++ | ++ | ++ | + | + | + | + | +b | (Hrabovszky et al., 2010) | ||
| Monkey | ++ | + | ++ | + | (Ramaswamy et al., 2008; Ramaswamy et al., 2010) | |||||||
| Sheep | ++ | + | ++ | + | + | (Franceschini et al., 2006; Goodman et al., 2007; Smith et al., 2008) | ||||||
| Rat | ++ | + | + | ++ | + | ++ | + | + | + | + | +c | (Adachi et al., 2007; Desroziers et al., 2010; Kinoshita et al., 2005; Takase, 2009) |
| Mouse | ++ | + | ++ | + | + | + | + | + | +d | (Clarkson and Herbison, 2006; Clarkson et al., 2009) | ||
| Hamster | ++ | + | ++ | (Mason et al., 2007) | ||||||||
| Goat | ++ | + | ++ | + | (Ohkura et al., 2009; Wakabayashi et al., 2010) | |||||||
| Horse | ++ | + | ++ | (Decourt et al., 2008) |
++, dense fibers; +, moderate or few fibers; PVN, paraventricular nucleus
Includes fibers in the median preoptic nucleus, AVPV, and PeN;
Other regions include: VMH, LHA;
Other regions include: suprachiasmatic nucleus, septohypothalamic area, medial septum;
Other regions include: medial septum, paraventricular nucleus of the thalamus, medial amygdala, periaqueductal gray, locus coeruleus.
The most consistent population of kisspeptin neurons identified across different mammalian species is the group located in the arcuate (ARC) nucleus (infundibular nucleus in humans). To date, this cell group has been identified, either by ISH or ICC, in humans, monkeys, sheep, rats, mice, hamsters, goats and horses (see Table 1 for references). In rodents, this population appears to be distributed throughout all rostral-caudal levels of the ARC (Gottsch et al., 2004; Smith et al., 2005), whereas in sheep, primates, goats and horses, they are located primarily at middle and caudal levels of the nucleus (Franceschini et al., 2006; Goodman et al., 2007; Ramaswamy et al., 2008).
In addition to the arcuate population, kisspeptin cell bodies have also been identified in the preoptic region by ISH or ICC in humans, monkeys, sheep, rats, mice and hamsters (Table 1). There are species differences in the precise location, and neurochemical phenotype, of kisspeptin cells in this region. In mice, rats and hamsters, preoptic kisspeptin cells are located in the anteroventral periventricular nucleus (AVPV) and extend as a continuum into the adjacent periventricular preoptic nucleus (PeN) (Gottsch et al., 2004; Smith et al., 2005; Smith et al., 2006); as discussed in more detail below, a subset of AVPV kisspeptin cells colocalize tyrosine hydroxylase (Kauffman et al., 2007b), galanin (Vida et al., 2009), which are present in other AVPV cells as well. In the sheep, monkey and human, kisspeptin cells are located at similar rostral-caudal levels in the preoptic area, although they are not directly adjacent to the third ventricle and appear to be more scattered than kisspeptin cells in the AVPV (Franceschini et al., 2006; Goodman et al., 2007; Hrabovszky et al., 2010; Smith et al., 2010). Furthermore, in the sheep, there appears to be no clear homolog to the AVPV, since the other neurochemical cell types that comprise this nucleus (e.g., dopamine, galanin (Herbison, 2008)) are not present as a well-defined cell group in the periventricular preoptic region (Lehman, unpublished observations). Whether the kisspeptin cells of the AVPV in rodents, and of the preoptic area (POA) in sheep and primates, are homologous to each other remains an open question and awaits use of additional markers. For the purpose of this review, we will refer to these subsets of neurons independently as the AVPV and POA populations, and consider both of them as cell groups in the ‘preoptic region’ (Table 1). It should be noted, however, there are a few species examined to date in which the presence of an AVPV, POA or other preoptic kisspeptin population has yet to be confirmed. For example, in goats, kisspeptin cells were not observed in the preoptic region despite the presence of large numbers of cells in the ARC in the same brains (Ohkura et al., 2009; Wakabayashi et al., 2010). However, these studies were performed using castrated male animals, and since kisspeptin expression in the AVPV and POA appears to be dependent on the presence of gonadal steroids (see below), these cells may not have expressed sufficient amounts of peptide to be detectable in castrated males. In the horse mare, one study (Magee et al., 2009) did identify POA neurons but used an antibody that has been questioned with regards to specificity (Goodman et al., 2007); another study in the female horse which used the more specific Caraty antibody (Decourt et al., 2008; Magee et al., 2009) failed to detect POA kisspeptin neurons. Hence the question of whether a kisspeptin cell population is present in the preoptic region of all mammals remains to be determined.
Total kisspeptin cell number appears to differ between the ARC and preoptic region populations, with greater numbers of cells seen in the ARC than POA in humans (Rometo et al., 2007) and sheep (Smith et al., 2007), in the ARC than AVPV in rats (Adachi et al., 2007; Kauffman et al., 2007b; Smith et al., 2006) based on ISH. It should be noted that the gonadal steroid, estradiol, has, in general, an opposite effect on each of these populations, stimulating Kiss1 mRNA and peptide in the preoptic region1 and inhibiting it in the ARC (Kauffman et al., 2007b; Smith et al., 2005; Smith et al., 2006; Smith et al., 2008). Thus differences between the number of preoptic region and ARC kisspeptin cells detected in these studies might simply be a reflection of the hormonal status of the animals used. However, even when the influence of steroidal milieu is taken into account (e.g., comparing kisspeptin cell number in the ARC of ovariectomized animals with preoptic region cell number in OVX animals treated with estradiol), the absolute number of detectable kisspeptin cells is higher in the arcuate nucleus than the preoptic region. For example, the POA of estradiol-treated OVX sheep during the breeding season contains approximately 100 Kiss1-expressing cells (Smith et al., 2008); by contrast, the ARC of OVX sheep contains more than 400 cells (Smith et al., 2008). Similarly in the rat, the number of Kiss1-mRNA expressing neurons in the AVPV of estradiol-treated OVX females contains approximately 120 cells (Kauffman et al., 2007b) whereas the ARC of OVX females without steroid treatment contains approximately 200 Kiss1 cells (Kauffman et al., 2007b). Thus, it appears that the ARC kisspeptin cell population contains consistently greater numbers of cells than the kisspeptin population in the preoptic region, even though the level of kisspeptin expression in these cells is influenced by gonadal hormones.
In addition to neuronal populations in the ARC and preoptic region, there are a few additional, smaller populations of kisspeptin cells that have been reported, and are variable among species. Perhaps the most controversial of these is a small group of scattered kisspeptin-immunoreactive neurons in the dorsomedial hypothalamus (DMH), that is seen in the brains of sheep (Franceschini et al., 2006), mice (Clarkson and Herbison, 2006; Clarkson et al., 2009) and horse mares (Decourt et al., 2008), but not in the rat (Desroziers et al., 2010; Kauffman et al., 2007b) or hamster (Greives et al., 2007). Although the genuine nature of kisspeptin localization in these cells was originally questioned because of their detection by non-specific antibodies that cross-reacted against RFRP3 cells in this region, as well as the failure to detect them by ISH, recent ICC studies have confirmed their presence in mice, sheep and horses, using the highly-specific Caraty antibody. The inability to detect these cells by ISH may be due to either their few number and scattered distribution, and/or to low levels of mRNA expression. In addition to kisspeptin cells in the DMH, in sheep and horses, a few kisspeptin-immunoreactive cells have also been reported in the ventromedial hypothalamic nucleus, however, in the sheep, these were detected using ISH and have not been confirmed with ICC using the Caraty antibody (Estrada, 2006), and in the horse (Magee et al., 2009), were detected with an antibody questioned for its specificity (Magee et al., 2009). In the monkey, a small number of kisspeptin cells extend from the ARC population directly into the median eminence (Ramaswamy et al., 2008), and in the human, kisspeptin neurons have been identified in the infundibular stalk (Hrabovszky et al., 2010). Finally, there is strong evidence from ISH studies in the mouse that a distinct population of kisspeptin cells exists outside the hypothalamus, in the medial amygdala (Gottsch et al., 2004; Kauffman, 2007) and BNST (Gottsch et al., 2004). Kisspeptin cells in the medial amygdala have not yet been identified in other species, however, a small number of Kiss1 expressing cells in the BNST have been recently reported in the female rhesus monkey (Smith et al., 2010). The presence of kisspeptin-immunoreactive cell bodies in the medial amygdala or BNST have not yet been reported, although there are kisspeptin-positive fibers in both regions of the mouse (Clarkson and Herbison, 2006; Clarkson et al., 2009).
In addition to hormonal influences, there is evidence of clear sexual dimorphism in kisspeptin expression in all species examined to date, and gender is a factor that needs to be taken into account when evaluating the presence or absence of specific populations using either ISH or ICC. In rodents, the AVPV kisspeptin population is sexually differentiated, with females expressing a significantly greater number of Kiss1/kisspeptin-ir neurons than males (Ansel et al., 2010; Clarkson and Herbison, 2006; Smith et al., 2006). This dimorphism cannot be accounted for by differences in the adult hormonal milieu, because both intact and gonadectomized males and females show this sex difference, as well as gonadectomized males and females replaced with the same gonadal steroid (Adachi et al., 2007; Kauffman et al., 2007b). Thus differences in the AVPV are likely due to the organizational influence of gonadal steroids during development (Kauffman, 2009). In contrast to the AVPV population, kisspeptin cells in the ARC of rodents show no sex difference in their number. However, in sheep, both POA and ARC kisspeptin populations show sex differences, with greater numbers of cells in ewes than rams (Cheng et al., 2010). The sex difference in the ARC population also appears to be due to organizational effects of gonadal steroids since ovariectomized pubertal ewes show greater numbers of cells than castrated rams (Nestor et al., 2010), but steroid replacement studies have yet to be done. In addition, sex differences in the same direction have recently been reported in the infundibular nucleus of humans with greater numbers of kisspeptin cells in females than males (Hrabovszky et al., 2010). In the human preoptic region, kisspeptin-immunoreactive neurons were consistently visualized in females, while none were seen in any of the male brains examined (Hrabovszky et al., 2010). Sex differences in kisspeptin cell number in other species, including monkeys, have not yet been reported, nor is it known whether the differences reported are due to gender-related cell death, as in the case of the sexual dimorphic nucleus of the preoptic area (Davis et al., 1996), or due to changes in gene/peptide expression. The reason for the difference in which kisspeptin populations are sexually differentiated between rodents, and sheep and humans, may lie in the functional roles that these areas play in the preovulatory GnRH surge, which is present in females, but not males. In rodents, the AVPV has been shown as a critical region driving the preovulatory GnRH surge, as lesions of this area prevent the estradiol-induced surge (Wiegand, 1980), whereas the ARC region has been implicated as essential for the steroid-induced preovulatory surge in sheep (Caraty et al., 1998) and primates (Hess et al., 1977; Krey et al., 1975). Thus, sexual differences in the ARC (infundibular) kisspeptin population in sheep and humans may reflect the importance of this cell group in the generation of the GnRH surge, compared to rodents in which the AVPV plays the predominant role.
Axonal fiber projections arising from kisspeptin cell populations have been analyzed by ICC in a range of species (Table 2). Kisspeptin fibers are reported consistently in the same regions where a majority of kisspeptin cells bodies are located, namely the ARC and preoptic region, with denser kisspeptin fibers reported in the ARC than in the preoptic region for all species. Besides the ARC, the densest accumulation of kisspeptin fibers is seen in the internal zone of the median eminence (Clarkson and Herbison, 2006; Decourt et al., 2008; Desroziers et al., 2010; Franceschini et al., 2006; Hrabovszky et al., 2010; Ramaswamy et al., 2010; Wakabayashi et al., 2010); In sheep (Franceschini et al., 2006), monkeys (Ramaswamy et al., 2008), rats (Desroziers et al., 2010) and goats (Wakabayashi et al., 2010), kisspeptin fibers have also been seen in the external zone of the median eminence where GnRH fibers terminate on portal vessels. However, it is noteworthy that in each of these species, axons and terminals in the external zone are much fewer in number and density than the kisspeptin fibers in the internal zone of the median eminence. A caveat is this observation may reflect more active release (and depletion) of peptide from fibers in the external than internal zone; comparison of kisspeptin fiber staining in the external zone under different endocrine conditions, presumably reflecting different patterns of endogenous kisspeptin release, might be useful in addressing this possibility.
Thus far, the mouse, rat and human, are the only species in which kisspeptin fibers have been thoroughly mapped outside of the ARC, POA and median eminence, and studies in each of these species have used the Caraty antibody. The overall comparison reveals many areas where kisspeptin fibers are found in common in mouse, rat, and human; these include the ARC, AVPV (including PeN), internal zone of the median eminence, PVN, DMH and lateral septum (Table 2). However, there are some differences: for example, in the mouse but in the rat, kisspeptin-positive fibers are seen in the paraventricular nucleus of the thalamus, medial amygdala, periaqueductal gray, and locus coeruleus (Clarkson et al., 2009; Desroziers et al., 2010). In the rat but not the mouse, kisspeptin fibers were reported in the suprachiasmatic nucleus and septohypothalamic area (Desroziers et al., 2010), while in humans, kisspeptin fibers are seen within the VMH (Hrabovszky et al., 2010) while in the rat (Desroziers et al., 2010) and mouse (Clarkson et al., 2009), fibers surround the VMH but do not enter it. Since these studies used antisera with the same specificity (Caraty anti-Kp-10 #564 and 566), and since the immunocytochemical protocols were largely the same, there may be genuine species difference in the distribution of kisspeptin fibers in these regions. As discussed below, the discovery of a unique set of neuropeptide markers of the ARC kisspeptin populations (Goodman et al., 2007; Navarro et al., 2009; Wakabayashi et al., 2010) has made it possible to use multiple-label ICC to map out fiber projections specific to the ARC population, along with identification of its postsynaptic targets.
3. Steroid Receptor Colocalization in Kisspeptin Neurons
A substantial body of work has implicated kisspeptin neurons as primary mediators of gonadal steroid feedback control of GnRH release in mammals (Lehman et al., 2010; Roseweir and Millar, 2008; Smith, 2008). One of the major pieces of evidence for this role is the high degree of colocalization of kisspeptin cells with gonadal steroid receptors, specifically those for estradiol, progesterone and testosterone (Table 3). In general, studies using multiple-label ISH or ICC to evaluate colocalization have revealed fairly similar pictures of the extent of colocalization in different species. For example, in the ARC, studies in rats, mice and sheep reveal a similar high degree of colocalization of estrogen receptor-alpha (ER-α), progesterone receptor (PR), and androgen receptor (AR) in kisspeptin neurons, ranging from 70–99% (Table 3; Fig. 1, A–C). In the sheep POA, approximately 50% of the kisspeptin neurons coexpress ER-α (Franceschini et al., 2006), and in the rodent AVPV, a range from 62–99% in colocalization of ER-α and PR has been reported (Adachi et al., 2007; Clarkson et al., 2008; Smith et al., 2005; Smith et al., 2006). The difference between sheep and rodents may be due to the different techniques employed (ISH in most rodent studies vs. ICC in sheep), or may reflect species differences in the functional roles that these populations may serve (i.e., the preovulatory GnRH surge). Nonetheless, there is a consistent, high degree of colocalization of ER-α, PR and AR in kisspeptin cells across species, supporting this feature as a key characteristic of the kisspeptin neuronal network. By contrast, the percentage of kisspeptin cells in both the ARC and preoptic region that colocalize estrogen receptor-beta (ER-β) is much less, ranging from 11–25% in the ARC and 21–43% in the AVPV (Table 3). Thus, the effects of estradiol on both ARC and AVPV kisspeptin populations are likely mediated primarily by ER-α, consistent with evidence that this isoform mediates physiological control of GnRH secretion by estradiol feedback (Smith et al., 2005; Wintermantel et al., 2006). For the most part, other nuclear steroid receptors have not yet been studied for colocalization in kisspeptin cells. One exception is the type II glucocorticoid receptor which has been shown to be present in approximately 50% of dynorphin neurons in the ARC (Oakley, 2009); given near complete colocalization of dynorphin and kisspeptin in the ARC (see below), glucocorticoid receptors are almost certainly co-expressed in ARC kisspeptin cells as well.
Table 3.
Percentage of Kisspeptin/Kiss1 Cells Colocalizing Gonadal Steroid Receptors in Female Mammals
| Species | ARC |
POA |
AVPV |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ER-α | ER-β | PR | AR | ER-α | ER-β | PR | ER-α | ER-β | PR | |
| Sheep | 93a | 86b, >95c | >85d | 50a | ||||||
| Rat | 92e, 70f | 11f | 90e, 62f | 21f | ||||||
| Mouse | >99g, 88h | 25g | 99g, 65i | 31g | 67i | |||||
Smith et al., 2007, ISH;
Cheng et al., 2010, ICC, data from males and females;
Lehman, unpublished, ICC;
Adachi et al., 2007, ISH;
Smith et al., 2006, ISH;
Smith et al., 2005a, ISH;
Smith et al., 2005b, ISH;
Fig. 1.
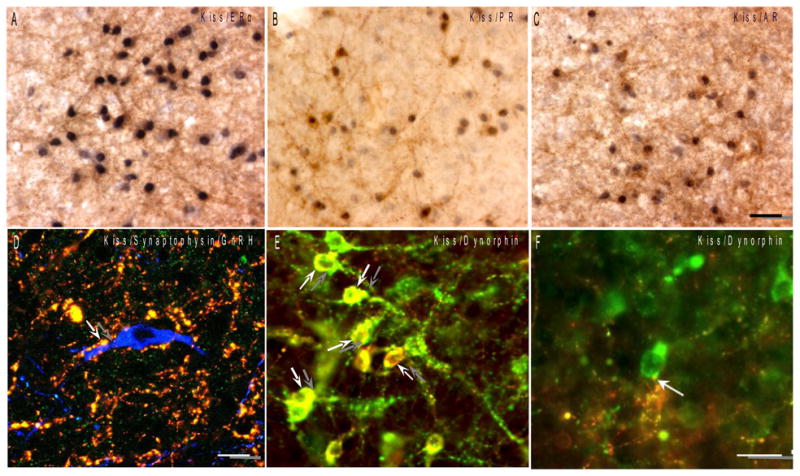
A–C: Colocalization of gonadal steroid receptors in kisspeptin neurons. Dual immunostained sections of sheep ARC showing high degree of colocalization of nuclear ER-α, PR and AR (blue-black) in kisspeptin cells (brown). Bar = 50 μm. D: Kisspeptin synaptic contacts onto a GnRH neuron. Confocal optical section (1 μm thick) of a triple-labeled section showing a terminal labelled with both kisspeptin (red) and synaptophysin (green) in direct contact with an ovine GnRH (blue) cell body (modified from Smith et al., 2008). Bar = 10 μm. E–F: Phenotypic heterogeneity between ARC and preoptic kisspeptin neurons. E: Colocalization of the endogenous opoid peptide, dynorphin (red), in kisspeptin neurons (arrows; green) of the sheep ARC. F: By contrast, kisspeptin neurons (green) in the sheep POA do not colocalize dynorphin even though they receive input from dynorphin-positive fibers (arrow; red). Bar = 20 μm. (modified from Goodman et al., 2007)
4. Anatomical Sites of Interaction between Kisspeptin and GnRH neurons
Given the expression of the Kiss1 receptor (Kiss1R) within GnRH neurons (Han et al., 2005; Herbison et al., 2010; Irwig et al., 2004), as well as the demonstration of direct stimulatory effects of kisspeptin upon GnRH cell electrophysiology (Han et al., 2005; Pielecka-Fortuna et al., 2008; Roseweir et al., 2009), it has been presumed that kisspeptin neurons must synapse directly upon GnRH neurons. Nonetheless, while a number of studies have shown contacts between kisspeptin fibers and GnRH neurons at a light microscopic level, there is currently no direct electron microscopic (EM) evidence of kisspeptin terminals synapsing directly on GnRH somas or dendrites. Perhaps the best evidence shy of EM comes from dual-label studies using the confocal microscope where optical sections of 1 micron or less in thickness can be analyzed for close associations between kisspeptin and GnRH neurons. Thus far, confocal images of kisspeptin terminals in direct apposition to GnRH cell bodies have demonstrated in monkeys, sheep, mice and horses (Clarkson and Herbison, 2006; Decourt et al., 2008; Ramaswamy et al., 2008; Smith et al., 2008). As in the case of other kisspeptin fibers, it should be noted that the detection of close contacts with GnRH neurons depends on the level of peptide present in those presynaptic terminals, and thus may vary according to gonadal hormone levels. Studies in monkeys (Ramaswamy et al., 2008; Smith et al., 2010), sheep (Smith et al., 2008), and mice (Clarkson and Herbison, 2006), have quantified the number of kisspeptin close contacts onto GnRH neurons located in either the POA and/or mediobasal hypothalamus (MBH). In the case of POA GnRH neurons, evidence from female mice (Clarkson and Herbison, 2006) and sheep (Smith et al., 2008) show that approximately 41–55% of GnRH cells receive at least one kisspeptin positive close contact; in the female sheep this percentage is much higher (95%) for GnRH cells located in the MBH. However, in female rhesus monkeys, the percentage of GnRH cells receiving input is much lower, with approximately 5–15% of POA GnRH neurons, and 20% of MBH GnRH neurons, receiving at least one kisspeptin-positive contact (Smith et al., 2010). In addition to species and regional differences, there is also evidence for sex differences in kisspeptin inputs onto GnRH neurons. Specifically, Clarkson and Herbison (2006) showed that a greater percentage of POA GnRH neurons in female brains (40%) receive direct kisspeptin contacts than in the male brain (10%) (Clarkson and Herbison, 2006). A sex difference in kisspeptin input to GnRH neurons may also be present in the monkey, where 33% of MBH GnRH neurons in the male receive one or more kisspeptin-positive inputs (Ramaswamy et al., 2008), as opposed to 20% of MBH GnRH neurons in the female (Smith et al., 2010). However, these observations are based on separate studies, and, as in the case of cell number comparisons, studies of sex difference in kisspeptin inputs need to be replicated with comparisons between gonadectomized animals, as well as gonadectomized animals with steroid replacement.
Confocal evidence of close contacts is not the same as direct EM level observations of synapses, but the additional detection of synaptic markers allows confocal multiple-label ICC to be used as a reliable proxy for the presence of synapses. For example, we have shown that direct contacts between synapsin-positive terminals and neurochemically-identified postsynaptic cells, seen under a light microscope in thin, 1 μm, sections, are always predictive of synapses when the same material is viewed at an EM level (Adams et al., 2006). Thus, analysis of 1 μm thick confocal sections that are triple-labeled for kisspeptin, synaptophysin (another synaptic marker), and GnRH, should provide strong evidence of synaptic inputs onto those cells. Using this approach, we have demonstrated that almost all kisspeptin close contacts on ovine GnRH neurons in the MBH are also synaptophysin-positive, and thus likely represent bona fide synaptic inputs (Smith et al., 2008; Fig. 1D). This same approach should be very useful for assessing the relative contribution of kisspeptin compared to other types of inputs (e.g., GABAergic) onto GnRH neurons, and the variation in that array of inputs with respect to sex, endocrine status, or age.
In addition to contact onto GnRH cell bodies, studies in the rhesus monkey and horse have noted the close associations of kisspeptin fibers with GnRH terminals in the external zone of the median eminence (Decourt et al., 2008; Ramaswamy et al., 2008). Anterograde tracing from neurokinin B cells in the ARC (that colocalize kisspeptin, see below) in rodents (Krajewski et al., 2010), as well as retrograde tracing studies from the median eminence in the sheep (Lehman et al., 2010), confirms this projection, and suggest that the ARC population is a major source of this kisspeptin input onto GnRH terminals. The ability of kisspeptin to affect the release of GnRH from murine hypothalamic slices lacking GnRH cell bodies has provided evidence for the median eminence as a potential site of action for kisspeptin in its control of GnRH secretion (d’Anglemont de Tassigny et al., 2008). Observations of close interactions between kisspeptin and GnRH fibers in the external zone provides an anatomical substrate for this site of action; however, as noted above, the number of kisspeptin-positive fibers in the external zone of the median eminence is sparse and variable among species, especially compared with the high density of these fibers in the internal zone (Table 2). It may be that if kisspeptin acts upon GnRH terminals in this region it does so via paracrine signaling, with kisspeptin released in the internal zone diffusing to the external zone. The possibility of paracrine signaling within the median eminence has been noted in older work where tancytes and other glial cells elements have been postulated to mediate the transport of molecules across internal and external zones (Agnati et al., 1995; Lehman, 2000).
5. Heterogeneity among Kisspeptin Cell Populations
Recent evidence suggests that not all kisspeptin neurons are the same phenotypically, and, that some of these anatomical differences may underlie functional differences in the role of specific kisspeptin population in positive and negative steroid feedback controls of GnRH secretion (Dungan et al., 2006; Kauffman et al., 2007a). In particular, there is consistent evidence in the mouse, rat, sheep, goat, and human that kisspeptin cells in the ARC, but not in the POA/AVPV, colocalize two other neuropeptides shown to be important in the control of GnRH secretion, neurokinin B (NKB) (Topaloglu et al., 2009) and dynorphin (DYN) (Goodman et al., 2004) (Fig. 1E–F; Fig. 2). Nearly all ARC kisspeptin neurons in these species colocalize NKB and DYN (Goodman et al., 2007; Lehman et al., 2010) because of this, and for convenience, we have termed this cell population, the KNDy (Kisspeptin. Neurokinin B, Dynorphin) cells (Cheng et al., 2010). KNDy cells likely play multiple roles in control of GnRH secretion. Evidence from sheep and rodents suggest they are critical for conveying the negative feedback influence of estradiol and progesterone onto GnRH neurons (Goodman et al., 2004; Smith et al., 2007); in the sheep they may also play a role in the positive feedback influence of estradiol to induce the preovulatory GnRH surge (Lehman et al., 2010; Smith et al., 2009). Another interesting characteristic of KNDy cells in the ARC is that they possess extensive reciprocal connections with each other, confirmed at both light microscopic and EM levels, forming what appears to be an interconnected network (Burke, 2006; Foradori, 2006; Krajewski et al., 2010). Reciprocal connections appear much less abundant among POA kisspeptin neurons in sheep (Lehman et al., 2010), which, if homologous to preoptic kisspeptin cells in the rodent, correlates with the lack of effect of kisspeptin on the electrophysiological firing of AVPV kisspeptin neurons in the mouse (Ducret et al., 2010). Thus, the reciprocal coupling of the ARC kisspeptin population, together with the colocalization of NKB and dynorphin receptors, may be critical in enabling these neurons to fire synchronously with each other. Observations of rhythmic, multiunit activity recorded from the location of kisspeptin neurons in the ARC is consistent with this (Ohkura et al., 2009), and the temporal association of these electrophysiological rhythms with GnRH/LH pulses has led to speculation that they comprise a critical component of the “GnRH pulse generator” (Lehman et al., 2010; Navarro et al., 2009; Wakabayashi et al., 2010). Observations of changes in the shape of each GnRH pulse in response to an opioid antagonist (Goodman, 1995) and alterations in both multi-unit activity (Wakabayashi et al., 2010) and LH pulse frequency induced by treatment with opioid (dynorphin) and NK3R (NKB) antagonists (Goodman et al., 2010) are consistent with this hypothesis.
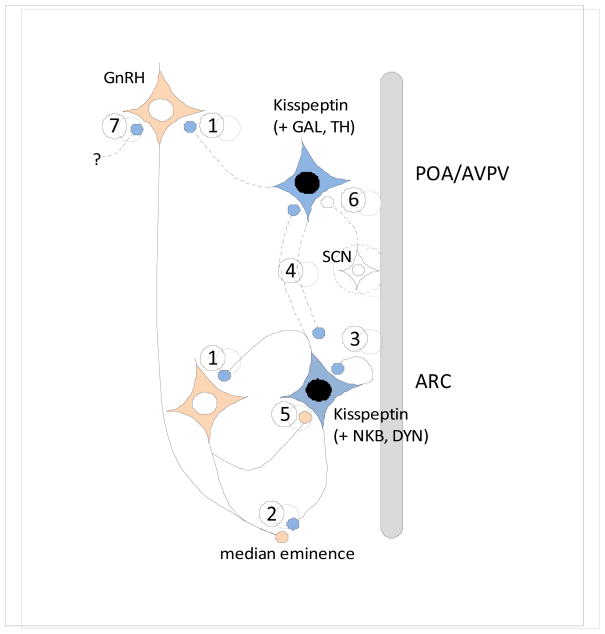
Fig. 2. Schematic horizontal section (top=rostral, bottom=caudal) showing the POA/AVPV and ARC kisspeptin populations in the mammalian hypothalamus, and their potential sites of interactions with GnRH neurons.
Virtually all ARC kisspeptin neurons co-express neurokinin B and dynorphin (Goodman et al., 2007; Navarro et al., 2009; Wakabayashi et al., 2010), while subsets of AVPV kisspeptin neurons in the preoptic region express either galanin (Vida et al., 2009) or tyrosine hydroxylase (Kauffman et al., 2007b). Connections (solid lines, published data; dotted lines, unpublished data) and sites of interactions between kisspeptin and GnRH systems include: 1) Direct projections from ARC and AVPV kisspeptin cells onto GnRH cell bodies (Clarkson and Herbison, 2006; Kinoshita et al., 2005; Krajewski et al., 2005; Lehman et al., 2010; Ramaswamy et al., 2008; Smith et al., 2008); 2) Inputs from ARC kisspeptin cells onto GnRH terminals in the median eminence (Burke, 2006; Krajewski et al., 2005; Lehman et al., 2010; Ramaswamy et al., 2008); 3) reciprocal connections among ARC kisspeptin cells that could be from the same or adjacent neurons (Burke, 2006; Foradori et al., 2002; Krajewski et al., 2010; Wakabayashi et al., 2010); 4) Projections from ARC kisspeptin neurons to POA kisspeptin cells in the sheep (Lehman, unpublished), and from AVPV kisspeptin neurons back to the ARC (Yeo and Herbison, 2009); and 5) projections from GnRH neurons back onto ARC kisspeptin cells (Ramaswamy et al., 2008). In addition, afferents to POA/AVPV kisspeptin cells from the suprachiasmatic nucleus (SCN) are indicated (6) (Vida et al., 2010), as well as the possibility that kisspeptin inputs to GnRH neurons in the POA or MBH may arise from other populations such as the DMH, BNST or medial amygdala (7).
The presence of the unique set of KNDy neuropeptides for the ARC kisspeptin population has provided the opportunity to use multiple-label ICC to analyze the projections of this population, including its potential inputs to GnRH neurons. Thus far, studies in the rat have used a combination of dynorphin and NKB (Burke et al, 2006), as well as the combination of kisspeptin and NKB (Kirigiti et al., 2009), to trace projections from KNDy cells. Dual-labeled projections from KNDy neurons have been reported in each of the areas shown in Table 2 where single-labeled kisspeptin fibers are found, albeit in lesser numbers. In addition, dual-labelled KNDy fibers are found in the external zone of the median eminence (Burke, 2006), and dynorphin fibers can be seen in direct contact with GnRH terminals in the median eminence at an electron microscopic level (Lehman et al., 2010). In addition, the colocalization of dynorphin and NKB in axon terminals has been used to show direct contacts of KNDy neurons onto GnRH cell bodies in the sheep (Lehman et al., 2010).
While kisspeptin cells in either the POA or the AVPV do not express either neurokinin B or dynorphin (Goodman et al., 2007; Navarro et al., 2009), there is evidence that a majority of AVPV kisspeptin population in the rodents colocalizes galanin, a neuropeptide implicated in female reproductive function (Vida et al., 2009), and that a subset also colocalizes tyrosine hydroxylase, a marker for dopaminergic neurons (Kauffman et al., 2007b) (Fig. 2). Subsets of AVPV neurons also express GABA and glutamate, and the extent to which either AVPV or POA kisspeptin neurons may colocalize these neurochemicals has not yet been examined. However, neurotensin, which is also expressed by AVPV neurons, is not colocalized with kisspeptin (Dungan Lemko et al., 2010). Kisspeptin cells of the amygdala (Kauffman, 2007) have not yet been examined for the co-expression of other neuropeptides/transmitters, although based on studies of the phenotype of cells in the medial amygdala that project to the preoptic area and GnRH cells, it seems likely that some of these may colocalize the neuropeptides, cholecystokinin and/or substance P (Simerly, 1989).
6. Summary and Future Directions
Key features of the kisspeptin neural network and its interactions with GnRH neurons, based on our current knowledge, are summarized in Fig. 2. Kisspeptin cell are found consistently in two major cell populations, one located in the ARC and the other in preoptic region, in either the AVPV or POA. While the ARC population is highly conserved among species, there is variation in the location and phenotype of preoptic kisspeptin neurons. In rodents, kisspeptin cells comprise a component of the AVPV, but in sheep, monkeys and humans, they appear to be more scattered and an AVPV homologue is not evident. In may be, in fact, that the connections and neurochemical features of the AVPV and its kisspeptin cells are critical for the functional role of this population in rodents, and may underlie differences between rodents and other species in the control the GnRH surge (Caraty et al., 1998; Krey et al., 1975; Wiegand, 1980). In addition to the ARC and preoptic populations, is also evidence for smaller populations of kisspeptin neurons in the DMH, BNST and medial amygdala, but it is not clear whether they are consistently seen across species, nor has their functional role(s) been identified. Kisspeptin cells of the ARC and preoptic populations differ in their neurochemical phenotype: virtually all ARC kisspeptin cells contain both neurokinin B and dynorphin, while subsets of AVPV kisspeptin cells express galanin and/or tyrosine hydroxylase.
There are several sites of demonstrated and potential direct interactions between the kisspeptin and GnRH neuronal networks (Fig. 2, numbers 1–2). Evidence from confocal, multiple-label studies using synaptic markers strongly supports the existence of direct synaptic connections onto GnRH cell bodies (Smith et al., 2008), some of which arise from the ARC kisspeptin population.. Transneuronal tracing studies (Wintermantel et al., 2006) and studies using conventional tracers (Gu and Simerly, 1997; Hahn and Coen, 2006; Polston and Simerly, 2006) have demonstrated that cells of the AVPV provide direct input to GnRH neurons; unpublished data (Herbison, 2008) suggests that these inputs arise at least in part from AVPV kisspeptin cells, although there are likely also inputs from other neurochemical subsets of the AVPV (glutamate, GABA). In addition, to direct inputs from kisspeptin cells onto GnRH cell bodies, there is also evidence for potential inputs at the level of GnRH terminals in the median eminence. EM observations have confirmed that dynorphin terminals, presumably arising from the ARC, establish direct contacts with GnRH terminals (Lehman et al., 2010). Nonetheless, evidence is still needed at an EM level to confirm that kisspeptin-positive terminals are in direct contact with GnRH terminals in the median eminence, as well as demonstration that Kiss1 receptors are present on the plasma membranes of those GnRH terminals.
A number of anatomical features support the view that kisspeptin cells form a reciprocally-innervated functional network, both within a given region and between different populations, and extending to include GnRH neurons. First, as noted above, kisspeptin cells of the ARC have extensive reciprocal connections with each other (Fig. 2, number 3) (Burke, 2006; Foradori, 2006; Goodman et al., 2007; Krajewski et al., 2010). Second, there is evidence that ARC kisspeptin cells (based on dual-labeling for kisspeptin and dynorphin) send direct projections to kisspeptin neurons in the sheep POA (Lehman, unpublished). Further, recent evidence suggests that approximately 40% of AVPV kisspeptin neurons in the mouse, in turn, project to the ARC (Yeo and Herbison, 2009), raising the possibility of reciprocal communication between these two populations (Fig. 2, number 4). Finally, in the monkey (Ramaswamy et al., 2008) and sheep (Lehman, unpublished), GnRH fibers have been shown to contact ARC kisspeptin neurons (Fig. 2, number 5), providing a route for two-way communication between GnRH and kisspeptin populations. Given that GnRH neurons are interconnected morphologically, at the level of their dendrites as well as axon terminals (Campbell et al., 2009), there is therefore potential for kisspeptin input to GnRH neurons, either at the level of their cell bodies or terminals, to influence the coordinated release of GnRH from many distributed neurons.
Many unanswered questions remain concerning the anatomical organization of this network. First, while some information has been obtained about the efferent connections of ARC and preoptic kisspeptin populations, projections from kisspeptin cells in the DMH, BNST and medial amygdala have not yet been examined, including the possibility that one or more of these populations also provides input to GnRH neurons. There is evidence from tract tracing studies that all three areas project to the preoptic region (Hahn and Coen, 2006; Simerly, 1986; Tillet et al., 1993) and specifically to GnRH neurons (Boehm et al., 2005; Pompolo et al., 2005; Yoon et al., 2005). Second, the sources of afferent inputs to each kisspeptin population need to be identified. One likely source of afferents to the AVPV kisspeptin population is the suprachiasmatic nucleus (SCN), given evidence that these kisspeptin neurons are involved in circadian regulation of the GnRH surge in rodents (Robertson et al., 2009) (Fig. 2). In fact, vasopressin and vasoactive intestinal peptide-expressing terminals originating from the SCN make contacts onto AVPV kisspeptin neurons, as shown recently by anterograde tract tracing combined with ICC (Vida et al., 2010). In the future, transgenic neuron-specific tracing, both anterograde and retrograde (transneuronal), could be used for defining the inputs to each kisspeptin population as it has for leptin-responsive cell populations (Leshan et al., 2010) and GnRH neurons (Boehm et al., 2005; Wintermantel et al., 2006; Yoon et al., 2005), respectively. Third, the identity of other postsynaptic targets of kisspeptin cells, besides GnRH, needs to be examined; for example, recent evidence suggests that kisspeptin plays a role in the regulation of prolactin via direct contacts of kisspeptin fibers onto A12 dopamine cells (Szawka et al., 2010). Finally, it will be important to know which cellular targets of kisspeptin cells actually express Kiss1R. While there is a considerable overlap between regions that express Kiss1R and kisspeptin fiber-immunoreactivity (Herbison et al., 2010), there are some examples of apparent receptor-ligand mismatch. For example, the dentate gyrus of the hippocampus is a region which contains a large number of Kiss1R-expressing cells (Herbison et al., 2010), although kisspeptin fibers have not been identified in that region. Conversely, some areas that have contain dense kisspeptin fibers, such as the ARC, are devoid of Kiss1R (Herbison et al., 2010). Whether these examples of receptor-ligand mismatch reflects the presence of other as yet unidentified receptors or ligands, or the influence of kisspeptin via extra-synaptic communication routes, remains to be seen.
In summary, converging data from a range of species suggests that the overall organization of the kisspeptin neuronal system in mammals is fairly consistent, and that direct anatomical projections to GnRH neurons, at the level of both cell bodies and terminals, are a common feature. In addition, there is growing recognition that kisspeptin is present as only one of several important peptides/neurotransmitters in this circuitry, and that the neural projections of the kisspeptin network are likely to include other neuroendocrine systems, as well as extend outside the preoptic-hypothalamic continuum. Indeed, the neurochemical and anatomical heterogeneity of the kisspeptin network is likely to be critical in defining the individual functional roles of subsets of kisspeptin neurons, and much important work remains to be done in order to define the structural framework for kisspeptin action in the brain.
Acknowledgments
This work was supported by NIH R01 HD39916 (M.N.L. and R.L.G.) and Canadian Institutes of Health Research Operating Grant 86744 (M.N.L.).
Abbreviations
- AR
androgen receptor
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- BNST
bed nucleus of the stria terminalis
- DMH
dorsomedial hypothalamus
- DYN
dynorphin
- EM
electron microscopy
- ER-α
estradiol receptor-alpha
- ER-β
estradiol receptor-beta
- ICC
immunocytochemistry
- ISH
in situ hybridization
- LH
luteinizing hormone
- LHA
lateral hypothalamic area
- MBH
mediobasal hypothalamus
- ME
median eminence
- NKB
neurokinin B
- OVX
ovariectomized
- PeN
periventricular nucleus
- POA
preoptic area
- PR
progesterone receptor
- PVN
paraventricular nucleus
- RFRP
RFamide-related peptides
- SCN
suprachiasmatic nucleus
- SON
supraoptic nucleus
- VMH
ventromedial hypothalamus
Footnotes
An exception to this is in the monkey, where quantitative PCR showed no difference in Kiss1 mRNA levels in the POA between ovariectomized and gonadally-intact female rhesus monkeys.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda KI. Involvement of Anteroventricular Periventricular Matastin/Kisspeptin Neurons in Estrogen Positive Feedback Action on Leutentizing Hormone Release in Female Rats. Journal of Reproduction and Development. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological Plasticity in the Neural Circuitry Responsible for Seasonal Breeding in the Ewe. Endocrinology. 2006;147:4843–4851. doi: 10.1210/en.2006-0408. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain: Wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- Ansel L, Bolborea M, Bentsen AH, Klosen P, Mikkelsen JD, Simonneaux V. Differential Regulation of Kiss1 Expression by Melatonin and Gonadal Hormones in Male and Female Syrian Hamsters. J Biol Rhythms. 2010;25:81–91. doi: 10.1177/0748730410361918. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback Loops Link Odor and Pheromone Signaling with Reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Siok LD, Masahiro O, Deling Y, Jun Y, Jaw Kang C, Eugen B, Nae JD. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. The Journal of Comparative Neurology. 2005;481:314–329. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. The Journal of Comparative Neurology. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proceedings of the National Academy of Sciences. 2009;106:10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence That the Mediobasal Hypothalamus Is the Primary Site of Action of Estradiol in Inducing the Preovulatory Gonadotropin Releasing Hormone Surge in the Ewe. Endocrinology. 1998;139:1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cell Population of the Arcuate Nucleus: Sex Differences and Effects of Prenatal Testosterone in Sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal Development of Kisspeptin Neurons in Mouse Hypothalamus; Sexual Dimorphism and Projections to Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 Signaling Is Essential for Preovulatory Gonadotropin-Releasing Hormone Neuron Activation and the Luteinizing Hormone Surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Tassigny XdAd, Colledge WH, Caraty A, Herbison AE. Distribution of Kisspeptin Neurones in the Adult Female Mouse Brain. Journal of Neuroendocrinology. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Fagg LA, Carlton MBL, Colledge WH. Kisspeptin Can Stimulate Gonadotropin-Releasing Hormone (GnRH) Release by a Direct Action at GnRH Nerve Terminals. Endocrinology. 2008;149:3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Research. 1996;734:10–18. [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus: Interactions with GnRH neuronal system. Journal of Chemical Neuroanatomy. 2008;36:131–137. doi: 10.1016/j.jchemneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. Mapping of kisspeptin fibres in the brain of the pro-oestrus rat. Journal of Neuroendocrinology. 2010 doi: 10.1111/j.1365–2826.2010.02053.x. Accepted Article. [DOI] [PubMed] [Google Scholar]
- Ducret E, Gaidamaka G, Herbison AE. Electrical and Morphological Characteristics of Anteroventral Periventricular Nucleus Kisspeptin and Other Neurons in the Female Mouse. Endocrinology. 2010;151:2223–2232. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–8. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Dungan Lemko HM, Naderi R, Adjan V, Jennes LH, Navarro VM, Clifton DK, Steiner RA. Interactions between neurotensin and GnRH neurons in the positive feedback control of GnRH/LH secretion in the mouse. Am J Physiol Endocrinol Metab. 2010;298:E80–88. doi: 10.1152/ajpendo.00380.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 Expression in the Arcuate Nucleus Prior to the Cyclic Preovulatory Gonadotrophin-Releasing Hormone/Lutenising Hormone Surge in the Ewe Suggests a Stimulatory Role for Kisspeptin in Oestrogen-Positive Feedback. Journal of Neuroendocrinology. 2006;18:806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of Progesterone Receptors in Parvicellular Dynorphin Neurons of the Ovine Preoptic Area and Hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of Dynorphin A and Neurokinin B Immunoreactivity in the Arcuate Nucleus and Median Eminence of the Sheep. Journal of Neuroendocrinology. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience Letters. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Goodman R, Parfitt DB, Evands NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136:2412–2420. doi: 10.1210/endo.136.6.7750462. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence That Dynorphin Plays a Major Role in Mediating Progesterone Negative Feedback on Gonadotropin-Releasing Hormone Neurons in Sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin Neurons in the Arcuate Nucleus of the Ewe Express Both Dynorphin A and Neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Nestor CC, Connors JM, Holaskova I, Lehman MN. Annual Meeting of the Society for Neuroscience. San Diego, CA: 2010. The actions of neurokinin B in the arcuate nucleus are important for episodic LH secretion in ewes. [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A Role for Kisspeptins in the Regulation of Gonadotropin Secretion in the Mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MAL, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental Control of Kisspeptin: Implications for Seasonal Reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. The Journal of Comparative Neurology. 1997;384:142–164. [PubMed] [Google Scholar]
- Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. The Journal of Comparative Neurology. 2006;494:190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V) Brain Research Reviews. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, d’Anglemont de Tassigny X, Doran J, Colledge WH. Distribution and Postnatal Development of Gpr54 Gene Expression in Mouse Brain and Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- Hess DL, Wilkins RH, Moossy J, Chang JL, Plant TM, McCormack JT, Nakai Y, Knobil E. Estrogen-Induced Gonadotropin Surges in Decerebrated Female Rhesus Monkeys with Medial Basal Hypothalamic Peninsulae. Endocrinology. 1977;101:1264–1271. doi: 10.1210/endo-101-4-1264. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. European Journal of Neuroscience. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin Activation of Gonadotropin Releasing Hormone Neurons and Regulation of KiSS-1 mRNA in the Male Rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Dhillo WS, Bloom SR. Kisspeptins and the control of gonadotropin secretion in humans. Peptides. 2009;30:76–82. doi: 10.1016/j.peptides.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007a;30:504–11. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual Differentiation of Kiss1 Gene Expression in the Brain of the Rat. Endocrinology. 2007b;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kauffman AS. Sexual differentiation and the Kiss1 system: Hormonal and developmental considerations. Peptides. 2009;30:83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Kim J, Clifton DK, Steiner RA. Society for Neuroscience 37th Annual Meeting. San Diego, CA: Society for Neuroscience; 2007. Regulation of Kiss1 gene expression by sex steroids in the medial amygdala of mice. [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda KI. Involvement of Central Metastin in the Regulation of Preovulatory Luteinizing Hormone Surge and Estrous Cyclicity in Female Rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kirigiti MA, True C, Ciofi P, Grove KL, Smith MS. Kisspeptin and NKB fiber distribution in the adult female rat: relationship to GnRH cell bodies and fibers/terminals in the median eminence. Endocrine society Abstr. 2009;2009:3–223. [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. The Journal of Comparative Neurology. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E. Surgical Disconnection of the Medial Basal Hypothalamus and Pituitary Function in the Rhesus Monkey. I Gonadotropin Secretion Endocrinology. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cells of the Arcuate Nucleus: A Central Node in the Control of Gonadotropin-Releasing Hormone Secretion. Endocrinology. 2010 doi: 10.1210/en.2010-0022. en 2010–0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R. CSF signaling in physiology and behavior. In: Agnati LF, Fuxe K, Nicholson C, Sykov E, editors. Progress in Brain Research. Vol. 125. 2000. pp. 415–33. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr Ventral Tegmental Area Leptin Receptor Neurons Specifically Project to and Regulate Cocaine- and Amphetamine-Regulated Transcript Neurons of the Extended Central Amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee C, Foradori CD, Bruemmer JE, Arreguin-Arevalo JA, McCue PM, Handa RJ, Squires EL, Clay CM. Biological and Anatomical Evidence for Kisspeptin Regulation of the Hypothalamic-Pituitary-Gonadal Axis of Estrous Horse Mares. Endocrinology. 2009;150:2813–2821. doi: 10.1210/en.2008-1698. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor CC, Seebaugh AM, Valent M, Connors JM, Goodman RL, Hileman SM. Annual Meeting of the Society for Neuroscience. San Diego, CA: 2010. Evaluation of neurokinin B and kisspeptin expression before and after puberty in sheep. [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Coolen LM, Lehman MN, Wagenmaker ER, Karsch FJ. Endocrine Society Meeting. Washington DC: 2009. Are dynorphin neurons in the arcuate nucleus responsive to cortisol and influenced by the combined presence of cortisol and estradiol? [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-Releasing Hormone Pulse Generator Activity in the Hypothalamus of the Goat. Journal of Neuroendocrinology. 2009;21:813–821. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin Acts Directly and Indirectly to Increase Gonadotropin-Releasing Hormone Neuron Activity and Its Effects Are Modulated by Estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston EK, Simerly RB. Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. The Journal of Comparative Neurology. 2006;495:122–132. doi: 10.1002/cne.20874. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Ischenko O, Pereira A, Iqbal J, Clarke IJ. Evidence that projections from the bed nucleus of the stria terminalis and from the lateral and medial regions of the preoptic area provide input to gonadotropin releasing hormone (GNRH) neurons in the female sheep brain. Neuroscience. 2005;132:421–436. doi: 10.1016/j.neuroscience.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural Interactions between Kisspeptin and GnRH Neurons in the Mediobasal Hypothalamus of the Male Rhesus Monkey (Macaca mulatta) as Revealed by Double Immunofluorescence and Confocal Microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B Stimulates GnRH Release in the Male Monkey (Macaca mulatta) and Is Colocalized with Kisspeptin in the Arcuate Nucleus. Endocrinology. 2010 doi: 10.1210/en.2010-0223. en 2010–0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian Regulation of Kiss1 Neurons: Implications for Timing the Preovulatory Gonadotropin-Releasing Hormone/Luteinizing Hormone Surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Lou Voytko M, Rance NE. Hypertrophy and Increased Kisspeptin Gene Expression in the Hypothalamic Infundibular Nucleus of Postmenopausal Women and Ovariectomized Monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Millar RP. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update. 2008:dmn058. doi: 10.1093/humupd/dmn058. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of Potent Kisspeptin Antagonists Delineate Physiological Mechanisms of Gonadotropin Regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 Gene as a Regulator of Puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of the neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Young BJ, Capozza MA, Swanson LW. Estrogen differentially regulates neuropeptide gene expression in a sexually dimorphic olfactory pathway. Proceedings of the National Academy of Sciences. 1989;86:4766–4770. doi: 10.1073/pnas.86.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 Neurons in the Forebrain as Central Processors for Generating the Preovulatory Luteinizing Hormone Surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 Messenger Ribonucleic Acid Expression in the Hypothalamus of the Ewe Is Regulated by Sex Steroids and Season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Smith JT. Kisspeptin signalling in the brain: Steroid regulation in the rodent and ewe. Brain Research Reviews. 2008;57:288. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in Kisspeptin and RFamide-Related Peptide (RFRP) Expression and Terminal Connections to Gonadotropin-Releasing Hormone Neurons in the Brain: A Novel Medium for Seasonal Breeding in the Sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin Neurons in the Ovine Arcuate Nucleus and Preoptic Area Are Involved in the Preovulatory Luteinizing Hormone Surge. Endocrinology. 2009;150:5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- Smith JT, Shahab M, Pereira A, Pau KYF, Clarke IJ. Hypothalamic Expression of KISS1 and Gonadotropin Inhibitory Hormone Genes During the Menstrual Cycle of a Non-Human Primate. Biology of Reproduction. 2010 doi: 10.1095/biolreprod.110.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szawka RE, Ribeiro AB, Leite CM, Helena CV, Franci CR, Anderson GM, Hoffman GE, Anselmo-Franci JA. Kisspeptin Regulates Prolactin Release through Hypothalamic Dopaminergic Neurons. Endocrinology. 2010 doi: 10.1210/en.2009-1414. [DOI] [PubMed] [Google Scholar]
- Tillet Y, Batallier M, Thibault J. Neuronal projections to the medial preoptic area of the sheep, with special reference to monoaminergic afferents: immunohistochemical and retrograde tract tracing studies. J Comp Neurol. 1993;330:195–220. doi: 10.1002/cne.903300205. [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida B, Hrabovsky E, Caraty A, Ciofi P, Coen CW, Liposits Z, Kallo I. Annual Meeting of the Society for Neuroscience. Chicago, I.L.: 2009. Gender differences in the co-localisation of neuropeptides with kisspeptin in the hypothalamic neurons of the mouse brain. Poster 865.9. [Google Scholar]
- Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen C, Liposits Z, Kalló I. Evidence for Suprachiasmatic Vasopressin Neurones Innervating Kisspeptin Neurones in the Rostral Periventricular Area of the Mouse Brain: Regulation by Oestrogen. Journal of Neuroendocrinology. 2010;22:1032–1039. doi: 10.1111/j.1365-2826.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda KI, Steiner RA, Okamura H. Neurokinin B and Dynorphin A in Kisspeptin Neurons of the Arcuate Nucleus Participate in Generation of Periodic Oscillation of Neural Activity Driving Pulsatile Gonadotropin-Releasing Hormone Secretion in the Goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of Estrogen Receptor Pathway Critical for Estrogen Positive Feedback to Gonadotropin-Releasing Hormone Neurons and Fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SH, Herbison A. Combio. Vol. 2009. Christchurch; New Zealand: 2009. Rostral periventricular kisspeptin neurons project to the arcuate nucleus in adult female mice; p. 167. [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory Inputs to Hypothalamic Neurons Controlling Reproduction and Fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]