SYNOPSIS
IscA is a key member of the iron-sulfur cluster assembly machinery in prokaryotic and eukaryotic organisms; however, the physiological function of IscA still remains elusive. Here we report the in vivo evidence demonstrating the iron binding activity of IscA in Escherichia coli cells. Supplement of exogenous iron (1μM) in the M9 minimal medium is sufficient to maximize the iron binding in IscA expressed in E. coli cells under aerobic growth conditions. In contrast, IscU, an iron-sulfur cluster assembly scaffold protein, or CyaY, a bacterial frataxin homologue, fails to bind any iron in E. coli cells under the same experimental conditions. Interestingly, the strong iron binding activity of IscA is greatly diminished in E. coli cells under anaerobic growth conditions. Additional studies reveal that oxygen in medium promotes the iron binding in IscA and that the iron binding in IscA in turn prevents formation of biologically inaccessible ferric hydroxide under aerobic conditions. Consistent with the differential iron binding activity of IscA under aerobic and anaerobic conditions, we find that IscA and its paralog SufA are essential for the iron-sulfur cluster assembly in E. coli cells under aerobic growth conditions but not under anaerobic growth conditions. The results provide the in vivo evidence that IscA may act as an iron chaperone for the biogenesis of iron-sulfur clusters in E. coli cells under aerobic conditions.
Keywords: Iron-sulfur cluster biogenesis, human IscA homologue, intracellular iron content
INTRODUCTION
In Escherichia coli, IscA is a key member of the iron-sulfur cluster assembly machinery [1–4], and is highly conserved from bacteria to humans [5]. Deletion of IscA and its paralog SufA in E. coli cells results in deficiency of the iron-sulfur cluster assembly in multiple proteins [6, 7] and a null-growth phenotype in the M9 minimal medium under aerobic conditions [8]. Depletion of IscA in Azotobacter vinelandii also causes a null-growth phenotype in the modified Burks minimal medium under elevated oxygen conditions [9]. In Saccharomyces cerevisiae, depletion of IscA homologues leads to iron accumulation in mitochondria and dependency on lysine and glutamate for the cell growth under aerobic conditions [10, 11]. In Schizosaccharomyces pombe, deletion of IscA homologues is lethal [12]. In cultured human HeLa cells, RNAi (RNA interference) knockdown of the IscA homologue (hIscA1) dramatically decreases the enzyme activity of iron-sulfur proteins in both mitochondrion and cytosol [13]. However, the specific function of IscA in the biogenesis of iron-sulfur clusters still remains controversial. One hypothesis suggests that IscA and SufA may act as regulatory proteins that control the iron homeostasis and redox stress responses in cyanobacterium Synechococcus sp. strain PCC 7002 [14], although the underlying molecular mechanism is not fully understood. The second hypothesis states that IscA and SufA are the alternative scaffold/carrier proteins that bind transient iron-sulfur clusters and transfer the assembled clusters to target proteins [15–22]. However, unlike other proposed iron-sulfur cluster assembly scaffold proteins such as IscU [23], purified E. coli IscA [24–28] and human IscA [29] have a strong iron binding activity with an iron association constant of 1.0 × 1019 M−1 in the presence of the thioredoxin/thioredoxin reductase system under aerobic conditions. Furthermore, the iron center in IscA can be readily mobilized by L-cysteine [30] and transferred for the iron-sulfur cluster assembly in a proposed scaffold IscU in vitro [27], suggesting that IscA/SufA may also act as iron chaperones to recruit intracellular iron [28] and deliver the iron for the iron-sulfur cluster assembly in proteins [27]. Nevertheless, the physiological relevance of iron binding in IscA for the biogenesis of iron-sulfur clusters has not been addressed.
In this study, we present the in vivo evidence demonstrating the strong iron binding activity of IscA in E. coli cells. Supplement of exogenous iron (1μM) in the M9 minimal medium is sufficient to maximize the iron binding occupancy of IscA expressed in E. coli cells under aerobic growth conditions. In contrast, the iron-sulfur cluster assembly scaffold protein IscU [23] or the bacterial frataxin homologue CyaY [31–34] fails to bind any iron in E. coli cells under the same experimental conditions. Importantly, the strong iron binding in IscA is greatly diminished in E. coli cells under anaerobic growth conditions. Additional studies indicate that oxygen in the medium promotes the iron binding in IscA by oxidizing ferrous iron to ferric iron in the iron binding site of IscA and that the iron binding in IscA in turn prevents formation of biologically inaccessible ferric hydroxide under aerobic conditions. Consistent with these observations, we find that IscA and SufA are largely dispensable under anaerobic growth conditions but are essential for the iron-sulfur cluster assembly in E. coli cells under aerobic growth conditions. The physiological roles of IscA/SufA in the biogenesis of iron-sulfur clusters will be discussed.
EXPERIMENTAL
Cell growth and protein expression
Overnight culture of E. coli (BL21(DE3) strain containing the expression vector pTISCA [24], pTSUFA [8], pTISCU [27], or pTCYAY [33] for expressing recombinant E. coli IscA, SufA, IscU, or CyaY, respectively) was diluted (1:100) in the M9 minimal medium containing glucose (0.2%), thiamin (5 μg/ml), and 20 amino acids (each at 10 μg/ml). After four hours of incubation at 37°C with aeration (250 rpm), the M9 minimal media were supplemented with or without ferric citrate 10 min before the protein expression was induced by adding isopropyl β-D-1-thiogalactopyranoside (200 μM). If the protein was expressed under anaerobic conditions, the E. coli cells were purged with pure argon gas for 20 min before isopropyl β-D-1-thiogalactopyranoside was added to the sealed flask. The cells were grown for additional one hour before being harvested and washed once with the protein purification buffer (20 mM Tris (pH 8.0), 500 mM NaCl). Protein was purified as described previously [27], and the purity of purified proteins was over 95% judging from the SDS polyacrylamide gel electrophoresis. The amounts of the acid-labile iron and sulfide in purified proteins were analyzed according to the Fischer’s method [35] and the Siegel’s method [36], respectively, using purified E. coli ferredoxin [2Fe-2S] cluster [37] as a standard.
Intracellular iron content measurements in E. coli cells
The intracellular iron content of E. coli cells was measured using the in vivo EPR (electron paramagnetic resonance) following the procedures described in [38, 39]. Briefly, overnight E. coli cells were diluted in the M9 minimal medium supplemented with different amounts of exogenous iron under aerobic conditions. The exponentially growing E. coli cells (O.D. at 600 nm = 0.5) were harvested, washed once, and re-suspended in the M9 minimal medium to O.D. at 600 nm = 20.0. After additional 30 min incubation, the cells were treated with a membrane permeable iron chelator desferrioxamine (20 mM) and a non-permeable iron chelator diethylenetriaminepentaacetic acid (10 mM) for 15 min. The cells were then chilled at 4°C, harvested, and washed twice with ice-cold buffer containing Tris (20 mM, pH 8.0) and NaCl (0.5 M), and re-suspended in the same ice-cold buffer. Iron standards for the EPR measurements were prepared by making serial dilutions of FeCl3 and desferrioxamine (1 mM) in Tris (20 mM, pH 8.0). The EPR spectra were recorded at X-band on a Bruker ESR-300 spectrometer using an Oxford Instruments ESR-9 flow cryostat. The routine EPR conditions were: microwave frequency, 9.45 GHz; microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 2.0 mT; sample temperature, 10 K; receive gain, 1.0×105.
Iron-sulfur cluster assembly in IscU in vitro
The E. coli IscU and cysteine desulfurase IscS were prepared as described previously [27]. Typically, purified IscU (50 μM) was incubated with IscS (1 μM), NaCl (200 mM), Tris (20 mM, pH 8.0) with various iron source in the presence of dithiothreitol (2 mM). L-cysteine (1 mM) was added to initiate the iron-sulfur cluster assembly reaction. The amount of the iron-sulfur clusters assembled in IscU was monitored at 456 nm [23] in a Beckman DU-640 UV-Visible spectrometer equipped with a temperature controller.
Aconitase activity assay in the cell extracts
The cell extracts were prepared from the E. coli cells containing recombinant aconitase B [40] by passing the cells through French press once. Aliquots were transferred to pre-incubation solutions containing Tris (50 mM, pH 8.0), MgCl2 (10 mM), and D,L-isocitrate (10 mM) at 30°C. The aconitase activity was monitored following the formation of cis-aconitate at 240 nm using an extinction coefficient of 3.6 mM−1cm−1 [40].
RESULTS
IscA is an iron binding protein in E. coli cells under aerobic growth conditions
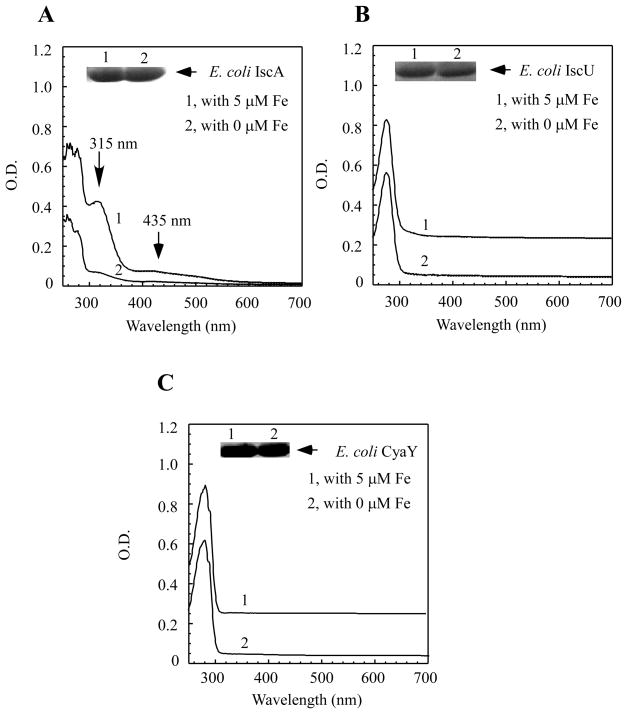
To explore the in vivo iron binding activity of IscA, we expressed recombinant IscA in E. coli cells grown in the M9 minimal medium under aerobic growth conditions. Figure 1A shows that IscA purified from E. coli cells grown in the M9 minimal medium without any exogenous iron had very little or no absorption peaks of the iron or iron-sulfur cluster binding in the protein. The acid-labile iron and sulfide content analyses also showed that purified IscA contained less than 0.02 iron atoms and 0.01 sulfide atoms per IscA dimer (n = 3). However, when the M9 minimal medium was supplemented with ferric citrate (5.0 μM), IscA purified from E. coli cells had a major absorption peak at 315 nm, indicative of iron binding in IscA [24] (Figure 1A). The acid-labile iron and sulfide content analysis further revealed that purified IscA contained 0.60±0.11 iron atoms and 0.03 sulfide atoms per IscA dimer (n = 3), suggesting that IscA binds iron, but not iron-sulfur clusters, in E. coli cells under the experimental conditions used.
Figure 1. IscA is an iron binding protein in E. coli cells under aerobic conditions.
A) Recombinant IscA was expressed in E. coli cells grown aerobically in the M9 minimal medium supplemented with (spectrum 1) or without (spectrum 2) 5.0μM ferric citrate and purified as described in the Experimental section. The protein concentrations were about 120 μM. B) Recombinant IscU was expressed in E. coli cells grown aerobically in the M9 minimal medium supplemented with (spectrum 1) or without (spectrum 2) 5.0μM ferric citrate and purified. The protein concentrations were about 60 μM. C) Recombinant CyaY was expressed in E. coli cells grown aerobically in the M9 minimal medium supplemented with (spectrum 1) or without (spectrum 2) 5μM ferric citrate and purified. The protein concentrations were about 20 μM. The insert in each panel is a photograph of the SDS/PAGE gel of the proteins purified from the E. coli cells grown in the M9 minimal medium supplemented with (lane 1) or without (lane 2) 5.0μM ferric citrate. The results are representatives from three independent experiments.
In parallel experiments, the proposed iron-sulfur cluster assembly scaffold protein IscU [23] was also expressed in E. coil cells grown in the M9 minimal medium supplemented with or without exogenous iron (5.0 μM ferric citrate). Figure 1B shows that supplement of exogenous iron in the M9 minimal medium did not increase the iron binding in E. coli IscU, consistent with the notion that IscU has a weak iron binding activity [23, 27]. We also expressed the bacterial frataxin homologue CyaY [33], a putative iron donor for the iron-sulfur cluster assembly [41], in E. coli cells under the same experimental conditions. As shown in Figure 1C, E. coli CyaY, like IscU, failed to bind iron whether or not the M9 minimal medium was supplemented with exogenous iron (5.0 μM ferric citrate) (Figure 1C). Thus, IscA has its unique iron binding activity in E. coli cells under aerobic growth conditions.
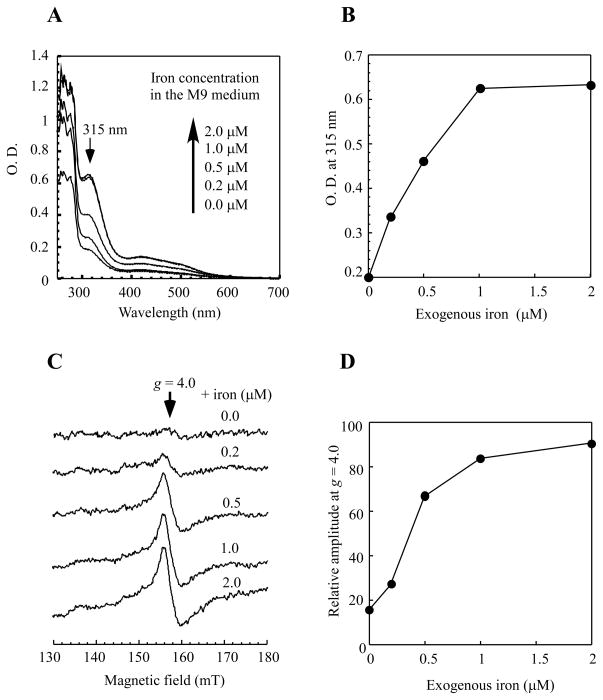
To further determine the iron binding activity of IscA in vivo, the E. coli cells expressing IscA were grown in the M9 minimal medium supplemented with increasing concentrations of exogenous iron under aerobic growth conditions. Figure 2A shows that the iron binding in IscA was almost linearly increased as the exogenous iron concentration in the M9 minimal medium was increased from 0 to 1.0μM, and appeared to be saturated (at approx.. 60% occupancy) above 1.0 μM iron (Figure 2B). Further increase of exogenous iron (up to 50 μM) in the M9 minimal medium failed to fully saturate the iron binding occupancy in IscA expressed in E. coli cells under aerobic growth conditions (data not shown).
Figure 2. Relative iron binding activity of IscA in E. coli cells under aerobic conditions.
A) UV-visible absorption spectra of recombinant IscA purified from E. coli cells grown aerobically in the M9 minimal medium supplemented with ferric citrate (0.0, 0.2, 0.5, 1.0, and 2.0μM). The protein concentration of purified IscA was about 250 μM. B) The apparent iron binding activity of IscA in E. coli cells. The amplitude of the absorption peak at 315 nm of purified IscA in A) was plotted as a function of the exogenous iron concentration supplemented in the M9 minimal medium. C) The EPR spectra of the E. coli cells treated with an iron indicator desferrioxamine. The E. coli cells grown in the M9 minimal medium supplemented with ferric citrate (0.0, 0.2, 0.5, 1.0, and 2.0 μM) were subject to the intracellular iron content measurements using the iron indicator desferrioxamine as described in the Experimental section. The amplitude of the EPR signal at g = 4.0 reflects the relative intracellular iron concentration in E. coli cells. D) The amplitudes of the EPR signal at g = 4.0 in C) were plotted as a function of the exogenous iron concentration supplemented in the M9 minimal medium. The data are representatives from three independent experiments.
Since elevated intracellular iron content will promote production of deleterious hydroxyl free radicals via the Fenton reaction [42], we reasoned that the intracellular iron concentration could be the limiting factor for the iron binding in IscA in E. coli cells. To test this hypothesis, we adopted the in vivo electron paramagnetic resonance (EPR) approach developed by the Imlay’s group [38] to probe the intracellular iron content of E. coli cells grown in the M9 minimal medium supplemented with exogenous iron under aerobic growth conditions. The amplitude of the EPR signal at g = 4.0 reflects the relative amount of the intracellular iron content in E. coli cells [38, 39]. Figure 2C shows that the intracellular iron content of E. coli cells grown in the M9 minimal medium without exogenous iron was very low, indicating that E. coli cells are under iron starvation in the M9 minimal medium. As the concentration of exogenous iron in the M9 minimal medium was increased, the amplitude of the EPR signal at g = 4.0 was progressively increased and plateaued at approx. 1 μM exogenous iron (Figure 2D). The saturation curve of the intracellular iron content in E. coli cells (Figure 2D) generally correlates with that of the iron binding of IscA in E. coli cells (Figure 2B). Thus, the partial occupancy (~60%) of the iron binding in IscA in E. coli cells could be due to the limited intracellular iron content under the experimental conditions.
The iron binding activity of IscA is greatly diminished in E. coli cells under anaerobic conditions
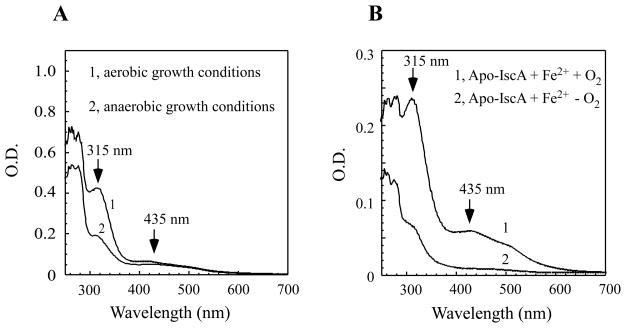
Because IscA has also been characterized as an alternative iron-sulfur cluster assembly scaffold/carrier protein [15–22], we decided to express IscA in E. coli cells grown in the M9 minimal medium under anaerobic growth conditions, hoping to purify an iron-sulfur cluster-bound IscA from the cells. To our surprise, IscA purified from the E. coli cells grown in the M9 minimal medium supplemented with 2 μM exogenous iron under anaerobic conditions had only a small absorption peak at 315 nm (Figure 3A). The acid-labile iron and sulfide content analyses further showed that purified IscA contained 0.12±0.03 iron atoms and 0.03 sulfide atoms per IscA dimer (n = 3), suggesting that the iron binding activity of IscA is greatly diminished in E. coli cells under anaerobic growth conditions.
Figure 3. The iron binding activity of IscA in vivo and in vitro under aerobic or anaerobic conditions.
A) The iron binding activity of IscA in vivo under aerobic and anaerobic conditions. UV-visible absorption spectra of IscA purified from the E. coli cells grown in the M9 minimal medium supplemented with 2.0μM ferric citrate under aerobic (spectrum 1) or anaerobic (spectrum 2) growth conditions. The protein concentration was about 150 μM. B) The iron binding activity of IscA in vitro under aerobic and anaerobic conditions. Apo-IscA (100 μM) was incubated with ferrous ammonium sulfite (50μM) and dithiothreitol (2 mM) under aerobic (spectrum 1) or anaerobic (spectrum 2) conditions at 25°C for 10 min. IscA was re-purified from the incubation solutions and subject to the UV-visible absorption measurements.
One of the likely explanations for the diminished iron binding in IscA in E. coli cells could be that the intracellular iron content is severely limited under anaerobic conditions. Using the whole cell EPR measurements as described above, we found that the intracellular iron content of E. coli cells grown in the M9 minimal medium supplemented with 2 μM exogenous iron under anaerobic conditions was similar to, if not higher than, that of E. coli cells under aerobic growth conditions (data not shown). We then asked whether oxygen could directly promote the iron binding in IscA in E. coli cells. To test this hypothesis, we prepared apo-IscA as described in [24] and incubated apo-IscA with ferrous iron and dithiothreitol under aerobic and anaerobic conditions. Figure 3B shows that while IscA had a strong iron binding activity in vitro under aerobic conditions as reported previously [24, 26], IscA had very little or no iron binding under anaerobic conditions, indicating that oxygen does have a crucial role for the strong iron binding in IscA. To directly test the binding affinity of apo-IscA for ferric iron, we incubated apo-IscA pre-reduced with dithiothreitol with an equal amount of ferric iron (FeCl3) under anaerobic conditions and found that apo-IscA was indeed converted into the iron-bound IscA after incubation (data not shown). Thus, oxygen is able to promote the iron binding in IscA probably by oxidizing ferrous iron to ferric iron in the iron binding site of IscA.
Role of IscA in the iron-sulfur cluster assembly under aerobic and anaerobic conditions
Our previous studies indicated that the iron-loaded IscA can readily provide iron for iron-sulfur cluster assembly in IscU in vitro under aerobic conditions [27]. However, since IscA has very little or no iron binding activity under anaerobic conditions (Figure 3), it is possible that IscA may be dispensable for iron-sulfur cluster assembly under anaerobic conditions. To test this hypothesis, we re-evaluated the role of IscA in the biogenesis of iron-sulfur clusters under aerobic and anaerobic conditions.
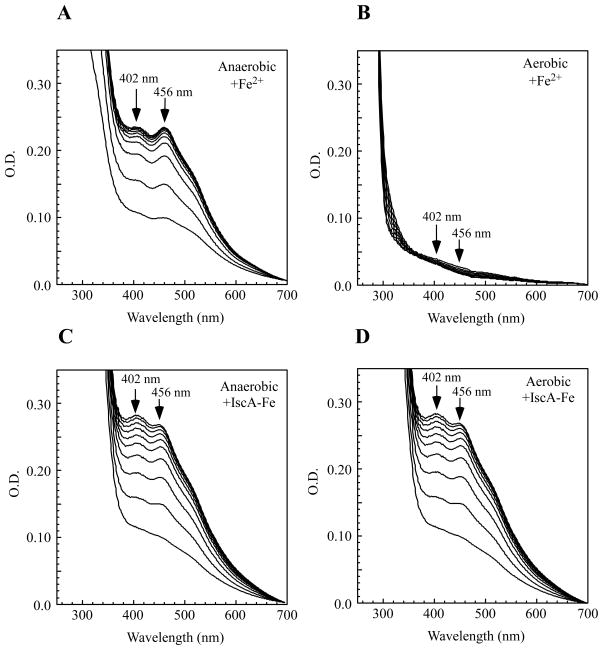
Figure 4 shows that under anaerobic conditions, “free” ferrous iron was able to provide iron for iron-sulfur cluster assembly in IscU in vitro (Figure 4A) as the absorption peaks at 402 nm and 456 nm reflecting the iron-sulfur cluster assembly in IscU [23] quickly appeared. Under the same anaerobic conditions, the iron-bound IscA had similar activity in providing iron for the iron-sulfur cluster assembly in IscU (Figure 4C). However, under aerobic conditions, pre-incubation of “free” ferrous iron resulted in deficiency of the iron-sulfur cluster assembly in IscU (Figure 4B), whereas the iron-bound IscA could still efficiently provide the iron for the iron-sulfur cluster assembly in IscU after the same pre-incubation under aerobic conditions (Figure 4D). If an equal amount of apo-IscA was mixed with “free” ferrous iron before pre-incubation under aerobic conditions, the iron also remained available for the iron-sulfur cluster assembly in IscU (data not shown). Thus IscA may have an essential role in preventing formation of biologically inaccessible iron and providing the iron for iron-sulfur cluster assembly under aerobic conditions, but not under anaerobic conditions.
Figure 4. IscA promotes in vitro iron-sulfur cluster assembly in IscU under aerobic conditions.
The iron-sulfur cluster assembly in IscU (50 μM) was carried out in vitro by incubating with cysteine desulfurase (IscS) (1 μM), dithiothreitol (2 mM), L-cysteine (1 mM), and different iron sources. The absorption peaks at 402 nm and 456 nm indicate the iron-sulfur cluster assembly in IscU. A) Ferrous ammonium sulfate (50 μM) was used for the iron-sulfur cluster assembly in IscU under anaerobic conditions. B) Ferrous ammonium sulfate (50 μM) was pre-incubated at 37°C for 3 hours under aerobic conditions before being used for the iron-sulfur cluster assembly in IscU. C) The iron-bound IscA (containing 50 μM iron) was used for the iron-sulfur cluster assembly in IscU under anaerobic conditions. D) The iron-bound IscA (containing 50 μM iron) was pre-incubated at 37°C for 3 hours under aerobic conditions before being used for the iron-sulfur cluster assembly in IscU. The experiments were repeated three times, and similar results were obtained.
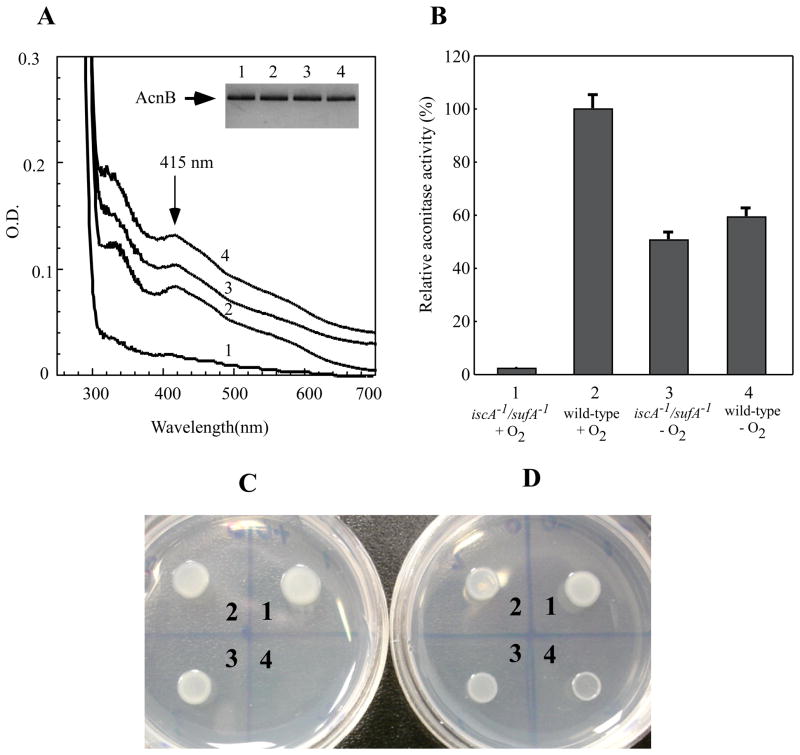
To re-evaluate physiological role of IscA in the biogenesis of iron-sulfur clusters in E. coli cells under aerobic and anaerobic conditions, we used an E. coli mutant in which both IscA and its paralog SufA were in-frame deleted [8]. A plasmid expressing recombinant iron-sulfur protein aconitase B [43] was introduced into the iscA−1/sufA−1 mutant and its parental wild-type cells as described in [8]. Recombinant aconitase B was expressed in the E. coli cells in rich LB medium under aerobic or anaerobic conditions and purified. Figure 5A shows that iron-sulfur clusters were assembled in recombinant aconitase B in the wild-type E. coli cells under both aerobic and anaerobic conditions. However, in the iscA−1/sufA−1 mutant cells, the iron-sulfur cluster assembly in aconitase B occurred only under anaerobic conditions, but not under aerobic conditions. Because the aconitase B activity requires an intact iron-sulfur cluster, we also analyzed the total aconitase activity in the cell extracts prepared from the wild-type and the iscA−1/sufA−1 mutant cells. As shown in Figure 5B, deletion of IscA/SufA in E. coli cells resulted in inactivation of aconitase B under aerobic conditions, but not under anaerobic conditions, further indicating that the iscA−1/sufA−1 mutant fails to assemble iron-sulfur clusters in aconitase B under aerobic conditions. Similar results were obtained when the iron-sulfur protein dihydroxyacid dehydratase [44] was expressed in the wild-type and the iscA−1/sufA−1 mutant cells (data not shown). Collectively, the results suggest that IscA and its paralog SufA are essential for the biogenesis of iron-sulfur clusters in E. coli cells under aerobic conditions but not under anaerobic conditions.
Figure 5. IscA and its paralog SufA are required for the iron-sulfur cluster assembly in aconitase B in E. coli cells under aerobic conditions.
A) Recombinant aconitase B was purified from the iscA−1/sufA−1 mutant E. coli cells grown in LB medium under aerobic (spectrum 1) and anaerobic (spectrum 3) conditions or from the wild-type E. coli cells grown under aerobic (spectrum 2) and anaerobic (spectrum 4) conditions. The absorption peaks at 415 nm of purified aconitase B indicates the iron-sulfur cluster in the protein. The insert is a photograph of the SDS/PAGE gel of purified aconitase B. B) Recombinant aconitase B was expressed in the iscA−1/sufA−1 mutant E. coli cells grown in LB medium under aerobic (sample 1) and anaerobic (sample 3) conditions or in the wild-type E. coli cells grown under aerobic (sample 2) and anaerobic (sample 4) conditions. The aconitase activity in the cell extracts was measured as described in the Experimental section. Values are the means ± S.D. for three independent experiments. C) About 2×105 cells of the wild-type (1), the iscA−1 mutant (2), the sufA−1 mutant (3), and the iscA−1/sufA−1 mutant (4) were spotted on the M9 minimal medium plates containing 0.2% glucose but without any amino acids and thiamin. The plate was incubated at 37°C overnight under aerobic conditions. D) Same as in C) except that the plate was incubated at 37°C overnight under anaerobic conditions.
If IscA and SufA were dispensable for the iron-sulfur cluster assembly in E. coli cells under anaerobic conditions, deletion of both IscA and SufA should have minimal effects on cell growth of E. coli in the M9 minimal medium under anaerobic conditions. Indeed, while deletion of IscA/SufA resulted in a null-growth phenotype of E. coli cells on the M9 minimal medium plate under aerobic conditions (Figure 5C) as reported previously [8], deletion of IscA/SufA did not prevent cell growth of E. coli under anaerobic conditions (Figure 5D).
DISCUSSION
In this study, we present the in vivo evidence demonstrating the iron binding activity of IscA in E. coli cells under aerobic conditions. Supplement of exogenous iron (1μM) in the M9 minimal medium is sufficient to maximize the iron binding occupancy of IscA in E. coli cells under aerobic growth conditions (Figures 1 and 2). IscU, a proposed iron-sulfur cluster assembly scaffold protein [23], and CyaY, a bacterial frataxin homolog that has been postulated as an iron donor for the iron-sulfur cluster assembly [41], fail to bind any iron in E. coli cells under the same experimental conditions (Figure 1). Importantly, the iron binding in IscA is greatly diminished in vivo and in vitro under anaerobic conditions (Figure 3). Additional studies reveal that oxygen promotes the iron binding in IscA likely by oxidizing ferrous iron to ferric iron in the binding site of the protein, and that the iron binding in IscA in turn prevents formation of biologically inaccessible ferric hydroxide and facilitates the iron-sulfur cluster assembly under aerobic conditions (Figure 4). Consistent with the differential iron binding activity of IscA under aerobic and anaerobic conditions, we find that IscA and its paralog SufA are essential for the iron-sulfur cluster assembly in E. coli cells under aerobic conditions but are dispensable under anaerobic conditions (Figure 5). Taken together, the results suggest that IscA/SufA may act as iron chaperones for the iron-sulfur cluster biogenesis under aerobic conditions.
We wish to emphasize that the results described in this study do not exclude the possibility that IscA/SufA may also act as alternative scaffold/carrier proteins for the biogenesis of iron-sulfur clusters as proposed by others [15–22]. Instead, we propose that two models for the function of IscA/SufA may be reconciled by suggesting that IscA/SufA could be bifunctional. When both the intracellular sulfide/L-cysteine and iron are abundant, iron-sulfur clusters may be assembled in IscA/SufA, and IscA/SufA act as alternative scaffold/carrier proteins for the biogenesis of iron-sulfur clusters as reported [15–22]. On the other hand, when sulfide/L-cysteine is limited in cells, IscA/SufA may act as iron chaperones to recruit intracellular iron for the iron-sulfur cluster assembly. The observation that supplement of exogenous iron (1 μM) in the M9 minimal medium is sufficient to maximize the iron binding occupancy of IscA expressing in E. coli cells (Figure 2) strongly suggests that IscA has a high iron binding affinity not only in vitro [24, 26] but also in vivo. Moreover, the bifunctional model for IscA/SufA is entirely consistent with the crystal structures of IscA [3, 4] and SufA [45] in which the conserved “cysteine pocket” could readily accommodate a mononuclear iron center or an iron-sulfur cluster without significant re-arrangements of protein structure.
The salient finding of this study is that IscA and its paralog SufA are essential for the iron-sulfur cluster assembly under aerobic conditions but not under anaerobic conditions (Figure 5). One interpretation could be that the biogenesis of iron-sulfur clusters is under high demand under aerobic conditions in such that deletion of IscA/SufA may result in severe deficiency of the iron-sulfur cluster assembly activity [7]. Alternatively, another IscA homologue ErpA [19] may substitute the function of IscA and SufA to support the iron-sulfur cluster assembly under anaerobic growth conditions. ErpA is involved in isoprenoid biosynthesis, and is required for cell growth of E. coli by either aerobic and anaerobic respiration but not fermentation [19]. Therefore, it cannot be ascertained as how ErpA could substitute IscA/SufA for the biogenesis of iron-sulfur clusters under anaerobic conditions but not under aerobic conditions [7]. In this context, we would like to offer new interpretation for the differential requirement of IscA/SufA for the biogenesis of iron-sulfur clusters in E. coli cells under aerobic and anaerobic conditions. We propose that under anaerobic conditions intracellular ferrous iron is readily available, and that IscA and SufA are not required to recruit intracellular iron for the biogenesis of iron-sulfur clusters (Figure 4). Consequently, deletion of both IscA and SufA has very little or no effect on the iron-sulfur cluster assembly in E. coli cells (Figure 5). Under aerobic conditions, however, the intracellular iron concentration is limited [38, 39] as elevated intracellular iron contents are highly toxic to cells [42], and IscA/SufA become essential to recruit intracellular iron and deliver iron for the biogenesis of iron-sulfur clusters (Figure 4B and D). Consistent with this notion, deletion of IscA/SufA results in deficiency of the iron-sulfur cluster assembly in aconitase B, dihydroxyacid dehydratase and several other iron-sulfur proteins in E. coli cells under aerobic conditions [6] (Figure 5). Nevertheless, additional experiments are needed to further illustrate the dynamic iron binding and physiological roles of IscA/SufA in cells under aerobic and anaerobic conditions.
Acknowledgments
This work was supported in part by the US Public Health Service Grant (CA107494) from the National Institutes of Health, the Chinese National Natural Science Foundation Grant (30770448), the Science and Technology Key Program of Zhejiang Province Grant (2006C14025), and the Natural Science Foundation of Zhejiang Province Grant (Y2081075 and 84107002).
Abbreviations
- AcnB
aconitase B
- CyaY
bacterial frataxin homologue
- EPR
electron paramagnetic resonance
Footnotes
Author Contributions: All authors contributed to the experimental design and data collections. H.D. wrote the paper.
References
- 1.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y, Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J Biochem (Tokyo) 1999;126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- 3.Bilder PW, Ding H, Newcomer ME. Crystal structure of the ancient, Fe-S scaffold IscA reveals a novel protein fold. Biochemistry. 2004;43:133–139. doi: 10.1021/bi035440s. [DOI] [PubMed] [Google Scholar]
- 4.Cupp-Vickery JR, Silberg JJ, Ta DT, Vickery LE. Crystal Structure of IscA, an Iron-sulfur Cluster Assembly Protein from Escherichia coli. J Mol Biol. 2004;338:127–137. doi: 10.1016/j.jmb.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLoS Genet. 2009;5:e1000497. doi: 10.1371/journal.pgen.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan G, Lu J, Bitoun JP, Huang H, Ding H. IscA/SufA paralogs are required for the [4Fe-4S] cluster assembly in enzymes of multiple physiological pathways in Escherichia coli under aerobic growth conditions. Biochem J. 2009;420:463–472. doi: 10.1042/BJ20090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mettert EL, Outten FW, Wanta B, Kiley PJ. The impact of O(2) on the FeS cluster biogenesis requirements of Escherichia coli FNR. J Mol Biol. 2008;384:798–811. doi: 10.1016/j.jmb.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Yang J, Tan G, Ding H. Complementary roles of SufA and IscA in the biogenesis of iron-sulfur clusters in Escherichia coli. Biochem J. 2008;409:535–543. doi: 10.1042/BJ20071166. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DC, Unciuleac MC, Dean DR. Controlled expression and functional analysis of iron-sulfur cluster biosynthetic components within Azotobacter vinelandii. J Bacteriol. 2006;188:7551–7561. doi: 10.1128/JB.00596-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen LT, Culotta VC. Role of Saccharomyces cerevisiae ISA1 and ISA2 in iron homeostasis. Mol Cell Biol. 2000;20:3918–3927. doi: 10.1128/mcb.20.11.3918-3927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaut A, Lange H, Diekert K, Kispal G, Lill R. Isa1p is a component of the mitochondrial machinery for maturation of cellular iron-sulfur proteins and requires conserved cysteine residues for function. J Biol Chem. 2000;275:15955–15961. doi: 10.1074/jbc.M909502199. [DOI] [PubMed] [Google Scholar]
- 12.Kim KD, Chung WH, Kim HJ, Lee KC, Roe JH. Monothiol glutaredoxin Grx5 interacts with Fe-S scaffold proteins Isa1 and Isa2 and supports Fe-S assembly and DNA integrity in mitochondria of fission yeast. Biochem Biophys Res Commun. 2010;392:467–472. doi: 10.1016/j.bbrc.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Song D, Tu Z, Lee FS. Human IscA1 interacts with IOP1/NARFL and functions in both cytosolic and mitochondrial iron-sulfur protein biogenesis. J Biol Chem. 2009;284:35297–35307. doi: 10.1074/jbc.M109.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balasubramanian R, Shen G, Bryant DA, Golbeck JH. Regulatory roles for IscA and SufA in iron homeostasis and redox stress responses in the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol. 2006;188:3182–3191. doi: 10.1128/JB.188.9.3182-3191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ollagnier-de-Choudens S, Mattioli T, Takahashi Y, Fontecave M. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J Biol Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- 16.Krebs C, Agar JN, Smith AD, Frazzon J, Dean DR, Huynh BH, Johnson MK. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry. 2001;40:14069–14080. doi: 10.1021/bi015656z. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto K, Yamashita E, Kondou Y, Lee SJ, Arisaka F, Tsukihara T, Nakai M. The asymmetric IscA homodimer with an exposed [2Fe-2S] cluster suggests the structural basis of the Fe-S cluster biosynthetic scaffold. J Mol Biol. 2006;360:117–132. doi: 10.1016/j.jmb.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Ghany SE, Ye H, Garifullina GF, Zhang L, Pilon-Smits EA, Pilon M. Iron-sulfur cluster biogenesis in chloroplasts. Involvement of the scaffold protein CpIscA. Plant Physiol. 2005;138:161–172. doi: 10.1104/pp.104.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, Sanakis Y, Teixeira de Mattos J, Fontecave M, Barras F. ErpA, an iron sulfur (Fe S) protein of the A-type essential for respiratory metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 2007;104:13626–13631. doi: 10.1073/pnas.0705829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng J, Geng M, Jiang H, Liu Y, Liu J, Qiu G. The IscA from Acidithiobacillus ferrooxidans is an iron-sulfur protein which assemble the [Fe4S4] cluster with intracellular iron and sulfur. Arch Biochem Biophys. 2007;463:237–244. doi: 10.1016/j.abb.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Chahal HK, Dai Y, Saini A, Ayala-Castro C, Outten FW. The SufBCD Fe-S scaffold complex interacts with SufA for Fe-S cluster transfer. Biochemistry. 2009;48:10644–10653. doi: 10.1021/bi901518y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta V, Sendra M, Naik SG, Chahal HK, Huynh BH, Outten FW, Fontecave M, Ollagnier de Choudens S. Native Escherichia coli SufA, coexpressed with SufBCDSE, purifies as a [2Fe-2S] protein and acts as an Fe-S transporter to Fe-S target enzymes. J Am Chem Soc. 2009;131:6149–6153. doi: 10.1021/ja807551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- 24.Ding H, Clark RJ. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem J. 2004;379:433–440. doi: 10.1042/BJ20031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding H, Clark RJ, Ding B. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions. J Biol Chem. 2004;279:37499–37504. doi: 10.1074/jbc.M404533200. [DOI] [PubMed] [Google Scholar]
- 26.Ding H, Harrison K, Lu J. Thioredoxin reductase system mediates iron binding in IscA and iron delivery for the iron-sulfur cluster assembly in IscU. J Biol Chem. 2005;280:30432–30437. doi: 10.1074/jbc.M504638200. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Bitoun JP, Ding H. Interplay of IscA and IscU in biogenesis of iron-sulfur clusters. J Biol Chem. 2006;281:27956–27963. doi: 10.1074/jbc.M601356200. [DOI] [PubMed] [Google Scholar]
- 28.Bitoun JP, Wu G, Ding H. Escherichia coli FtnA acts as an iron buffer for reassembly of iron-sulfur clusters in response to hydrogen peroxide stress. Biometals. 2008;21:693–703. doi: 10.1007/s10534-008-9154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Bitoun JP, Tan G, Wang W, Min W, Ding H. Iron binding activity of human iron-sulfur cluster assembly protein hIscA-1. Biochem J. 2010;428:125–131. doi: 10.1042/BJ20100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding B, Smith ES, Ding H. Mobilization of the iron centre in IscA for the iron-sulphur cluster assembly in IscU. Biochem J. 2005;389:797–802. doi: 10.1042/BJ20050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li DS, Ohshima K, Jiralerspong S, Bojanowski MW, Pandolfo M. Knockout of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Lett. 1999;456:13–16. doi: 10.1016/s0014-5793(99)00896-0. [DOI] [PubMed] [Google Scholar]
- 32.Bou-Abdallah F, Adinolfi S, Pastore A, Laue TM, Dennis Chasteen N. Iron Binding and Oxidation Kinetics in Frataxin CyaY of Escherichia coli. J Mol Biol. 2004;341:605–615. doi: 10.1016/j.jmb.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 33.Ding H, Yang J, Coleman LC, Yeung S. Distinct iron binding property of two putative iron donors for the iron-sulfur cluster assembly: IscA and the bacterial frataxin ortholog CyaY under physiological and oxidative stress conditions. J Biol Chem. 2007;282:7997–8004. doi: 10.1074/jbc.M609665200. [DOI] [PubMed] [Google Scholar]
- 34.Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, Martin SR, Bonomi F, Pastore A. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat Struct Mol Biol. 2009;16:390–396. doi: 10.1038/nsmb.1579. [DOI] [PubMed] [Google Scholar]
- 35.Fischer DS. A method for the rapid detection of acute iron toxicity. Clin Chem. 1967;13:6–11. [PubMed] [Google Scholar]
- 36.Siegel LM. A Direct Microdetermination of Sulfide. Anal Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- 37.Rogers PA, Ding H. L-cysteine-mediated destabilization of dinitrosyl iron complexes in proteins. J Biol Chem. 2001;276:30980–30986. doi: 10.1074/jbc.M101037200. [DOI] [PubMed] [Google Scholar]
- 38.Woodmansee AN, Imlay JA. Quantitation of intracellular free iron by electron paramagnetic resonance spectroscopy. Methods Enzymol. 2002;349:3–9. doi: 10.1016/s0076-6879(02)49316-0. [DOI] [PubMed] [Google Scholar]
- 39.Jacques JF, Jang S, Prevost K, Desnoyers G, Desmarais M, Imlay J, Masse E. RyhB small RNA modulates the free intracellular iron pool and is essential for normal growth during iron limitation in Escherichia coli. Mol Microbiol. 2006;62:1181–1190. doi: 10.1111/j.1365-2958.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- 40.Duan X, Yang J, Ren B, Tan G, Ding H. Reactivity of nitric oxide with the [4Fe-4S] cluster of dihydroxyacid dehydratase from Escherichia coli. Biochem J. 2009;417:783–789. doi: 10.1042/BJ20081423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layer G, Ollagnier-de Choudens S, Sanakis Y, Fontecave M. Iron-sulfur cluster biosynthesis: characterization of Escherichia coli CyaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU. J Biol Chem. 2006;281:16256–16263. doi: 10.1074/jbc.M513569200. [DOI] [PubMed] [Google Scholar]
- 42.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varghese S, Tang Y, Imlay JA. Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J Bacteriol. 2003;185:221–230. doi: 10.1128/JB.185.1.221-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flint DH, Emptage MH, Finnegan MG, Fu W, Johnson MK. The role and properties of the iron-sulfur cluster in Escherichia coli dihydroxy-acid dehydratase. J Biol Chem. 1993;268:14732–14742. [PubMed] [Google Scholar]
- 45.Wada K, Hasegawa Y, Gong Z, Minami Y, Fukuyama K, Takahashi Y. Crystal structure of Escherichia coli SufA involved in biosynthesis of iron-sulfur clusters: Implications for a functional dimer. FEBS Lett. 2005;579:6543–6548. doi: 10.1016/j.febslet.2005.10.046. [DOI] [PubMed] [Google Scholar]