Abstract
Objective
Prior research demonstrates that hydroxychloroquine (HCQ) lowers glycated hemoglobin (HbA1c) in non-rheumatic diabetic patients. We examined medical records of patients with diabetes mellitus (DM) and concomitant rheumatic illness to measure changes in HbA1c after starting HCQ or methotrexate (MTX).
Methods
We utilized electronic medical records to identify patients initiating either HCQ or MTX, with a diagnosis of DM or HbA1c ≥ 7%, and with at least one HbA1c measurement before and within 12 months after initiation. A structured medical record abstraction examined rheumatologic diagnosis, use of oral glucocorticoids, body mass index, age, gender. Adjusted linear regression models determined changes in HbA1c from pre-drug values to the lowest post-drug values within twelve months.
Results
We identified 45 HCQ users and 37 MTX users who met inclusion criteria. Half in each group carried a rheumatoid arthritis diagnosis. Age, gender, and mean pre-drug HbA1c levels were similar across groups. Mean BMI for HCQ users (35.4 kg/m2) was slightly higher than MTX users (32.2 kg/m2) (p = 0.13). Glucocorticoid use appeared more common in MTX (46%) than HCQ (29%) users (p = 0.17). The mean reduction in HbA1c between pre- and post-HCQ was 0.66% (95% CI 0.26–1.05) versus 0.11% (95% CI −0.18 – 0.40) for MTX. In fully adjusted analyses, the reduction in HbA1c among HCQ users was 0.54% greater than the drop among MTX users (p = 0.041).
Conclusion
HCQ initiation was associated with a significantly greater reduction in HbA1c as compared to MTX initiation among diabetic patients with rheumatic disease.
People with rheumatic diseases, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE), are at an increased risk of developing cardiovascular disease (CVD) [1–4]. Cardiovascular disease is a leading cause of death in individuals with rheumatic diseases; however the risk factors for CVD in this population are not clear. Both traditional risk factors and inflammation likely contribute to CVD [5–7]. Current management guidelines for CVD in rheumatic disease suggest measures applicable to the general population, with the addition of slightly more aggressive risk stratification [8–10]. It would be ideal if management strategies for CVD and its risk factors could be tailored for rheumatic disease patients.
Several risk factors – both traditional and those specific to rheumatic conditions – may indicate an elevated risk of CVD in rheumatic disease patients [5–7]. Although there is no consensus regarding the risk of diabetes mellitus (DM) in RA [11, 12], it is clear that patients with rheumatic disease experience a substantially elevated risk of insulin resistance [13, 14]. Insulin resistance refers to a state of impaired insulin sensitivity and glucose metabolism, which commonly precedes development of DM [15]. It is strongly associated with the metabolic syndrome, defining one of the WHO criteria for this constellation of CVD risk factors, and predicts future CVD events [16].
It has been shown that inflammation directly influences insulin and glucose metabolism through cytokines such as TNFα and IL-6 [13, 17–19]. Considering this, several disease modifying anti-rheumatic drugs (DMARDS) – agents prescribed to primarily treat rheumatic diseases – have also been tested as interventions to improve insulin and glucose metabolism. Anakinra – an IL-1 receptor antagonist FDA approved for the treatment of RA – was examined as a therapy for DM, and associated with significantly lower glycated hemoglobin (HbA1c) [20]. Several TNFα antagonists have been shown to improve insulin metabolism in patients with RA or ankylosing spondylitis [17–19]. In addition, hydroxchloroquine (HCQ), an FDA approved DMARD for the treatment of RA and lupus, has been found in at least two randomized controlled trials to improve diabetes control in non-rheumatic subjects [21, 22]. One large epidemiologic study also showed that HCQ reduces the risk of DM among patients with RA [23].
It is unclear whether the effects of HCQ seen among diabetics would generalize to rheumatic disease patients. As well, it is not clear whether other, more potent DMARDs, such as methotrexate (MTX), would affect HbA1c. Considering these questions, we compared the effect of HCQ with that of MTX on HbA1c in diabetic adults under treatment for a rheumatologic condition
PATIENTS AND METHODS
Study Cohort
Adult subjects were identified from a large academic medical center’s clinical data repository. This clinical data repository includes information on patients’ charges, diagnoses, procedures, laboratory values, prescribed medications, medical history, and sociodemographics.
One author (LRR) abstracted the medical records of potentially eligible subjects using a structured review. Items of interest included age, gender, body mass index (BMI), rheumatologic diagnosis, DM diagnosis, history of insulin use or non-insulin DM medication, and history of oral corticosteroid use.
We searched the repository for patients aged 18 years and older, with a diagnosis of DM (or a pre-treatment HbA1c of 7% or greater) [24] with two or more HbA1c values available; and who had initiated HCQ but not MTX, OR had initiated MTX but not HCQ. From this group of potentially eligible subjects (n = 537) we reviewed full medical records, and excluded: those without one pre-treatment HbA1c and without one post treatment HbA1c (n = 391); those taking both HCQ and MTX (n = 26); and those who lacked a pre-treatment HbA1c of 7% or higher or a clinical diagnosis of DM (n = 38).
The Partners HealthCare System Institutional Review Board approved all aspects of this study.
Exposure
The exposures of interest were initiation of either HCQ or MTX; concomitant users were excluded. Information about HCQ or MTX initiation was derived from physician notes as well as the electronic medication list. We made no distinctions based on the dosage of these medications. While simultaneous use of HCQ and MTX was not permitted, subjects were commonly prescribed other anti-rheumatic therapies during the same period; these cases were included. In addition, glucocorticoid use was not excluded.
Outcome of Interest
The primary outcome of interest was change in HbA1c, from the measurement most proximal prior to HCQ or MTX initiation to at least 12 weeks after initiation. Two different post-exposure HbA1c values were examined: the most proximal to HCQ or MTX initiation and the lowest within 12 months following initiation. The HbA1c values were derived from routine clinical laboratory practice, which uses a Tosoh HLC-723G8 Analyzer instrument to measure HbA1c (coefficient of variation < 0.5%) [25].
Statistical Analysis
Descriptive statistics such as mean, median, standard deviation for each continuous variable, and frequencies for each categorical variable were used to summarize the data. The normality of the distribution of primary outcomes of interest was examined with a normal probability plot. Two sample t-tests, Chi-squared tests, Fisher’s exact tests or non-parametric tests were used for baseline comparisons between the two exposure groups when applicable. The change in HbA1c was calculated separately for HCQ and MTX groups, as was HbA1cPRE – HbA1cPOST. First, the change in HbA1c within each group was assessed using paired t-tests or Wilcoxon signed rank tests. We then compared changes in HbA1c across drugs using general linear regression adjusted for rheumatologic diagnosis, cumulative steroid use, duration (months) between drug initiation and lowest HbA1c, a change in DM medication, body mass index, age, and gender. Data analyses were performed using SAS 9.2 (SAS Institute, Inc. Cary, North Carolina).
RESULTS
The baseline characteristics of the 82 subjects (45 HCQ users, and 37 MTX users) who are included in the analyses are shown in Table 1. The mean age for both groups was 61 years and most patients were female: 82% for HCQ and 73% for MTX. Approximately half of the subjects in each treatment group carried a diagnosis of RA (53% for HCQ and 57% for MTX); 7% of the HCQ treated subjects were diagnosed with SLE, compared with 0% for MTX. There was a trend toward more frequent oral glucocorticoid use in the twelve months following drug initiation in MTX (46%) than in HCQ (29%) (p = 0.17), as well as a slightly higher cumulative dose in MTX (612±982 grams) than in HCQ (464±809 grams) (p = 0.46). Mean BMI for HCQ users (35.4 kg/m2) was slightly higher than MTX users (32.2 kg/ m2) (p = 0.13). A physician’s diagnosis of DM was recorded in 89% of HCQ users and 97% of MTX users, with roughly 30% in both groups prescribed insulin therapy and close to 60% in both groups prescribed non-insulin DM medication.
Table 1.
Baseline Characteristics of Diabetic Adults with Rheumatic Disease at Time of New Initiation of Hydroxychloroquine or Methotrexate
| Hydroxychloroquine n = 45 | Methotrexate n = 37 | P value | |
|---|---|---|---|
| Mean ± SD or % | |||
| Age, mean ± SD, years | 61 ± 13 | 61 ± 13 | 0.79 |
| Female gender, % | 82% | 73% | 0.20 |
| Body mass index | 35.4±8.5 | 32.2±6.1 | 0.13 |
| Rheumatoid arthritis, % | 53.3% | 56.8% | 0.95 |
| Systemic lupus erythematosus, % | 6.7% | 0 | 0.50 |
| Diabetes mellitus, by physician diagnosis % | 88.9% | 97.3% | 0.22 |
| Prescribed insulin, n, % | 33.3% | 29.7% | 0.75 |
| Prescribed non-insulin diabetes medication, n, % | 62.2% | 59.5% | 0.82 |
| Cumulative* steroid use, % | 28.9% | 46.0% | 0.17 |
| Cumulative* steroid use, grams | 464±809 | 612±982 | 0.46 |
Cumulative refers to the 12 months after initiation of HCQ or MTX.
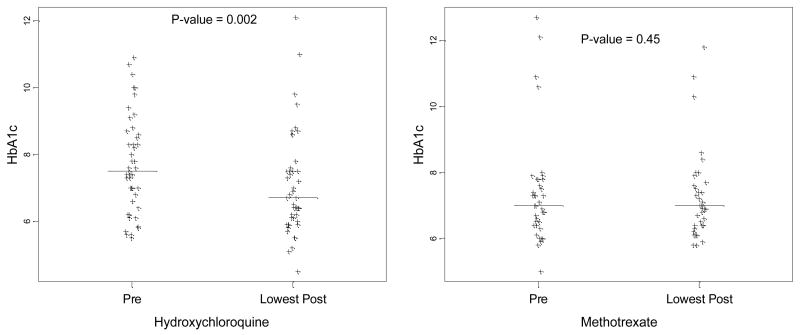
Mean pre-drug initiation HbA1c values were similar in both groups (HCQ 7.71% and MTX 7.38%, p = 0.35) (Table 2). The most proximally recorded HbA1c after initiation of drug were similar (HCQ 7.28% and MTX 7.47%, p = 0.58), as were the absolute values for the lowest HbA1c values within twelve months after treatment initiation (HCQ 7.05% and MTX 7.27%, p = 0.49). However, a comparison of the change in HbA1c from pre treatment to lowest values post treatment revealed a significant reduction with HCQ (0.66%; 95% CI 0.26 – 1.05), as compared to the significantly smaller reduction observed with MTX (0.11%; 95% CI −0.18 – 0.40) (p = 0.04) (Figure 1). In adjusted analyses this drop in HbA1c among HCQ users was 0.54% greater than that in MTX users (p = 0.041).
Table 2.
Change in HbA1c with Initiation of Hydroxychloroquine or Methotrexate, in Diabetic Adults with Rheumatic Disease
| Hydroxychloroquine n = 45 | Methotrexate n = 37 | P value* | |
|---|---|---|---|
| Mean ± SD | |||
| Pre-drug HbA1c | 7.71±1.46 | 7.38±1.66 | 0.35 |
| Most proximal post-drug HbA1c | 7.28±1.63 | 7.47±1.40 | 0.58 |
| Lowest post-drug HbA1c within 12 mos. | 7.05±1.54 | 7.27±1.34 | 0.49 |
| ΔHbA1c (Pre-drug minus lowest post-drug) | 0.66±1.31 | 0.11±0.87 | 0.04 |
P-values from general linear regression adjusted for rheumatologic diagnosis, cumulative steroid use, duration (months) between drug initiation and lowest HbA1c, a change in DM medication, body mass index, age, and gender.
Figure 1.
Effect of HCQ versus MTX on HbA1c within 12 months post drug initiation in diabetic adults with rheumatologic condition. P-value compares pre-treatment HbA1c to the lowest HbA1c within 12 months post-treatment; p-value calculated from a paired t-test. Horizontal bar indicates sample median.
DISCUSSION
The literature suggests that HCQ may possess unique hypoglycemic effects, in addition to its remittive actions for rheumatic diseases. To better understand whether the hypoglycemic effects of HCQ are in part related to its anti inflammatory properties or another mechanism, we examined the potential hypoglycemic benefits of MTX, another DMARD commonly used to treat RA and other rheumatic diseases. In this study, we examined the change in HbA1c among patients with rheumatic diseases initiating either drug; all patients had DM with a pre-drug HbA1c of 7% or higher. Comparing pre-drug levels with those within one year following drug initiation, HCQ produced a significant reduction in HbA1c that was larger than the change associated with MTX. Patients starting MTX did not experience a significant reduction in HbA1c, but relatively few subjects starting MTX were studied.
While inflammation is known to be associated with impaired glucose control, the mechanism by which HCQ exerts a hypoglycemic effect is not entirely clear. Chloroquine has been shown to increase C-peptide response, which Gerstein et al. cite as a potential effect of improved beta cell functioning with decreased blood glucose [22, 26]. Additionally, HCQ’s inhibitory effect on insulin metabolism has been demonstrated in animal models, with effects including reductions in intracellular insulin degradation, and increases in insulin accumulation [27, 28]. Whether due to a novel mechanism or the established anti-inflammatory effect of this therapy, the results from this study align with the clinically observed responses detailed in epidemiologic studies and case reports.
Data from our study represent novel findings, highlighting HCQ’s potential ability to decrease HbA1c in diabetic persons with systemic inflammatory disease. Several previous studies in non-rheumatic patients with DM have demonstrated the hypoglycemic effect of HCQ as well. HCQ therapy contributed to significant improvements in type 2 DM management, as compared with a placebo, in a small group of treatment refractory individuals in a randomized trial [21]. Subsequently, HCQ was found in another randomized trial to improve glucose control and HDL cholesterol in a larger cohort of type 2 diabetics [22]. In addition, a large epidemiologic study among patients with RA found that HCQ was associated with a reduced risk of incident DM, even after controlling for disease activity and glucocorticoid use [23]. Considering these prior data and the significant effect we observed with HCQ, our findings generate interesting questions about the potential long-term role of this therapy, apart from its effect on arthritis symptoms, in treating systemic rheumatic disease.
Our study has several limitations. As a non-randomized retrospective chart review, there is the possibility for confounding by unmeasured factors, including changes in diabetes medications and BMI. The data was collected on a limited number of patients at one academic medical center, potentially limiting its generalizability. In addition, information on rheumatic disease activity was not collected, and only extremely limited data on concomitant glucocorticoid use was available. Finally, the study was small and not specifically powered to detect a difference in MTX; the null effect may be an artifact of the small sample size.
In the last decade, there has been a growing awareness of the increased cardiovascular morbidity associated with RA and SLE. The reasons for this observation are in part related to inflammation as well as to traditional risk factors like diabetes mellitus and impaired insulin resistance. We wanted to better understand whether HCQ might improve glucose control in rheumatic disease patients and begin to explore whether HCQ’s effects were related to a reduction in inflammation, or to another, independent effect of HCQ. We examined the effects of HCQ and MTX, two commonly used DMARDs, on HbA1c among rheumatic disease patients. HCQ significantly lowered glycated hemoglobin; MTX did not, but the sample size was limiting. These findings add to an existing literature suggesting beneficial effects of HCQ on insulin and glucose metabolism, perhaps distinct from MTX. Comprehensive, larger, and prospective studies are clearly warranted to determine the potential role of HCQ in mitigating cardiovascular morbidities in patients with systemic rheumatic diseases.
Acknowledgments
Support:Dr. Solomon’s work on this project was supported by the NIH (K24 AR-055989; R21 AR-057924-01).
Footnotes
Disclosures: Dr. Solomon receives salary support from research grants from Amgen and Abbott regarding inflammatory arthritis. He has also received a medical education grant from BMS.
WORKS CITED
- 1.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003 Mar 11;107(9):1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 2.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis and rheumatism. 2001 Dec;44(12):2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 3.Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford, England) 2007 Feb;46(2):350–7. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- 4.Goodson N, Marks J, Lunt M, Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Annals of the rheumatic diseases. 2005 Nov;64(11):1595–601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis and rheumatism. 2002 Aug;46(8):2010–9. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 6.Navarro-Cano G, Del Rincon I, Pogosian S, Roldan JF, Escalante A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis and rheumatism. 2003 Sep;48(9):2425–33. doi: 10.1002/art.11127. [DOI] [PubMed] [Google Scholar]
- 7.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis and rheumatism. 2005 Feb;52(2):402–11. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 8.Boers M, Dijkmans B, Gabriel S, Maradit-Kremers H, O’Dell J, Pincus T. Making an impact on mortality in rheumatoid arthritis: targeting cardiovascular comorbidity. Arthritis and rheumatism. 2004 Jun;50(6):1734–9. doi: 10.1002/art.20306. [DOI] [PubMed] [Google Scholar]
- 9.Hall FC, Dalbeth N. Disease modification and cardiovascular risk reduction: two sides of the same coin? Rheumatology (Oxford, England) 2005 Dec;44(12):1473–82. doi: 10.1093/rheumatology/kei012. [DOI] [PubMed] [Google Scholar]
- 10.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Annals of the rheumatic diseases. 2009 Sep 22; doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Annals of the rheumatic diseases. 2008 Jan;67(1):64–9. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 12.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. The Journal of rheumatology. 2006 Nov;33(11):2167–72. [PubMed] [Google Scholar]
- 13.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis and rheumatism. 2008 Jul;58(7):2105–12. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis and rheumatism. 2006 Sep;54(9):2765–75. doi: 10.1002/art.22053. [DOI] [PubMed] [Google Scholar]
- 15.Riserus U, Arnlov J, Berglund L. Long-term predictors of insulin resistance: role of lifestyle and metabolic factors in middle-aged men. Diabetes care. 2007 Nov;30(11):2928–33. doi: 10.2337/dc07-0360. [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. Journal of the American College of Cardiology. 2007 Jan 30;49(4):403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clinical rheumatology. 2007 Sep;26(9):1495–8. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 18.Rosenvinge A, Krogh-Madsen R, Baslund B, Pedersen BK. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFalpha therapy. Scandinavian journal of rheumatology. 2007 Mar-Apr;36(2):91–6. doi: 10.1080/03009740601179605. [DOI] [PubMed] [Google Scholar]
- 19.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Annals of the rheumatic diseases. 2005 May;64(5):765–6. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007 Apr 12;356(15):1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 21.Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, Ceriello A, et al. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. A new job for an old drug? Annals of internal medicine. 1990 May 1;112(9):678–81. doi: 10.7326/0003-4819-112-9-678. [DOI] [PubMed] [Google Scholar]
- 22.Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas--a randomized trial. Diabetes research and clinical practice. 2002 Mar;55(3):209–19. doi: 10.1016/s0168-8227(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 23.Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. Jama. 2007 Jul 11;298(2):187–93. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 24.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2008 Jul;93(7):2447–53. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 25.Chapelle JP, Teixeira J, Maisin D, Assink H, Barla G, Stroobants AK, et al. Multicentre evaluation of the Tosoh HbA1c G8 analyser. Clin Chem Lab Med. 2009 Dec 18; doi: 10.1515/CCLM.2010.062. [DOI] [PubMed] [Google Scholar]
- 26.Powrie JK, Smith GD, Shojaee-Moradie F, Sonksen PH, Jones RH. Mode of action of chloroquine in patients with non-insulin-dependent diabetes mellitus. The American journal of physiology. 1991 Jun;260(6 Pt 1):E897–904. doi: 10.1152/ajpendo.1991.260.6.E897. [DOI] [PubMed] [Google Scholar]
- 27.Emami J, Pasutto FM, Mercer JR, Jamali F. Inhibition of insulin metabolism by hydroxychloroquine and its enantiomers in cytosolic fraction of liver homogenates from healthy and diabetic rats. Life sciences. 1999;64(5):325–35. doi: 10.1016/s0024-3205(98)00568-2. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Webb P, Bonser AM. Insulin binding and degradation in isolated hepatocytes from streptozotocin injected rats. Biochemical and biophysical research communications. 1985 Apr 30;128(2):487–93. doi: 10.1016/0006-291x(85)90073-7. [DOI] [PubMed] [Google Scholar]