Abstract
Objective To improve HIV treatment in China by determining changes over time of patient characteristics (geographic, clinical and route of HIV infection) among patients enrolled in the China National Free Antiretroviral Treatment Program.
Methods Patients in the national treatment database from 1 June 2002 to 1 June 2009 were eligible. Patients were excluded if <18 years old, not previously treatment-naïve, missing initial treatment date or not initiated on triple drug therapy.
Results About 62 919 patients were included, located across 54.8% of counties/districts throughout mainland China; 86.4% were concentrated in 11.1% of counties/districts. Median age was 38 years, 41% female, 45.4% former plasma donors (FPDs), 33.9% sexually infected and 15.5% injection drug users (IDUs). Median baseline CD4 cell count was 129/µl. In 2002, 100% of treated were FPDs with no CD4 cell counts. By 2009, 59% of the treated were sexually infected and 96% had baseline CD4 cell counts. Injection drug users remained a minority of those treated.
Conclusions Limited treatment resources can be focused on areas with more patients. Greater emphasis needs to be placed on earlier HIV diagnosis and treatment. New strategies must be identified to bring HIV-infected IDUs into treatment. Routine HIV testing would identify those at risk earlier.
Keywords: China, HIV, national treatment program, baseline characteristics, injection drug users, routine testing
Introduction
The HIV epidemic in China has been concentrated primarily in the high-risk cohorts of former plasma donors (FPDs), injection drug users (IDUs), female sex workers (FSWs), and men who have sex with men (MSM).1–3 The FPDs were estimated to have been infected predominantly in the early- to mid-1990s and thus developed AIDS in large numbers by the early 2000s. In response, the China Ministry of Health created the Division of Treatment and Care within the National Centre for AIDS/STD Control and Prevention (NCAIDS) of the Chinese Centre for Disease Control and Prevention (China CDC) in 2001 with the mandate to establish a national HIV treatment program.4–6
The China National Free Antiretroviral Treatment Program (NFATP), which began in 2002, has expanded each year since and has now treated over 80 000 patients across the entire country.7,8 Over these past 8 years, the program has changed as different resources have become available. For instance, drugs initially available through the NFATP included Chinese-produced generic zidovudine, stavudine, didanosine, nevirapine and indinavir. Branded lamivudine and efavirenz drugs became more widely available from 2005 onwards. The objectives of this article are to document the dramatic changes over time of patient characteristics (geographic, clinical and route of HIV infection) among patients enrolled in the NFATP, and to highlight implications for improving the quality of HIV care and treatment in China.
Methods
Patients who met the national treatment guidelines of CD4 cell count <200/µl, total lymphocyte count <1200/µl, or the World Health Organization (WHO) stage III/IV disease were initiated on highly active antiretroviral therapy (HAART).9 All patients treated through the NFATP were prospectively captured in a previously described10 ongoing observational database maintained at the NCAIDS. Baseline data captured included patient demographics, self-reported route of HIV infection, laboratory results including CD4 cell count and viral load when available and initial treatment regimen. Because of the difficulty in making definitive diagnoses of opportunistic infections in rural settings, easily diagnosed signs and symptoms were used as a proxy (syndromic surveillance). The categories were as follows: (i) fever; (ii) pulmonary (cough, dyspnoea, chest pain, night sweats or lymphadenopathy); (iii) gastrointestinal (nausea, vomiting or diarrhoea); (iv) skin or mucosal (rash, thrush or oral hairy leukoplakia); and (v) central nervous system (headache or visual changes). This information was collected as part of a general review of systems and physical examination during the baseline patient visit. Because Henan Province did not participate in the national treatment database until 1 July 2006, baseline CD4 cell counts for Henan patients before then were collected instead from the national HIV epidemiology database, also maintained by the NCAIDS.
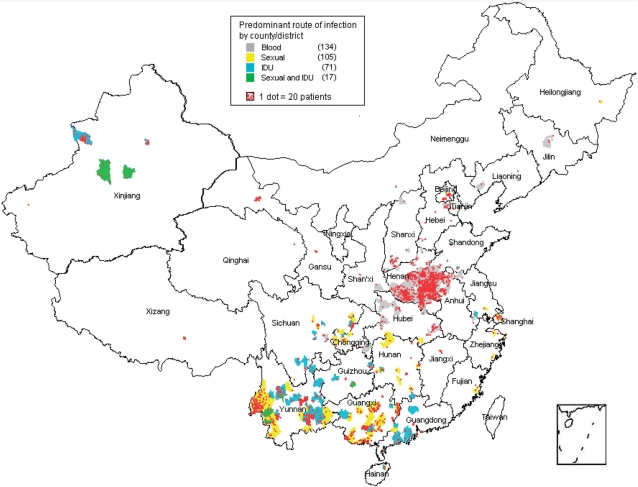
All patients enrolled in the database from 1 June 2002 to 1 June 2009 were eligible for this analysis. Patients were excluded if they were <18 years old, were not previously treatment naïve, were not initially treated through the NFATP, had a missing initial treatment date or were not initiated on triple drug therapy. Overall baseline characteristics between routes of infection were compared with α = 0.05, using the Pearson χ2 test for categorical variables and the non-parametric Kruskal–Wallis test for continuous variables because none fulfilled the Kolmogorov–Smirnov test for normality. Pairwise comparisons between routes of infection used Pearson χ2 for categorical variables and the Mann–Whitney test for continuous variables when the overall test is significant, using a Bonferroni adjusted α = 0.0083 (0.05/6). Baseline characteristics by time were compared using the Pearson χ2 test, with time categorized as 2002–04, 2005–06 and 2007–09. All hypotheses testing were two-sided. Data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). To present a geographical distribution of the routes of infection, a map was generated using MapInfo Professional, version 6.5 (Pitney Bowes Software Inc., Troy, NY, USA) using county and district level data. Each dot represented 20 patients and each county or district with at least 20 patients was stratified by colour into route of transmission, based on whichever route(s) represented >50% of transmissions in that area. Some provinces did not have one route comprising >50% of transmissions but had sexual and IDU transmissions together comprising >50% of transmissions and were classified as sex-IDU mixed. Data analyses were approved by the institutional review board of the NCAIDS.
Results
As of 1 June 2009, a cumulative total of 66 694 patients had begun HAART through the NFATP. After excluding patients previously treated (N = 1802), age <18 years or unknown (N = 1335), who started on only one or two drugs (N = 1134, most likely due to data transcription errors) or with missing treatment initiation date (N = 496, above categories not mutually exclusive), 62 919 (94.3%) patients were included in this analysis. Among all treated patients, 45.4% were FPDs, 15.5% IDUs and 33.9% sexually infected (Table 1), among whom 1245 (5.8%) were reported as MSM. At baseline, median age was 38 years and 41% were female, with FPDs being older and IDUs predominantly male. Overall, 74.2% had a baseline CD4 cell count, comprising only 50.1% of FPDs but 96.5% of IDUs and 93.4% of those infected sexually. Those infected sexually had a significantly lower median CD4 cell count and a significantly higher proportion with CD4 cell counts <50/µl than patients with other routes of transmission. However, if the other 50% of FPDs who were predominantly treated in the earlier years had also had CD4 cell counts done, their overall median CD4 cell count would likely be lower because significantly more FPDs had more baseline sign/symptom categories consistent with severe immunosuppression. Only 5% had a baseline viral load performed. Among the 86.3% with baseline liver function tests, IDUs had significantly more abnormal tests and proportion with alanine aminotransferase > 80 U/l. Of note, the majority of variables between the sexually transmitted cohort and the unknown transmission cohort were not statistically different, suggesting that the majority of those with unknown HIV transmission routes may have acquired their infection sexually.
Table 1.
Baseline characteristics of the 62 919 eligible adult patients, stratified by HIV transmission route, included in the China NFATP, June 2002–June 2009
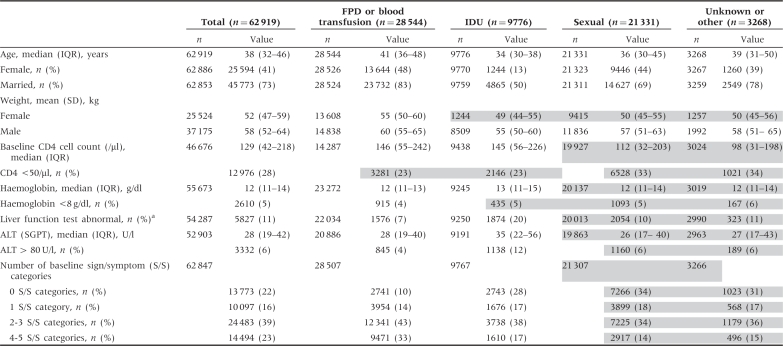 |
IQR = interquartile range; SD = standard deviation; ALT = alanine aminotransferase; SGPT = serum glutamic pyruvic transaminase. Overall, all characteristics were statistically significantly different from each other by route of transmission (P < 0.001). In pairwise comparisons of all characteristics by route of transmission, using Bonferroni adjusted P < 0.0083, all variables were statistically significantly different from each other except for those shaded together.
aLiver function test was considered abnormal when AST or ALT was >80 U/l (2× upper limit of normal) or total bilirubin was >27.36 µmol/l (1.5 × upper limit of normal).
Among a total of 2950 counties and districts across mainland China, the 62 919 treated patients were located in 1617 (54.8%) counties/districts, showing the broad spread of HIV across mainland China. Of these, only 327 counties/districts, encompassing 54 338 (86.4%) patients, had at least 20 patients, demonstrating that the vast majority of patients were concentrated in only 11.1% of the counties/districts. These 327 counties/districts and patients were mapped and stratified by route of transmission (Figure 1). The largest concentration of treated patients was FPDs located in and around Henan Province. Southern China, particularly Yunnan, Guangxi and Guangdong Provinces, had a mixture of sexual and IDU transmissions. Xinjiang Province, to the West, was predominantly IDU-driven, with some sexual transmissions reported as well. Overall, when stratifying the 327 counties by primary route(s) of transmission, transmission by blood encompassed 134 (41%) of counties; by sex, 105 (32%); by IDU, 71 (22%) and by sex-IDU mixed, 17 (5%).
Figure 1.
Among 62 919 patients included in this analysis, the 54 338 (86.4%) of patients located in the 327 counties/districts with at least 20 patients each were plotted on this map, with each county/district stratified by predominant mode of HIV transmission
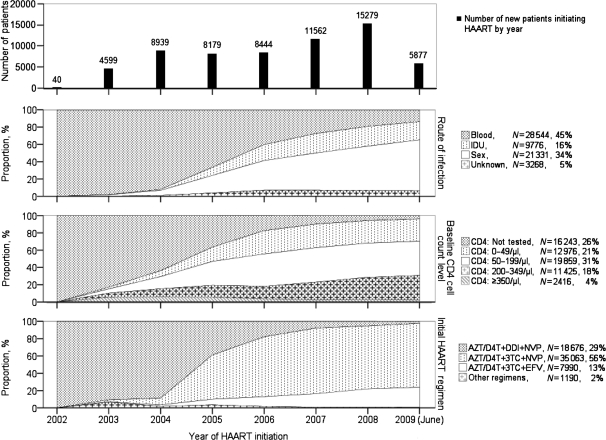
The baseline characteristics of those treated changed significantly over time (Figure 2). Although almost 100% of those initiating treatment in 2002–03 were FPDs, this changed to 14% infected by blood, 59% sexually infected and 21% IDUs during the first half of 2009. No baseline CD4 cell counts were done when the NFATP first began but, by 2009, 96% had a baseline CD4 cell count. Of these, the proportion entering care with known CD4 cell count <50/µl has remained relatively constant since 2006 at ∼25%. In 2002–03, the predominant first-line antiretroviral drugs available were zidovudine, stavudine, didanosine and nevirapine, with a very small amount of lamivudine and efavirenz. Consequently, almost everyone received a didanosine-based regimen. Lamivudine became widely available in 2005 and, since then, has essentially replaced didanosine. During the first half of 2009, 74% of patients initiated treatment with zidovudine/stavudine + lamivudine + nevirapine. Zidovudine and stavudine usage rates (49 and 51%, respectively) have been about equal over time, with stavudine having been used more before 2007 and zidovudine used predominantly starting in 2007. Efavirenz has been used as initial therapy primarily for those diagnosed with HIV–tuberculosis co-infection. The changes in all four baseline comparisons (new patients, route of infection, CD4 cell count and initial treatment regimen) over time were statistically significant (P < 0.0001).
Figure 2.
Baseline characteristics among all 62 919 patients included in this analysis, stratified over time by the number of new patients initiating highly active antiretroviral therapy (HAART), route of transmission, baseline CD4 cell count and treatment regimen. The number of new patients included by year is shown in the top graph. Note: 2009 data are only to June 1 2009. P < 0.0001 for the change in each comparison (number of new patients, route of infection, baseline CD4 cell count and initial HAART regimen) over time
Discussion
Our analysis of the baseline characteristics of patients entering the China NFATP between 2002–09 reflects a developing country’s programme in transition as it scaled up to meet the needs of the patient population. Overall, the 62 919 patients included in this analysis at baseline were predominantly young, majority male and predominantly infected via plasma donation. These patients were spread across 55% of all counties/districts across mainland China but 86% were concentrated in only 11% of all counties/districts. When these demographics were stratified by year, however, a number of stark changes were noted in the baseline characteristics of patients entering the NFATP. The predominant route of transmission shifted from 100% FPDs in 2002 to 59% sexual, 21% IDU and only 14% by blood in 2009. Baseline CD4 cell count testing increased from none to 96%, with overall median CD4 cell count of 129/µl. The treatment regimens used transitioned from a didanosine-based to a lamivudine-based regimen. As we consider the ramifications of these changes, clear public health implications emerge, which can be used to guide and improve HIV treatment practices in mainland China and in other developing countries’ HIV treatment programmes.
One of the most striking changes is the increase in numbers and geographic distribution of treated patients over 7 years, from 40 patients in one county in 2002 to 62 919 in 55% of all counties/districts across mainland China in 2009. Scaling up the NFATP to cover such a vast area over a relatively short amount of time was no small feat.11 From 2002 to 2005, treatment was more of an emergency response to the medical needs among FPDs. A training curriculum was designed for all levels of clinicians, from infectious diseases specialists to village doctors,4 with a national training manual developed to provide uniform teaching.9 Data from all treated patients were collected on standardized forms and maintained centrally in an observational database to facilitate research and analysis.10 After 2005, treatment became more standardized and was broadened to other cohorts. Starting in 2008, the national HIV treatment guidelines were revised to initiate treatment among patients with CD4 cell count from <200/µl. Analyses of treatment outcomes to date have shown the success of the NFATP, with dramatically reduced mortality rates among treated patients, from 22.6/100 person-years at treatment initiation to 4–5/100 person-years after 6 months, sustained across 5 years.8,12
Two other profound baseline changes in the NFATP are the shift in route of transmission of treated patients and availability of CD4 cell counts over time. When the programme began in 2002–03, the extent of the FPD epidemic was just beginning to be understood and treatment was initiated in this cohort first. At that time, the treatment infrastructure and laboratory capacity were limited, particularly in the rural areas where the FPDs were located. Treatment was begun predominantly based on reported symptoms and total lymphocyte count. As the programme matured and the NFATP expanded nationwide, the primary route of transmission for those initiating treatment shifted away from FPDs and more towards sexual transmission, consistent with national epidemiology data.13 CD4 cell count capacity expanded such that, in the first half of 2009, 96% of patients initiated treatment based on a pre-treatment CD4 cell count. The CD4 cell count results, however, indicated an important area of clinical and public health improvement––initiating HAART before patients become too immunosuppressed. Median baseline CD4 cell count among all patients was only 129/µl, with 28% having a CD4 cell count <50μl. Among those infected sexually, median CD4 cell count was only 112/µl. We and others have shown that initiating treatment at low CD4 cell counts has worse long-term outcomes than initiating treatment earlier;8,14–17 thus, this combination of increasing numbers of patients infected sexually but with low presenting CD4 cell counts is of great concern. The change in national treatment criteria from CD4 cell count of 200–350/µl will be of little benefit if patients continue to present so with such low CD4 counts. Additional outreach is needed among the high-HIV-risk cohorts in China to encourage increased testing and to educate them regarding the benefits of earlier treatment, with free treatment offered to those eligible. This is particularly important because the majority of those initiating treatment in recent years are IDUs and those infected sexually, including FSWs and MSM. These populations are marginalized and stigmatized in China and may not come in for care until they are extremely ill. China previously made HIV testing routine among FPDs, which caused some controversy.18 Yet, the subsequent survival benefit to those identified and treated is now documented.7,8 Routine HIV testing is also offered among other high-risk groups in China19 but, despite this, most patients continue to initiate treatment at very immunosuppressed levels, suggesting routine testing alone has been insufficient in identifying patients earlier. Consideration should be given to making HIV testing routine to the general public in areas with a known high prevalence of HIV infection, similar to what has been successfully done elsewhere.20,21
Injection drug use has been one of the primary routes of HIV infection in China, with the first reported HIV outbreak in China among IDUs in 1989.2 In prevalence estimates of HIV in China, injecting drugs was always the primary route of transmission until 2007 when IDUs, accounting for 38% of reported routes of transmission, were second only to those infected via heterosexual transmission, at 41%.13 Yet, despite IDUs being likely the earliest infected cohort and comprising the largest infected cohort in China until the 2007 estimate, only a cumulative total of 15.5% of treated patients were IDUs. The reasons for this are likely multifactorial, including social–political (stigma, incarceration), individual (fear of side effects, low HAART self-efficacy, addiction-related instability) and provider-based (physician perception, inexperience) reasons.22 More research needs to be done to explore strategies to increase access to treatment in this population in China, such as possibly combining and expanding antiretroviral treatment and methadone maintenance treatment clinics.19
Among all patients in our analysis, the IDUs stood out as having significantly more abnormal liver function test results. Co-infection of HIV with hepatitis C virus (HCV) is a well-known complication with this cohort,23–26 and a number of studies have demonstrated high rates of co-infection with HIV, hepatitis B virus (HBV) and HCV.27–30 Among the FPDs, studies have shown high rates of HCV co-infection31–33 but relatively low rates of HBV infection,34,35 despite the endemicity of HBV in China, because HBV-positive plasma donors were screened out before donation. This has implications for the long-term treatment of HIV and hepatitis in China. Hepatitis screening, unfortunately, has not been done systematically within the NFATP but will be incorporated into standard baseline testing at treatment initiation in the future. Hepatic adverse effects of antiretroviral therapy need to be monitored, particularly among the IDUs. With mortality now significantly reduced among treated HIV patients in China,7,8 plans for treating HIV/hepatitis co-infections need to be considered. Tenofovir and lamivudine will be introduced as first-line treatment for those co-infected with hepatitis B. How to treat patients co-infected with hepatitis C is a significant challenge because the treatments are expensive, complex and have significant side effects.
There are limitations associated with this analysis. First, the data come from an observational database and therefore may have inherent biases, such as reporting or recall biases, associated with the collection of non-random data. However, the large number of patient observations in the database should help mitigate any single bias in any one direction. Secondly, route of infection is self-reported and not always straightforward to ascertain. Although the FPDs as a cohort may have few additional HIV risk factors, sexual transmission and IDU cohorts often overlap.36 Sex workers may inject drugs and IDUs may sell sex to purchase drugs. Despite this overlap, baseline differences between these two cohorts in our data suggest that this overlap was not that great. Some of these differences were clinically meaningful, such as higher abnormal liver function test results with the concern about higher rates of viral hepatitis co-infection in IDUs, while others were not, such as the need to promote earlier HIV testing and treatment among all cohorts.
As the NFATP moves forward, lessons and trends from the initial 7 years can guide the program to allocate more effectively the limited resources available. Although HIV infections are spread across more than half of China, 86% of treated patients are concentrated in only 11% of counties/districts; thus, the bulk of resources will need to focus on these areas. Creative strategies must be developed to identify and treat earlier those infected sexually and through injecting drugs, with consideration given to encourage routine HIV testing for all who live in high-prevalence areas. CD4 cell counts have already been scaled up and viral load testing and hepatitis screening are scaling up now. Although these lessons are specific to China, the principles on which they are based are applicable to other developing countries with limited resources. Monitoring and evaluation of treatment outcomes is critically important to understanding the effectiveness of treatment programmes and usage rates and trends for planning purposes. For example, national programmes may not need to target the entire country. Geographic locations and risk cohorts that were hot spots several years ago may not be so today. Programmes must be flexible enough to adapt to changing trends and needs. This would not be possible without standardized data collection procedures put in place on a national or other large-scale database. Analyses should be performed routinely to ensure that limited resources are allocated effectively and that the cohorts most in need of treatment are reached. China’s NFATP has improved through careful analyses of its data and will continue to adapt in response to new data trends and scientific results.
Funding
This work was supported by the Chinese National Antiretroviral Treatment Program, and by the China Ministry of Science and Technology (MOST) Chinese National Basic Research Program (973:2006CB504201). Preparation of the manuscript was partly supported by US National Institutes of Health Research Grants (U2RTW06918-04S1 and 1R03TW008203-01).
Acknowledgement
The authors acknowledge the helpful review and comments from Dr. Marc Bulterys.
Conflict of interest: None declared.
KEY MESSAGES.
The vast majority of patients are concentrated in a few areas and limited treatment resources can be focused in these areas.
Patients still present very late in their disease course and greater emphasis needs to be placed on earlier HIV diagnosis and treatment.
Injecting drug users (IDU) comprise a disproportionately low percent of those treated, compared to the overall national epidemiology. New strategies must be identified to bring HIV-infected IDUs into treatment.
References
- 1.Zhang FJ, Chen RY, Lo SN, Ma Y. Country review: China. In: Zuniga JM, Whiteside A, Ghaziani A, Bartlett JG, editors. A Decade of HAART. Oxford: Oxford University Press; 2008. [Google Scholar]
- 2.Qian HZ, Vermund SH, Wang N. Risk of HIV/AIDS in China: subpopulations of special importance. Sex Transm Infect. 2005;81:442–7. doi: 10.1136/sti.2004.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He N, Detels R. The HIV epidemic in China: history, response, and challenge. Cell Res. 2005;15:825–32. doi: 10.1038/sj.cr.7290354. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21(Suppl. 8):S143–48. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Hsu M, Yu L, Wen Y, Pan J. Initiation of the national free antiretroviral therapy program in rural China. In: Kaufman J, Kleinman A, Saich T, editors. AIDS and Social Policy in China. Cambridge, MA: Harvard University Asia Center; 2006. pp. 96–104. [Google Scholar]
- 6.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China's free ART program. Cell Res. 2005;15:877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Dou Z, Yu L, et al. The effect of highly active antiretroviral therapy on mortality among HIV-infected former plasma donors in China. Clin Infect Dis. 2008;47:825–33. doi: 10.1086/590945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241–51, W-52. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, editor. China Free ART Manual. Beijing: Chinese Center for Disease Control and Prevention; 2005. [Google Scholar]
- 10.Ma Y, Zhang F, Zhao Y, et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol. 2010;39:973–79. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulterys M, Vermund SH, Chen RY, Ou CY. A public health approach to rapid scale-up of free antiretroviral treatment in China: an ounce of prevention is worth a pound of cure. Chin Med J (Engl) 2009;122:1352–55. [PubMed] [Google Scholar]
- 12.Ma Y, Zhao D, Yu L, et al. Predictors of virologic failure in HIV-1-infected adults receiving first-line antiretroviral therapy in 8 provinces in China. Clin Infect Dis. 2010;50:264–71. doi: 10.1086/649215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A Joint Assessment of HIV/AIDS Prevention, Treatment and Care in China (2007) Beijing: State Council AIDS Working Committee Office, UN Theme Group on AIDS in China; 2007. [Google Scholar]
- 14.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 15.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–26. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 17.Collaboration ATC. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46:607–15. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Sun X, Sullivan SG, Detels R. Public health. HIV testing in China. Science. 2006;312:1475–76. doi: 10.1126/science.1120682. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369:679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandisarewa W, Stranix-Chibanda L, Chirapa E, et al. Routine offer of antenatal HIV testing (“opt-out” approach) to prevent mother-to-child transmission of HIV in urban Zimbabwe. Bull World Health Organ. 2007;85:843–50. doi: 10.2471/BLT.06.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creek TL, Ntumy R, Seipone K, et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. J Acquir Immune Defic Syndr. 2007;45:102–7. doi: 10.1097/QAI.0b013e318047df88. [DOI] [PubMed] [Google Scholar]
- 22.Wood E, Kerr T, Tyndall MW, Montaner JS. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–56. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 23.Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18:352–58. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta-analysis. Public Health. 2008;122:990–1003. doi: 10.1016/j.puhe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Yang R, Xia X, et al. High prevalence of HIV-1 and hepatitis C virus coinfection among injection drug users in the southeastern region of Yunnan, China. J Acquir Immune Defic Syndr. 2002;29:191–96. doi: 10.1097/00042560-200202010-00014. [DOI] [PubMed] [Google Scholar]
- 26.Garten RJ, Zhang J, Lai S, Liu W, Chen J, Yu XF. Coinfection with HIV and hepatitis C virus among injection drug users in southern China. Clin Infect Dis. 2005;41(Suppl. 1):S18–24. doi: 10.1086/429491. [DOI] [PubMed] [Google Scholar]
- 27.Ruan Y, Qin G, Yin L, et al. Incidence of HIV, hepatitis C and hepatitis B viruses among injection drug users in southwestern China: a 3-year follow-up study. AIDS. 2007;21(Suppl. 8):S39–46. doi: 10.1097/01.aids.0000304695.54884.4f. [DOI] [PubMed] [Google Scholar]
- 28.Wang YC, Xu SH, Li XH, Song AJ, Jia XR, Zhuang H. A study on the prevalence rates of human immunodeficiency virus, hepatitis B virus and hepatitis C virus infections in intravenous drug users. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27:777–79. [PubMed] [Google Scholar]
- 29.Garten RJ, Lai SH, Zhang JB, Liu W, Chen J, Yu XF. Factors influencing a low rate of hepatitis C viral RNA clearance in heroin users from Southern China. World J Gastroenterol. 2008;14:1878–84. doi: 10.3748/wjg.14.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JR, Gong RY, Tian KL, Wang J, Wang YX, Huang HJ. Study on the blood-borne virus co-infection and T lymphocyte subset among intravenous drug users. World J Gastroenterol. 2007;13:2357–62. doi: 10.3748/wjg.v13.i16.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P, Xiang K, Tang H, et al. Molecular epidemiology of human immunodeficiency virus type 1 and hepatitis C virus in former blood donors in central China. AIDS Res Hum Retroviruses. 2008;24:1–6. doi: 10.1089/aid.2007.0144. [DOI] [PubMed] [Google Scholar]
- 32.Qian HZ, Vermund SH, Kaslow RA, et al. Co-infection with HIV and hepatitis C virus in former plasma/blood donors: challenge for patient care in rural China. AIDS. 2006;20: 1429–35. doi: 10.1097/01.aids.0000233577.33973.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian HZ, Yang Z, Shi X, et al. Hepatitis C virus infection in former commercial plasma/blood donors in rural Shanxi Province, China: the China Integrated Programs for Research on AIDS. J Infect Dis. 2005;192:1694–700. doi: 10.1086/497148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Y, Zhao QX, Ren YJ, Ding LM. Coinfection with HBV and HCV in 128 AIDS patients infected through blood transmission. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:662–64. [PubMed] [Google Scholar]
- 35.Xu JQ, Wang JJ, Han LF, et al. Epidemiology, clinical and laboratory characteristics of currently alive HIV-1 infected former blood donors naive to antiretroviral therapy in Anhui Province, China. Chin Med J (Engl) 2006;119:1941–48. [PubMed] [Google Scholar]
- 36.Wang H, Chen RY, Ding G, et al. Prevalence and predictors of HIV infection among female sex workers in Kaiyuan City, Yunnan Province, China. Int J Infect Dis. 2009;13:162–69. doi: 10.1016/j.ijid.2008.05.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]