Abstract
Background Accumulating evidence implicates insufficient oxidative capacity in the development of type 2 diabetes. This notion has not been well tested in large, population-based studies.
Methods To test this hypothesis, we assessed the cross-sectional association of plasma lactate, an indicator of the gap between oxidative capacity and energy expenditure, with type 2 diabetes in 1709 older adults not taking metformin, who were participants in the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study.
Results The prevalence of type 2 diabetes rose across lactate quartiles (11, 14, 20 and 30%; P for trend <0.0001). Following adjustment for demographic factors, physical activity, body mass index and waist circumference, the relative odds of type 2 diabetes across lactate quartiles were 0.98 [95% confidence interval (CI) 0.59–1.64], 1.64 (95% CI 1.03–2.64) and 2.23 (95% CI 1.38–3.59), respectively. Furthermore, lactate was associated with higher fasting glucose among non-diabetic adults.
Conclusions Plasma lactate was strongly associated with type 2 diabetes in older adults. Plasma lactate deserves greater attention in studies of oxidative capacity and diabetes risk.
Keywords: Diabetes mellitus, type 2, lactic acid, epidemiology, oxidative phosphorylation, glycolysis, insulin resistance
Introduction
Accumulating evidence implicates insufficient oxidative capacity in the development of insulin resistance and type 2 diabetes.1–20 This evidence includes the association of insulin resistance and type 2 diabetes with maternally inherited forms of diabetes,1,2 increased glycolysis in muscle,3–5 decreased mitochondrial size and density,6–10 decreased oxidative gene expression,10–14 decreased oxidative phosphorylation14–18 and decreased aerobic capacity.13,19,20 However, clinical research on oxidative capacity as a mediator the physiological effects of obesity has been limited by the absence of a marker of oxidative capacity for use in populations.
Blood lactate is a measure of the gap between energy expenditure and oxidative capacity. Lactate is used clinically to indicate energy imbalance associated with vigorous exercise, hypoxia and ischaemia.21,22 Prior work also suggests that lactate is elevated among obese, insulin-resistant subjects23,24 and decreases with weight loss.25 These studies were limited by small, highly selected samples, however. One prospective study further suggested that serum lactate may be an independent risk factor for the development of type 2 diabetes.26 This study included only white men.
Given lactate’s association with insulin resistance and type 2 diabetes in earlier studies and the accumulating evidence linking oxidative capacity to insulin resistance, we hypothesized that decreased oxidative capacity, as assessed by higher levels of plasma lactate, is associated with type 2 diabetes. We therefore examined the cross-sectional association of plasma lactate, obesity and type 2 diabetes in the Atherosclerosis Risk in Communities (ARIC) Carotid MRI (CAR–MRI) study, a community-based cohort of 2066 older white and African American adults.
Material and methods
Study population and design
The ARIC study was initiated in 1987 to study the development and progression of cardiovascular disease in African American and white men and women (n = 15792), aged 45–64 years, selected from four communities in the USA (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN and Washington County, MD).27 The ARIC CAR–MRI study, described herein, enrolled participants from the ARIC cohort, now aged 60–84 years. The study was designed to investigate the genomic, metabolic and cellular correlates of carotid artery atherosclerotic plaque characterized using high-resolution contrast-enhanced magnetic resonance imaging (MRI). The study was approved by the institutional review boards of the participating institutions, and all participants gave their informed consent.
ARIC participants were selected using a stratified sampling design that oversampled those subjects with the thickest carotid artery intima-media thickness (IMT) at the most recent ultrasound examination (visits 3 or 4, 1993–98). Site-specific carotid artery IMT cut-points were adjusted to achieve targeted group sizes of ∼1200 with thick walls (e.g. high IMT) and ∼800 participants without high IMT. The cut-points ranged from 1.00 to 1.28 mm (69th to 73rd percentile) to allow for an approximately equal distribution of participants across field centres. The cut points were chosen in order to maximize the number of individuals with detectable plaque, while still being able to make generalizable inferences to the ARIC base population. A total of 4307 persons were invited to participate. Of these, 1404 refused, 837 were ineligible and 2066 participated (48%). The final ARIC CAR–MRI sample included 1250 high-IMT individuals and 816 individuals randomly sampled from the remainder of the IMT distribution.
The measurement of lactate was approved by the ARIC Steering Committee as an ARIC ancillary study. Non-fasting individuals and individuals with missing variables of interest (e.g. lactate, diabetes and potential confounders) were excluded from the final analysis. Since metformin increases blood lactate levels, an additional 143 subjects with type 2 diabetes (26%) were excluded if they were taking metformin during the 4 weeks prior to the CAR–MRI examination.28 After exclusions, the final analysis sample included 1709 CAR–MRI participants.
Baseline variables and data collection
The core examination procedures are identical to those previously established by ARIC.27 Briefly, a 90-min examination was performed in the morning after a 12-h fast. After informed consent, trained technicians performed anthropometry measurements and obtained urine and blood samples. Blood samples were collected in 10-ml potassium–EDTA (lavender top) tubes (Becton, Dickinson and Company). Following each blood draw, blood was iced immediately and kept between 0 and 8°C prior to centrifugation (3000 g for 20 min at 4°C). Within 30 min of the blood draw, plasma was aliquoted and stored at –70°C at the field centres. Frozen samples where then shipped by Federal Express Priority Overnight mail to the ARIC central laboratories for long-term storage and analysis.
Exposure
Plasma lactate was measured using an enzymatic reaction to convert lactate to pyruvate using a Roche Hitachi 911 auto-analyzer.29 The Roche analyzer uses the enzyme lactate oxidase (LOD) to convert l-lactate to pyruvate and hydrogen peroxide (H2O2):
The hydrogen peroxide subsequently reacts with peroxidase to generate a coloured dye. This is the preferred method because it has better reagent stability than previous enzymatic methods.29
In order to assess the reliability of the lactate measurements, we performed within-visit and repeat-visit assessments of plasma lactate. The quality control analysis of blind replicate samples in 117 pairs demonstrated a within-visit coefficient of reliability of 0.93 with a coefficient of variation of 9.2%. The quality-control analysis of samples drawn from 61 individuals on separate days demonstrated a day-to-day coefficient of repeatability of 0.55 with a coefficient of variation across the two visits of 24.7%. The mean difference between the repeat and study measurement was small (mean = –0.11 mg/dl) and not significantly different from zero [95% confidence interval (CI) –0.82, 0.60 mg/dl]. Furthermore, no systematic bias was detected (test for the proportion of positive differences = 50%, P = 0.694).
Outcome
Type 2 diabetes was defined as a fasting glucose ≥7.0 mmol/l (≥126 mg/dl; reported a minimum of 8 h of fasting prior to visit), a self-reported physician diagnosis, or diabetes treatment in the 4 weeks prior to the clinic visit.
Covariates
Other variables of interest included age, race, sex, ARIC field centre, triglycerides, high-density lipoprotien (HDL) and low-density lipoprotien (LDL) cholesterol, diabetes medication use, body mass index (BMI), waist circumference, prevalent coronary heart disease, smoking status (never, former, current) and leisure-time physical activity. Details have been previously described for measurement of plasma lipids,30 fasting glucose31 and determination of BMI (kg/m2).32 Physical activity was assessed using the Baecke physical activity questionnaire.33 To ascertain medication use, participants were asked to bring containers of current medications to the visit. Fasting insulin was not assessed during this visit.
Statistical analyses
Data are expressed as means and 95% CIs unless otherwise specified. All statistical analyses incorporated the stratified random sampling design, for estimation, testing and CIs, using Stata 9.2.34 Sampling weights were based on the probability of being selected from each field centre based upon the high-IMT status of each participant. The sampling weight among high-IMT subjects was approximately equal to 1 for all field centres. The sampling weights for individuals in the remainder of the IMT distribution varied according to field centre, ranging from 3.9 to 6.1. The advantage of the stratified sampling design is that it allowed for oversampling of high-IMT subjects, while maintaining balanced proportions of subjects from each centre, enabling us to make generalizable inferences to the ARIC base population; however, the precision of the estimates is decreased relative to analysis of the entire study population.
Characteristics of subjects participating in the ARIC CAR–MRI study were first compared across lactate quartiles. To assess for linear trends across quartiles, accounting for the sampling distribution and non-linearity in the lactate distribution, survey-weighted logistic regression and survey-weighted linear regression were utilized, treating the variable of interest as the dependent variable and the median lactate value for each quartile as a continuous independent variable. Multivariate survey-weighted logistic regression analysis was then conducted to assess the cross-sectional association of type 2 diabetes with lactate quartile. Initial models adjusted for ethnicity, gender, age, high-IMT status, ARIC field centre and leisure-time physical activity. BMI and waist circumference were added to the model to assess the impact of adiposity. The final model further adjusted for triglycerides. Subsequent analyses were conducted among non-diabetics to assess the association of lactate with fasting glucose.
Results
Distribution of lactate
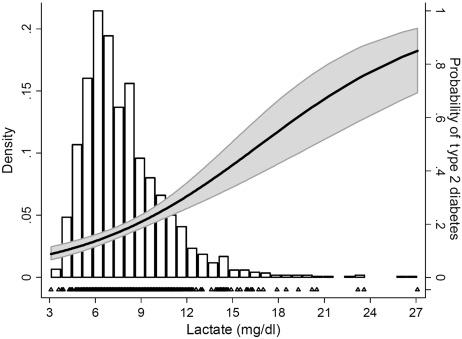
Plasma lactate was distributed approximately log-normally with a median value of 7.2 mg/dl [inter-quartile range (IQR) 5.9–9.0; Figure 1]. Clinically, plasma lactate is routinely used to assess poor tissue oxygenation associated with ischaemic bowel and circulatory collapse. In the presence of these disorders, plasma lactate commonly rises to values >36 mg/dl. By contrast, most ARIC CAR–MRI participants (95.7%) fell well within the normal range (4.5–19.8 mg/dl).35 The distribution of plasma lactate did not differ by age or gender, but did vary by ethnicity [whites 7.1 mg/dl (IQR 5.9–8.7); African Americans 7.7 mg/dl (IQR 6.5–9.9), P < 0.0001].
Figure 1.
Probability of type 2 diabetes across the distribution of blood lactate among 1749 older adults. Bars represent the distribution of lactate. The solid line denotes the predicted probability of type 2 diabetes (right axis); 95% CIs are shaded in grey; Triangles (bottom) represent the individual cases of type 2 diabetes
CAR–MRI study characteristics by lactate quartile
Overall, 56% of ARIC CAR–MRI participants were women, 19% were African American and the mean age was 70.4 years (range 60–84). Higher lactate was associated with both BMI (P < 0.0001) and waist circumference (P < 0.0001) (Table 1). In the first quartile of lactate, 29% of subjects were obese (mean BMI 27.9 kg/m2), whereas 48% of subjects in the fourth quartile were obese (mean BMI 30.4 kg/m2; Ptrend < 0.0001). Higher lactate also was associated with higher triglycerides, lower HDL and a higher prevalence of type 2 diabetes.
Table 1.
Characteristics among 1709 older adults in the ARIC CAR–MRI studya
| Lactate quartile units |
||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall (n = 1709) | <5.9 (n = 378) | 5.9–7.2 (n = 480) | 7.3–9.1 (n = 458) | ≥9.2 (n = 393) | Ptrendb |
| Age (years) | 70.4 (70.0–70.7) | 70.2 (69.5–70.9) | 70.6 (70.0–71.3) | 70.2 (69.6–70.8) | 70.4 (69.7–71.1) | 0.858 |
| Female (%) | 56 | 62 | 53 | 55 | 55 | 0.296 |
| African American (%) | 19 | 12 | 19 | 18 | 28 | <0.0001 |
| BMI (kg/m2) | 28.8 (28.5–29.1) | 27.9 (27.2–28.5) | 28.5 (28.0–29.1) | 28.7 (28.2–29.2) | 30.4 (29.7–31.1) | <0.0001 |
| Obese (≥30 kg/m2) (%) | 35 | 29 | 32 | 34 | 48 | <0.0001 |
| Waist circumference (cm) | 100.9 (100.1–101.7) | 97.2 (95.4–99.0) | 100.7 (99.1–102.3) | 100.9 (99.5–102.3) | 105.2 (103.2–107.1) | <0.0001 |
| Triglycerides | 131 [94–184] | 107 [82–147] | 121 [90–167] | 139 [102–186] | 174 [117–232] | <0.0001 |
| HDL cholesterol (mg/dl) | 50.3 (49.4–51.2) | 54.2 (52.2–56.1) | 50.4 (48.7–52.1) | 48.9 (47.2–50.6) | 47.7 (45.8–49.7) | <0.0001 |
| Trig/HDL ratio | 2.79 [1.75–4.31] | 1.98 [1.40–3.10] | 2.53 [1.64–3.82] | 3.05 [1.87–4.49] | 3.81 [2.30–5.85] | <0.0001 |
| Trig/HDL r: ≥3 (%) | 45 | 27 | 40 | 53 | 63 | <0.0001 |
| Trig/HDL r: ≥3 / ≥2 (%) | 51 | 32 | 46 | 57 | 71 | <0.0001 |
| Glucose (mg/dl) | 106.9 (105.5–108.3) | 101.8 (99.0–104.7) | 104.0 (101.6–106.4) | 107.2 (104.9–109.5) | 115.8 (112.2–119.3) | <0.0001 |
| Type 2 diabetes (%)c | 18 | 12 | 14 | 19 | 30 | <0.0001 |
Trig: Triglyceride.
aResults displayed as percentages, means (95% CIs) and medians [IQRs].
bPtrend takes into account sampling weights for both categorical and continuous variables. Survey-weighted logistic and linear regression were utilized, treating the variable of interest as the dependent variable and the median lactate values by quartile as a continuous independent variable.
cExcluding type 2 diabetics taking metformin.
Association of lactate with prevalent type 2 diabetes
The relationship between lactate and type 2 diabetes is illustrated in Figure 1. A strong, linear and graded association between lactate quartile and prevalent type 2 diabetes was seen, increasing from 12% in the first quartile to 30% in the fourth quartile (Ptrend < 0.0001; Table 2). In the unadjusted logistic model, the fourth quartile of lactate was associated with a 3.25-fold increase in the odds of prevalent type 2 diabetes when compared with the first quartile (95% CI 2.05–5.14). Following adjustment for age, gender, ethnicity, field centre, high-IMT status, prevalent coronary heart disease, smoking status, leisure-time activity, BMI and waist circumference (Model 3), the odds ratio estimates in the second, third and fourth quartiles were somewhat attenuated, but the linear trend remained highly significant (Ptrend < 0.0001): 0.98 (95% CI 0.59–1.64), 1.64 (95% CI 1.03–2.64) and 2.23 (95% CI 1.38–3.59). The linear trend across lactate quartiles remained significant (P = 0.002) after further adjustment for triglycerides. Adjustment for use of anti-hypertensive medications and HMG-CoA reductase inhibitors attenuated the association only slightly (data not shown).
Table 2.
Odds of prevalent type 2 diabetes by plasma lactate quartile among 1709 older adults in the ARIC CAR–MRI studya
| Modelb |
|||||
|---|---|---|---|---|---|
| Lactate quartile units | Diabetes prevalence | 1 | 2 | 3 | 4 |
| <5.9 | 12 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 5.9–7.2 | 14 | 1.23 (0.76–2.01) | 1.10 (0.67–1.79) | 0.98 (0.59–1.64) | 0.94 (0.56–1.57) |
| 7.3–9.1 | 19 | 1.84 (1.15–2.95) | 1.78 (1.12–2.84) | 1.64 (1.03–2.64) | 1.47 (0.91–2.36) |
| ≥9.2 | 30 | 3.25 (2.05–5.14) | 2.74 (1.71–4.37) | 2.23 (1.38–3.59) | 1.82 (1.12–2.95) |
| P (trend)c | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.002 |
aExcluding type 2 diabetics taking metformin.
bModel 1 is the unadjusted model. Model 2 adjusts for age, gender, ethnicity, high-IMT status, ARIC field centre, prevalent coronary heart disease, smoking status and leisure time activity. Model 3 additionally adjusts for BMI and waist circumference. Model 4 further adjusts for triglycerides.
cPtrend was calculated using survey-weighted logistic regression, treating the median lactate values by quartile as a continuous independent variable.
Additional analyses were stratified by ethnicity to assess potential differences in the distribution of lactate by race. Among whites, a strong, linear and graded association between lactate quartile and prevalent type 2 diabetes was seen. No association was seen between lactate quartile and prevalent type 2 diabetes among African Americans. This difference by ethnicity was largely due to a higher prevalence of type 2 diabetes in the first quartile of lactate (33.1%) relative to the second quartile (17.1%) among African Americans.
Analysis of lactate among non-diabetic adults
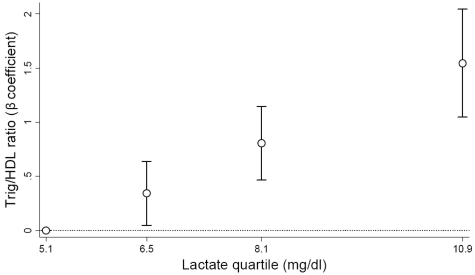
The trends by lactate quartile among non-diabetic adults paralleled those seen in the full cohort (Table 3). BMI, waist circumference and triglycerides all increased with higher lactate levels. In particular, the triglyceride/HDL ratio increased in a graded fashion by lactate quartile, from 1.97 in the first quartile to 3.35 in the fourth quartile (Ptrend < 0.0001). Similarly, the frequency of having a triglyceride/HDL ratio of ≥3, a marker of insulin resistance, increased from 27 to 57% across lactate quartiles (Ptrend < 0.0001). The association between lactate quartile and glucose, triglyeride and triglyceride/HDL ratios remained after adjustment for BMI and waist circumference (Table 3, last column). Furthermore, the association was minimally attenuated in the model adjusting for demographic factors, high IMT, BMI and fasting glucose level (Figure 2). Unlike the heterogeneity seen in the association of lactate with prevalent type 2 diabetes across ethnic groups, the association of lactate with triglyceride/HDL ratio among non-diabetic individuals was strong and linear for African Americans and whites. Parallel analyses with lactate and fasting glucose showed that lactate levels were lowest among subjects with normal fasting glucose levels, intermediate among subjects with impaired fasting glucose (100–125 mg/dl), and highest among subjects with type 2 diabetes in both whites and African Americans (data not shown).
Table 3.
Characteristics among 1388 non-diabetic adults in the ARIC CAR–MRI studya
| Lactate quartile units |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Overall (n = 1388) | <5.9 (n = 335) | 5.9–7.2 (n = 409) | 7.3–9.1 (n = 367) | ≥9.2 (n=277) | Ptrendb | Ptrend adjustedc |
| Age (years) | 70.2 (69.9–70.6) | 70.1 (69.3–70.8) | 70.4 (69.7–71.1) | 70.1 (69.4–70.8) | 70.3 (69.5–71.1) | 0.791 | 0.528 |
| Female (%) | 57 | 63 | 52 | 59 | 55 | 0.287 | 0.678 |
| African American (%) | 16 | 9 | 18 | 16 | 24 | <0.0001 | 0.002 |
| BMI (kg/m2) | 28.3 (28.0–28.6) | 27.4 (26.8–28.1) | 28.2 (27.6–28.8) | 28.3 (27.8–28.8) | 29.6 (28.8–30.4) | <0.0001 | – |
| Obese (≥30 kg/m2) (%) | 31 | 26 | 30 | 31 | 42 | 0.001 | – |
| Waist circumference (cm) | 99.3 (98.4–100.2) | 95.8 (94.0–97.6) | 99.7 (98.0–101.4) | 99.6 (98.1–101.2) | 102.8 (100.5–105.1) | <0.0001 | – |
| Triglycerides | 127 [92–177] | 107 [82.5–147] | 120 [89–164] | 138 [101–181] | 161 [107–218] | <0.0001 | <0.0001 |
| HDL cholesterol (mg/dl) | 51.5 (50.4–52.5) | 54.3 (52.4–56.3) | 50.9 (49.0–52.7) | 50.5 (48.6–52.4) | 49.9 (47.4–52.4) | 0.011 | 0.278 |
| Trig/HDL ratio | 2.60 [1.68–3.97] | 1.97 [1.39–3.10] | 2.49 [1.59–3.64] | 2.95 [1.87–4.18] | 3.35 [2.10–5.07] | <0.0001 | <0.0001 |
| Trig/HDL r: ≥3 (%) | 42 | 27 | 37 | 50 | 57 | <0.0001 | <0.0001 |
| Trig/HDL r: ≥3/≥2 (%) | 46 | 29 | 43 | 53 | 63 | <0.0001 | <0.0001 |
| Glucose (mg/dl) | 99.8 (99.1–100.5) | 97.2 (95.8–98.6) | 100.0 (98.6–101.4) | 100.8 (99.5–102.1) | 101.5 (99.8–103.3) | <0.0001 | 0.010 |
Trig: Triglyceride.
aResults displayed as percentages, means (95% CIs) and medians [IQRs].
bPtrend takes into account sampling weights for both categorical and continuous variables. Survey-weighted logistic and linear regression were utilized, treating the variable of interest as the dependent variable and the median lactate values by quartile as a continuous independent variable.
cPtrend after adjustment for BMI and waist circumference.
Figure 2.
Association of triglyceride–HDL ratio with plasma lactate quartile among non-diabetics. Beta coefficients are adjusted for age, sex, high-IMT status, field centre, BMI and fasting glucose. Lactate quartiles are represented by the median lactate value within each quartile
Discussion
Our results show that lactate’s association with prevalent type 2 diabetes is strong, graded and independent. The graded association occurred across the normal clinical range of lactate values. The strength of the association was robust and independent of measures of adiposity. Among non-diabetics, plasma lactate was associated with higher fasting glucose, triglycerides and triglyceride–HDL ratio, a marker of insulin resistance.36,37
Our results are consistent with previous studies reporting an association between lactate, adiposity and type 2 diabetes. Small clinical studies have shown that lactate is higher among obese subjects23,24 and decreases with weight loss.25 Work by Chen and colleagues demonstrated that lactate is low in lean subjects, higher in non-diabetic obese subjects and still higher in obese diabetics.38 In two small, cross-sectional studies, lactate was correlated with insulin resistance independent of obesity.39,40 In a longitudinal analysis of Swedish men, elevated serum lactate was associated with a 2.4-fold higher incidence of type 2 diabetes.26 The association was attenuated after adjusting for BMI and other factors, however, and lactate was not included in the final model based on a step-wise regression analysis.26 Taken together, these studies suggest that lactate is associated with both obesity and its downstream complications including insulin resistance and type 2 diabetes. Although compelling, these findings were from small clinical studies or of limited generalizability. Our study is the largest population-based study to date investigating the association of lactate and type 2 diabetes. Strengths of our study include the large community-based sample of white and African American men and women, and extensive data on potential confounders.
Adipose tissue is responsible for a large portion of the lactate produced in obesity.25,41 Among obese subjects, decreased blood flow to adipose tissue leads to local hypoxia and increased lactate production.42 Furthermore, adipocyte production of lactate increases as adipocyte size increases,24,41,43 approaching the diffusion limit of oxygen.42 Therefore, decreased oxygen availability in adipocytes may drive a major portion of the excess lactate production associated with obesity. There is also evidence that hypoxia drives adipocytokine dysregulation42 and decreased insulin signalling44 in adipocytes from obese individuals. In this study, lactate’s association with type 2 diabetes was independent of BMI, however, suggesting that lactate is not solely a marker of adiposity. Alternatively, blood lactate may indicate the activity of adipose tissue, or its degree of hypoxia and decreased oxidative capacity, which is not captured by BMI and other measures of adiposity.
Oxidative capacity may also be decreased in insulin-resistant skeletal muscle. The evidence supporting this notion includes the association of insulin resistance and type 2 diabetes with increased glycolysis in muscle,3–5 decreased mitochondrial size and density,6–10 decreased oxidative gene expression,10–14 decreased oxidative phosphorylation14–18 and decreased aerobic capacity.13,19,20 The decrease in oxidative capacity may account for the markedly altered lactate metabolism in insulin-resistant muscle, where lactate concentration is increased and the lactate–pyruvate interconversion rates are enhanced as much as 3- to 4-fold.45,46 The expression of the lactate transporter monocarboxylate transport protein 1 (MCT1) is altered as well.46 In skeletal muscle, lactate functions to shuttle oxidative precursors from glycolytic fibres to oxidative fibers.47 MCT4 facilitates lactate’s transfer out of glycolytic fibres and MCT1 facilitates lactate’s transfer into oxidative fibres where it is oxidized.48 The expression of MCT1 is markedly decreased in insulin-resistant muscle.46 Although the reason for the decreased expression is unknown, these findings are consistent with a decreased capacity to oxidize exogenous lactate in oxidative fibres, leading to decreased expression of MCT1. Taken together, the association of blood lactate with type 2 diabetes may result from a global decrease in oxidative capacity which leads to altered lactate metabolism in insulin-resistant muscle and increased lactate release from adipose tissue.
Lactate’s association with type 2 diabetes may also be causal. DiGirolamo and colleagues have argued that the elevated lactate associated with obesity may be at least partially responsible for insulin resistance.24 Elevated lactate may promote hepatic gluconeogenesis and interfere with glucose uptake in muscle by substituting for glucose utilization.49 The evidence in support of this notion is mixed; lactate infusion decreases glucose oxidation, for example, but does not increase the rate of glucose production.50 Lactate’s potential causal role in insulin resistance needs further study.
Several limitations of our study deserve mention. First, because our study was cross-sectional, it was not possible to determine whether elevated lactate is a cause or consequence of type 2 diabetes. Therefore, we cannot rule out the possibility that type 2 diabetes leads to higher lactate levels. However, lactate was associated with markers of fasting glucose among non-diabetics, suggesting that lactate elevation is not just a consequence of type 2 diabetes. A specific mechanism potentially linking diabetes with elevated lactate is poor tissue oxygenation due to cardiovascular disease in diabetic participants. Adjustment for prevalent coronary heart disease, however, attenuated the association between lactate and prevalent diabetes only slightly. Second, lactate can be artificially elevated because of ongoing glycolysis following the blood draw. This effect was minimized through the rapid processing and cooling of blood samples and would have biased our results toward the null; it is highly unlikely that glycolysis in the samples would produce the large and graded associations here described. Third, the day-to-day repeatability of resting blood lactate is moderate, reflecting known diurnal variation in lactate levels and sensitivity of lactate to changes in the metabolic state. Despite this variation, lactate was strongly associated with type 2 diabetes. Finally, we recognize that lactate is only an indirect indicator of oxidative capacity.
Our results show that plasma lactate is strongly associated with prevalent type 2 diabetes among non-diabetics. The association of lactate with type 2 diabetes supports the potential role of decreased oxidative capacity in the aetiology of insulin resistance. Plasma lactate deserves greater attention in epidemiologic and physiologic studies of oxidative capacity and diabetes risk. Further work must be carried out to reassess the prospective association of plasma lactate and type 2 diabetes in a modern cohort. If confirmed, blood lactate measurement could be used as a marker of oxidative capacity in clinical and population studies.
Funding
National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases T32 training grant to S.C. J.H.Y. was supported by National Institutes of Health through a research career award, American Heart Association through a scientist development award, and the American Diabetes Association through an Innovation Award. The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021 and N01-HC-55022.
KEY MESSAGES.
Insufficient oxidative capacity, also referred to as mitochondrial dysfunction, may play a role in the development of type 2 diabetes.
Blood lactate, a commonly used marker of decreased tissue oxygenation in circulatory collapse and of oxidative capacity during exercise, may be a good indicator of oxidative capacity at rest.
In a large population-based sample of older adults, blood lactate’s association with prevalent type 2 diabetes was strong, graded and independent of measures of obesity. The graded association occurred across the normal range of lactate values and supports the notion that decreased oxidative capacity is related to type 2 diabetes.
Acknowledgements
The authors thank the staff of the ARIC study for their important contributions.
Conflict of interest: None declared.
References
- 1.Gerbitz KD, Gempel K, Brdiczka D. Mitochondria and diabetes—genetic, biochemical, and clinical implications of the cellular energy circuit. Diabetes. 1996;45:113–26. doi: 10.2337/diab.45.2.113. [DOI] [PubMed] [Google Scholar]
- 2.Robinson BH. Lactic acidemia and mitochondrial disease. Mol Genet Metab. 2006;89:3–13. doi: 10.1016/j.ymgme.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–78. [PubMed] [Google Scholar]
- 4.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–15. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 5.Del PS, Bonadonna RC, Bonora E, et al. Characterization of cellular defects of insulin action in type 2 (non-insulin-dependent) diabetes mellitus. J Clin Invest. 1993;91:484–94. doi: 10.1172/JCI116226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–42. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 8.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288:E818–25. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 9.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–94. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 10.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–33. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 12.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 14.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Short KR, Nair KS, Stump CS. Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:2419–21. doi: 10.1056/NEJM200406033502320. [DOI] [PubMed] [Google Scholar]
- 16.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce CR, Anderson MJ, Carey AL, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88:5444–51. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- 18.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–87. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 19.Wisloff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–20. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson KF, Lindgarde F. Impaired glucose tolerance in a middle-aged male urban population: a new approach for identifying high-risk cases. Diabetologia. 1990;33:526–31. doi: 10.1007/BF00404139. [DOI] [PubMed] [Google Scholar]
- 21.Jansen TC, van BJ, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37:2827–39. doi: 10.1097/CCM.0b013e3181a98899. [DOI] [PubMed] [Google Scholar]
- 22.Sabatine MS, Liu E, Morrow DA, et al. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation. 2005;112:3868–75. doi: 10.1161/CIRCULATIONAHA.105.569137. [DOI] [PubMed] [Google Scholar]
- 23.Doar JW, Wynn V, Cramp DG. Blood pyruvate and plasma glucose levels during oral and intravenous glucose tolerance tests in obese and non-obese women. Metabolism. 1968;17:690–701. doi: 10.1016/0026-0495(68)90053-x. [DOI] [PubMed] [Google Scholar]
- 24.DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 1992;6:2405–12. doi: 10.1096/fasebj.6.7.1563593. [DOI] [PubMed] [Google Scholar]
- 25.DiGirolamo M, Newby FD, Hill JO. Blood lactate levels in human obesity (Abstract) Int J Obes. 1989;13:394. [Google Scholar]
- 26.Ohlson LO, Larsson B, Bjorntorp P, et al. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. 1988;31:798–805. doi: 10.1007/BF00277480. [DOI] [PubMed] [Google Scholar]
- 27.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 28.Davis TM, Jackson D, Davis WA, Bruce DG, Chubb P. The relationship between metformin therapy and the fasting plasma lactate in type 2 diabetes: The Fremantle Diabetes Study. Br J Clin Pharmacol. 2001;52:137–44. doi: 10.1046/j.0306-5251.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barhan D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 97. 1972;142 doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 30.ARIC Coordinating Center. Atherosclerosis Risk in Communities Study Protocol: Lipid and Lipoprotein Determinations, Manual 8. ARIC study website; 1994. http://www.cscc.unc.edu/aric/pubuse/manual/Lipid_and_Lipoprotein_Determinations.1_8.pdf. [Google Scholar]
- 31.ARIC Coordinating Center. Atherosclerosis Risk in Communities Study Protocol: Clinical Chemistry Determinations, Manual 10. ARIC study website; 1991. http://www.cscc.unc.edu/aric/pubuse/manual/Clinical_Chemistry_Determinations.1_10.pdf. [Google Scholar]
- 32.ARIC Coordinating Center. Atherosclerosis Risk in Communities Study Protocol: Cohort Component Procedures, Manual 2. ARIC study website; 1997. http://www.cscc.unc.edu/aric/pubuse/manual/Cohort_Procedures.1_2.pdf. [Google Scholar]
- 33.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 34.UCLA Academic Technology Services. Analyzing Data with a Stratified Sampling Design: Stata 9. Stata Computing, College Station, Texas, 2006. [Google Scholar]
- 35.Bakerman S. ABC'S of Interpretive Laboratory Data. 2nd. (Eight printing editions) Interpretive Laboratory Data, Inc., Greenville, NC, 1984; 271. [Google Scholar]
- 36.Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med. 1992;231:25–30. doi: 10.1111/j.1365-2796.1992.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–9. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 38.Chen YD, Varasteh BB, Reaven GM. Plasma lactate concentration in obesity and type 2 diabetes. Diabetes Metab. 1993;19:348–54. [PubMed] [Google Scholar]
- 39.Lovejoy J, Newby FD, Gebhart SS, DiGirolamo M. Insulin resistance in obesity is associated with elevated basal lactate levels and diminished lactate appearance following intravenous glucose and insulin. Metabolism. 1992;41:22–27. doi: 10.1016/0026-0495(92)90185-d. [DOI] [PubMed] [Google Scholar]
- 40.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–24. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 41.Jansson PA, Smith U, Lonnroth P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia. 1990;33:253–56. doi: 10.1007/BF00404805. [DOI] [PubMed] [Google Scholar]
- 42.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 43.Sandqvist MM, Eriksson JW, Jansson PA. Increased lactate release per fat cell in normoglycemic first-degree relatives of individuals with type 2 diabetes. Diabetes. 2001;50:2344–48. doi: 10.2337/diabetes.50.10.2344. [DOI] [PubMed] [Google Scholar]
- 44.Regazzetti C, Peraldi P, Gremeaux T, et al. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58:95–103. doi: 10.2337/db08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avogaro A, Toffolo G, Miola M, et al. Intracellular lactate- and pyruvate-interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J Clin Invest. 1996;98:108–15. doi: 10.1172/JCI118754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juel C, Holten MK, Dela F. Effects of strength training on muscle lactate release and MCT1 and MCT4 content in healthy and type 2 diabetic humans. J Physiol. 2004;556(Pt 1):297–304. doi: 10.1113/jphysiol.2003.058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philp A, Macdonald AL, Watt PW. Lactate—a signal coordinating cell and systemic function. J Exp Biol. 2005;208(Pt 24):4561–75. doi: 10.1242/jeb.01961. [DOI] [PubMed] [Google Scholar]
- 48.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–64. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 49.Consoli A, Nurjhan N, Reilly JJ, Jr, Bier DM, Gerich JE. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990;86:2038–45. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller BF, Fattor JA, Jacobs KA, et al. Metabolic and cardiorespiratory responses to "the lactate clamp". Am J Physiol Endocrinol Metab. 2002;283:E889–98. doi: 10.1152/ajpendo.00266.2002. [DOI] [PubMed] [Google Scholar]