Abstract
Background Domestic and international wars continue to be pervasive in the 21st century. This study summarizes the effects of war-related stress on all-cause mortality using meta-analyses and meta-regressions.
Methods A keyword search was performed, supplemented by extensive iterative hand-searches for observational studies of war-related stress and mortality. Two hundred and twenty mortality risk estimates from 30 studies were extracted, providing data on more than 9 million persons.
Results The mean hazard ratio (HR) was 1.05 [95% confidence interval (CI) 0.98–1.13] among HRs adjusted for age and additional covariates. The mean effect for men was 1.14 (CI 1.00–1.31), and for women it was 0.92 (CI 0.66–1.28). No differences were found for various follow-up durations or for various types of war stress. Neither civilians nor military personnel had an elevated mortality risk. Those exposed to a combat zone during the Vietnam War had a slightly higher chance of death (HR 1.11; 95% CI 1.00–1.23).
Conclusions The results show that, over all, exposure to war-stress did not increase the risk of death when studies were well controlled. Effects were small when found. This lack of substantial effect may be the result of selection processes, developed resiliency and/or institutional support.
Keywords: Meta-analysis, systematic review, psychological stress, combat-neurosis, mortality determinants
Introduction
On 7 October 2001, less than 1 month after the 11 September attacks, the USA launched Operation Enduring Freedom and invaded Afghanistan. A year and a half later a multinational force led by the USA and the UK invaded Iraq. The two wars continue at present, generating loss of human life, and exposing millions to the stress of the conflicts. According to recent estimates, there are more than 300 000 US veterans from both wars, with more than 4000 casualties and 30 000 wounded among US troops in the Iraq War alone. Although fiercely debated, sources estimate the number of violent deaths among Iraqis to be at 100 000 to more than 1 million.1 The wars in Afghanistan and Iraq (especially the latter) have also generated millions of refugees and internally displaced persons. Recent reports by the UN estimate that there are up to 4.7 million Iraqis who have become uprooted, more than 2 million being refugees in other countries, the rest displaced within Iraq.2
The wars in Iraq and Afghanistan, as well as in other places around the globe, make studies of the impact of war-related stress on mortality especially important. Former studies have covered both civilians and military personnel and examined myriad stress types, including whether a person spent time in a combat zone,3–6 was a prisoner of war,7–10 had been confined in a concentration camp,11–13 lost family members during a conflict14 or became a refugee.15 The conflicts that were studied include World War II,16–18 the Korean7 and Vietnam Conflicts,19–21 the 1982 Lebanon War,14 the 1991 Iraq War22–24 and the Balkan Wars of the mid-1990s.25
Despite this growing literature, a meta-analysis of the effect of war stress on mortality has not been conducted. Such an analysis is especially needed given the large number of active militarized conflicts, which continue to expose both military and civilian populations to the stress of war. Furthermore, while theory suggests that stressful experiences have deleterious health effects and lead to higher mortality rates, research on war stress and all-cause mortality has not provided homogeneous results. Some studies suggest that the experience of war stress has a clear negative impact on life span.4,9,14 However, other studies could not find a significant effect,16,19,24 and some have even found lower (although mostly not statistically significant) death rates among those who experienced war-related stress.11,17,26 Meta-analysis is well suited to address this research problem and examine whether, and under which conditions, war stress has an effect on the risk of mortality.
We have conducted meta-analyses and meta-regressions based on an extensive literature search. Meta-analyses by gender, age group, duration of follow-up, type of respondent (i.e. civilian vs military), type of war stress and conflict are presented. In addition, multivariate meta-regression analyses were conducted in an effort to assess various study-level factors that affect the estimated magnitude of the risk.
Search strategy and coding procedures
In June 2005, we conducted a sensitive search of electronic bibliographic databases to retrieve publications concerning psychosocial stress, including war-related stress and all-cause mortality. The full search algorithm is available from the authors upon request. We identified 1570 publications from MEDLINE, CINAHL, EMBASE, PsycINFO and ISI Web of Science. Using these results as a base, we iteratively hand-searched the bibliographies of eligible publications, the lists of sources citing an eligible publication, and the sources identified as ‘similar to’ an eligible publication. We exhausted the literature after eight iterations. We re-ran the electronic keyword searches in July 2008 and completed the search and coding stages in January 2009.
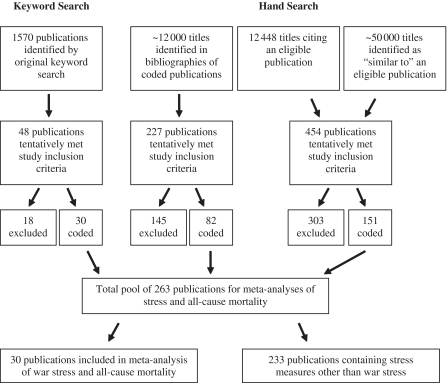
Two authors (E.S. and D.R.) determined publication eligibility, extracted the data from the articles, and assessed study quality. Unpublished work encountered was considered for study inclusion. Figure 1 summarizes the number of publications considered at each step of the search process. The full database contains 262 publications examining the effects of various stressful events on all-cause mortality. To evaluate coding accuracy, we randomly selected and recoded 40 of these publications (including 446 point estimates). Of the point estimates, 98.65% were error-free. The present analysis uses the subset of articles (n = 30) that reported the effect of war-related stress on all-cause mortality. Of these publications, 28 appeared in peer-reviewed journals and 2 appeared in state and governmental records (Table 1).
Figure 1.
Search strategy and yield
Table 1.
Studies included in the analysis
| Source | Data source | Country | War | Sample size | Years | Exposure type | Comparison group | Average HR | Number of HRs |
|---|---|---|---|---|---|---|---|---|---|
| Adena et al.4 | Australian army personnel files | Australia | Vietnam | 44 882 | 1966–1982 | Combat zone | Other military | 1.29 | 1 |
| Anderson et al.19 | National Personnel Records Center | USA | Vietnam | 122 238 | 1975–1984 | Combat zone | Other military | 1.01 | 2 |
| Ayalon and Covinsky16 | Original data | Israel | World War II | 4179 | 1997–2004 | European Jew exposed to Nazi Regime | Jews outside of Nazi Europe | 1.04 | 2 |
| Boehmer et al.6 | National Personnel Records Center | USA | Vietnam | 18 313 | 1964–2000 | Combat zone | Other military | 1.15 | 8 |
| Boscarino3 | Vietnam Experience Study | USA | Vietnam | 15 288 | 1985–2000 | Combat zone (high) | Combat zone (low) | 1.20 | 1 |
| Boyle20 | National Personnel Records Center | USA | Vietnam | 18 313 | 1965–1983 | Combat zone | Other military | 1.23 | 5 |
| Collins et al.11 | Kiryat Yovel Community Health Study | Israel | World War II | 774 | 1985–1996 | Concentration camp | Jews outside of Nazi Europe | 0.84 | 6 |
| Costa10 | Union army pension records | USA | US Civil | 1447 | 1868–1986 | Prisoner of war | Other military | 1.73 | 1 |
| Dent et al.9 | Australian army personnel files | Australia | World War II | 1705 | 1945–1983 | Prisoner of war | Other military | 1.57 | 32 |
| DesMeules et al.15 | Longitudinal Immigration Database | Canada | Various conflicts | 369 936 | 1980–1998 | Refugee | Non-refugee | 1.23 | 16 |
| Eitinger12 | Original data | Norway | World War II | 4570 | 1946–1966 | Concentration camp | General population | 1.16 | 5 |
| Fett et al.39 | Australian Veterans Health Studies Mortality Studies | Australia | Vietnam | 44 882 | 1966–1982 | Combat zone | Other military | 1.22 | 15 |
| Fett et al.40 | Australian army personnel files | Australia | Vietnam | 46 166 | 1966–1982 | Combat zone | Other military | 1.23 | 16 |
| Gale et al.17 | War Pensions Agency | UK | World War II | 11 134 | 1952–1997 | Prisoner of war | General population | 0.75 | 4 |
| Hearst et al.21 | Defense Manpower Data Center | USA | Vietnam | 14 145 | 1970–1983 | High draft number for Vietnam | Low draft number | 1.04 | 1 |
| Kang and Bullman5 | National Personnel Records Center | USA | First Gulf War | 1 441 807 | 1991–1993 | Combat zone | Other military | 1.17 | 6 |
| Kang and Bullman22 | National Personnel Records Center | USA | First Gulf War | 1 368 150 | 1991–1997 | Combat zone | Other military | 0.96 | 12 |
| Kark et al.23 | Census, 1991 | Israel | First Gulf War | 5 059 000 | 1991–1991 | War zone exposure | No war zone exposure | 1.42 | 4 |
| Keehn8 | National Personnel Records Center | USA | World War II | 19 066 | 1946–1976 | Prisoner of war | Other military | 1.01 | 35 |
| Macfarlane et al.24 | UK Ministry of Defence | UK | First Gulf War | 106 924 | 1991–9199 | Combat zone | Other military | 1.00 | 9 |
| Macfarlane et al.41 | UK Ministry of Defence | UK | First Gulf War | 53 462 | 1991–2004 | Combat zone | Other military | 1.03 | 1 |
| Mollica et al.25 | Original data | Croatia | Balkan Wars | 529 | 1996–1999 | Civilians held in captivity | Other civilians | 1.32 | 4 |
| Nefzger7 | National Personnel Records Center | USA | World War II and Korea | 19 158 | 1942–1965 | Prisoner of war | Other military | 1.06 | 16 |
| O'Toole et al.42 | Australian Veterans Health Studies Mortality Studies | Australia | Vietnam | 2309 | 1965–1982 | Combat zone | Other military | 1.29 | 1 |
| Sibai et al.14 | Original data | Lebanon | 1982 Lebanese War | 300 | 1983–1994 | Loss of family or property | Other civilians with no losses | 2.12 | 6 |
| Stessman et al.13 | Jerusalem Longitudinal Cohort Study | Israel | World War II | 458 | 1990–1998 | Concentration camp | Jews outside of Nazi Europe | 1.09 | 4 |
| Thomas et al.26 | National Personnel Records Center | USA | Vietnam | 9568 | 1965–1987 | Combat zone | Other military | 0.80 | 2 |
| Visintainer43 | Michigan Department of Management and Budget’s Vietnam-era bonus list | USA | Vietnam | 377 028 | 1974–1989 | Combat zone | Other military | 0.91 | 1 |
| Watanabe and Kang44 | National Personnel Records Center | USA | Vietnam | 20 062 | 1973–1991 | Combat zone | Other Military | 1.15 | 2 |
| Williams et al.18 | Israeli Ischemic Heart Disease Study | Israel | World War II | 3012 | 1965–1986 | Concentration camp | Not in camp | 1.03 | 2 |
Methods and inclusion criteria
For the present analyses, a study was included if a clear comparison was made between a group of people that experienced war-related stress and another group that either did not experience war stress at all or experienced it to a lesser degree. As shown in Table 1, most of the studies examined war stress among combatants, a group that is predominately male, younger, healthier, and of lower socio-economic status than the general population. Therefore, publications that compared combatants to civilians or to the general population were excluded from the study. The two most studied types of war stress were having been in a combat zone and having been held captive during a conflict.
Statistical methods varied from study to study, necessitating the conversion of odds ratios (OR), rate ratios, standardized mortality ratios, relative risks and hazard ratios (HRs) into a common form (see Section 1 of Supplementary Appendix 1, available at IJE online). All non-HR point estimates were converted to HRs (the most frequently reported type). As is standard practice, we used the standard errors (SEs) reported in the publications to calculate the inverse variance weights (see Section 2 of Supplementary Appendix 2, available at IJE online).
Q-test results from preliminary analyses revealed substantial heterogeneity across studies. Because of this, all meta-analyses and meta-regressions were calculated by maximum likelihood using a random effects model. For a clear introduction on the logic and use of these methods, see Lipsey and Wilson.27 For more details on the specific procedures adopted in the current research, see Roelfs et al. (2010)28. Analysis was performed with Statistical Package for the Social Sciences 16.0 using matrix macros provided by Lipsey and Wilson.27 The possibility of selection bias was examined using a funnel plot of the log HRs against sample size. Analyses performed include meta-analyses of subgroups and multivariate meta-regressions. The following covariates were used in the analyses: (i) a variable indicating if the SE was estimated; (ii) proportion of respondents who were male; (iii) mean age of sample at baseline; (iv) time elapsed between the end of baseline and the beginning of follow-up; (v) follow-up duration; (vi) the respondent’s identity (military or civilian); (vii) the type of war stress; (viii) the conflict in which the respondent participated; (ix) the country in which the respondent resided; and (x) a series of variables indicating whether gender, age, and health were statistically controlled.
Results
Table 2 provides descriptive statistics on the 220 HRs included in this study. Data were obtained from 30 studies covering eight countries, published between 1973 and 2008, and representing more than 9 million persons. The majority of persons analysed were men. The median follow-up duration across all studies was 14 years.
Table 2.
Distribution of mortality risk estimates in the analyses by selected variables
| Variable | Distribution |
|---|---|
| Publication date | |
| 1970–79 | 9.5 |
| 1980–89 | 49.1 |
| 1990–99 | 10.0 |
| 2000–08 | 31.4 |
| Statistical adjustment level | |
| Unadjusted | 45.9 |
| Adjusted for age only | 39.5 |
| Adjusted multiple covariates | 14.5 |
| Sex | |
| Women | 7.7 |
| Men | 70.5 |
| Both (predominantly men) | 21.8 |
| Mean age at baseline (years) | |
| <40 | 73.2 |
| 40–64 | 11.8 |
| ≥65 | 15.0 |
| Comparison group | |
| General population | 4.1 |
| Appropriate comparison group | 95.9 |
| Nation where study was done | |
| USA | 41.8 |
| UK | 6.4 |
| Australia | 29.5 |
| Canada | 7.3 |
| Croatia | 1.8 |
| Israel | 8.2 |
| Lebanon | 2.7 |
| Norway | 2.3 |
| Conflict | |
| US Civil War | 0.5 |
| World War II | 46.4 |
| Korean War | 1.8 |
| Vietnam War | 25.0 |
| 1982 Lebanon War | 2.7 |
| 1991 Gulf War | 14.5 |
| Balkan Wars | 1.8 |
| Unknown conflict (Canadian refugees) | 7.3 |
| Type of conflict exposure | |
| Concentration camp | 6.8 |
| Severe conflict experience (civilians) | 12.6 |
| Prisoner of War | 31.4 |
| Severe combat exposure (military) | 6.9 |
| Military service in conflict zone | 42.3 |
| Identity of respondent | |
| Military | 77.3 |
| Civilian | 22.7 |
| Follow-up duration (years) | |
| First quartile | 11.6 |
| Median | 19.0 |
| Third quartile | 30.0 |
| Standard error estimated? | |
| Yes | 61.8 |
| No | 38.2 |
All numbers shown are percentages (n = 219) unless otherwise indicated.
Table 3 presents the meta-analyses results (see Table 4 for sample size and heterogeneity test information). All analyses were stratified by level of statistical adjustment. The maximum likelihood random effects variance component for the meta-analysis of all 220 HRs was significant (P < 0.001), indicating that important moderating variables exist and supporting the decision to use random effects models and conduct subgroup meta-analyses. However, as shown by the results of the Q-tests in Table 4, heterogeneity was adequately accounted for by the use of a random effects model. Since the discussion of the meta-analysis focused on HRs adjusted for age and additional covariates, the results presented below are clearly not an artefact of heterogeneity in the data.
Table 3.
Meta-analyses of war-related stress and all-cause mortality
| Unadjusted | Adjusted for age only | Adjusted for age and additional covariatesa | |
|---|---|---|---|
| All available data | 1.07 (1.00, 1.15) | 1.08* (1.00, 1.16) | 1.05 (0.98, 1.13) |
| Non-estimated SE only | 1.08* (1.01, 1.16) | 0.98 (0.90, 1.08) | 1.05 (0.98, 1.13) |
| Sex | |||
| Women | 0.94 (0.70, 1.26) | 1.35** (1.11, 1.65) | 0.92 (0.66, 1.28) |
| Men | 1.15* (1.03, 1.27) | 1.03 (0.94, 1.13) | 1.14* (1.00, 1.31) |
| Time elapsed between conflict and end of follow-up (years) | |||
| ≤5 | 1.03 (0.89, 1.19) | 1.29*** (1.11, 1.50) | 1.01 (0.90, 1.14) |
| 5.1–15 | 1.07 (0.95, 1.19) | 1.10 (0.96, 1.27) | 1.08 (0.93, 1.25) |
| >15 | 1.09 (0.99, 1.20) | 0.97 (0.88, 1.07) | 1.07 (0.96, 1.18) |
| Respondent type | |||
| Civilian | 1.15 (0.98, 1.35) | 1.27*** (1.15, 1.40) | 1.00 (0.86, 1.16) |
| Military | 1.05 (0.98, 1.12) | 0.91 (0.83, 1.00) | 1.06 (0.99, 1.14) |
| Type of war stress | |||
| Concentration camp | 1.03 (0.78, 1.34) | 0.93 (0.64, 1.36) | 0.96 (0.81, 1.15) |
| Prisoner of war | 1.22 (0.80, 1.86) | 0.82*** (0.75, 0.91) | 1.73 (0.97, 3.09) |
| Other severe conflict (civilian or military) | 1.17 (0.97, 1.40) | 1.30*** (1.14, 1.49) | 1.10 (0.92, 1.31) |
| Military service in conflict zone | 1.05 (0.99, 1.11) | 1.18*** (1.07, 1.29) | 1.04 (0.98, 1.11) |
| Conflict | |||
| US Civil War | … | … | 1.73 (0.97, 3.08) |
| World War II | 1.06 (0.90, 1.25) | 0.84*** (0.78, 0.92) | 0.99 (0.86, 1.13) |
| Korean War | … | 0.94 (0.54, 1.62) | … |
| Vietnam War | 1.08 (1.00, 1.17) | 1.13 (0.97, 1.32) | 1.11* (1.00, 1.23) |
| 1982 Lebanon War | 1.34* (1.00, 1.78) | 4.03** (1.58, 10.26) | … |
| 1991 Gulf War | 1.01 (0.93, 1.10) | 1.28*** (1.11, 1.48) | 1.01 (0.94, 1.09) |
| Balkan Wars | 1.20 (0.71, 2.02) | … | 1.63 (0.72, 3.67) |
| Unknown (Canadian refugees) | … | 1.36*** (1.17, 1.57) | … |
All meta-analyses calculated by maximum likelihood using a random effects model. See Table 4 for information on sample sizes for each analysis. Numbers reported are the mean HR (95% CI). Ellipses indicate situations where n ≤ 1 and a meaningful mean HR could not be calculated.
aThe number and type of covariates vary between studies.
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Table 4.
Number of HRs analysed in the meta-analyses reported in Table 3 and Q-tests
|
Unadjusted |
Adjusted for age only |
Adjusted for age and additional covariates |
||||
|---|---|---|---|---|---|---|
| n | Q-test P-value | n | Q-test P-value | n | Q-test P-value | |
| All available data | 101 | 0.999 | 87 | 0.668 | 32 | 0.999 |
| Non-estimated SE only | 39 | 0.971 | 13 | 0.000 | 32 | 0.998 |
| Sex | ||||||
| Women | 4 | 0.064 | 11 | 0.052 | 2 | 0.905 |
| Men | 76 | 0.999 | 68 | 0.968 | 11 | 0.823 |
| Time elapsed between conflict and end of follow-up | ||||||
| ≤5 years | 11 | 0.965 | 21 | 0.725 | 7 | 0.847 |
| 5.1–15 years | 66 | 0.999 | 41 | 0.999 | 7 | 0.767 |
| >15 years | 24 | 0.982 | 25 | 0.003 | 18 | 0.987 |
| By respondent type | ||||||
| Civilian | 9 | 0.558 | 30 | 0.173 | 11 | 0.976 |
| Military | 92 | 0.999 | 57 | 0.980 | 21 | 0.958 |
| By type of war stress | ||||||
| Concentration camp | 1 | … | 7 | 0.994 | 7 | 0.961 |
| Prisoner of war | 34 | 0.999 | 34 | 0.686 | 1 | … |
| Other severe conflict (civilian or military) | 6 | 0.283 | 32 | 0.520 | 5 | 0.772 |
| Military service in conflict zone | 60 | 0.999 | 14 | 0.360 | 19 | 0.951 |
| By conflict | ||||||
| US Civil War | 0 | … | 0 | … | 1 | … |
| World War II | 34 | 0.999 | 58 | 0.997 | 10 | 0.991 |
| Korean War | 0 | … | 4 | 0.332 | 0 | … |
| Vietnam War | 43 | 0.999 | 2 | 0.178 | 10 | 0.968 |
| 1982 Lebanon War | 4 | 0.191 | 2 | 0.592 | 0 | … |
| 1991 Gulf War | 17 | 0.892 | 4 | 0.017 | 10 | 0.801 |
| Balkan Wars | 3 | 0.737 | 0 | … | 1 | … |
| Unknown (Canadian refugees) | 0 | … | 16 | 0.275 | 0 | … |
Table 3 shows that, except for a weak effect among HRs statistically adjusted for age alone, persons who experienced war stress were not more likely to die than the comparison group. The mean HR was 1.07 [95% confidence interval (CI) 1.00–1.15; two-sided P = 0.0579; n = 101] for unadjusted HRs, 1.08 (95% CI 1.00–1.16; two-sided P = 0.0488; n = 87) for age-adjusted HR, and 1.05 (95% CI 0.98–1.13; two-sided P = 0.1802; n = 32) for HRs adjusted for age and additional covariates. The second row of Table 3 shows the results did not change when HRs with estimated SEs were excluded.
Subgroup analyses and multivariate meta-regression
Subgroup analyses confirmed the initial findings (see again Table 3), showing that war stresses tended to have no significant effect on all-cause mortality regardless of factors such as gender, time elapsed since the conflict occurred, whether the respondent was a civilian or combatant, the type of stress and the specific conflict. While in many of the cases a weak effect seemed to emerge when studies controlled for a limited number of variables (mostly age and gender), once studies controlled for a larger set of covariates (such as health measures and socio-economic status) the effects diminished.
Mean HRs were similar for women and men. The risk of death was elevated among age-adjusted studies of women (HR 1.35, 95% CI 1.11–1.65; two-sided P = 0.003; n = 11), but the mean HR diminished when controlling for additional variables such as pre-existing health conditions and socio-economic status (HR 0.92, 95% CI 0.66–1.28; two-sided P = 0.6124; n = 2). For men there was an increase in the risk of death among studies adjusted for multiple covariates (HR 1.14, 95% CI 1.00–1.31; two-sided P = 0.0493; n = 11), but the magnitude of the effect was quite weak. Meta-regression results (Table 5) confirm the lack of substantive difference in the risk of death for men and women.
Table 5.
Multivariate meta-regression analyses predicting the magnitude of the effect of war stress on all-cause mortality
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Standard error imputed (1, yes; 0, no) | 1.18* (1.03–1.35) | 1.25** (1.06–1.48) | 1.00 (0.86–1.16) |
| Proportion of sample that is male | 1.11 (0.94–1.30) | 1.14 (0.97–1.34) | 1.07 (0.91–1.26) |
| Mean age of sample at baseline (in decades) | 1.05* (1.02–1.09) | 1.05** (1.02–1.09) | 1.05* (1.01–1.09) |
| Decades between conflict and beginning of follow-up | 0.98 (0.94–1.02) | 1.01 (0.96–1.07) | 0.97* (0.94–1.00) |
| Follow-up duration (in decades) | 1.01 (0.96–1.05) | 1.00 (0.95–1.05) | 0.98 (0.94–1.02) |
| Controlled for gender | 0.95 (0.84–1.07) | 0.98 (0.87–1.09) | 0.95 (0.85–1.07) |
| Controlled for age | 1.01 (0.92–1.11) | 1.03 (0.94–1.12) | 1.02 (0.93–1.11) |
| Controlled for health | 1.08 (0.91–1.29) | 1.03 (0.87–1.21) | 1.06 (0.90–1.25) |
| Respondent identity (1, civilian; 0, military personnel) | 1.06 (0.91–1.22) | 1.05 (0.92–1.19) | 1.02 (0.84–1.24) |
| Stress type | |||
| Military service in conflict zone | Reference | … | … |
| Concentration camp | 0.75* (0.58–0.97) | … | … |
| Prisoner of war | 0.78* (0.66–0.92) | … | … |
| Other severe conflict (civilian or military) | 0.92 (0.75–1.13) | … | … |
| Conflict | |||
| US Civil War | … | 1.67 (0.92–3.05) | … |
| World War II | … | Reference | … |
| Korean War | … | 0.99 (0.56–1.76) | … |
| Vietnam War | … | 1.44*** (1.20–1.72) | … |
| 1982 Lebanon War | … | 1.53 (0.98–2.38) | … |
| 1991 Gulf War | … | 1.37** (1.10–1.72) | … |
| Balkan Wars | … | 1.55 (0.80–3.01) | … |
| Unknown conflict (Canadian refugees) | … | 1.21 (0.96–1.52) | … |
| Country | |||
| USA | … | … | Reference |
| UK | … | … | 0.82** (0.71–0.93) |
| Australia | … | … | 1.18* (1.02–1.37) |
| Canada | … | … | 1.20 (0.88–1.64) |
| Croatia | … | … | 1.09 (0.56–2.11) |
| Israel | … | … | 0.93 (0.71–1.21) |
| Lebanon | … | … | 1.11 (0.70–1.77) |
| Norway | … | … | 0.98 (0.42–2.29) |
| Constant | 0.82 (0.66–1.01) | 0.56*** (0.40–0.78) | 0.87 (0.71–1.06) |
| R2 | 0.3709 | 0.4399 | 0.4327 |
We present the results for differences in stress type (Model 1), conflict (Model 2) and country (Model 3) in three separate models to account for the problem of multicollinearity between these three important variables.
All meta-regressions calculated by maximum likelihood using a random effects model (n = 219 for each model). Numbers reported are the exponentiated regression coefficients (95% CI). Ellipses indicate where a variable was not included in a model.
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Table 3 also shows that, among HRs adjusted for age and additional covariates, follow-up duration does not impact the risk of all-cause mortality, be it <5 years (HR 1.01, 95% CI 0.90–1.14; two-sided P = 0.8405; n = 7), 5–15 years (HR 1.08, 95% CI 0.93–1.25; two-sided P = 0.3421; n = 7) or >15 years (HR 1.07, 95% CI 0.96–1.18; two-sided P = 0.2122; n = 18). The meta-regressions (Table 5) confirm that follow-up duration is not a significant factor. They also show that this is not the result of differences in the amount of time elapsed between the end of a conflict and the start of a study. Table 3 further shows no excess risk among both civilians (HR 1.00, 95% CI 0.86–1.16; two sided P = 0.9861; n = 11) and military personnel (HR 1.06, 95% CI 0.99–1.14; two-sided P = 0.1148; n = 21), and the meta-regressions (Table 5) confirm that there is no statistical difference between the two groups.
Our study distinguished between four distinct types of war-related stress: (i) the experience of being in a Nazi concentration camp, (ii) the experience of being a prisoner of war, (iii) experiencing combat as a soldier and (iv) other high-level exposures to war. The multivariate meta-regression (Model 1 of Table 5) shows important differences between the magnitudes of the HRs. The exponentiated meta-regression coefficients, which are ratios of HRs (RHRs), show that when compared to persons engaged in regular military service in a combat zone, the HR was lower for those in concentration camps (RHR 0.75, 95% CI 0.58–0.97; two-sided P = 0.0289) and also lower for prisoners of war (RHR 0.78, 95% CI 0.66–0.92; two-sided P = 0.0035). However, the subgroup meta-analyses (Table 3) show that the risk of death for the various types of war-related stress had no effect among HRs adjusted for multiple covariates. The small differences between the various measures of war stress are important, because they shed light on the issue of exposure assessment. For example, the measure ‘military service in a conflict zone’ is a somewhat less precise measurement of war exposure as it does not differentiate between frontline and rear-duty personnel. The fact that there was no substantial difference between the effect sizes of this measure and the other more narrowly defined measures suggests that the various measures of exposure were valid.
Among mortality risk estimates that were most statistically controlled, only the Vietnam War showed an effect (HR 1.11, 95% CI 1.00–1.23; two-sided P = 0.0412; n = 10). However, as in other cases where effects existed, the magnitude of this effect was quite weak. The meta-regression results (Table 5) confirm the elevated risk of death associated with the Vietnam War (in Model 2 RHR 1.44, 95% CI 1.20–1.72; two-sided P = 0.0001). They also suggest an increased risk for the 1991 Gulf War (RHR 1.37, 95% CI 1.10–1.72; two-sided P = 0.0060).
Two other results from the meta-regressions (Table 5) are notable. First, the mean age of the study sample had a small effect on the magnitude of the HR, being somewhat elevated among samples with a higher mean age. For each additional 10 years of age, the HR increased by between 5% (Model 3) and 7% (Models 1 and 2). Second, the HR was 16% lower for those coming from the UK, but 18% higher for those coming from Australia when compared to persons from the USA (Model 3).
Discussion
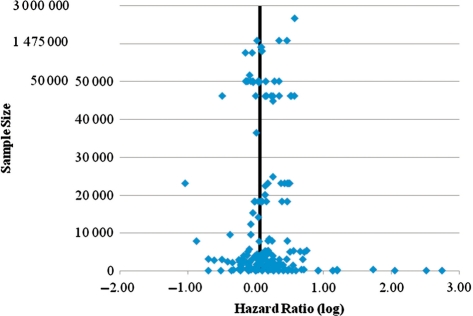
Our findings show that war stress had no significant impact on a person’s risk of all-cause mortality. Based on 220 mortality risk estimates from 30 publications, we showed that after adjustment for multiple covariates, the risk of death among those who experienced war-related stress was not different from the risk for those who did not experience this type of stress. A funnel plot of the log HRs against sample size was symmetric around the mean HR, suggesting no selection bias (see Figure 2). The meta-analyses and meta-regressions showed that the lack of relationship between war stress and mortality was almost completely uniform across all subgroups. Only in two instances did a risk of death emerge: among men and among those who experienced Vietnam War–related stress. Even for these groups, the magnitude of the effect is very weak and is not robust across different levels of adjustment. When considering that researchers sometimes avoid publishing non-significant findings (the file drawer effect), such weak results should be treated with caution.
Figure 2.
Funnel plot of logarithmic HRs vs sample size. Vertical line denotes the mean log HR of 0.653. Scale is less condensed from 0 to 50 000 to make dispersion of points more visible
Interestingly, our study found that many of the differences among subgroups were no longer substantial once additional factors (such as health and socio-economic status) were introduced. One possible explanation for these results may be that many of the SEs for the minimally controlled risk estimates in this analysis were estimated. The meta-regressions show that HRs with estimated SEs were higher (18% in Model 1 and 25% in Model 2). These findings suggest a cautious interpretation of minimally adjusted risk estimates and that future studies take pains to control for key covariates such as health, socio-economic status and the level of social support.
There are a few possible explanations for the lack of differences found in this study. First, the results may be influenced by a selection effect. For example, those who survived confinement in a Nazi concentration camp during the Holocaust or in a prisoner of war camp may be persons who are especially hardy. This unmeasured attribute may have countered the deleterious effects of stress. This explanation seems especially convincing when considering the well-known fact that in concentration camps the Nazis often conducted what scholars of eugenics call ‘a negative selection process’, in which those deemed less healthy were chosen to be executed first.
A somewhat related explanation, which applies to war stress in general, could be that going through hard experiences may increase a person’s resiliency and sense of self-preservation.29,30 Those who went through life-threatening events may have become less sensitive to future stressful life events. In addition, they may have become more aware of the fragility of life, and therefore avoid risky behaviours and pay better attention to their health.
This latter explanation, however, is not supported by the findings of studies which showed that Gulf War veterans are more prone to late-night car crashes, especially alcohol-related ones,31 and are more likely to suffer from heavy weekly drinking, binge drinking and alcohol-related problems.32 In fact, some scholars have suggested that physical and psychological traumas experienced during wartime may result in the post-war adoption of ‘coping’ behaviours that increase the risk for injuries.33,34 Furthermore, recent studies have found either higher rates of suicide among former US active duty soldiers,35 or no differences between soldiers and the general population,36,37 suggesting that soldiers may be equally or less psychologically resilient than the general population.
A final explanation for the results presented here may be that survivors, especially in Western countries, were quite likely to receive increased institutional support. In many Western nations, veterans receive life-long subsidized medical care, as well as psychological and social welfare counselling. Many of them also obtain a certain degree of social capital and elicit respect based on their service. Such benefits may help counter the deleterious effects of the stressful experience.
This study has important limitations. As noted above, a healthy survivor effect may apply to these findings. Some studies have noted increased myocardial infarction38 or sudden death15 in the hours or days immediately after exposure to an acute stressor. Those vulnerable to immediate death may not have survived to participate in the observational studies included here. Second, many of the studies presented only statistically unadjusted or age-adjusted models. Thus, the number of HRs that were well controlled is quite small, and conclusions from these analyses should be made cautiously. Third, all of the data in the analysis came from studies conducted among respondents from the developed world, except for three studies of Lebanon,14 Croatia25 and Canadian refugees.15 In these three studies, the effects of war-induced stress on mortality is clearly deleterious. A possible explanation for these results is that systems of emotional and instrumental support for those who experienced severe war stress are less available in the developing world. Future studies should further investigate the effects of war stress on mortality in the developing world.
This study shows that war-related stresses and traumas do not substantially increase the risk of overall mortality. Future research should focus on the specific conditions under which war stress may yet have differential impacts, especially in developing nations, and on the factors that might moderate or counter any deleterious effect. Future studies should also be careful to adjust for covariates such as health status, socio-economic conditions and social support, because not controlling for these may result in overestimating the effects of stress on mortality. Lastly, this study suggests that researchers should shift their attention towards studying the effects of war stress on more specific causes of death, such as coronary heart disease or suicide.
Supplementary data
Supplementary data are available at IJE online.
Funding
The authors are grateful for the support provided by grant HL-76857 from the National Institutes of Health. The funding source had no involvement in the collection, analysis and interpretation of the data, in the writing of the report, and in the decision to submit the paper for publication.
Conflict of interest: None declared.
KEY MESSAGES.
This study summarizes the effects of war-related stress on all cause mortality using meta-analyses and meta-regressions.
No differences were found between most of those who experienced war stress and comparable populations who did not when studies were well-controlled.
This lack of substantial effect may be the result of selection processes, developed resiliency, and/or institutional support.
References
- 1.White D. Iraq War Facts, Results & Statistics at June 24, 2009. [cited 20 July 2009]. Available at: http://usliberals.about.com/od/homelandsecurit1/a/IraqNumbers.htm (24 June 2007, date last accessed) [Google Scholar]
- 2.Fink S. Iraqi Refugees Problem Persists. [cited 20 July 2009]; Available from: http://www.propublica.org/article/iraqi-refugee-problem-persists-401 (1 April 2009, date last accessed) [Google Scholar]
- 3.Boscarino JA. Posttraumatic stress disorder, mortality among US army veterans 30 years after military service. Ann Epidemiol. 2006;16:248–56. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Adena MA, Cobbin DM, Fett MJ, et al. Mortality among Vietnam veterans compared with non-veterans and the Australian population. Med J Aust. 1985;143:541–44. doi: 10.5694/j.1326-5377.1985.tb119945.x. [DOI] [PubMed] [Google Scholar]
- 5.Kang HK, Bullman TA. Mortality among U.S. veterans of the Persian Gulf War. N Engl J Med. 1996;335:1498–504. doi: 10.1056/NEJM199611143352006. [DOI] [PubMed] [Google Scholar]
- 6.Boehmer TKC, Flanders WD, McGeehin MA, Boyle C, Barrett DH. Postservice mortality in Vietnam veterans: 30-year follow-up. Arch Intern Med. 2004;164:1908–16. doi: 10.1001/archinte.164.17.1908. [DOI] [PubMed] [Google Scholar]
- 7.Nefzger MD. Follow-up studies of World War II and Korean War prisoners: I. study plan and mortality findings. Am J Epidemiol. 1970;91:123–38. doi: 10.1093/oxfordjournals.aje.a121120. [DOI] [PubMed] [Google Scholar]
- 8.Keehn RJ. Follow-up studies of World War II and Korean conflict prisoners. Am J Epidemiol. 1980;111:194–211. doi: 10.1093/oxfordjournals.aje.a112887. [DOI] [PubMed] [Google Scholar]
- 9.Dent OF, Richardson B, Wilson S, Goulston KJ, Murdoch CW. Postwar mortality among Australian World War II prisoners of the Japanese. Med J Aust. 1989;150:378–82. doi: 10.5694/j.1326-5377.1989.tb136529.x. [DOI] [PubMed] [Google Scholar]
- 10.Costa DL. Height, weight, wartime stress, and older age mortality: evidence from the union army records. Explorations Econ History. 1993;30:424–49. [Google Scholar]
- 11.Collins C, Burazeri G, Gofin J, Kark JD. Health status and mortality in holocaust survivors living in Jerusalem 40-50 years later. J Traum Stress. 2004;17:403–11. doi: 10.1023/B:JOTS.0000048953.27980.18. [DOI] [PubMed] [Google Scholar]
- 12.Eitinger L. A Follow-up study of the Norwegian concentration camp survivors' mortality and morbidity. Isr Ann Psychiatr Relat Discip. 1973;11:199–209. [PubMed] [Google Scholar]
- 13.Stessman J, Cohen A, Hammerman-Rozenberg R, et al. Holocaust survivors in old age: the Jerusalem longitudinal study. J Am Geriatr Soc. 2008;56:470–77. doi: 10.1111/j.1532-5415.2007.01575.x. [DOI] [PubMed] [Google Scholar]
- 14.Sibai AM, Fletcher A, Armenian HK. Variation in the impact of long-term wartime stressors on mortality among the middle-aged and older population in Beirut, Lebanon, 1983–1993. Am J Epidemiol. 2001;154:128–37. doi: 10.1093/aje/154.2.128. [DOI] [PubMed] [Google Scholar]
- 15.DesMeules M, Gold J, McDermott S, et al. Disparities in mortality patterns among Canadian immigrants and refugees, 1980–1998: results of a National Cohort Study. J Immigr Minor Health. 2005;7:221–32. doi: 10.1007/s10903-005-5118-y. [DOI] [PubMed] [Google Scholar]
- 16.Ayalon L, Covinsky KE. Late-life mortality in older Jews exposed to the Nazi regime. J Am Geriatr Soc. 2007;55:1380–86. doi: 10.1111/j.1532-5415.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 17.Gale CR, Braidwood EA, Winter PD, Martyn CN. Mortality from Parkinson's disease and other causes in men who were prisoners of war in the Far East. Lancet. 1999;354:2116–18. doi: 10.1016/S0140-6736(99)06264-9. [DOI] [PubMed] [Google Scholar]
- 18.Williams RL, Medalie JH, Zyzanski, Flocke SA, Yaari S, Goldbourt U. Long-term mortality of Nazi concentration camp survivors. J Clin Epidemiol. 1993;46:573–75. doi: 10.1016/0895-4356(93)90130-s. [DOI] [PubMed] [Google Scholar]
- 19.Anderson HA, Hanrahan LP, Jensen M, Laurin D, Yick W-Y, Wiegman P. Wisconsin Vietnam Veteran Mortality Study: Final Report. Wisconsin: Department of Health and Human Services, State of Wisconsin; 1986. [Google Scholar]
- 20.Boyle CA, Decoufle P, Delaney RJ, et al. Postservice mortality among Vietnam veterans. JAMA. 1987;257:790–95. [PubMed] [Google Scholar]
- 21.Hearst N, Newman TB, Hulley SB. Delayed effects of the military draft on mortality: a randomized natural experiment. N Engl J Med. 1986;314:620–24. doi: 10.1056/NEJM198603063141005. [DOI] [PubMed] [Google Scholar]
- 22.Kang HK, Bullman TA. Mortality among US Veterans of the Persian Gulf War: 7-year follow-up. Am J Epidemiol. 2001;154:399–405. doi: 10.1093/aje/154.5.399. [DOI] [PubMed] [Google Scholar]
- 23.Kark JD, Goldman S, Epstein L. Iraqi missle attacks on Israel: the association of mortality with a life-threatening stressor. JAMA. 1995;273:1208–10. doi: 10.1001/jama.273.15.1208. [DOI] [PubMed] [Google Scholar]
- 24.Macfarlane G, Thomas E, Cherry N. Mortality among UK Gulf War veterans. Lancet. 2000;356:17–21. doi: 10.1016/S0140-6736(00)02428-4. [DOI] [PubMed] [Google Scholar]
- 25.Mollica RF, Srajlic N, Chernoff M, Lavelle J, Vukovic IS, Massagli MP. Longitudinal study of psychiatric symptoms, disability, mortality, and emigration among Bosnian refugees. JAMA. 2001;286:546–54. doi: 10.1001/jama.286.5.546. [DOI] [PubMed] [Google Scholar]
- 26.Thomas TL, Kang HK, Dalager NA. Mortality among Women Vietnam Veterans, 1973-1987. Am J Epidemiol. 1991;134:973–80. doi: 10.1093/oxfordjournals.aje.a116182. [DOI] [PubMed] [Google Scholar]
- 27.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 28.Roelfs DJ, Eran S, Louise F, Karina WD, Joseph ES. Meta Analysis for Sociology: A Bottom-Up Approach. Paper presented at the Annual Meeting of the Eastern Sociological Society in Boston, MA, March 18–21, 2010. [Google Scholar]
- 29.Pietrzak RH, Johnson DC, Goldstein MB, et al. Psychosocial buffers of traumatic stress, depressive symptoms, and psychosocial difficulties in veterans of operations enduring freedom and Iraqi freedom: the role of resilience, unit support, and postdeployment social support. J Affect Disord. 2010;120:188–92. doi: 10.1016/j.jad.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Meichenbaum D. Stress inoculation training for coping with stressors. Clin Psychol. 1996;49:4–7. [Google Scholar]
- 31.Lincoln AE, Hooper TI, Kang HK, Debakey SF, Cowan DN, Gackstetter GD. Motor vehicle fatalities among Gulf War era veterans: characteristics, mechanisms and circumstances. Traffic Inj Prev. 2006;7:31–37. doi: 10.1080/15389580500412028. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson IG, Hooper TI, Boyko EJ, Wells TS. Alcohol use and alcohol-related problems before and after military combat deployment. JAMA. 2008;300:663–75. doi: 10.1001/jama.300.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell NS, Amoroso PJ, Wegman DH, Senier L. Proposed explanations for excess injury among veterans of the Persian Gulf War and a call for greater attention from policymakers and researchers. Inj Prev. 2001;7:4–9. doi: 10.1136/ip.7.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray GC, Gackstetter GD, Kang HK, Graham JT, Scott KC. After more than 10 years of Gulf War veteran medical evaluations, What have we learned? Am J Prev Med. 2004;26:443–52. doi: 10.1016/j.amepre.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Kang HK, Bullman TA. Risk of suicide among US veterans after returning from the Iraq or Afghanistan war zones. JAMA. 2008;300:652–53. doi: 10.1001/jama.300.6.652. [DOI] [PubMed] [Google Scholar]
- 36.Kapur N, While D, Blatchley N, Bray I, Harrison K. Suicide after leaving the UK armed forces – a cohort study. PLoS Med. 2009;6:e1000026. doi: 10.1371/journal.pmed.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fear NT, Ward VR, Harrison K, Davison L, Williamson S, Blatchley NF. Suicide among male regular UK armed forces personnel, 1984-2007. Occup Environ Med. 2009;66:438–41. doi: 10.1136/oem.2008.040816. [DOI] [PubMed] [Google Scholar]
- 38.Culic V, Eterovi D, Miric D. Meta-analysis of possible external triggers of acute myocardial infarction. Int J Cardiol. 2004;99:1–8. doi: 10.1016/j.ijcard.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Fett MJ, Dunn M, Adena MM, Cobbin DM. Australian Veterans—Health Studies: The Mortality Report (Part1) Canberra, Australia: Australian Government Publishing Service; 1984. [Google Scholar]
- 40.Fett MJ, Adena MM, Cobbin DM, Dunn M. Mortality among Australian conscripts of the Vietnam conflict era. I. death from all causes. Am J Epidemiol. 1987;125:869–77. doi: 10.1093/oxfordjournals.aje.a114603. [DOI] [PubMed] [Google Scholar]
- 41.Macfarlane GJ, Hotopf M, Maconochie N, Blathley N, Richards A, Lunt M. Long-term mortality amongst Gulf War veterans: is there a relationship with experiences during deployment and subsequent morbidity? Int J Epidemiol. 2005;34:1403–8. doi: 10.1093/ije/dyi205. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole BI, Adena MA, Jones MP. Risk factors for mortality in Australian Vietnam-era national servicemen: a case-control study. Commun Health Stud. 1988;12:408–17. doi: 10.1111/j.1753-6405.1988.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 43.Visintainer PF, Barone M, McGee H, Peterson EL. Proportionate mortality study of Vietnam-era veterans of Michigan. J Occup Environ Med. 1995;37:423–28. doi: 10.1097/00043764-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe KK, Kang HK. Military service in Vietnam and the risk of death from trauma and selected cancers. Ann Epidemiol. 1995;5:407–12. doi: 10.1016/1047-2797(95)00039-a. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–91. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]