Abstract
Background The association between diagnostic X-ray exposures early in life and increased risk of childhood leukaemia remains unclear.
Methods This case–control study included children aged 0–14 years diagnosed with acute lymphoid leukaemia (ALL, n = 711) or acute myeloid leukaemia (AML, n = 116) from 1995 to 2008. Controls were randomly selected from the California birth registry and individually matched to cases with respect to date of birth, sex, Hispanic ethnicity and maternal race. Conditional logistic regression analyses were performed to assess whether ALL or AML was associated with self-reported child’s X-rays after birth (post-natal), including number of X-rays, region of the body X-rayed and age at first X-ray, as well as maternal X-rays before and during pregnancy (preconception and prenatal).
Results After excluding X-rays in the year prior to diagnosis (reference date for matched controls), risk of ALL was elevated in children exposed to three or more post-natal X-rays [odds ratio (OR) = 1.85, 95% confidence interval (CI) 1.12–2.79]. For B-cell ALL specifically, any exposure (one or more X-rays) conferred increased risk (OR = 1.40, 95% CI 1.06–1.86). Region of the body exposed was not an independent risk factor in multivariable analyses. No associations were observed between number of post-natal X-rays and AML (OR = 1.05, 95% CI 0.90–1.22) or T-cell ALL (OR = 0.84, 95% CI 0.59–1.19). Prevalence of exposure to prenatal and preconception X-rays was low, and no associations with ALL or AML were observed.
Conclusions The results suggest that exposure to post-natal diagnostic X-rays is associated with increased risk of childhood ALL, specifically B-cell ALL, but not AML or T-cell ALL. Given the imprecise measures of self-reported X-ray exposure, the results of this analysis should be interpreted with caution and warrant further investigation.
Keywords: Childhood leukaemia, diagnostic X-rays, California
Introduction
Leukaemia is the most common childhood cancer, accounting for nearly one-third of all cancers among children aged <15 years.1 Acute leukaemia accounts for the majority of paediatric cases, with 80% acute lymphoid leukaemia (ALL) and ∼20% acute myeloid leukaemia (AML).2
Only a few risk factors for childhood leukaemia have been identified, including genetic disorders such as Down syndrome, some chemotherapeutic agents and exposure to ionizing radiation.3 Although the association between high doses of ionizing radiation and leukaemia is well established, influences of low-dose sources such as diagnostic X-ray exposures remain disputed.4,5 The risk of childhood leukaemia associated with in utero exposure from maternal abdominal diagnostic X-rays has been studied extensively. The results of several case–control studies from the 1950s to 1980s6–8 showed a ∼1.3- to 1.5-fold increased risk of leukaemia. Cohort studies from the same time period, which showed mixed results, were generally composed of small case series.5,9,10 The largest of these reported risk estimates differing from those in case control studies, but results were thought to be unreliable.12 Two systematic reviews of the literature published after 1990 obtained summary risk estimates that were non-elevated14 or attenuated compared with prior estimates,4 perhaps because of the marked decline of both prevalence and dose of prenatal diagnostic irradiation since the mid-20th century4 or because of design limitations of recent studies.13 Maternal preconception diagnostic X-ray exposures have been studied less commonly, and findings in the literature are inconsistent.15 Generally, there is little evidence to support a relationship between maternal preconception exposure to ionizing radiation and childhood leukaemia.16 More attention has been focused on paternal occupational exposure,17 but early findings have not been reproduced.18
With respect to post-natal diagnostic X-ray exposures in childhood, early studies reported greater exposure among cases than controls,6,19,20 but studies from the past 20 years have produced inconsistent results. Several found no increased risk associated with post-natal X-rays, even for multiple exposures, or among different study populations.16,21,22 Conversely, others found a slightly elevated risk for childhood ALL, specifically pre-B-cell ALL,23 associated with exposure to two or more X-rays.24 To our knowledge, only one study has assessed risk of childhood AML associated with post-natal diagnostic X-ray exposures,22 and no increased risk was observed.
The objective of this study was to examine the association of childhood leukaemia subtypes (ALL overall and by immunophenotype, and AML) and diagnostic irradiation in an individually matched case–control study conducted in Northern and Central California. Exposures included maternal preconception and pregnancy diagnostic X-rays and child's diagnostic X-rays after birth (post-natal X-rays). Child's X-rays were assessed by number of X-rays received, region of the body exposed and age at first X-ray exposure.
Methods
Study population
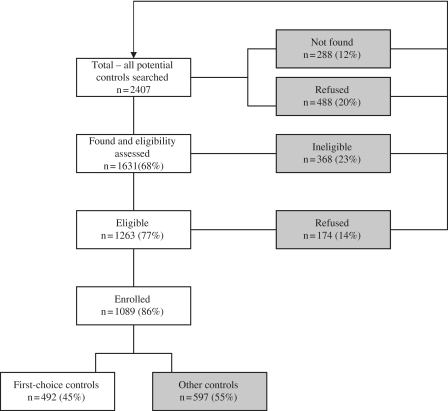
The Northern California Childhood Leukemia Study (NCCLS) is a population-based, matched case–control study. Phase I of the study (1995–99) included 17 counties in Northern California and Phase II (1999–2008) an additional 18 counties (35 counties in total); therefore, Phase II comprises a larger study area than Phase I. Cases were ascertained within 72 h after diagnosis at seven (Phase I) or nine (Phase II) Northern and Central California hospitals. Case subjects were considered eligible for participation if they were <15 years of age at diagnosis, had an English- or Spanish-speaking parent or guardian, lived in one of the 35 counties that comprised the population base at the time of diagnosis and had never been previously diagnosed with leukaemia. Comparison of case ascertainment in the 35-county study area with the California Cancer Registry (1997–2003) showed that the NCCLS ascertained 96% of children diagnosed with leukaemia in the seven Phase I participating hospitals and 93% in the nine Phase II hospitals. When considering both participating and non-participating hospitals within the 35 study counties, cases ascertained represented 76% of all diagnosed cases. A total of 86% of case subjects determined eligible consented to participate. The control subjects were randomly selected from groups of four birth certificates obtained through the California Office of Vital Records, and one (Phase I) or two (Phase II) control subjects were matched to case subjects on child's date of birth (within 10 days), sex, Hispanic status (defined as either one or both parents being Hispanic, as indicated on the birth certificate record) and maternal race (as indicated on the birth certificate record). The control selection process is presented in Figure 1, and is also described elsewhere.25 Among those contacted and considered eligible, 86% participated; 45% were first-choice control subjects, meaning that they were the first control identified in the random sampling of four birth certificate records. If the first-choice control subject was not available to participate, another birth certificate from the remaining three was chosen. Additional sets of four birth certificates were requested if there were no available participants in the first set. Of the ‘other’, non-first-choice control subjects, 90% were identified from the first group of four birth certificates sampled, whereas an additional 8% came from the second group of four sampled and the remaining 2% came from groups three (1.87%) and four (0.13%).
Figure 1.
Selection of controls for the NCCLS from August 1995 to July 2008. In instances when first-choice controls were not available, i.e. they refused, were ineligible or could not be located, alternative controls were selected (indicated with shading in the flow diagram). If controls refused participation after enrolment or were later found to be ineligible, additional controls were not identified (n = 26, or 2% of enrolled subjects)
Using NCCLS study data, Ma et al. compared birth certificate control subjects with ‘ideal’ control subjects (California birth certificated records that were exactly population based, for individuals that did not need to be traced) and found little difference in demographic characteristics between the two, suggesting that the NCCLS is approximately population based.26 In the present analysis, case and control participants are similar with respect to matching characteristics, but differ by household income, maternal education and maternal age at birth, all higher among controls (Table 1). The study was approved by the University of California Committee for the Protection of Human Subjects, the California Health and Human Services Agency Committee for the Protection of Human Subjects and the institutional review boards of all participating hospitals. Written, informed consent was obtained from the parents of all participating subjects.
Table 1.
Selected characteristics of NCCLS participating cases and controls by leukaemia subtype
| ALL |
AML |
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| n = 711 | n = 960 | n = 116 | n = 147 | |
| n (%) | n (%) | n (%) | n (%) | |
| Child's sexa | ||||
| Male | 406 (57.1) | 558 (58.1) | 63 (54.3) | 79 (53.7) |
| Female | 305 (42.9) | 402 (41.9) | 53 (45.7) | 68 (46.3) |
| Child's ethnicitya | ||||
| Hispanic | 326 (45.6) | 433 (44.8) | 41 (38.7) | 49 (36.8) |
| Non-Hispanic | 387 (54.1) | 534 (55.2) | 65 (61.3) | 84 (63.2) |
| Don't know | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| Age in years at diagnosis/reference datea | ||||
| 0.0–1.0 | 17 (2.4) | 20 (2.1) | 11 (9.5) | 14 (9.5) |
| 1.1–5.0 | 392 (55.1) | 532 (55.4) | 37 (31.9) | 51 (34.7) |
| 5.1–14.9 | 302 (42.5) | 408 (42.5) | 68 (58.6) | 82 (55.8) |
| Annual household income in $ | ||||
| <15 000 | 115 (16.2) | 95 (9.9) | 23 (19.8) | 12 (8.2) |
| 16 000–29 000 | 122 (17.2) | 118 (12.3) | 23 (19.8) | 20 (13.6) |
| 30 000–44 000 | 112 (15.8) | 123 (12.8) | 13 (11.2) | 16 (10.9) |
| 45 000–59 000 | 85 (11.9) | 102 (10.6) | 10 (8.6) | 18 (12.2) |
| 60 000–74 000 | 53 (7.5) | 106 (11.1) | 11 (9.5) | 15 (10.2) |
| >75 000 | 201 (28.3) | 387 (40.3) | 33 (28.5) | 63 (42.9) |
| Don't know | 23 (3.23) | 29 (2.1) | 3 (2.6) | 3 (2.1) |
| Maternal education | ||||
| Less than high school | 90 (12.7) | 71 (7.4) | 12 (10.3) | 15 (10.2) |
| High school/some college | 218 (30.7) | 270 (28.1) | 40 (34.5) | 37 (25.2) |
| Bachelor's degree | 200 (28.1) | 295 (30.7) | 26 (22.4) | 37 (25.2) |
| Post-baccalaureate degree | 203 (28.6) | 323 (33.7) | 38 (32.8) | 58 (39.5) |
| Don't know | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Maternal age in years at child's birth | ||||
| <20 | 70 (9.9) | 74 (7.8) | 13 (11.4) | 9 (6.2) |
| 20–24.9 | 168 (23.9) | 175 (18.5) | 25 (21.9) | 16 (11.1) |
| 25–29.9 | 175 (24.9) | 253 (26.8) | 41 (35.9) | 48 (33.1) |
| 30–34.9 | 180 (25.6) | 262 (27.7) | 20 (17.5) | 44 (30.3) |
| ≥35 | 110 (15.7) | 181 (19.2) | 15 (13.2) | 28 (19.3) |
| Don't know | 8 (0.01) | 15 (0.02) | 2 (0.02) | 2 (0.01) |
| Mean (SD) | 28.3 (6.3) | 29.2 (6.1) | 27.1 (5.7) | 29.8 (5.7) |
| Median (IQR) | 28.5 (23.1–32.9) | 29.2 (24.7–33.8) | 27.3 (23.2–30.9) | 29.9 (26.6–33.8) |
IQR: inter-quartile range.
aMatching variables.
The present analysis includes all NCCLS study participants (Phases I and II, 1995–2008). Excluded from analysis were nine subjects with missing X-ray exposure data, and 31 subjects with Down syndrome, as Down syndrome was associated with both case status and increased exposure to X-rays. After the application of these exclusionary criteria, 711 ALL and 116 AML matched case–control sets (pairs and trios) were available for analysis (Phase 1: 363 ALL and 56 AML cases; Phase II: 348 ALL and 60 AML cases).
Data collection
X-ray exposure information was collected through home-based, in-person interviews. The median time between date of diagnosis for cases and interview was slightly more than 4 months (range 1–35 months). For controls, the average time between reference date (comparable with date of diagnosis of corresponding matched case) and interview was ∼14 months (range 1–73 months). A total of 98% of respondents were the biological mothers of the children, who provided information about their own X-ray exposure(s) during the year prior to conception and pregnancy, and their child's X-ray exposure(s) prior to the date of diagnosis, or corresponding reference date for matched controls (post-natal X-rays). If the biological father was the respondent (20 cases and 21 controls), information on maternal X-rays received prior to conception and during pregnancy was not collected. For post-natal X-rays, all exposures, with the exception of dental X-rays, were reported by the following broadly defined regions of the body: chest, skull, broken bone and ‘other’. In Phase II, X-ray exposure questions were refined to better capture region of the body exposed for broken bone and ‘other’ X-rays, and were reported by region of the body exposed (abdomen, extremities, chest, back, head or whole body). In Phase II, 85% of ‘broken bone’ X-rays were to the extremities, whereas ‘other’ X-rays were mixed, with no one region of the body predominantly represented. In order to assess the impact of exposure misclassification, we conducted a sensitivity analysis where broken bone X-rays given to the chest were re-classified into the chest X-ray group, those given to the head re-classified into the skull group etc. As a result, the following revised categories of region exposed were created: chest, head/skull, extremities and ‘other’. The point estimates using original vs revised categories (i.e. all broken bone vs extremities only) varied by <10%. Therefore, findings were reported using the original categories by which the information was obtained in both phases of the study. Respondents were also asked the age of the child when the first exposure occurred and the number of X-rays that the child received, for each region exposed. X-ray exposures that occurred after the date of diagnosis or reference date were not included in the analysis.
Immunophenotype classification
Immunophenotype was determined for ALL using flow cytometry profiles (CD10 and CD19 for B-cell lineage and CD2, CD3, CD4, CD5, CD7 or CD8 for T-cell lineage) and has been described previously.27 In the present study, there were 469 B-cell ALL cases and 50 T-cell ALL cases. This represents a subset of the total ALL cases in the study because immunophenotype classification was not readily available for all cases at the time of analysis.
Statistical analysis
Analyses were conducted separately for ALL and AML, and for immunophenotypes of ALL. Conditional estimation was used to obtain the parameters of logistic regression models for estimated odds ratios (ORs) and 95% confidence intervals (CIs) measuring the frequency of X-rays among cases and controls. Demographic characteristics, including household income, maternal age, maternal education, illness during pregnancy and child's early life health characteristics (infections during the first year of life, visits to the paediatrician for assessment of infection) were assessed as confounders but not included in the final models because they did not affect the OR by >10% and did not improve the fit of the models, assessed using chi-square log likelihood ratio tests (P > 0.10).
We conducted statistical analyses to assess the risk of childhood leukaemia by: (i) any exposure to X-rays during the preconception, pregnancy or post-natal periods, e.g. mother or child received ≥1 X-ray during these time windows; (ii) for the post-natal period, the number of X-rays received and (iii) for the post-natal period, risk by the region of the body exposed.
Any exposure to X-rays during the preconception, pregnancy or post-natal periods was modelled as a binary variable (yes/no). Because of the scarcity of exposures during the preconception and prenatal periods, there were few subjects with both prenatal or preconception, and post-natal X-rays, and the joint effect of exposures during these time periods could not be assessed. Therefore, any exposure to X-rays was modelled separately by each exposure period.
X-rays by the region of the body were grouped in the following manner, to create mutually exclusive exposure categories: (i) only one region of exposure (chest only, broken bone only, skull only or ‘other’ exposures only); (ii) multiple regions with at least one chest X-ray and (iii) multiple regions but no chest X-rays (e.g. ‘broken bone’ and ‘skull’). The multiple exposure groups were constructed in this manner because univariate analyses suggested that chest X-rays conferred greater risk than X-rays at other regions of the body.
Total number of X-rays was modelled both as a categorical variable (zero, one to two and three or more X-rays) and directly as a count. For number of X-rays, we assessed departure from linearity by adding a quadratic term to the regression model and comparing the models with and without the quadratic term. There was no evidence of a departure from linearity.
Interaction between matching covariates and selected demographic characteristics on the relationship between X-ray exposure and childhood leukaemia was also assessed using likelihood ratio tests. Interaction terms of all matching covariates (age at diagnosis, sex, child’s Hispanic status and maternal race) and X-ray exposure, and selected demographic characteristics (income, maternal age and maternal education) did not improve model fit, so no interaction terms were included in the final models. Therefore, all final models are unadjusted, with confounding by age, sex, Hispanic ethnicity and maternal race addressed through the individual matching process.
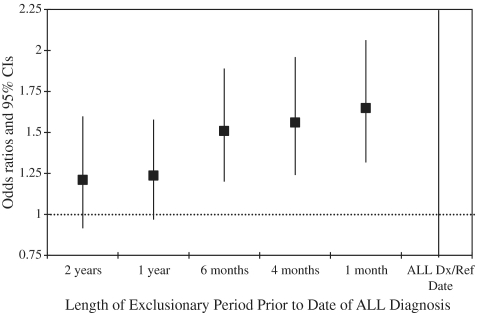
X-ray exposures that occurred <1 year prior to the date of diagnosis or reference date for controls were excluded from analysis. There is no consensus in the literature regarding an appropriate length for an ‘exclusionary period’, and recent studies have used periods of 1 month up to 2 years prior to diagnosis (reference date).16,23,24 Therefore, we examined the influence of excluding diagnostic X-ray exposures given before the diagnostic/reference date from 1 month up to 2 years on the risk estimates for childhood ALL (Figure 2). As the exclusionary period lengthened, the OR for X-ray exposure decreased from ∼1.7-fold at 1 month to 1.2-fold at 1 year, where it remained constant. We chose 1 year as the appropriate exposure exclusionary length because this period excludes any X-rays case children may have received as part of the leukaemia diagnostic process, and is sufficiently long enough to partially account for latency, which, for childhood leukaemia, is unknown.
Figure 2.
Exposure to post-natal diagnostic X-rays and risk of ALL; ORs and 95% CIs for different length of exclusionary period
Results
Selected demographic characteristics by leukaemia subtype are presented in Table 1. Overall, the distributions of ALL cases and controls differed with respect to distribution of maternal age at birth (P = 0.02), maternal education (P = 0.001) and household income (P < 0.001). In contrast, the distributions of AML cases and controls only differed by distribution of maternal age at birth (P = 0.01). Distribution of X-ray exposure (number of X-rays) by leukaemia subtype is presented in Table 2. For children with ALL, the number of X-rays ranged from 0 to 20 in cases, compared with 0 to 10 in controls. Among the AML group, the distribution of X-ray exposure was similar for cases and controls.
Table 2.
Characteristics of diagnostic X-ray exposure among cases and controls participating in the NCCLS, by leukaemia subtypea
| ALL |
AML |
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| n = 711 | n = 960 | n = 116 | n = 147 | |
| n (%) | n (%) | n (%) | n (%) | |
| Preconception | 99 (12.9) | 100 (11.2) | 20 (15.2) | 16 (10.6) |
| Pregnancy | 32 (4.2) | 33 (3.4) | 7 (5.3) | 8 (5.3) |
| Post-natal | 278 (39.1) | 301 (31.4) | 41 (35.3) | 46 (31.5) |
| Body region | ||||
| Chest | 128 (17.9) | 119 (12.4) | 18 (17.0) | 18 (13.7) |
| Broken bone | 82 (11.5) | 101 (10.5) | 12 (11.3) | 22 (16.5) |
| Skull | 38 (5.3) | 58 (6.0) | 3 (2.8) | 8 (6.0) |
| Other | 47 (6.6) | 46 (4.8) | 3 (2.8) | 4 (3.0) |
| Number of X-rays | ||||
| Zero | 495 (69.3) | 701 (72.7) | 74 (69.8) | 83 (78.3) |
| One to two | 155 (21.7) | 210 (21.8) | 23 (21.7) | 31 (23.3) |
| Three or more | 62 (8.7) | 50 (5.2) | 9 (8.5) | 10 (7.5) |
| Mean (SD) | 0.7 (1.8) | 0.5 (1.2) | 0.8 (2.2) | 0.7 (1.4) |
| Range | 0–20 | 0–10 | 0–10 | 0–9 |
aExcludes X-rays that were received <1 year prior to diagnosis for cases and the corresponding reference date for controls.
Maternal preconception and in utero X-ray exposures
The ORs and 95% CIs for the risk of leukaemia associated with X-ray exposures during the preconception and pregnancy periods are presented in Tables 3 and 4 for ALL and AML, respectively. No association between maternal history of diagnostic X-rays and ALL was observed. A 2-fold increased risk for AML associated with exposures during the preconception was noted; however, the CI for this estimate overlapped the null.
Table 3.
Exposure to diagnostic X-ray and risk of ALL by immunophenotype and period of exposure
| Number of cases | Discordant pairs/triplets n (%) | OR (95% CI) | |
|---|---|---|---|
| All ALL combined | |||
| Preconception | 649 | 167 (25.7) | 1.17 (0.85–1.61) |
| Pregnancy | 652 | 60 (9.2) | 1.20 (0.71–2.04) |
| Post-natal | 711 | 318 (44.5) | 1.21 (0.96–1.51) |
| Number of X-rays (category) | |||
| Zero | 313 (44.0) | 1.00 | |
| One to two | 284 (40.0) | 1.06 (0.83–1.36) | |
| Three or more | 100 (14.0) | 1.85 (1.22–2.79) | |
| Number of X-rays (continuous) | 1.10 (1.03–1.18) | ||
| B-cell ALL | 472 | ||
| Post-natal | 205 (43.5) | 1.40 (1.06–1.86) | |
| Number of X-rays (continuous) | 1.13 (1.04–1.23) | ||
| T-cell ALL | 52 | ||
| Post-natal | 21 (40.4) | 0.54 (0.21–1.35) | |
| Number of X-rays (continuous) | 0.84 (0.59–1.19) |
Table 4.
Exposure to diagnostic X-ray and risk of AML by period of exposure
| Number of cases | Discordant pairs/triplets n (%) | OR (95% CI) | |
|---|---|---|---|
| Preconception | 111 | 26 (23.4) | 1.94 (0.80–4.69) |
| Pregnancy | 111 | 13 (11.8) | 0.85 (0.26–2.78) |
| Post-natal | 116 | 36 (31.0) | 0.78 (0.38–1.61) |
| Number of X-rays (category) | |||
| Zero | 36 (35.0) | 1.00 | |
| One to two | 35 (33.0) | 0.89 (0.43–1.84) | |
| Three or more | 19 (18.0) | 0.95 (0.36–2.49) | |
| Number of X-rays (continuous) | – | 1.05 (0.90–1.22) |
Post-natal X-ray exposures
Matched analyses for risk of childhood ALL associated with post-natal X-ray exposure are presented in Table 3. Although having any exposure to X-rays (e.g. one or more X-ray) was not associated with increase in risk of ALL (OR = 1.21, 95% CI 0.96–1.51), children who had ever received three or more X-rays at any body region had a 1.85-fold elevated risk of ALL (95% CI 1.22–2.79). An increased OR was observed for B-cell ALL subjects who had received one or more X-rays (OR = 1.40, 95% CI 1.06–1.86) but not for T-cell ALL subjects (OR = 0.54, 95% CI 0.28–1.70). The disparity in point estimates and range of CIs suggests a difference in the influence of X-ray exposure by immunophenotype, despite the smaller number of subjects in the T-cell ALL group. The distribution of age at first X-ray was similar between cases and controls for ALL (data not shown).
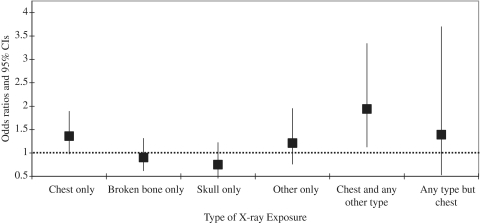
In univariate analysis, we observed suggested elevated risks of ALL associated with chest X-rays only (OR = 1.36, 95% CI 0.99–1.89) and chest X-rays combined with other regions exposed (OR = 1.94, 95% CI 1.13–3.34), indicating that risk of exposure may differ by region of body exposed (Figure 3). In multivariable analysis adjusting for number of X-rays, the association with chest X-rays only or in combination with other X-rays remained elevated, with a CI overlapping the null (OR = 1.46, 95% CI 0.83–2.59). Therefore, there was a slight indication that the dose relationship observed with number of X-ray differed between children who had received chest X-rays and those who did not.
Figure 3.
ORs and 95% CIs for ever X-ray exposure, by region of the body exposed for the following, mutually exclusive, combinations of exposure: (i) chest only; (ii) broken bone only; (iii) skull only; (iv) ‘other’ only; (v) multiple regions exposed, including chest and (vi) multiple regions exposed, not including chest. The estimates are for ever exposure, not for numbers of X-rays, e.g. ‘chest only’ includes all subjects who received at least one chest X-ray and no other types of X-rays
In contrast to ALL, post-natal X-ray exposure was not associated with AML (Table 3). Like ALL, the distribution of age at first exposure was similar for AML cases and controls, and the effect did not differ by region of the body exposed (data not shown). Overall, the results for ALL and AML were similar in Hispanic and non-Hispanic White children, the two largest racial/ethnic groups in our study.
Discussion
In the current analysis, we found an association between exposure to ≥3 X-rays and ALL and an association between B-cell ALL and ever exposure. Using an additive model, number of X-rays was associated with ALL. Although history of chest X-rays was associated with elevated risk of ALL in univariate analyses, this association diminished after accounting for number of X-rays. We did not observe associations between post-natal X-rays and AML or T-cell ALL, nor between maternal diagnostic X-rays before and during pregnancy and risk of ALL or AML.
The current literature is not consistent with regard to an association between post-natal X-rays and childhood leukaemia. A case–control study conducted in Shanghai among 172 ALL cases and 92 acute non-lymphocytic leukaemia cases (primarily AML) and 618 controls diagnosed from 1974 to 1986 did not report elevated risk with ever exposure or by number of X-rays.22 A US case–control study with 1842 cases (diagnosed 1989–93) and 1986 controls found no overall association between post-natal X-ray exposure and ALL, but an increased risk of pre-B cell ALL was reported following exposure to three or more X-rays received >2 years prior to the date of diagnosis, among children aged >6 years (OR = 3.8, 95% CI 1.1–13.3).23 However, given the acute nature of ALL and AML, an exclusionary period of 2 years may be conservative, excluding etiologically important exposures. Another large case–control study from the German Childhood Cancer Registry (1184 cases diagnosed 1992–94, and 2588 controls) reported a reduced risk for leukaemia (all types) among subjects exposed to three or fewer X-rays, particularly for children born between 1975 and 1987 (OR = 0.67, 95% CI 0.53–0.85), but no test for trend was indicated.16 In the German study, X-rays given during the year prior to diagnosis were excluded from analysis; however, all leukaemia subtypes were analysed together, which may have obscured differences by subtype. In a Canadian case–control study (491 cases diagnosed from 1980 to 1993, and 491 controls) exposure to two or more X-rays was associated with increased risk of ALL, but this effect was limited to girls (OR = 2.26, 95% CI 1.20–4.23).24 In the present analysis, we did not observe a difference in the association between X-ray exposure and case–control status by sex. Finally, a large cohort study of children exposed to diagnostic X-rays in Germany (n = 92 957) between 1976 and 2003 identified 33 incident leukaemia cases (24 ALL, 5 AML). This study did not find an increased risk for either leukaemia subtype (combined standardized incidence ratio (SIR) = 1.08, 95% CI 0.74–1.52) or any trend associated with magnitude of exposure.30 Consistent with two studies examining leukaemia risk for ALL immunophenotype23 and acute non-lymphocytic leukaemia (ANLL),22 we did not observe associations between post-natal X-ray exposures and T-cell ALL or AML. Given the small numbers of cases with these subtypes, we are unable to state definitively whether the observed negative association for T-cell ALL is due to differing susceptibility by immunophenotype or insufficient sample size to detect exposure differences by case status. Further investigation is warranted.
The dose of diagnostic organ-specific radiation received differs by region of the body exposed and by procedure32 and these factors were not accurately captured in the present analysis. Here, the regions of the body exposed were broadly defined and misclassification in self-reports may have occurred. For example, an X-ray to a broken clavicle or rib could have been interpreted by a respondent as a chest X-ray or a broken bone X-ray. Further, information about the specific type of X-ray received was not collected in this analysis. A recent report of the National Council for Radiation Protection and Measurements (no. 160) indicates that the effective dose of radiation from medical procedures increased 7-fold for the average American between the early 1980s and 2006, and that a substantial source of this increased exposure is computed tomographic (CT) scans.28 Although the report does not provide information on exposures specific to the paediatric population, it has been estimated that the proportion of total CT scans given to children is 6–11%.29 Radiation doses from a CT scan can be ≥50 times that for a plain X-ray, in the case of abdominal X-rays,29 and may be more variable for children than adults. Appropriate dose reduction for children is neither well established, nor consistent among hospitals.31 Unfortunately, the NCCLS data do not distinguish whether the X-rays received were plain or CT, so risk by type of X-ray could not be explored in the present analysis. Further, the target tissue for radiation-induced leukaemia is red bone marrow, and some of the highest estimated doses for this tissue type are from CT scans to the chest, abdomen and pelvis.32 The results of the present analysis suggest a possible modest risk associated with chest X-rays compared with other regions of the body exposed, after adjusting for number of X-rays. However, additional studies with more precise exposure information must be conducted to confirm these results.
Consistent with recent large studies,23,33 the present investigation did not find evidence to support elevated risks of ALL or AML associated with preconception or in utero exposure to diagnostic X-rays. Prevalence of these exposures was low during the study period, and very few were to the abdominal area. Furthermore, modern medical practices, such as shielding during exposure, are more widespread, in keeping with the International Commission on Radiological Protection recommendations to keep radiation doses as low as reasonably achievable.34 Therefore, it may be that these X-rays do not pose an increased risk, or that prevalence of exposure was too small to detect any risk.
Challenges in assessing the causal relationship between X-ray exposure and leukaemia have been discussed in the literature,15 and several apply to the current analysis. The major limitation of this analysis is the use of a questionnaire to ascertain participant diagnostic X-ray exposure. Although recall bias is always a concern in case–control studies, its effect may be of particular concern in these analyses because the study participants are children and the relationship between cancer and radiation is well known. On the other hand, parents of controls as well as cases may be more likely to accurately remember X-rays given for illness or trauma, as compared with daily exposures, such as dietary intake. Because of these different and conflicting factors, it is difficult to assess the magnitude of differential exposure misclassification. Respondents may also have inaccurately recalled the number of X-rays given, particularly for older children who received X-rays many years prior to the date of the study interview. Because this study did not have access to study participant hospital or physician records, we were unable to confirm the numbers or type of X-rays that the child received, as previously noted. The fact that we observed both positive and negative findings by histological and immunophenotypic subtype (B-cell ALL, T-cell ALL vs AML) may be an indication that recall bias has little influence on the results seen in this study.
In the NCCLS study population, controls have significantly higher household income and maternal education levels than cases, which may be a function of participation rates and ability to effectively trace control subjects. However, we did not find X-ray exposure to be correlated with socio-economic indicators such as income, maternal education or maternal age at birth, nor did ORs differ by race or ethnicity. Therefore, these factors are unlikely to account for the relationship between ALL and X-ray exposure observed. Furthermore, if socio-economic status (SES) did confound the relationship between X-ray exposures and childhood leukaemia, we would expect this relationship to be mediated through health-care access, which would be greater among the higher SES controls, and an inverse relationship between X-rays and case status would be observed. Therefore, it is unlikely that the observed association is due to confounding by SES.
Despite some limitations, the NCCLS has several advantages. First, it uniquely represents a large and diverse population in Northern and Central California, allowing differences in risk to be assessed for distinct subpopulations. Second, because of the rapid case ascertainment method used, cases are identified often within days of diagnosis, expediting the interview and control selection process, which may help to reduce recall bias, especially for more recent exposures. As noted earlier, in this analysis, the median time between date of diagnosis (reference date) and date of interview was 4 months for cases and 14 months for controls. Finally, as noted, although case ascertainment is hospital-based, the vast majority of children diagnosed with leukaemia at the participating hospitals and the study region were ascertained through this study.
In summary, the results of this study provide support for a modest association of post-natal X-ray exposures with childhood ALL, specifically B-cell ALL, and suggest that the risk increases with increasing number of post-natal X-rays. Similar increased risk was not observed for either AML or T-cell ALL, nor for exposures received during the preconception and prenatal periods. Given the imprecision of the exposure metric employed in this study, the results should be interpreted with caution. Further investigation is needed to assess exposure level more accurately, particularly the role of CT scans, and to identify other factors that may modify the risk of X-ray exposure.
Funding
National Institute of Environmental Health Sciences (Grant numbers R01ES009137 and P42ES0470518).
KEY MESSAGES.
Although a statistical association between in utero irradiation and childhood leukaemia is well established, risk associated with post-natal X-ray exposure and childhood leukaemia is not well characterized.
The findings of the present analysis suggest increased risk for ALL, particularly B-cell ALL, associated with post-natal X-ray exposure and number of X-rays received. No associations were observed for AML or T-cell ALL. Furthermore, no increased risk was observed for prenatal or preconception X-ray exposures, though these exposures were uncommon in this study population.
Given the lack of precision in the exposure estimates, the results of this study should be interpreted with caution, but they warrant further investigation.
Acknowledgements
The authors thank the families for their participation. They also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr Jonathan Ducore), University of California San Francisco (Dr Mignon Loh and Dr Katherine Matthay), Children’s Hospital of Central California (Dr Vonda Crouse), Lucile Packard Children’s Hospital (Dr Gary Dahl), Children’s Hospital Oakland (Dr James Feusner), Kaiser Permanente Roseville (Dr Kent Jolly and Dr Vincent Kiley), Kaiser Permanente Santa Clara (Dr Alan Wong and Dr Carolyn Russo), Kaiser Permanente San Francisco (Dr Kenneth Leung) and Kaiser Permanente Oakland (Dr Daniel Kronish and Dr Stacy Month). Finally, we acknowledge the entire Northern California Childhood Leukemia Study staff and the UCB Survey Research Center for their effort and dedication.
Conflict of interest: None declared.
References
- 1.Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute; SEER Program. NIH Pub. No. 99-4649. Bethesda, MD, 1999. [Google Scholar]
- 2.Pui CH, editor. Childhood Leukemias. New York, NY: Cambridge University Press; 1999. [Google Scholar]
- 3.Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environ Health Perspect. 2007;115:138–45. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakeford R. Childhood leukemia following medical diagnostic exposure to ionizing radiation in utero or after birth. Radiat Prot Dosimetry. 2008;132:66–74. doi: 10.1093/rpd/ncn272. [DOI] [PubMed] [Google Scholar]
- 5.Boice JD, Miller RW. Childhood and adult cancer after intrauterine exposure to ionizing radiation. Teratology. 1999;59:227–33. doi: 10.1002/(SICI)1096-9926(199904)59:4<227::AID-TERA7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Stewart A, Webb J, Hewitt D. A survey of childhood malignancies. BMJ. 1958;1:1495–508. doi: 10.1136/bmj.1.5086.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bithell JF, Stewart AM. Pre-natal irradiation and childhood malignancy: a review of British data from the Oxford survey. Brit J Cancer. 1975;31:271–87. doi: 10.1038/bjc.1975.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mac Mahon B. Prenatal x-ray exposure and childhood cancer. J Natl Cancer Inst. 1962;28:1173–91. [PubMed] [Google Scholar]
- 9.Oppenheim BE, Griem ML, Meier P. The effects of diagnostic X-ray exposure on the human fetus: an examination of the evidence. Radiology. 1975;114:529–34. doi: 10.1148/114.3.529. [DOI] [PubMed] [Google Scholar]
- 10.Court Brown WM, Doll R, Hill RB. Incidence of leukaemia after exposure to diagnostic radiation in utero. BMJ. 1960;2:1539–45. doi: 10.1136/bmj.2.5212.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monson RR, MacMahon B. Prenatal x-ray exposure and cancer in children. In: Boice JD, Fraumeni JF, editors. Radiation Carcinogenesis: Epidemiology and Biological Significance. New York, NY: Raven Press; 1984. pp. 97–105. [Google Scholar]
- 12.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–39. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 13.Wakeford R. On pre- or post-natal diagnostic X-rays as a risk factor for childhood cancer. Radiat Environ Biophys. 2009;48:237–39. doi: 10.1007/s00411-009-0214-3. [DOI] [PubMed] [Google Scholar]
- 14.Schulze-Rath R, Hammer GP, Blettner M. Are pre- or post-natal diagnostic X-rays a risk factor for childhood cancer? A systematic review. Radiat Environ Biophys. 2008;47:301–12. doi: 10.1007/s00411-008-0171-2. [DOI] [PubMed] [Google Scholar]
- 15.Boice JD. Ionizing radiation. In: Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. 3rd. New York, NY: Oxford University Press; 2006. p. 259. [Google Scholar]
- 16.Meinert R, Kaletsch U, Kaatsch P, Schuz J, Michaelis J. Associations between childhood cancer and ionizing radiation: Results of a population-based case–control study in Germany. Cancer Epidemiol Biomarkers Prev. 1999;8:793–99. [PubMed] [Google Scholar]
- 17.Gardner MJ, Snee MP, Hall AJ, Powell CA, Downes S, Terrell JD. Results of case–control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ. 1990;300:423–29. doi: 10.1136/bmj.300.6722.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakeford R. The risk of childhood cancer from intrauterine and preconceptional exposure to ionizing radiation. Environ Health Perspect. 1995;103:1018–25. doi: 10.1289/ehp.951031018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham S, Levin ML, Lilienfeld AM, et al. Preconception, intrauterine, and postnatal irradiation as related to leukemia. Natl Cancer Inst. 1966;19:347–71. [PubMed] [Google Scholar]
- 20.Polhemus DW, Koch R. Leukemia and medical radiation. Pediatrics. 1959;23:453–61. [PubMed] [Google Scholar]
- 21.Linabery AM, Olshan AF, Gamis AS. Exposure to medical test irradiation and acute leukemia among children with Down syndrome: a report from the children's oncology group. Pediatrics. 2006;118:1499–508. doi: 10.1542/peds.2006-0644. [DOI] [PubMed] [Google Scholar]
- 22.Shu XO, Gao YT, Brinton LA, et al. A population-based case–control study of childhood leukemia in Shanghai. Cancer. 1988;62:635–44. doi: 10.1002/1097-0142(19880801)62:3<635::aid-cncr2820620332>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Shu XO, Potter JD, Linet MS. Diagnostic X-rays and ultrasound exposure and risk of childhood acute lymphoblastic leukemia by immunophenotype. Cancer Epidemiol Biomarkers Prev. 2002;11:177–85. [PubMed] [Google Scholar]
- 24.Infante-Rivard C, Mathonnet G, Sinnett D. Risk of childhood leukemia associated with diagnostic irradiation and polymorphisms in DNA repair genes. Environ Health Perspect. 2000;108:495–98. doi: 10.1289/ehp.00108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163:1091–100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Buffler PA, Layefsky M, Does MB, Reynolds P. Control selection strategies in case–control studies of childhood diseases. Am J Epidemiol. 2004;159:915–21. doi: 10.1093/aje/kwh136. [DOI] [PubMed] [Google Scholar]
- 27.Aldrich MC, Zhang L, Wiemels JL, et al. Cytogenetics of Hispanic and White children with acute lymphoblastic leukemia in California. Cancer Epidemiol Biomarkers Prev. 2006;15:578–81. doi: 10.1158/1055-9965.EPI-05-0833. [DOI] [PubMed] [Google Scholar]
- 28.National Council on Radiation Protection and Measurements. Ionizing Radiation Exposure of the Population of the United States. Report No. 160. Bethesda, MD: NCRP; 2009. [Google Scholar]
- 29.Brenner D, Hall E. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 30.Hammer GP, Seidenbusch MC, Schneider K, et al. A cohort study of childhood cancer incidence after postnatal diagnostic x-Ray exposure. Radiation Research. 2009;171:504–12. doi: 10.1667/RR1575.1. [DOI] [PubMed] [Google Scholar]
- 31.Robbins E. Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer. 2008;51:453–57. doi: 10.1002/pbc.21599. [DOI] [PubMed] [Google Scholar]
- 32.Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–51. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 33.Rodvall Y, Pershagen G, Hrubec Z, Ahlbom A, Pedersen N, Boice JD. Prenatal X-ray exposure and childhood cancer in Swedish twins. Int J Cancer. 2006;46:362–65. doi: 10.1002/ijc.2910460304. [DOI] [PubMed] [Google Scholar]
- 34.Wrixon AD. New International Commission on Radiological Protection (ICRP) recommendations. J Radiol Prot. 2008;28:161–68. doi: 10.1088/0952-4746/28/2/R02. [DOI] [PubMed] [Google Scholar]