Abstract
Background
Though an increased efficacy of carmustine and temozolomide (TMZ) has been demonstrated by inactivation of O6-methylguanine-DNA methyltransferase (MGMT) with O6-benzyl-guanine (BG) in human pancreatic tumors refractive to alkylating agents, the regulatory mechanisms have not been explored.
Methods
The effects of TMZ and BG on apoptosis, cell growth, the mitotic index, cell cycle distribution, and protein expression were studied by TUNEL, cell counting, flow cytometry, and Western blot analysis, respectively.
Results
The wt-p53 human pancreatic tumor cell line Capan-2 and p53-efficient mouse embryonic fibroblasts (MEFs) were more responsive to treatment with TMZ + BG than mutant p53 Capan-1 and p53-null MEFs. S phase delay with a subsequent G2/M arrest was observed in Capans in response to BG + TMZ. The G1-to-S transition delay in Capan-2 was associated with p53-dependent apoptosis and was distinctly different from the presumed mismatch repair (MMR) killing operative during the G2/M arrest. The effect of p53 on BG + TMZ toxicity was supported by a marked change in apoptosis when p53 function was restored/inactivated. There was an early induction of MMR proteins in p53-efficient lines.
Conclusion
p53 provokes a classic proapoptotic response by delaying G1-to-S progression, but it may also facilitate cell killing by enhancing MMR-related cell cycle arrest and cell death.
Key Words: O6-Benzylguanine, Cell cycle arrest, Mismatch repair, p21, p53, Pancreatic cancer, Temozolomide
Introduction
Pancreatic cancer is the fourth and fifth most frequent cause of cancer mortality in men and women respectively in the US [1]. Estimated new pancreatic cancer cases and deaths in the US in 2005 are 32,160 and 31,800, respectively [2]. Surgery is an option in less than 1 of 5 patients, and even with tumor resection the majority of patients succumb to the disease [3]. Chemotherapy for advanced pancreatic cancer is palliative in nature. The use of 5-FU in combination with radiation in the locally advanced setting has been shown to enhance survival; however, results have been inconsistent [4,5]. Gemcitabine emerged as the cornerstone of current chemotherapy for pancreatic cancer based on the results of a randomized trial comparing it with 5-FU in patients with advanced unresectable disease [6]. Current gemcitabine- or 5-FU-containing combinations may result in longer survival times than those observed with single-agent therapy; however, results to date remain preliminary [6]. The unresponsiveness of pancreatic tumors to chemotherapy [7] may reflect extensive activation of prosurvival pathways [8] by the activation of K-ras (90%) and the inactivation of tumor suppressors p16/Rb (>90%), p53 (75%), and SMAD4/DPC4 (>50%). Furthermore, the failure to design effective treatments against pancreatic tumors is due to the silencing of several proapoptotic and cell cycle control mechanisms by a wide spectrum of mutations.
Genotoxic alkylating agents, including nitrosoureas, have been used unsuccessfully against pancreatic cancer [9] presumably because of the presence of high levels of O6-methylguanine-DNA methyltransferase (MGMT) protein [10]. An impressive improvement in the efficacy of alkylating drugs against pancreatic tumor xenografts has been demonstrated following the inactivation of MGMT by O6-benzyl-guanine (BG) prior to treatment with either the DNA cross-linking agent carmustine (BCNU) or the methylating agent temozolomide (TMZ) [11]. MGMT depletion enhances cell killing via preservation of the O6-MeG adducts which trigger DNA mismatch repair (MMR)-related cell cycle arrest and killing [12]. Such killing is not well understood, but it has been postulated that O6-MeG causes single-strand and double-strand DNA breaks due to its mismatched pairing with T or C and the subsequent recognition of the mismatch by Mut-S [13,14,15]. In turn, DNA breaks induce the ataxia-telangiectasia mutated (ATM)/ATR response, the phosphorylation/activation of Chk1, and the possible activation of p53 and the FAS receptor which lead to G2/M arrest [16,17,18]. A G2 arrest may also be derived from the activation of p38 and may involve CDC25 and CDC2 [19,20,21,22]. In addition, O6-MeG could trigger the induction of p21 signaling, but there are reports that the synthesis of this cell cycle inhibitor is delayed or not involved in cell cycle arrest in certain tumors [23]. A possible consequence of p21 induction could be mediated by G1/S arrest, the abrogation of which prevents the functional induction of apoptotic response by the BAX/BCL-2 pathway and leads to survival [24,25,26]. Further improvement in the efficacy of DNA-methylating drugs, such as TMZ, against pancreatic neoplasms is possible based on the discovery of secondary post-MGMT mechanisms of tumor resistance [10]. Preliminary data suggest that one such mechanism is associated with a loss of p53 and probably of other tumor suppressor genes that regulate cell cycle check points in response to DNA damage [27]. In this communication, we further examine the involvement of p21 in conjunction with other p53-inducible genes in uncovering the functional role of p53 in the induction of cell cycle check points that are likely to be involved in the toxicity of TMZ + BG against pancreatic neoplasms. Isogenic mouse fibroblast lines differing in the expression of p53 are also used to underline p53-related differences and similarities between normal and malignant cells.
Methods
Cell Lines
Pancreatic cell lines Capan-1 (mut-p53) and Capan-2 (wt-p53) were purchased from the American Type Culture Collection (Rockville, Md., USA) and grown in DMEM with high glucose (Gibco-BRL) supplemented with 10% fetal bovine serum and 1% penicillin streptomycin at 37°C and 5% CO2. Primary wild-type, p53(+/+), p53(+/–), and p53(–/–) mouse fibroblasts (MEFs) from normal mice at passage 3, provided by Dr. Tyler Jacks (Department of Biology, Center for Cancer Research, and Howard Hughes Medical Institute, Massachusetts Institute of Technology, Cambridge, Mass., USA), were grown under similar conditions.
Plasmids and Transient Transfection
Human plasmid pCMV-p53 (Clontech Laboratories, Mountain View, Calif., USA) and pCMV-mouse verified full-length p53 cDNA clone (Open Biosystems, Huntsville, Ala., USA) that express the human wild-type p53 and mouse wild-type p53 tumor suppressor proteins, respectively, under the constitutive CMV promoter were used for restoration of the p53 function in Capan-1 (that harbor endogenously mutated p53) and MEF [p53 (–/–)] cells by transient transfections. Cells were transiently transfected immediately after trypinsinzation and replated using the improved transfection technique for adherent cells with Effectene reagent (Qiagen, Valencia, Calif., USA) [28]. Briefly, the cells were incubated with lipid-DNA complexes at 37°C and 5% CO2. Ten micrograms of the p53 expression plasmids or 10 μg of the empty vector were used. After transfection for 48 h, cells were exposed to BG/TMZ (30/500 μM).
Treatments
The TMZ was generously donated by Schering Plough, Inc. (Kenilworth, N.J., USA). BG was synthesized [11] and donated by Dr. Robert Moschel (Frederick Cancer Center, Frederick, Md., USA). A 30-mM stock solution of BG in 40% PEG in phosphate-buffered saline (PBS) stored at 4°C was diluted 1:1,000 times in growth medium at a final concentration of 30 μM. A 100-mM stock solution of TMZ in DMSO was stored at −20°C until used. Cultures were treated with DMSO (–BG controls), BG + DMSO (+BG controls), TMZ (TMZ-treated), or TMZ + BG (BG/TMZ-treated). Treatment was performed as follows: Cultures at about 40% confluence were preincubated in control medium or in BG containing medium 24 h before TMZ treatment (50–1,000 μM). The medium was aspirated and cultures washed twice with PBS. Serum-free medium without BG was added to all the flasks and the required volume of TMZ or DMSO was added and mixed vigorously in less than 5 s. Cultures were incubated at 37°C for 30 min, the medium was aspirated, and serum containing medium with BG (BG/TMZ-treated and +BG controls) or without BG (TMZ-treated and –BG controls) was added to the cultures which were subsequently incubated at 37°C for a maximum of 8 days. DMSO concentrations varied from 0.2 to 1% v/v during the treatment with TMZ; however, exposure to this agent was transient and lasted only 30 min.
Flow Cytometric Analysis
Flow cytometry was performed as previously described [29]. Untreated, BG-treated, TMZ-treated, and BG/TMZ-treated cells (1 × 106) were washed in PBS and counted using a Neubauer chamber at various time intervals following treatment to determine cell growth. Cells were then fixed in ice-cold ethanol. Fixed cells were pelleted and resuspended in 500 μl of PBS. RNA was eliminated by treating cells with RNAse A (Sigma, St. Louis, Mo., USA). Cells were then stained with propidium iodide in PBS and analyzed using a FACStar Calibur (Becton Dickinson) cell sorter. The cell cycle distribution at each time point was determined in triplicate.
Quantitation of Apoptosis
Apoptosis was quantified by TUNEL staining. An in situapoptosis detection kit (Roche Diagnostics Corporation, Indianapolis, Ind., USA) which detects the DNA strand breaks in single cells by terminal transferase-mediated fluorescein-dUTP end labeling was used as per the instructions provided by the manufacturer [30]. Briefly, cells were seeded in chamber slides (Nunc, Inc., Naperville, Ill., USA) and 24 h later were preexposed to 30 μM BG for 24 h followed by TMZ treatment for 30 min; they were then harvested at 48 and 96 h, respectively, and stained for apoptosis. The stained specimens were observed in a triple band-pass filter using a Nikon microphoto epifluorescence microscope. To determine the percentage of cells showing apoptosis, 2 experiments in total were performed and approximately 1,000 cells were counted in each experiment at 5 different locations. The number of cells containing visible chromosomes was also counted and divided by the total number of cells in the field of view to determine the mitotic index.
Immunofluorescence Assay
Pancreatic tumor cells cultured on Lab-Tek chamber slides (Nunc) were left untreated or treated with BG/TMZ or BG alone and 48 h after exposure were fixed in buffered formalin. Nonspecific sites were blocked with 3% BSA in PBS (pH 7.4) with 0.25% Tween 20 for 30 min. Slides were then incubated overnight at 4°C in primary antibody rabbit polyclonal anti-p21 (Santa Cruz Biotechnology, Santa Cruz, Calif., USA) that was diluted to 1:100 with the blocking buffer. After washes in PBS (pH 7.4) with 0.25% Tween 20 (3 times each for 15 min), the cells were exposed to secondary antibody Cy3-conjugated anti-rabbit IgG that was diluted to 1:1,000 in blocking buffer. After 3 washes in PBS (pH 7.4) with 0.25% Tween 20 (3 times each for 15 min) and 1 wash in PBS for 15 min, the slides were mounted with aqueous mounting media using antifade and 4′,6-diamidino-2-phenylindole (DAPI) (VectaShield; Vector, Burlingame, Calif., USA) and visualized using a triple band-pass filter in a Nikon epifluorescence microscope.
siRNA Assay
The siGENE SMARTpool TP53 targeting human p53 mRNA was purchased from Dharmacon Research (Lafayette, Colo., USA). Transient transfections in Capan-2 were performed as described above either with the siRNA for p53 or the control siRNA. The cells were left untreated or were treated with 30 μM BG plus 500 μM TMZ or BG alone after 48 h of transfection. TUNEL and Western blot analysis for the p53 target genes were performed 48 h after treatment.
Western Blot Analysis
Total proteins were extracted from untreated, BG-treated, TMZ-treated, and BG/TMZ-treated cultures at various time points and subjected to Western blot analysis as described earlier [30]. Total proteins were electrophoresed in SDS-PAGE and transferred to a PVDF membrane. After blocking by milk, the membrane was incubated in a milk solution containing antibodies specific to p21 (sc-817), Bcl-2 (sc-509), Bax (sc-493), p53 (Sc-126) or p53 (Pab-246 in case of MEFs), hPMS2 (E-19), hPMS1 (C-20), hMSH2 (N-20) or MLH1 (C-20), and p27 (sc-528) supplied by Santa Cruz. The bound antigen-antibody complex was detected by the secondary antibody (monoclonal or polyclonal, depending on the primary antibody) and the chemiluminescence kit (Amersham BioSciences UK Ltd., Little Chalfont, UK). The same membrane was used for β-actin levels detected by anti-β-actin antibody (Sigma) as an internal loading control. Protein expression by Western blot analysis was performed in triplicate.
Statistical Analysis
Values are expressed as means ± standard error and were compared by ANOVA analysis. Data were considered significantly different when p < 0.05.
Results
Effects of BG/TMZ Treatment on Cell Proliferation, the Mitotic Index, and Apoptosis in MEFs
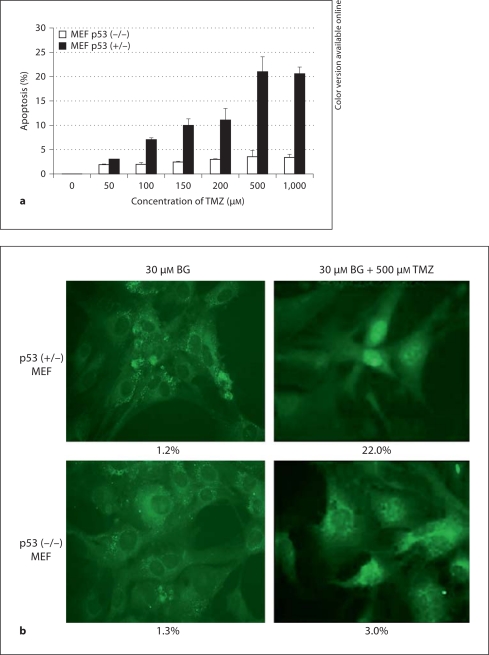
The rate of cell growth as well as cell death determined by the induction of apoptosis was dependent on p53 status in MEFs (fig. 1). Induction of apoptosis was assessed as a function of increasing concentrations of TMZ 48 h posttreatment by TUNEL assay in the presence of BG (fig. 1a). An enhanced cell death was observed with increasing concentrations of TMZ in the presence of BG in MEFs having a functional p53 (fig. 1a, b). Induction of apoptosis, however, was saturated after 500 μM TMZ in both MEF p53 (–/–) and MEF p53 (+/+) cells. Overall growth and apoptotic indices (apoptotic figures per 1,000 cells) in MEF cell cultures were not significantly affected by the presence of PEG (1%), DMSO (up to 1%), or BG (30 μM) (fig. 1c). TMZ alone inhibited the growth of MEF (+/+) cells at 96 h and of MEF (+/–) and MEF (–/–) cells at 48 h. However, cells resumed growth after 48 h. Inhibition of growth in MEFs by TMZ alone was not due to increased apoptosis (fig. 1c). Treatment of cells with BG/TMZ resulted in a 2.5- to 22-fold increase in apoptosis compared to untreated cells at 48 h with a maximum induction in MEF (+/–) cells (fig. 1c). At 96 h posttreatment the apoptotic indices, though still higher than in the untreated groups, were reduced significantly in all cells. Impairment of the growth of MEF (+/+) and MEF (+/–) by TMZ/BG was due to a loss of mitotic activity between 48 and 96 h (data not shown) and increased apoptosis between 0 and 48 h (fig. 1). Although, the apoptotic indices were statistically greater in BG/TMZ-treated MEF (–/–) than in the controls or TMZ-treated (p < 0.05) cells, the extent of apoptosis was lower in MEF (–/–) than in MEF (+/+) or MEF (+/–). The weak effect of BG/TMZ treatment on the induction of apoptosis allowed the growth of BG/TMZ-treated MEF (–/–) cells at rates comparable to those in the untreated controls (fig. 1c).
Fig. 1.
Concentration-dependent effects of TMZ on the induction of apoptosis and effects of TMZ and BG on the growth and apoptotic indices of mouse embryonic fibroblasts p53 (+/+, +/–, –/–). a Induced apoptosis in MEF p53 (+/–) and (–/–) cells in response to BG/TMZ treatment. Cells were treated with 0–1,000 μM TMZ plus 30 μM BG and subjected to TUNEL analysis at 48 h after treatment. Bars depict the average from 2 independent experiments ± standard error of 5 measurements per sample. b Micrographs showing apoptotic death using TUNEL on MEF p53 (+/–) and (–/–) cells in response to to BG/TMZ (30/500 μM) 48 h after treatment. c Effects of TMZ and BG on growth and apoptotic indices. Cells were untreated or treated with PEG (continuously; control for BG), BG (30 μM-continuously), DMSO (30 min-control for TMZ), TMZ (30 min with 250 μM), or BG + TMZ (30 min with 30/250 μM). The total number of cells and the number of apoptotic cells were determined at 48 and 96 h after treatment. Apoptotic indices are defined as the number of apoptotic bodies counted per 1,000 cells. Bars depict the average from 2 independent experiments ± standard error of 5 measurements per sample. UT = Untreated.
Effects of BG/TMZ Treatment on Cell Proliferation, the Mitotic Index, and Apoptosis in Capan-1 and Capan-2 Cells
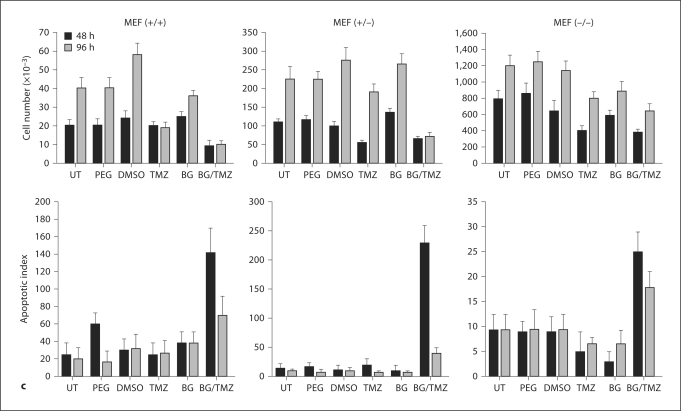
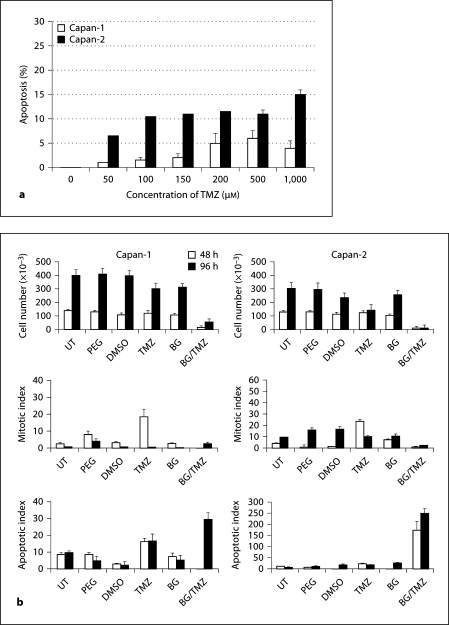
Induction of apoptosis assessed by TUNEL assay as a function of increasing concentrations of TMZ showed that BG/TMZ treatment markedly enhanced apoptosis in both Capan-1 and Capan-2; this is true particularly for the later, which showed no significant changes at TMZ concentrations between 100 and 500 μM but did display a further increase at 1,000 μM (fig. 2a). The growth of Capan cells, like that of MEFs, was not affected by the presence of PEG, DMSO, or BG (fig. 2b). TMZ had a marginal inhibitory effect on the growth of Capan-2 and no effect on the growth of Capan-1 (fig. 2b). A marked inhibition of growth was observed in both Capan-1 and Capan-2 cells 48 h after treatment with BG + TMZ. At 96 h after treatment, Capan-1 cells started to recover as an increase in cell number was observed, but growth inhibition was extended up to 96 h in Capan-2 cells (fig. 2b). A greater responsiveness of Capan-2 versus Capan-1 to BG/TMZ was primarily due to robust apoptosis in Capan-2 which was maintained for at least 96 h although a marginal reduction in the mitotic index was also observed in these cells. In Capan-1 cells, however, mitotic indices remained unchanged with BG/TMZ treatment, suggesting a role of only apoptosis in the observed growth inhibition effects (fig. 2b). The absence of apoptosis in pancreatic tumor cells treated with TMZ alone indicated that unrepaired O6-MeG is necessary for p53-dependent cell death in the first 48 h of exposure.
Fig. 2.
Effect of TMZ and BG on the growth and mitotic and apoptotic indices of pancreatic cell lines Capan-1 (mut p53) and Capan-2 (wt-p53). a Induced apoptosis in Capan-1 and Capan-2 cells in response to BG/TMZ treatment. Cells were treated with 0–1,000 μM TMZ plus 30 μM BG and subjected to TUNEL analysis at 48 h after treatment. Bars depict the average from 2 independent experiments ± standard error of 5 measurements per sample. b Effects of TMZ and BG on growth and mitotic and apoptotic indices. Cells were untreated or treated with PEG (continuously; control for BG), BG (30 μM; continuously), DMSO (30 min; control for TMZ), TMZ (30 min with 250 μM), or BG + TMZ (30 min with 30/250 μM). The total number of cells and the number of apoptotic/mitotic cells were determined at 48 and 96 h after treatment. Apoptotic/mitotic indices are defined as the number of apoptotic/mitotic bodies counted per 1,000 cells, respectively. Bars depict the average from 2 independent experiments ± standard error of 5 measurements per sample. UT = Untreated.
Effects of BG/TMZ Treatment on Cell Cycle Distribution in Capan-1 and Capan-2 Cells
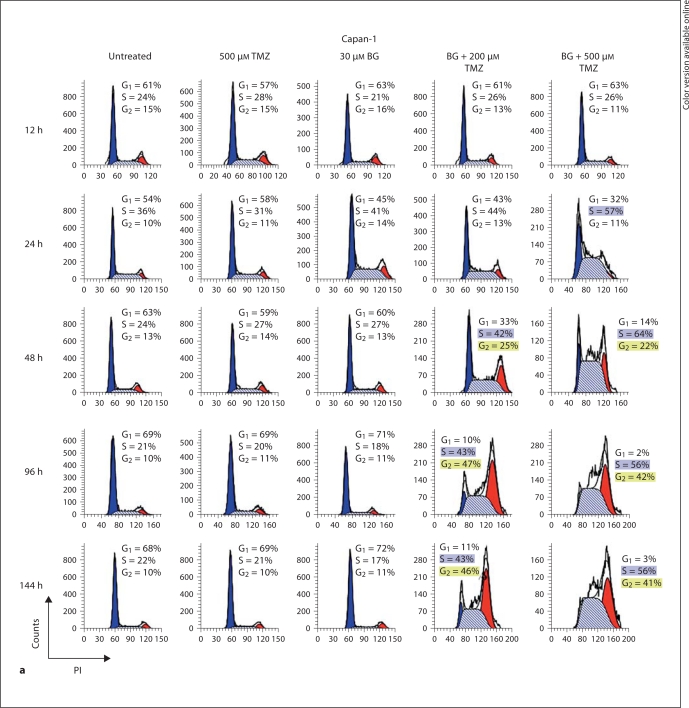
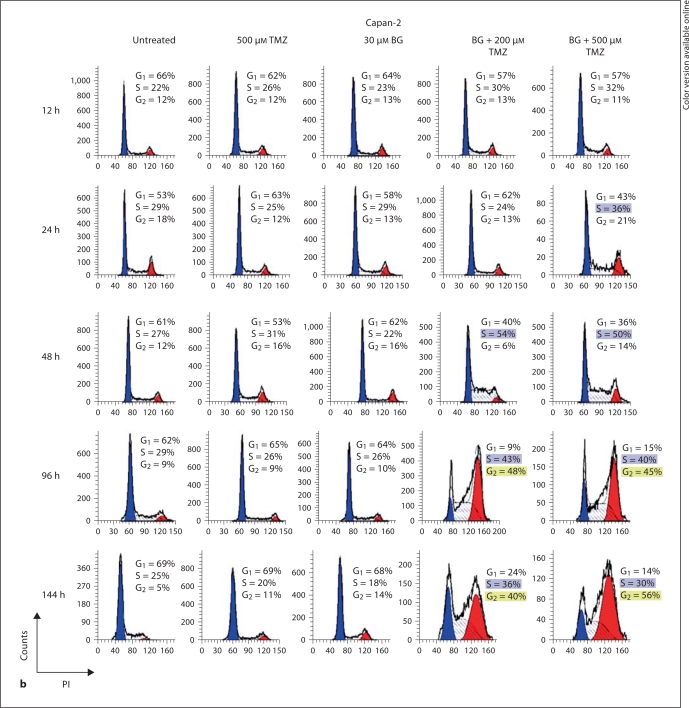
The absence of an effect of TMZ alone in pancreatic tumor cells is further demonstrated in figure 3 by cell cycle kinetics studied by flow cytometry. Cell cycle kinetics in both Capan-1 and Capan-2 remained nearly unaltered after treatment with TMZ at concentrations as high as 500 μM (fig. 3). The continuous treatment of cell cultures with BG, starting 1 day prior to TMZ exposure, resulted in the severe inhibition of S phase progression and subsequently led to a G2/M block in both Capan-1 and Capan-2 cells (fig. 3). The robust G2/M block observed in both the cell lines coupled with the apparent greater extent of the inhibition of mitosis and the greater apoptotic indices in Capan-2 compared to Capan-1 (fig. 2b) indicated a stricter G2/M cell cycle block in Capan-2. The higher G2 population in Capan-1 compared to Capan-2 at 48 h supports our thesis that the higher incidence of apoptosis in Capan-2 is dependent on p53- and not MMR-related processes that operate in G2.
Fig. 3.
BG/TMZ-induced cell cycle kinetics. Kinetics of the cell cycle inhibition of human pancreatic tumor cell lines mut-p53 Capan-1 (a) and wt-p53 Capan-2 (b) in response to treatment with TMZ (500 μM), BG (30 μM), or TMZ + BG. The absence of G1/S inhibition leading to S accumulation and a G2 cell cycle arrest 2 days after treatment with BG/TMZ, but not with TMZ alone, can be observed. TMZ alone had a marginal effect on Capan-2 by arresting progression from G1 to S at 24 h. However, the absence of an inhibition of S phase progression and of G2 arrest in cells treated with TMZ alone signifies the importance of O6-MeG in the onset of S phase inhibition and of a cell cycle block at G2. The following differences between Capan-1 and Capan-2 are observed following treatment with BG/TMZ: In Capan-1 cells, the percentage of cells declined sharply in G1 compared to Capan-2, indicating a role of G1/S; the increase in cells in the S phase is faster and more profound in Capan-1 than in Capan-2, signifying a more rapid transition from G1 to S in Capan-1, and there is a difference in the kinetics for the accumulation of cells in G2 between Capan-1 and Capan-2 with an earlier accumulation of Capan-1 cells (at 48 h). PI = Propidium iodide.
Effects of BG/TMZ Treatment on the Expression of Proteins Involved in Cell Cycle Arrest, Apoptosis, and DNA Repair in MEFs and Capan-1 and Capan-2 Cells
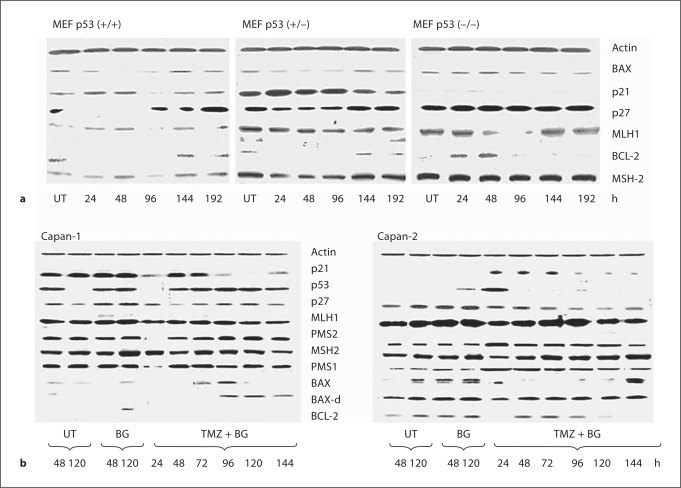
The kinetics of the expression of proteins involved in cell cycle arrest, i.e. p21 and p27, antiapoptotic BCL-2, and proapoptotic BAX, was analyzed in p53 (+/+), (+/–), and (–/–) MEF cells at different time intervals following treatment with BG/TMZ (fig. 4a). Levels of β-actin were assessed as loading controls (fig. 4a). Basal levels of p21 were significantly lower in untreated MEF (–/–) than in MEF (+/–) and MEF (+/+) cells. The p21 levels did not change in response to BG/TMZ in the p53-null cell line, but were markedly upregulated in p53-efficient MEFs up to 48 h after drug exposure. The proapoptotic BAX was weakly present in MEF (+/+, +/–), while it was modestly expressed in p53 (–/–) MEF cells. Its expression was enhanced at late stages following treatment in p53 (+/–) and (+/+) cells. On the other hand, expression of BCL-2 was markedly downregulated immediately after treatment with BG/TMZ in p53-efficient lines and was found to be upregulated in p53-null MEFs. These results suggest that induction of p21 by BG/TMZ may be linked with apoptosis via a decrease in BCL-2/BAX ratios. Unlike p21, which was upregulated in MEF (+/+, +/–) cells, p27 was significantly downregulated, suggesting the facilitation of a G1-to-S transition.
Fig. 4.
Changes in the treatment-induced expression of proteins involved in the cell cycle, DNA MMR, apoptosis, and survival. a Determination of the effect of p53 on the BG/TMZ-induced expression of BAX, BCL-2, p21, p27, MLH-1, and MSH-2 using isogenic lines MEF (+/+), MEF (+/–), and MEF (–/–). BG/TMZ treatment elevates p21 levels in p53-efficient MEF lines and downregulates the expression of p27, while it has no effect on the expression of these proteins in p53-null cells. Antiapoptotic BCL-2 is lost while proapoptotic BAX is elevated in p53-efficient MEFs in response to BG/TMZ treatment. BCL-2 is elevated while BAX remains unchanged in p53-null MEF under the same conditions. MLH-1 is downregulated between 48 and 96 h in MEF (+/–) and MEF (–/–), while MSH-2 is downregulated between 24 and 96 h in MEF (+/–). Changes in MMR protein levels are not related to apoptosis. b Determination of the effects of BG/TMZ treatment on the expression of p53, BAX, BAX-d, BCL-2, p21, p27, MLH1, PMS1, PMS2, and MSH2 in Capan-1 and Capan-2. The expression of p53 and p21 is lost for 24 h in Capan-1, but it is induced for 24 and 72 h in Capan-2 in response to BG/TMZ treatment. p27 is downregulated in both Capan-1 and Capan-2 in response to BG/TMZ treatment. Although BAX is downregulated by BG/TMZ, its levels are higher in Capan-2 than in Capan-1 at 24 and 144 h after treatment. BCL-2 is highly expressed in Capan-2 and is severely suppressed at 24, 120, and 144 h following BG/TMZ treatment. Expression of PMS1, PMS2 and MLH1 was enhanced, while that of MSH2 reached a zenith at 144 h in response to BG/TMZ treatment in Capan-2. Suppression of PMS1 and PMS2 at 24 h was observed in Capan-1 in response to BG/TMZ. UT = Untreated.
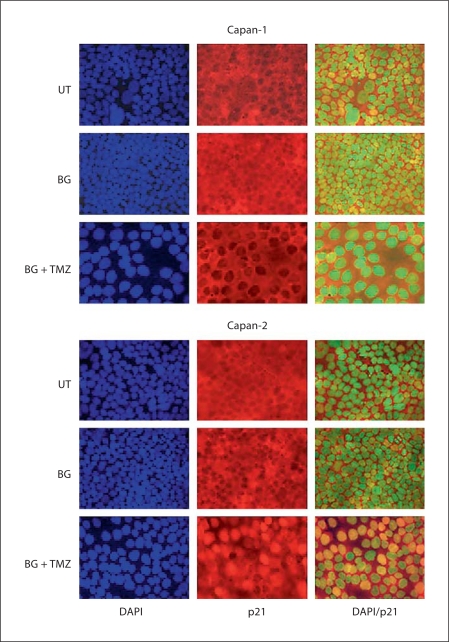
The kinetics of p53, p21, BCL-2, and BAX expression in Capan-1 and Capan-2 in response to BG/TMZ treatment are shown in figure 4b. Capan-1 showed elevated basal levels of p53 and p21. In Capan-2, p53 was upregulated in response to BG/TMZ treatment. Such upregulation was retained for 24 h with a concomitant increase in p21 that lasted 72 h. This coincided with a decline in the G1 population from 57 to 36% and a concomitant increase in the S population from 32 to a maximum of 50% with stable G2 (fig. 3b). Although p21 function was restored at 48 h and was maintained for 72 h at near-control levels in Capan-1, G1 was suppressed and cells accumulated exclusively in S (64%) and G2 (22%). After 72 h, there was a marked loss of p21 and the reemergence of p27 that was suppressed at 48 h (fig. 4b). To further substantiate the role of p21 in apoptosis in response to BG/TMZ treatment, the nuclear translocation of p21 was analyzed by immunofluorescence in Capan-1 and Capan-2 cells (fig. 5). An increased nuclear presence of p21 was observed in Capan-2 cells treated with BG/TMZ at 48 h, but not in those treated with BG alone, and this translocation was absent in Capan-1 cells (fig. 5). Since cytoplasmic p21 may protect cells from growth arrest, it is suggested that the high expression of p21 in p53-deficient MEF and Capan-1 is related to the protection of these cells from apoptosis.
Fig. 5.
Effects of BG/TMZ treatment on the intracellular distribution of p21. Nuclear translocation of p21, as determined by the yellow color in the nucleus in superimposed images of p21 (red) and the DAPI-stained nucleus (pseudo blue color), was present in Capan-2 cells but not in Capan-1 cells in response to 30/500 μM BG/TMZ treatment after 48 h. BG alone shows the absence of nuclear translocation of p21 in both cell lines. UT = Untreated.
Analysis of the expression of BAX and BCL-2 in pancreatic cancer cells showed that BAX-d expression remained nearly constant in Capan-2 following treatment with BG/TMZ, while BCL-2 was eliminated at 24 h posttreatment (fig. 4b). These results are similar to those observed in MEF (+/–, +/+) lines (fig. 4a), indicating that marked changes in BAX/BCL-2 ratios immediately after BG/TMZ treatment may be linked to early apoptotic death in Capan-2 but not in Capan-1, in which BAX-d is elevated 96 h after treatment. Transition of cells from G1 to S may be facilitated in Capan-2 by a reduction of p27 levels in response to BG/TMZ treatment similar to MEFs (+/+, +/–). Since induction of p21 and loss of p27 is likely to result in S phase accumulation, it is postulated that the less severe S phase block in Capan-2 is due to a p21-dependent apoptosis of S phase cells having O6-MeG adducts. Moreover, these results indicate that mitosis may be inhibited by p27, while cells die in G2 in response to a marked elevation of BAX/BCL-2 ratios.
The onset of an MMR response to O6-MeG:C(T) pairs that was maintained by the continuous presence of BG was manifested by the marked G2 arrest which was associated with increased death rates in both p53-efficient and p53-deficient cell cultures at time intervals from 96 to 144 h for Capan-2 and from 48 to 144 h for Capan-1 (fig. 2b, 3). The magnitude and kinetics of the MMR response were examined by assessing the induction of the MMR proteins, MLH1, PMS1 and PMS2, and MSH2 in these 2 cell lines in response to BG/TMZ (fig. 4b). PMS1 and PMS2 were expressed highly in untreated Capan-1. Their levels declined 24 h after treatment and resurged at 48 h. MSH2 and MLH-1, however, remained unchanged in response to BG/TMZ. In contrast to Capan-1, PMS1 and PMS2 were induced in Capan-2 at 24 h after treatment and remained highly expressed. MSH2 reached a zenith at 144 h, while MLH-1 was upregulated 24 h after treatment but declined afterwards. The levels of MMR proteins fluctuated in a manner that may show a possible loss of MMR control early on after treatment in Capan-1, but an increased MMR control in both Capan-1 and Capan-2 at time intervals exceeding 72 h after treatment. This fluctuation in MMR proteins which occurs primarily in p53-efficient cells that arrest in G1 in response to DNA methylation needs to be further investigated in order to assess its significance in MMR-related late cellular death.
The Role of p53 in Cellular Response to BG/TMZ Treatment in MEF Cells
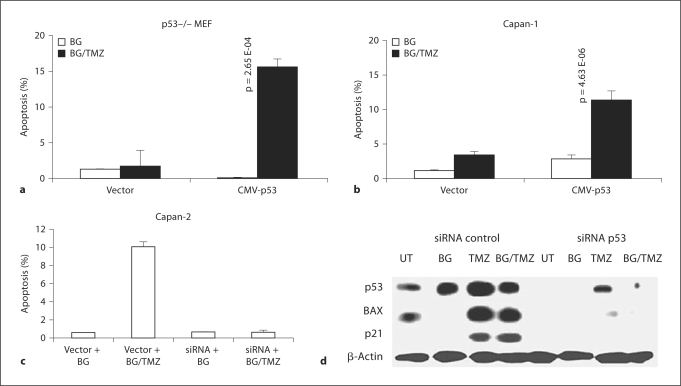
In order to further examine the effect of p53 on the cell response to TMZ treatment, p53 (–/–) MEF cells were transiently transfected using the CMV-p53 or vector-alone constructs. Transiently transfected cells were treated with either BG or with BG/TMZ and then TUNEL assay was performed at 48 h after treatment (fig. 6a). MEF (–/–) cells transfected with CMVp53 showed a marked increase in cell death (16.0%) in response to BG/TMZ treatment (fig. 6a).
Fig. 6.
Role of p53 in induction of apoptosis in MEF p53 (–/–) and Capan-1 and Capan-2 cells. a Restoration of TMZ sensitivity in p53 (–/–) MEF transiently transfected with CMV-p53. TMZ-induced apoptosis in p53 (–/–) transfectants was determined by TUNEL assay. Transfectants were treated with 30 μM BG or 500 μM TMZ plus 30 μM BG and subjected to TUNEL analysis. Bars depict the average from 2 independent experiments ± standard error of approximately 1,000 cells scored in each experiment. b Restoration of TMZ sensitivity in Capan-1 cells transiently transfected with CMV-p53. TMZ-induced apoptosis in the transfectants was determined by TUNEL assay. Transfectants were treated with 30 μM BG or 500 μM TMZ plus 30 μM BG and subjected to TUNEL analysis. Bars depict the average from 2 independent experiments ± standard error of approximately 1,000 cells scored in each experiment. c Reversal of TMZ sensitivity in Capan-2 cells transiently transfected with p53 siRNA. TMZ-induced apoptosis in Capan-2 transfectants was determined by TUNEL assay. Transfectants (control siRNA or p53 siRNA) were treated with 30 μM BG alone or 500 μM TMZ plus 30 μM BG and subjected to TUNEL analysis. Approximately 1,000 cells in total were scored in each experiment (p < 0.05). d Western blot analysis of the transient transfectants of Capan-2 (vector siRNA or p53 siRNA) left untreated or treated with BG alone, 500 μM TMZ alone, and 500 μM TMZ plus 30 μM BG for p53, BAX, and p21 proteins. β-Actin was used as the loading control. UT = Untreated.
The Role of p53 in Cellular Response to BG/TMZ Treatment in Capan-1 and Capan-2 Cells
TUNEL assay performed at 48 h after treatment with BG/TMZ showed that sensitivity to the treatment was restored in mut-p53 Capan-1 cells that were transiently transfected with pCMV-p53 (fig. 6b). Thus, the p53 gain-of-function in MEF (–/–) and Capan-1 resulted in an induction of apoptosis equal to its normal endogenous p53-efficient counterparts MEF (+/–) and MEF (+/+) cells (fig. 6a, 1a) and Capan-2 (fig. 6b, 2a), respectively.
The role of p53 in the induction of apoptosis in response to treatment with BG/TMZ was further confirmed with a loss-of-function approach. The disruption of p53 function in Capan-2 cells by transient transfections with p53 siRNA construct (loss of p53, BAX, and p21 expressions; fig. 6d) resulted in a marked reduction in apoptosis during the first 48 h after treatment with BG/TMZ (fig. 6c).
Discussion
In this study, we have demonstrated that DNA-methylating antineoplastic agent TMZ is ineffective against highly MGMT-expressing pancreatic tumor cells. This is in agreement with the previously demonstrated failure of BCNU and TMZ to elicit a response in human pancreatic tumor xenografts without the aid of MGMT inactivators [11]. Based on the high levels of MGMT in human pancreatic neoplasms [10], it has been postulated that such neoplasms are immune to treatment with agents that exert toxicity via the alkylation of the O6 position of guanine. MGMT is the primary and most effective line of defense of tumors against DNA-alkylating agents, and its depletion with MGMT pseudosubstrates such as BG results in a marked increase in the efficacy of such drugs [12,31,32,33]. However, this does not necessarily imply that the inactivation of MGMT renders tumors entirely susceptible to treatment with alkylating agents [27]. The response to DNA damage induced by methylating agents is dependent on the mutation spectrum of the tumor cell [27] which determines whether the tumor will undergo translational DNA replication, leading to mutations, or die. Immerging research has demonstrated that methyl DNA adducts activate cell cycle checkpoints and various signal transduction pathways that are generally believed to coordinate cell cycle progression with DNA repair and apoptosis [34]. It is now established that ATM and ATR (ATM and Rad3 related) act as check point transducers by phosphorylating numerous target proteins involved in cell cycle arrest, DNA repair, and apoptosis [35,36,37]. ATM phosphorylates p53 on Ser15 in response to DSBs, while ATR is the principal signal transducer that phosphorylates p53 on Ser15 and Ser37 when cells are challenged with agents that interfere with DNA replication and also enforces and sustains cell cycle arrest and the checkpoints induced by DSBs [35,36,37]. ATM and ATR also phosphorylate Chk1 and Chk2 [35,36,37,38], which in turn phosphorylate p53 on Ser20 and the Cdc25A and Cdc25C phosphatases on Ser123 and 216, respectively [39,40]. Phosphorylation of Cdc25A prevents the activation of cyclin-dependent kinases CDK2 and CDK1, contributing to the G1, S, and G2 checkpoints [39,40]. In addition, ATR and/or ATM phosphorylate a number of target proteins, including BRCA1, NBS1, and SMC1 [35,36,37], which participate in DNA damage-induced checkpoints.
In order to activate cell cycle checkpoints in response to DNA damage, the cell must possess a functional MMR system [33,41,42,43,44,45,46]. MMR-deficient cells do not phosphorylate p53 on Ser15 and Ser392 when treated with methylating agents N-methyl-N′-nitro-N-nitrosoguanidine (MNNG), N-methyl-N-nitrosourea, and TMZ. These cells fail to arrest at the G2/M phase and do not undergo apoptosis in response to drug concentrations that are highly effective in killing MMR-proficient cells [33,41,42,43]. Abrogation of MSH2 expression in HeLa cells leads to the impaired phosphorylation of Chk1 and SMC1, as well as defective S phase checkpoint activation after treatment with MNNG [46]. Moreover, mitogen-activated protein kinase p38 which is involved in drug-induced G2/M arrest is activated in MMR-proficient, but not in MMR-deficient, cells in response to TMZ [45].
An immediate response to p53 phosphorylation and nuclear translocation is the upregulation of p21. In spite of the overwhelming data showing the importance of p21 in apoptosis and cell cycle inhibition in response to radiation damage [47], the role of this and other CDK inhibitors induced by alkylating agents is poorly understood. Upregulation of p21 is likely to inhibit CDK2 and possibly CDK4 in combination with p27, thus resulting in G1 arrest. Similarly, p21 is likely to inhibit CDK1, leading to G2/M arrest. Existing data show a controversial role for p21 in the response of tumors to treatment with methylating agents [27,45]. This controversy may result from variations in the localization of p21 in the cell, which is known to determine its function [48]. Cytoplasmic localization of p21 is highly correlated with the overexpression of phospho-p21 (T145) due to higher AKT activity since both cytoplasmic p21 and the overexpression of phospho-p21 (T145) are associated with a high expression of HER2/neu and phospho-Akt. The observation that cytoplasmic localization of p21 and the overexpression of phospho-p21 (T145), HER2/neu, and phospho-AKT are all associated with a worse overall survival [49] suggests that cells with a prevalent prosurvival signal transduction silence their p21 as a cell cycle inhibitor via its phosphorylation and, subsequently, its cytoplasmic translocation. This is further supported by accumulating evidence suggesting that p21 located in the cytoplasm might play a role in promoting transformation and tumor progression in ras-transformed cells where there is an increase of cytoplasmic p21 that is related to increased MEK activity [50]. Furthermore, inhibition of the PI3-kinase pathway results in the loss of cytoplasmic p21 expression. Accordingly, localization of p21 in the cytoplasm in transformed cells has been shown to contribute to pathways that favor not only cell proliferation but also cell motility, invasion, and metastasis [50]. Our results show that TMZ reverses the cytoplasmic accumulation of p21, thus contributing to the loss of prosurvival characteristics and the facilitation of apoptosis. In particular, treatment of Capan-2 with BG/TMZ results in the induction of p53, as expected, and the upregulation of p21. The persistence of p21 in Capan-2 for 72 h coincides with a slower G1-to S-transition in these cells as compared with Capan-1. The S phase reaches a zenith at 48 h and then declines, while G2 increases gradually to a zenith at 144 h. Because of the presence of p21 and p27 it is suggested that Capan-2 is blocked both in G1/S and G2/M and that cells die as a result of these 2 blocks. A gradual increase in BAX from 72 to 144 h and a concomitant decline of BCL-2 in the same time interval suggest that these 2 proteins participate in cell death following the disappearance of p21 and the progression of Capan-2 into S and G2. This delayed killing related to BAX/BCL-2 is p53-dependent and is not present in Capan-1.
The complexity of DNA damage caused by methylating agents is generally due to the variety of lesions induced and the various levels of toxicity such lesions exert. It is generally accepted that DNA damage, other than O6-methylguanine, that is induced by methylating agents is easily repaired by base or nucleotide excision. This view is in accordance with our observation that, in the absence of BG, pancreatic tumor cells having high levels of MGMT activity neither arrest nor die when treated with TMZ alone. It is, therefore, postulated that all the cell cycle effects observed in response to treatment with BG/TMZ are caused by or are at least related to O6-MeG, which is kept unrepaired throughout the experimental interval studied here. Previous experiments [51,52] suggest that the use of high levels of TMZ results in the activation of ATM/ATR and the imposition of an MMR-independent cell cycle arrest possibly viathe expression of Chk2, Chk1, and p38. In contrast to this situation, when cells are treated with low doses of TMZ they undergo a single cycle before arresting in G2 in an MMR-dependent manner. Obviously, 1 cycle of translation (containing O6-MeG lesions) DNA replication will result in the accumulation of O6-MeG:T mismatches that could lead to C:C to A:T mutagenesis if the O6-MeG is repaired at this stage. In order to avoid mutations, a strict inhibition of the MGMT-mediated repair of O6-MeG should be enforced in G2, and this is achieved by the continuous administration of BG.
The experiments presented here show that, in spite of the high concentrations of TMZ used, pancreatic tumor cells eventually undergo a p53-independent MMR-mediated G2 arrest that is associated with a marked upregulation of MMR proteins. Such a G2 arrest follows a p53-mediated delay in the G1-to-S transition that coincides with the nuclear accumulation of p21 in p53-efficient Capan-2. The upregulation of p21 and BAX activities in p53-efficient cells leads to a G1/S arrest and apoptosis, respectively. These events do not occur in p53-defective cells, possibly due to the presence of high levels of basal p21 which remains cytoplasmic and fails to translocate to the nucleus in response to DNA damage. A failure to arrest in G1/S may result in the replication of damaged DNA in p53-deficient cells and the appearance of O6-MeG:T mismatches which, in the absence of an effective MMR, could result in mutations. This is supported by the observation that many MMR proteins are not immediately upregulated in response to BG/TMZ treatment in p53-deficient cells, as they are in their p53-efficient counterparts. Significant levels of MMR proteins may be reached after several days following TMZ treatment in p53-defective cells, at which time the cell cycle is blocked in G2. As a consequence of a slow activation of MMR and a failure to arrest in G1/S, p53-inefficient cells may undergo mutagenic transformation instead of apoptosis. This is in accordance with our previous observation that mutagenic transformation in response to TMZ treatment is greater in p53-defective tumors as compared to wt-p53 tumors.
The findings of this study have important implications for the treatment of pancreatic and other neoplasms expressing high levels of MGMT activity. DNA-damaging agents, including TMZ, induce a G1-to-S transition delay in p53-efficient pancreatic tumor cells which allows for the repair of O6-MeG mismatches with thymine instead of cytosine, even if MGMT is initially inactivated by a single treatment of BG. Thus an extensive G1/S arrest in p53-efficient tumors may allow the repair of mismatches, which could lead to mutagenesis and survival. Such repair in p53-efficient tumors is prevented if MGMT is kept suppressed for several days following exposure to TMZ. Providing that MGMT is suppressed throughout a G1/S arrest, the efficacy of TMZ is enhanced in p53-efficient tumors due to the upregulation of the BAX/BCL-2 ratios that facilitate apoptosis (MGMT-independent/p53-dependent) and the upregulation of MMR proteins, which may enhance tumor cell killing in G2 (MGMT dependent/p53 independent).
Acknowledgements
This work was supported by NIH grant CA86937 to M.M.A.
References
- 1.Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol. 2004;34:238–244. doi: 10.1093/jjco/hyh045. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Hennig R, Ding XZ, Adrian TE. On the role of the islets of Langerhans in pancreatic cancer. Histol Histopathol. 2004;19:999–1011. doi: 10.14670/HH-19.999. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Rubio E, Evans TR, Tabemero J, Cassidy J, Sastre J, Eatock M, Bisset D, Regueiro P, Baselga J. Capecitabine (xeloda) in combination with oxaliplatin: A phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol. 2002;13:558–565. doi: 10.1093/annonc/mdf065. [DOI] [PubMed] [Google Scholar]
- 5.Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Rubio E. New chemotherapeutic advances in pancreatic, colorectal, and gastric cancers. Oncologist. 2004;9:282–294. doi: 10.1634/theoncologist.9-3-282. [DOI] [PubMed] [Google Scholar]
- 7.Oster MW, Gray R, Panasci L, Perry MC. Chemotherapy for advanced pancreatic cancer. A comparison of 5-fluorouracil, adriamycin, and mitomycin (FAM) with 5-fluorouracil, streptozotocin, and mitomycin (FSM) Cancer. 1986;57:29–33. doi: 10.1002/1097-0142(19860101)57:1<29::aid-cncr2820570108>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Hilgers W, Kern SE. Molecular genetic basis of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 1999;26:1–12. [PubMed] [Google Scholar]
- 9.Frey C, Twomey P, Keehn R, Elliott D, Higgins G. Randomized study of 5-FU and CCNU in pancreatic cancer: report of the Veterans Administration Surgical Adjuvant Cancer Chemotherapy Study Group. Cancer. 1981;47:27–31. doi: 10.1002/1097-0142(19810101)47:1<27::aid-cncr2820470106>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Kokkinakis DM, Ahmed MM, Delgado R, Fruitwala MM, Mohiuddin M, Albores-Saavedra J. Role of O6-methylguanine-DNA methyltransferase in the resistance of pancreatic tumors to DNA alkylating agents. Cancer Res. 1997;57:5360–5368. [PubMed] [Google Scholar]
- 11.Kokkinakis DM, Ahmed MM, Chendil D, Moschel RC, Pegg AE. Sensitization of pancreatic tumor xenografts to carmustine and temozolomide by inactivation of their O6-methylguanine-DNA methyltransferase with O6-benzylguanine or O6-benzyl-2’-deoxyguanosine. Clin Cancer Res. 2003;9:3801–3807. [PubMed] [Google Scholar]
- 12.Preuss I, Thust R, Kaina B. Protective effect of O6-methylguanine-DNA methyltransferase (MGMT) on the cytotoxic and recombinogenic activity of different antineoplastic drugs. Int J Cancer. 1996;65:506–512. doi: 10.1002/(SICI)1097-0215(19960208)65:4<506::AID-IJC19>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Karran P, Stephenson C. Mismatch binding proteins and tolerance to alkylating agents in human cells. Mutat Res. 1990;236:269–275. doi: 10.1016/0921-8777(90)90010-3. [DOI] [PubMed] [Google Scholar]
- 14.Kat A, Thilly WG, Fang WH, Longley MJ, Li GM, Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci USA. 1993;90:6424–6428. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckett DR, Drummond JT, Murchie AI, Reardon JT, Sancar A, Lilley DM, Modrich P. Human mutSalpha recognizes damaged DNA base pairs containing O6-methylguanine, O4methylthymine, or the cisplatin-d(GpG) adduct. Proc Natl Acad Sci USA. 1996;93:6443–6447. doi: 10.1073/pnas.93.13.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamane K, Taylor K, Kinsella TJ. Mismatch repair-mediated G2/M arrest by 6-thioguanine involves the ATR-chk1 pathway. Biochem Biophys Res Commun. 2004;318:297–302. doi: 10.1016/j.bbrc.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Dodson GE, Shi Y, Tibbetts RS. DNA replication defects, spontaneous DNA damage, and ATM-dependent checkpoint activation in replication protein A-deficient cells. J Biol Chem. 2004;279:34010–34014. doi: 10.1074/jbc.C400242200. [DOI] [PubMed] [Google Scholar]
- 18.Yan T, Desai AB, Jacobberger JW, Sramkoski RM, Loh T, Kinsella TJ. CHK1 and CHK2 are differentially involved in mismatch repair-mediated 6-thioguanine-induced cell cycle checkpoint responses. Mol Cancer Ther. 2004;3:1147–1157. [PubMed] [Google Scholar]
- 19.O’Connor PM, Fan S. DNA damage checkpoints: implications for cancer therapy. Prog Cell Cycle Res. 1996;2:165–173. doi: 10.1007/978-1-4615-5873-6_16. [DOI] [PubMed] [Google Scholar]
- 20.Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Li J, Booher RN, Kraker A, Lawrence T, Leopold WR, Sun Y. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res. 2001;61:8211–8217. [PubMed] [Google Scholar]
- 22.Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- 23.Hirose Y, Berger MS, Pieper RO. P53 affects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61:1957–1963. [PubMed] [Google Scholar]
- 24.Li X, Ding X, Adrian TE. Arsenic trioxide causes redistribution of cell cycle, caspase activation, and GADD expression in human colonic, breast, and pancreatic cancer cells. Cancer Invest. 2004;22:389–400. doi: 10.1081/cnv-200029068. [DOI] [PubMed] [Google Scholar]
- 25.Shukla S, Gupta S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol Carcinog. 2004;39:114–126. doi: 10.1002/mc.10168. [DOI] [PubMed] [Google Scholar]
- 26.Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20:3806–3813. doi: 10.1038/sj.onc.1204487. [DOI] [PubMed] [Google Scholar]
- 27.Bocangel DB, Finkelstein S, Schold SC, Bhakat KK, Mitra S, Kokkinakis DM. Multifaceted resistance of gliomas to temozolomide. Clin Cancer Res. 2002;8:2725–2734. [PubMed] [Google Scholar]
- 28.Escobedo J, Koh TJ. Improved transfection technique for adherent cells using a commercial lipid reagent. Biotechniques. 2003;35:936–938. doi: 10.2144/03355bm06. 940. [DOI] [PubMed] [Google Scholar]
- 29.Chendil D, Das A, Dey S, Mohiuddin M, Ahmed MM. Par-4, a pro-apoptotic gene, inhibits radiation-induced NF kappa B activity and Bcl-2 expression leading to induction of radiosensitivity in human prostate cancer cells PC-3. Cancer Biol Ther. 2002;1:152–160. doi: 10.4161/cbt.61. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed MM, Sells SF, Venkatasubbarao K, Fruitwala SM, Muthukkumar S, Harp C, Mohiuddin M, Rangnekar VM. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1. J Biol Chem. 1997;272:33056–33061. doi: 10.1074/jbc.272.52.33056. [DOI] [PubMed] [Google Scholar]
- 31.Kaina B, Christmann M. DNA repair in resistance to alkylating anticancer drugs. Int J Clin Pharmacol Ther. 2002;40:354–367. doi: 10.5414/cpp40354. [DOI] [PubMed] [Google Scholar]
- 32.Meikrantz W, Bergom MA, Memisoglu A, Samson L. O6-alkylguanine DNA lesions trigger apoptosis. Carcinogenesis. 1998;19:369–372. doi: 10.1093/carcin/19.2.369. [DOI] [PubMed] [Google Scholar]
- 33.Kaina B, Ziouta A, Ochs K, Coquerelle T. Chromosomal instability, reproductive cell death and apoptosis induced by O6-methylguanine in Mex-, Mex+ and methylation-tolerant mismatch repair compromised cells: facts and models. Mutat Res. 1997;381:227–241. doi: 10.1016/s0027-5107(97)00187-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 35.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 36.Khanna KK, Lavin MF, Jackson SP, Mulhern TD. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ. 2001;8:1052–1065. doi: 10.1038/sj.cdd.4400874. [DOI] [PubMed] [Google Scholar]
- 37.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 38.Gatei M, Sloper K, Sorensen C, Syljuasen R, Falck J, Hobson K, Savage K, Lukas J, Zhou BB, Bartek J, Khanna KK. Ataxia-telangiectasia-mutated (ATM) and nbs1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J Biol Chem. 2003;278:14806–14811. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- 39.Rhind N, Russell P. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J Cell Sci. 2000;113:3889–3896. doi: 10.1242/jcs.113.22.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–122. doi: 10.1016/s0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- 41.D’Atri S, Tentori L, Lacal PM, Graziani G, Pagani E, Benincasa E, Zambruno G, Bonmassar E, Jiricny J. Involvement of the mismatch repair system in temozolomide-induced apoptosis. Mol Pharmacol. 1998;54:334–341. doi: 10.1124/mol.54.2.334. [DOI] [PubMed] [Google Scholar]
- 42.Duckett DR, Bronstein SM, Taya Y, Modrich P. HmutSalpha-and hmutLalpha-dependent phosphorylation of p53 in response to DNA methylator damage. Proc Natl Acad Sci USA. 1999;96:12384–12388. doi: 10.1073/pnas.96.22.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickman MJ, Samson LD. Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc Natl Acad Sci USA. 1999;96:10764–10769. doi: 10.1073/pnas.96.19.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown KD, Rathi A, Kamath R, Beardsley DI, Zhan Q, Mannino JL, Baskaran R. The mismatch repair system is required for S-phase checkpoint activation. Nat Genet. 2003;33:80–84. doi: 10.1038/ng1052. [DOI] [PubMed] [Google Scholar]
- 45.Hirose Y, Katayama M, Stokoe D, Haas-Kogan DA, Berger MS, Pieper RO. The p38 mitogen-activated protein kinase pathway links the DNA mismatch repair system to the G2 checkpoint and to resistance to chemotherapeutic DNA-methylating agents. Mol Cell Biol. 2003;23:8306–8315. doi: 10.1128/MCB.23.22.8306-8315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Yang Y, Schofield MJ, Du C, Fridman Y, Lee SD, Larson ED, Drummond JT, Alani E, Hsieh P, Erie DA. DNA bending and unbending by MutS govern mismatch recognition and specificity. Proc Natl Acad Sci USA. 2003;100:14822–14827. doi: 10.1073/pnas.2433654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HS, Cho HJ, Park SJ, Park KW, Chae IH, Oh BH, Park YB, Lee MM. The essential role of p21 in radiation-induced cell cycle arrest of vascular smooth muscle cell. J Mol Cell Cardiol. 2004;37:871–880. doi: 10.1016/j.yjmcc.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Chen T, Turner J, McCarthy S, Scaltriti M, Bettuzzi S, Yeatman TJ. Clustering-mediated apoptosis is regulated by adenomatous polyposis coli and is p21 dependent but p53 independent. Cancer Res. 2004;64:7412–7419. doi: 10.1158/0008-5472.CAN-04-2077. [DOI] [PubMed] [Google Scholar]
- 49.Xia W, Chen JS, Zhou X, Sun PR, Lee DF, Liao Y, Zhou BP, Hung MC. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res. 2004;10:3815–3824. doi: 10.1158/1078-0432.CCR-03-0527. [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Helfman DM. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem. 2004;279:1885–1891. doi: 10.1074/jbc.M306968200. [DOI] [PubMed] [Google Scholar]
- 51.Caporali S, Falcinelli S, Starace G, Russo MT, Bonmassar E, Jiricny J, D’Atri S. DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol. 2004;66:478–491. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 52.Hirose Y, Katayama M, Berger MS, Pieper RO. Cooperative function of Chk1 and p38 pathways in activating G2 arrest following exposure to temozolomide. J Neurosurg. 2004;100:1060–1065. doi: 10.3171/jns.2004.100.6.1060. [DOI] [PubMed] [Google Scholar]