Abstract
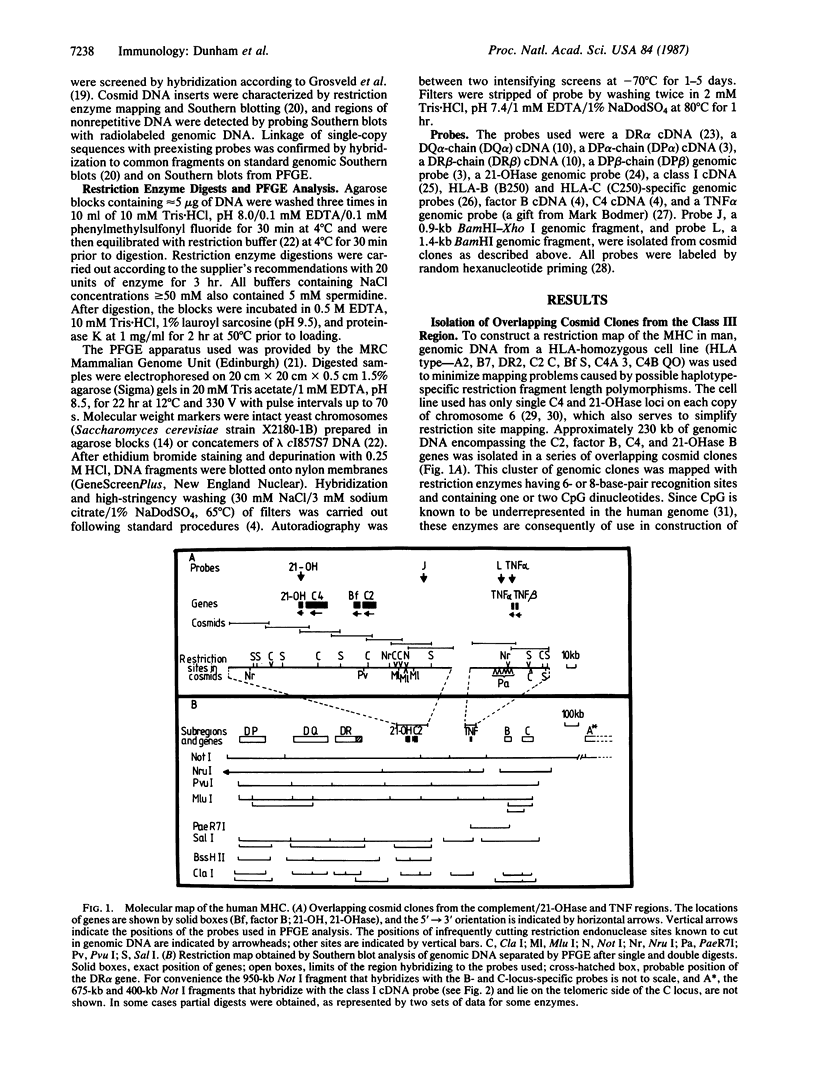
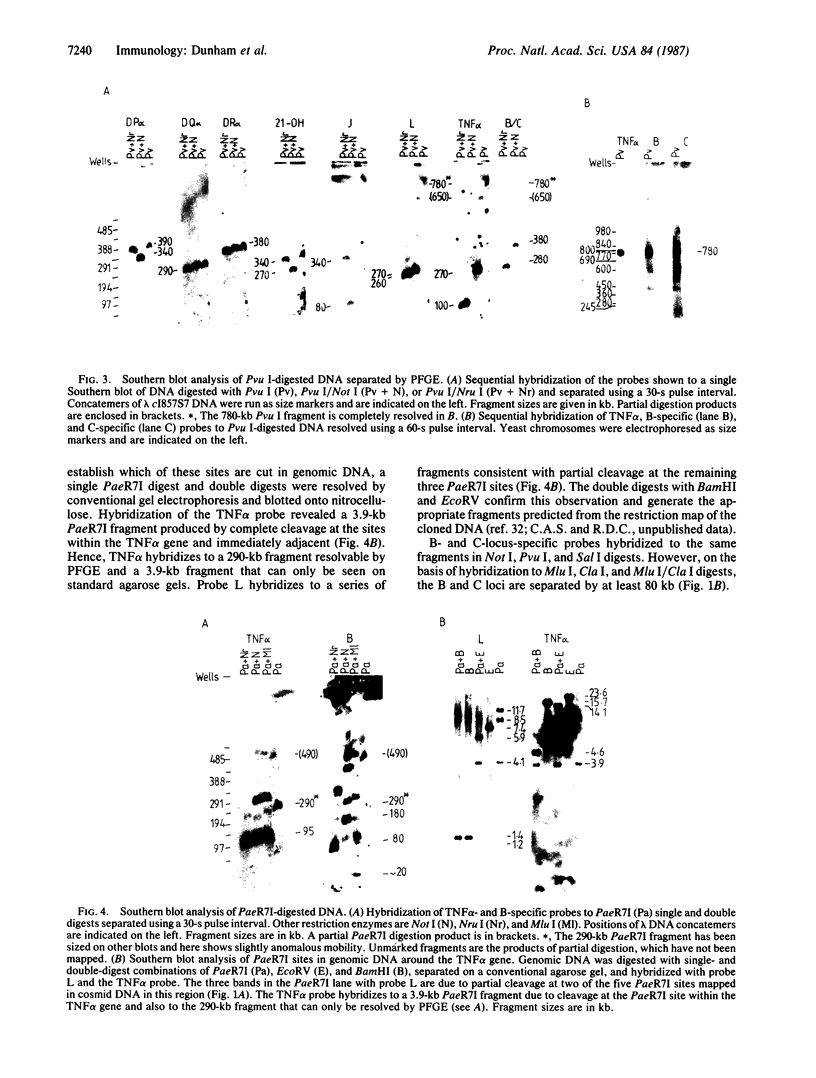
Pulsed-field gel electrophoresis and "cosmid walking" have been used to establish a molecular map of the human major histocompatibility complex (MHC). We have isolated approximately equal to 230 kilobases (kb) of genomic DNA in overlapping cosmid clones covering the genes for the second and fourth components of complement (C2 and C4, respectively), factor B, and steroid 21-hydroxylase, and approximately equal to 82 kb of genomic DNA surrounding the genes for the tumor necrosis factors alpha and beta. Single-copy hybridization probes isolated from these cosmid clusters and probes for the known MHC gene loci were hybridized to Southern blots of genomic DNA that had been digested with infrequently cutting restriction endonucleases and separated on pulsed-field gels. The data obtained allowed the construction of a long-range genomic restriction map and indicated that the MHC spans 3800 kb. This map orients the MHC class III gene cluster with respect to the DR subregion; the C2 gene is on the telomeric side of the 21-hydroxylase B gene. In addition we have defined the positions of the genes for the tumor necrosis factors alpha and beta in the human MHC. Genes for the alpha chain of DR and 21-hydroxylase B are separated by at least 300 kb, while the distance between the genes for C2 and tumor necrosis factor alpha is 390 kb. The HLA-B locus lies approximately equal to 250 kb on the telomeric side of the tumor necrosis factor genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Bird A. P. Long-range restriction site mapping of mammalian genomic DNA. 1986 Jul 31-Aug 6Nature. 322(6078):477–481. doi: 10.1038/322477a0. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Porter R. R. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985 Jan;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Palsdottir A., Belt K. T., Porter R. R. Deletion of complement C4 and steroid 21-hydroxylase genes in the HLA class III region. EMBO J. 1985 Oct;4(10):2547–2552. doi: 10.1002/j.1460-2075.1985.tb03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. A., Bell J. I., Long E. O., Lindsten T., McDevitt H. O. Mapping of the class II region of the human major histocompatibility complex by pulsed-field gel electrophoresis. Nature. 1986 Oct 2;323(6087):453–455. doi: 10.1038/323453a0. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. A general method for preparing chromatin containing intact DNA. EMBO J. 1985 Apr;4(4):913–918. doi: 10.1002/j.1460-2075.1985.tb03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm L. U., Olaisen B. Report of the Committee on the Genetic Constitution of Chromosomes 5 and 6. Cytogenet Cell Genet. 1985;40(1-4):128–155. doi: 10.1159/000132172. [DOI] [PubMed] [Google Scholar]
- Lawrance S. K., Smith C. L., Srivastava R., Cantor C. R., Weissman S. M. Megabase-scale mapping of the HLA gene complex by pulsed field gel electrophoresis. Science. 1987 Mar 13;235(4794):1387–1390. doi: 10.1126/science.3029868. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Travers P. J., Carey J., Grosveld F., Jenkins J., Bodmer W. F. Sequence of an HLA-DR alpha-chain cDNA clone and intron-exon organization of the corresponding gene. Nature. 1982 Oct 21;299(5885):750–752. doi: 10.1038/299750a0. [DOI] [PubMed] [Google Scholar]
- Müller U., Jongeneel C. V., Nedospasov S. A., Lindahl K. F., Steinmetz M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature. 1987 Jan 15;325(6101):265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- Müller U., Stephan D., Philippsen P., Steinmetz M. Orientation and molecular map position of the complement genes in the mouse MHC. EMBO J. 1987 Feb;6(2):369–373. doi: 10.1002/j.1460-2075.1987.tb04764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedospasov S. A., Shakhov A. N., Turetskaia R. L., Mett V. A., Georgiev G. P. Molekuliarnoe klonirovanie genov cheloveka, kodiruiushchikh faktory nekroza opukholei: tandemnoe raspolozhenie al'fa- i beta-genov v korotkom segmente (6 tys. par nukleotidov) genoma cheloveka. Dokl Akad Nauk SSSR. 1985;285(6):1487–1490. [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Ragoussis J., van der Bliek A., Trowsdale J., Ziegler A. Mapping of HLA genes using pulsed-field gradient electrophoresis. FEBS Lett. 1986 Aug 11;204(1):1–4. doi: 10.1016/0014-5793(86)81376-x. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Dunham I., Yu C. Y., Carroll M. C., Porter R. R., Campbell R. D. Molecular characterization of the HLA-linked steroid 21-hydroxylase B gene from an individual with congenital adrenal hyperplasia. EMBO J. 1987 Jun;6(6):1653–1661. doi: 10.1002/j.1460-2075.1987.tb02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Shirai T., Yamaguchi H., Ito H., Todd C. W., Wallace R. B. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. 1985 Feb 28-Mar 6Nature. 313(6005):803–806. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- Smith D. I., Golembieski W., Gilbert J. D., Kizyma L., Miller O. J. Overabundance of rare-cutting restriction endonuclease sites in the human genome. Nucleic Acids Res. 1987 Feb 11;15(3):1173–1184. doi: 10.1093/nar/15.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spies T., Morton C. C., Nedospasov S. A., Fiers W., Pious D., Strominger J. L. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8699–8702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Strachan T., Dodge A. B., Smillie D., Dyer P. A., Sodoyer R., Jordan B. R., Harris R. An HLA-C-specific DNA probe. Immunogenetics. 1986;23(2):115–120. doi: 10.1007/BF00377971. [DOI] [PubMed] [Google Scholar]
- Strachan T. Molecular genetics and polymorphism of class I HLA antigens. Br Med Bull. 1987 Jan;43(1):1–14. doi: 10.1093/oxfordjournals.bmb.a072166. [DOI] [PubMed] [Google Scholar]
- Trowsdale J. Genetics and polymorphism: class II antigens. Br Med Bull. 1987 Jan;43(1):15–36. doi: 10.1093/oxfordjournals.bmb.a072168. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Lee J., Kelly A., Carey J., Jenkins J., Travers P., Bodmer W. F. Isolation and sequencing of a cDNA clone for a human HLA-ABC antigen. Mol Biol Med. 1984 Feb;2(1):53–61. [PubMed] [Google Scholar]
- Yu C. Y., Campbell R. D. Definitive RFLPs to distinguish between the human complement C4A/C4B isotypes and the major Rodgers/Chido determinants: application to the study of C4 null alleles. Immunogenetics. 1987;25(6):383–390. doi: 10.1007/BF00396104. [DOI] [PubMed] [Google Scholar]