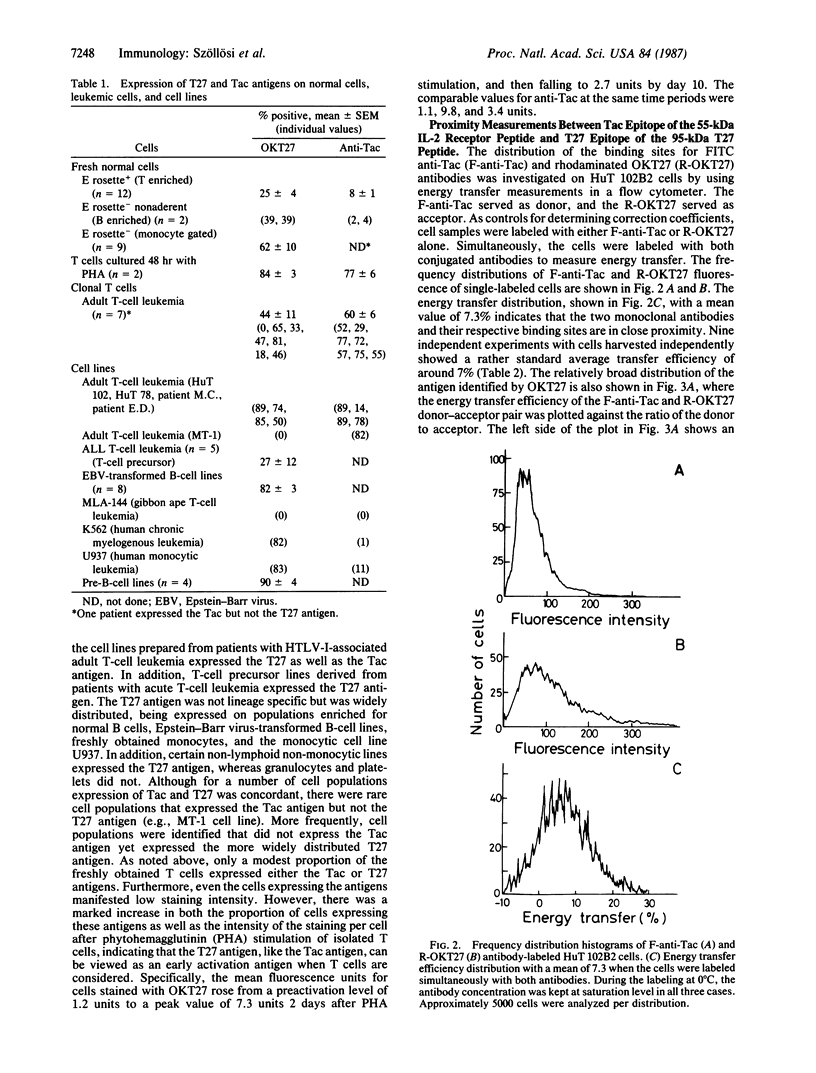
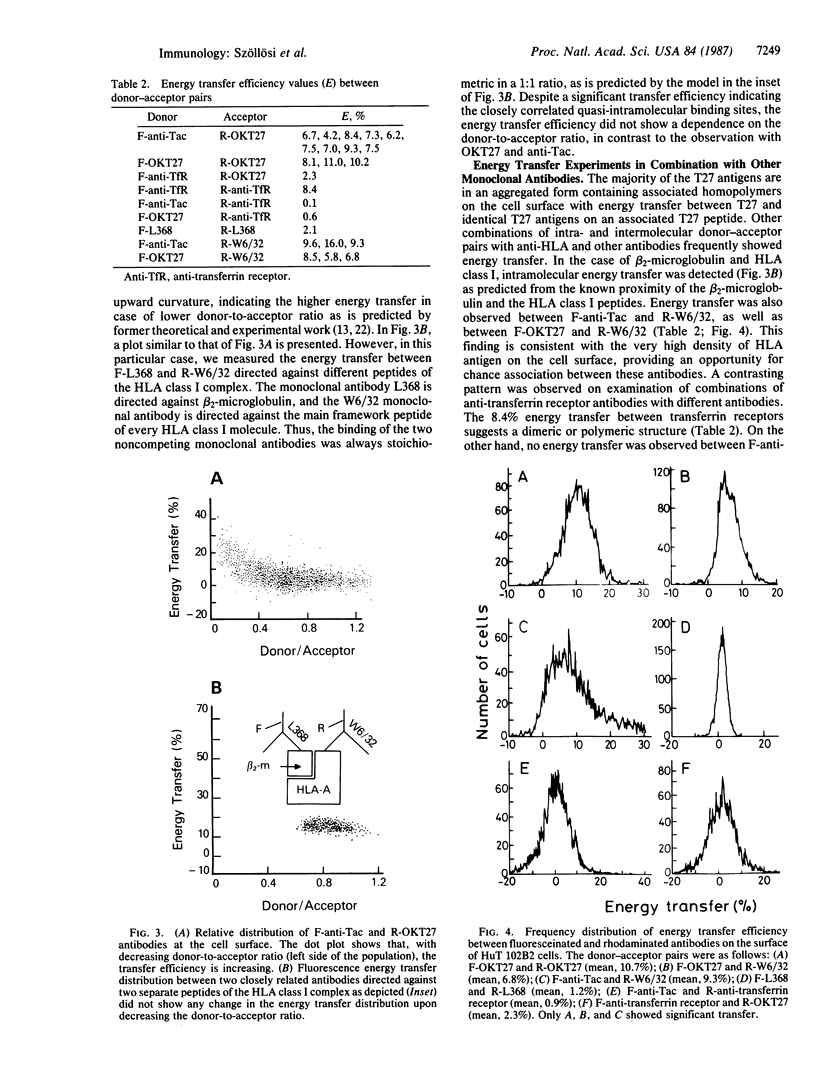
Abstract
Two monoclonal antibodies (OKT27 and OKT27b) have been produced that react with distinct epitopes of a 95-kDa peptide. The T27 antigen is widely distributed, being expressed on B lymphocytes, monocytes, and adult T-leukemic cells but not on polymorphonuclear leukocytes or platelets. There was a low level of T27 expression on resting T cells that increased on T-cell activation. In preliminary studies, the OKT27b antibody coprecipitated a 55-kDa peptide, as well as the 95-kDa peptide, from the radiolabeled cells of the HuT 102B2 cell line. Preclearance with anti-Tac, a monoclonal antibody to the 55-kDa peptide of the multichain interleukin 2 receptor, removed the 55-kDa but not the 95-kDa peptide from subsequent OKT27b immunoprecipitates of HuT 102B2 extracts, suggesting the possibility that the T27 peptide was associated with the Tac peptide. However, the precipitation of the p55 Tac peptide by OKT27b was quite inconsistent. Thus, additional information was sought using a flow cytometric energy transfer technique to provide a physical estimation of the proximity between the Tac and the T27 peptides. The flow cytometric version of the fluorescence resonance energy transfer technique permits the determination of inter- and intramolecular distances at 2- to 10-nm levels on a cell-by-cell basis. Using this approach, there was a mean energy transfer of 7.3% with HuT 102B2 cells when fluorescein isothiocyanate anti-Tac served as the donor and tetramethylrhodamine isothiocyanate OKT27 served as the acceptor. In contrast, there was no energy transfer in comparable studies observed when fluorescein anti-Tac and rhodamine anti-transferrin receptor antibodies were used. These observations support the conclusion that there is a close nonrandom proximity in HuT 102B2 cells between the 95-kDa peptide identified by the OKT27 monoclonal antibody and the p55 Tac peptide of the multichain interleukin 2 receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovich S., Trón L., Szöllösi J., Zidovetzki R., Vaz W. L., Regateiro F., Arndt-Jovin D. J., Jovin T. M. Distribution and mobility of murine histocompatibility H-2Kk antigen in the cytoplasmic membrane. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5985–5989. doi: 10.1073/pnas.80.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The use of singlet-singlet energy transfer to study macromolecular assemblies. Methods Enzymol. 1978;48:347–379. doi: 10.1016/s0076-6879(78)48019-x. [DOI] [PubMed] [Google Scholar]
- Goldman N. D., Liu T. Y. Biosynthesis of human C-reactive protein in cultured hepatoma cells is induced by a monocyte factor(s) other than interleukin-1. J Biol Chem. 1987 Feb 15;262(5):2363–2368. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson B. J., Sharrow S. O., Campbell P. S., Asofsky R. An Lyt differentiated thymocyte subpopulation detected by flow microfluorometry. Nature. 1979 Feb 8;277(5696):478–480. doi: 10.1038/277478a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Spack E. G., Jr, Packard B., Wier M. L., Edidin M. Hydrophobic adsorption chromatography to reduce nonspecific staining by rhodamine-labeled antibodies. Anal Biochem. 1986 Oct;158(1):233–237. doi: 10.1016/0003-2697(86)90614-7. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Trón L., Damjanovich S., Helliwell S. H., Arndt-Jovin D., Jovin T. M. Fluorescence energy transfer measurements on cell surfaces: a critical comparison of steady-state fluorimetric and flow cytometric methods. Cytometry. 1984 Mar;5(2):210–216. doi: 10.1002/cyto.990050216. [DOI] [PubMed] [Google Scholar]
- Trón L., Szöllósi J., Damjanovich S., Helliwell S. H., Arndt-Jovin D. J., Jovin T. M. Flow cytometric measurement of fluorescence resonance energy transfer on cell surfaces. Quantitative evaluation of the transfer efficiency on a cell-by-cell basis. Biophys J. 1984 May;45(5):939–946. doi: 10.1016/S0006-3495(84)84240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys J. 1979 Nov;28(2):197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]