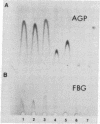
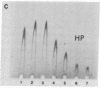
Abstract
One of the oldest and most preserved of the homeostatic responses of the body to injury is the acute phase protein response associated with inflammation. The liver responds to hormone-like mediators by the increased synthesis of a series of plasma proteins called acute phase reactants. In these studies, we examined the relationship of hepatocyte-stimulating factor derived from peripheral blood monocytes to interferon beta 2 (IFN-beta 2), which has been cloned. Antibodies raised against fibroblast-derived IFN-beta having neutralizing activity against both IFN-beta 1 and -beta 2 inhibited the major hepatocyte-stimulating activity derived from monocytes. Fibroblast-derived mediator elicited the identical stimulated response in human HepG2 cells and primary rat hepatocytes as the monocyte cytokine. Finally, recombinant-derived human B-cell stimulatory factor type 2 (IFN-beta 2) from Escherichia coli induced the synthesis of all major acute phase proteins studied in human hepatoma HepG2 and primary rat hepatocyte cultures. These data demonstrate that monocyte-derived hepatocyte-stimulating factor and IFN-beta 2 share immunological and functional identity and that IFN-beta 2, also known as B-cell stimulatory factor and hybridoma plasmacytoma growth factor, has the hepatocyte as a major physiologic target and thereby is essential in controlling the hepatic acute phase response.
Full text
PDF


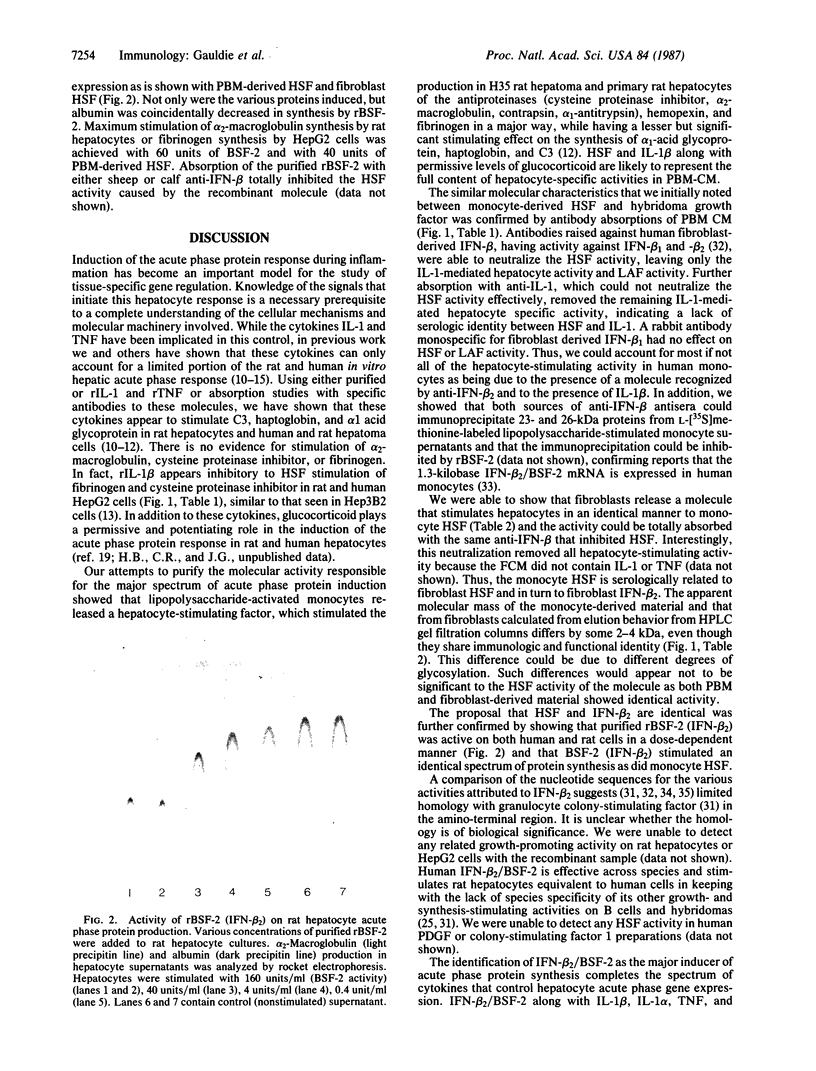

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy T. J., Avery O. T. THE OCCURRENCE DURING ACUTE INFECTIONS OF A PROTEIN NOT NORMALLY PRESENT IN THE BLOOD : I. DISTRIBUTION OF THE REACTIVE PROTEIN IN PATIENTS' SERA AND THE EFFECT OF CALCIUM ON THE FLOCCULATION REACTION WITH C POLYSACCHARIDE OF PNEUMOCOCCUS. J Exp Med. 1941 Jan 31;73(2):173–182. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Baumann H., Onorato V., Gauldie J., Jahreis G. P. Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. J Biol Chem. 1987 Jul 15;262(20):9756–9768. [PubMed] [Google Scholar]
- Bornstein D. L. Leukocytic pyrogen: a major mediator of the acute phase reaction. Ann N Y Acad Sci. 1982;389:323–337. doi: 10.1111/j.1749-6632.1982.tb22147.x. [DOI] [PubMed] [Google Scholar]
- Content J., De Wit L., Poupart P., Opdenakker G., Van Damme J., Billiau A. Induction of a 26-kDa-protein mRNA in human cells treated with an interleukin-1-related, leukocyte-derived factor. Eur J Biochem. 1985 Oct 15;152(2):253–257. doi: 10.1111/j.1432-1033.1985.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Sauder D. N., McAdam K. P., Dinarello C. A. Purified interleukin-1 (IL-1) from human monocytes stimulates acute-phase protein synthesis by rodent hepatocytes in vitro. Immunology. 1987 Feb;60(2):203–207. [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Content J., Volckaert G., Derynck R., Tavernier J., Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986 Sep 15;159(3):625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Jordana M., Newhouse M. T., Gauldie J. Alveolar macrophage/peripheral blood monocyte-derived factors modulate proliferation of primary lines of human lung fibroblasts. J Leukoc Biol. 1987 Jul;42(1):51–60. doi: 10.1002/jlb.42.1.51. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F. Leukocytic endogenous mediator. J Reticuloendothel Soc. 1978 Apr;23(4):287–297. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Regoeczi E., Sauder D. N., Sweeney G. D. The acute-phase response of cultured rat hepatocytes. System characterization and the effect of human cytokines. Biochem J. 1984 Dec 1;224(2):505–514. doi: 10.1042/bj2240505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Sweeney G. D., Regoeczi E., Sauder D. N. A simple bioassay for monocyte-derived hepatocyte stimulating factor: increased synthesis of alpha 2-macroglobulin and reduced synthesis of albumin by cultured rat hepatocytes. J Immunol Methods. 1985 Feb 11;76(2):317–327. doi: 10.1016/0022-1759(85)90309-6. [DOI] [PubMed] [Google Scholar]
- Koj A., Kurdowska A., Magielska-Zero D., Rokita H., Sipe J. D., Dayer J. M., Demczuk S., Gauldie J. Limited effects of recombinant human and murine interleukin 1 and tumour necrosis factor on production of acute phase proteins by cultured rat hepatocytes. Biochem Int. 1987 Mar;14(3):553–560. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- May L. T., Helfgott D. C., Sehgal P. B. Anti-beta-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the beta 2 interferon involved. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8957–8961. doi: 10.1073/pnas.83.23.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Zilberstein A. Interferon-beta 2 living up to its name. Nature. 1987 Feb 12;325(6105):581–582. doi: 10.1038/325581b0. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. An in vitro bioassay for leukocytic endogenous mediator(s) using cultured rat hepatocytes. Inflammation. 1981 Dec;5(4):275–287. doi: 10.1007/BF00911093. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., May L. T., Tamm I., Vilcek J. Human beta 2 interferon and B-cell differentiation factor BSF-2 are identical. Science. 1987 Feb 13;235(4790):731–732. doi: 10.1126/science.3492764. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Sagar A. D. Heterogeneity of poly(I) x poly(C)-induced human fibroblast interferon mRNA species. Nature. 1980 Nov 6;288(5786):95–97. doi: 10.1038/288095a0. [DOI] [PubMed] [Google Scholar]
- Tillett W. S., Francis T. SEROLOGICAL REACTIONS IN PNEUMONIA WITH A NON-PROTEIN SOMATIC FRACTION OF PNEUMOCOCCUS. J Exp Med. 1930 Sep 30;52(4):561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Cayphas S., Opdenakker G., Billiau A., Van Snick J. Interleukin 1 and poly(rI).poly(rC) induce production of a hybridoma growth factor by human fibroblasts. Eur J Immunol. 1987 Jan;17(1):1–7. doi: 10.1002/eji.1830170102. [DOI] [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero C., Sanceau J., Weissenbach J., Beranger F., Falcoff R. Regulation of human gamma-interferon and beta-interferon gene expression in PHA-activated lymphocytes. J Interferon Res. 1986 Apr;6(2):161–170. doi: 10.1089/jir.1986.6.161. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloski B. M., Fuller G. M. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1443–1447. doi: 10.1073/pnas.82.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]