Abstract
Stromal fibroblasts modify the initial recruitment of leucocytes by endothelial cells (EC), but their effects on subsequent transendothelial migration remain unclear. Here, EC and dermal or synovial fibroblasts were cultured on opposite surfaces of 3-μm pore filters and incorporated in static or flow-based migration assays. Fibroblasts had little effect on tumour necrosis factor-α-induced transendothelial migration of neutrophils, but tended to increase the efficiency of migration away from the endothelium. Surprisingly, similar close contact between EC and fibroblasts strongly reduced lymphocyte migration in static assays, and nearly abolished stable lymphocyte adhesion from flow. Fibroblasts did not alter endothelial surface expression of adhesion molecules or messenger RNA for chemokines. Inhibition of attachment did not occur when EC-fibroblast contact was restricted by using 0·4-μm pore filters, but under these conditions pre-treatment with heparinase partially inhibited adhesion. In the 3-μm pore co-cultures, inhibition of metalloproteinase activity partially recovered lymphocyte adhesion, but addition of CXCL12 (SDF-1α) to the endothelial surface did not. Hence, the ability of EC to present activating chemokines for lymphocytes may have been enzymatically inhibited by direct contact with fibroblasts. To avoid contact, we cultured EC and fibroblasts on separate 3-μm pore filters one above the other. Here, fibroblasts promoted the transendothelial migration of lymphocytes. Fibroblasts generate CXCL12, but blockade of CXCL12 receptor had no effect on lymphocyte migration. While stromal cells can provide signal(s) promoting leucocyte migration away from the sub-endothelial space, direct cell contact (which might occur in damaged tissue) may cause disruption of chemokine signalling, specifically inhibiting lymphocyte rather than neutrophil recruitment.
Keywords: endothelial cells, fibroblasts, lymphocytes, migration, neutrophils
Introduction
Extravasation of circulating leucocytes is required for immune surveillance and the inflammatory response to tissue injury and infection. After exposure to inflammatory cytokines, endothelial cells (EC) present selectins and vascular cell adhesion molecule-1 (VCAM-1), which can capture flowing neutrophils and lymphocytes (reviewed in ref. 1). Endothelial surface-presented chemokines may then cause activation of leucocyte integrins (e.g. integrins α4β1 or β2), which support stable attachment and onward migration. For flowing neutrophils, CXC-chemokines induce stable attachment to EC which have been stimulated with tumour necrosis factor-α (TNF-α),2 but we have recently found that a subsequent signal from prostaglandin D2 is also required for efficient transendothelial migration.3 Flow studies in vitro reveal that lymphocytes can also migrate rapidly through activated EC.4–7 In the case of treatment with TNF-α plus interferon-γ (IFN-γ), chemokines acting through CXCR3 were shown to stabilize lymphocyte attachment, but the signals inducing transmigration were not defined.5,8 Exogenous CXCL12 (SDF1-α) added to the surface of EC promoted transmigration,9 but this agent is not produced by the EC themselves. Recently, we reported the continuous migration of lymphocytes back and forth across cytokine-treated endothelial monolayers in a ‘frustrated’ manner.7 In addition, lymphocytes showed little penetration of substrates (such as collagen gel) underneath cultured EC even after hours.7,10,11 On the other hand, neutrophils quickly infiltrated collagen gels underneath cytokine-treated endothelium.7 We proposed that lymphocytes might be actively retained by EC until a separate stromal signal induces migration into the underlying tissue.7
There is increasing evidence that interactions between various stromal cells and EC influence the recruitment of leucocytes.8,12–17 However, the role of pericytes as regulators of the endothelial-mediated leucocyte recruitment remains ambiguous. We showed that co-culturing EC with secretory smooth muscle cells augmented cytokine-induced capture of flowing leucocytes.14 Furthermore, hepatocytes promoted lymphocyte adhesion to hepatic sinusoidal EC in response to lymphotoxin.15 More recently we reported that rheumatoid synovial fibroblasts directly induced endothelial capture of flowing neutrophils and lymphocytes.8,16,17 In contrast, dermal fibroblasts cultured with EC reduced lymphocyte adhesion induced by TNF-α + IFN-γ.8 None of these studies addressed the ability of the recruited leucocytes to migrate through the EC and stroma, because the co-cultured cells were on either side of 0·4-μm pore filters, which did not allow passage of cells. Others have shown that culturing endothelial and epithelial cells on opposite sides of 3-μm pore filters marginally enhanced neutrophil chemotaxis to interleukin-8 (IL-8).13 On the other hand, renal tubular epithelial cells inhibited migration of neutrophils through TNF-α treated EC.18 Dermal fibroblasts from scleroderma patients promoted the migration of a T-cell line through an immortalized EC line coated on an 8-μm pore filter,12 when the fibroblasts were cultured on the plate underneath.
Here we examined whether fibroblasts isolated from the skin or the synovium of patients with rheumatoid arthritis could provide signals to recruited leucocytes, specifically releasing lymphocytes from the endothelium and/or improving their migration efficiency. Initially, the cells were cultured on opposite surfaces of 3-μm pore filters to enable studies of migration through the complete construct. Because we found unexpected inhibition of recruitment of lymphocytes when fibroblasts and EC were brought into such close contact, we also carried out studies using smaller pore filters, and using EC and fibroblasts on closely juxtaposed but separate filters. These studies revealed that fibroblasts could have different effects on neutrophil or lymphocyte recruitment, depending on the degree of the contact with the EC.
Methods
Isolation of human leucocytes, fibroblasts and endothelial cells
Venous blood from healthy individuals was collected in EDTA tubes (Sarstedt, Leicester, UK). Neutrophils were isolated by centrifugation using a two-step density gradient as described.19 Peripheral blood lymphocytes (PBL) were isolated using Histopaque 1077 followed by panning on culture plastic to remove contaminating monocytes.7,8,20 Isolated cells were washed, counted and adjusted to the desired concentration in phosphate-buffered saline containing Ca2+, Mg2+ and 5 mm glucose or Medium 199 (Gibco Invitrogen Compounds, Paisley, UK), both supplemented with 0·15% bovine serum albumin (Sigma-Aldrich, Poole, UK) (PBSA or M199BSA respectively). The PBSA was used for short perfusions in flow-based assays, while M199BSA was used for longer cultures of leucocytes with EC. In some experiments, lymphocytes were treated for 15 min with 1 mg/ml of the CXCR4 inhibitor, AMD3100 (AnorMED Inc., Langley, BC, Canada). We have previously demonstrated that this treatment inhibits lymphocyte recruitment from flow to EC co-cultured with synovial fibroblasts.8
Tissue samples from synovium and overlying skin were obtained from the same patients with rheumatoid arthritis and fibroblasts were isolated as previously described.21 Cells were cultured in RPMI-1640 (Gibco Invitrogen Compounds) supplemented with 10% heat inactivated fetal calf serum, minimum essential medium–non-essential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Sigma) and used between passages 5 and 9.8
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords using collagenase as previously described22 and cultured in M199 supplemented with 20% fetal calf serum, 1 ng/ml epidermal growth factor, 35 μg/ml gentamycin, 1 μg/ml hydrocortisone (all from Sigma) and 2·5 μg/ml amphotericin B (Gibco Invitrogen Compounds).8 All human tissue was obtained with informed consent and with approval from the South Birmingham Local Research Ethics Committee.
Culture of endothelial cells and fibroblasts on Transwell filters
Fibroblasts and EC were dissociated using trypsin/EDTA (Sigma) and co-cultured on the opposite sides of 24-well or six-well, low-density, 3·0 μm or 0·4 μm pore Transwell filter inserts (BD Pharmingen, Cowley, UK) as described.8 Fibroblasts were seeded onto inverted filters at densities based on previous reports8,16 and cultured for 24 hr. HUVEC were then seeded on the inner surface of filters at a concentration that yielded confluent monolayers within 24 hr. For comparison, parallel cultures of HUVEC were seeded inside filters without fibroblasts. The cells were cultured in fibroblast medium for 24 hr or 4 days, after which 100 U/ml TNF-α (Sigma) was added alone or in combination with 10 ng/ml IFN-γ (Peprotech Inc., London, UK) for 4 or 24 hr. In some experiments, a neutralizing antibody against IL-6 (clone 6708, 5 μg/ml; R&D Systems, Abingdon, Oxfordshire, UK) was added when co-culture was established and was present during cytokine treatment. We have previously demonstrated that this treatment inhibits the ability of fibroblasts to modulate EC recruitment of leucocytes from flow.8,16 Alternatively, cytokine-treated endothelial mono- and co-cultures were treated with 100 ng/ml CXCL12 (SDF-1; Peprotech) for 30 min before assay. In another series, EC were cultured alone for 4 days to establish a stable monolayer before co-culture with fibroblasts for 24 hr.
The following enzymes or enzyme inhibitors were added to co-cultures when desired, and were present during the cytokine treatment: 10 μm galardin [a broad-spectrum inhibitor of matrix metalloproteinase (MMP) and some ADAM (a disintegrin and metalloproteinase) family members; BioMed International, Exeter, UK]; 10 mU/ml heparinase I (bacterial homologue to human heparinase23) (Oxford Glycosciences, Abingdon, UK); 30 U/ml hyaluronidase (broad spectrum enzyme which cleaves hyaluronan and chrondroitin sulphate); 100 μg/ml α1-antitrypsin (inhibits trypsin, chymotrypsin, pancreatic and granulocytic elastase and acrosin); 50 μg/ml aprotinin (broad-spectrum serine protease inhibitor) (all from Sigma).
For studies to investigate soluble mediators, conditioned medium was collected from unstimulated fibroblasts or co-cultures. Subsequently, an endothelial monolayer was cultured in this conditioned medium along with TNF-α + IFN-γ for 24 hr. Alternatively, conditioned medium was collected from endothelial mono- or co-cultures following 24 hr of stimulation with TNF-α + IFN-γ, and transferred to a second endothelial monolayer for 24 hr.
Migration of leucocytes through Transwell filters – static assay
Leucocyte migration was assessed using 24-well format Transwell filters as previously described.7,24 Co-cultures were washed to remove residual cytokines, fresh M199BSA was placed in the lower chamber and purified leucocytes (200 μl at 2 × 106cells/ml) were added to the upper chamber. The cells were allowed to settle, adhere and migrate at 37° in a CO2 incubator for the desired period, typically 2 hr for neutrophils and 24 hr for PBL. Migration was stopped at the chosen time by transferring the filter into a fresh well, leaving the transmigrated cells in the original lower chamber. The leucocytes suspended in the upper chamber were removed, and pooled with cells obtained when the filter was washed twice. These cells were taken to represent non-adherent cells.
For neutrophils, the non-adherent and transmigrated cells were counted using a Coulter Multisizer II (Coulter Electronics Ltd, Essex, UK). Adherent cells (representing all cells that bound to the culture whether they transmigrated or not) were calculated by subtracting the non-adherent population from the total number of cells added to each well. From the known number of added neutrophils, the percentages that adhered or transmigrated were calculated. For lymphocytes, cells were counted and their surface phenotypes were analysed by flow cytometry. Freshly isolated, non-adherent or transmigrated cells were labelled with phycoerythrin-conjugated anti-CD4, fluorescein isothiocyanate-conjugated anti-CD8 (Becton Dickinson, Oxford, UK) or Cy5-conjugated anti-CD45RA (Serotec, Oxford, UK) for 30 min on ice. Fixed volume counts for positively labelled cells were made using a Coulter XL flow cytometer and analysed using WinMDI software. In this way, we calculated the percentages of CD4+, CD8+ and memory T-cells that adhered and transmigrated. Again, adherent cells were calculated by subtracting the non-adherent counts from the total number of cells added.
In a variant on this assay, EC or fibroblasts were cultured on the inner surface of 24- or 12-well 3-μm pore Transwell filter inserts respectively. After 24 hr, the 24-well inserts were fitted into the 12-well inserts and cells were cultured together for 24 hr. This arrangement brought the EC within about 250 μm of the fibroblasts but did not allow contact. Co-cultures were treated with cytokines as above. The PBL were added and allowed to adhere for 10 min after which non-adherent cells were removed and analysed by flow cytometry as above. After 24 hr, migrated cells suspended in the medium between the two filters or in the lower chamber were collected. Filters were treated with Accutase (Gibco) to dissociated migrated cells associated with the endothelial or fibroblast monolayers, and washed twice. Cells from each compartment were pooled with their respective washes and analysed by flow cytometry as above. Migration was quantified as the sum of the lymphocytes located in the compartments beneath the endothelial monolayer; i.e. in medium between the two filters, attached to the fibroblasts or in the lowest chamber.
Leucocyte recruitment from flow and subsequent migration through co-cultures
Filters were cut from the six-well format Transwell holders, placed on a 75 × 25 mm coverslip and incorporated into a parallel-plate flow chamber; the chamber was attached to a perfusion system and mounted on the stage of a phase-contrast videomicroscope, all at 37°, as described previously.8,25 At one end, the chamber was connected to a Harvard withdrawal syringe pump, which delivered flow at a rate equivalent to a wall shear stress of 0·1 Pa. At the other end, it was connected to an electronic valve (Lee Products, Gerrards Cross, UK) which selected flow from reservoirs containing purified leucocytes or cell-free PBSA. A 4-min bolus of leucocytes was perfused over the EC followed by cell-free wash buffer. Video recordings were made of five microscope fields along the centreline of the flow channel after 2 and 11 min of washout. By adjusting the focus, recordings were made of endothelial surface and then of the underside of the filter, the fibroblast layer and the coverslip surface. Between these times, a single recording was made of the endothelial surface to allow lymphocyte migration velocity to be analysed.
The video recordings were digitized and analysed offline using Image-Pro Plus software (Media Cybernetics UK, Marlow, UK). Leucocytes bound to the endothelium were counted, and each was classified as either: (i) rolling adherent (spherical cells moving over the surface much slower than free-flowing cells); (ii) stationary or firmly adherent (typically with distorted shape and actually migrating slowly on the surface); (iii) transmigrated under the endothelial monolayer (phase dark and spread). Cells migrated into the layers below the filter were also counted. Transmigrated cells were subdivided into cells located above or beneath the filter. The total number of adherent leucocytes (i.e. all behaviours) was averaged per field and expressed /mm2/106 cells perfused.26
The migration velocities of phase-dark neutrophils underneath the endothelium were measured by digitizing a sequence of images 10 seconds apart for 5 min. Individual cells were outlined and the position of their centroid was determined each minute. Migration velocity (μm/min) was the average distance moved by the centroid per minute.
Gene expression by EC
Trypsin/EDTA was used to detach HUVEC from the inside of filters, and messenger RNA (mRNA) was isolated from the cells using the RNeasy Mini Kit (Qiagen, Crawley, UK). The mRNA levels of CXCL9, -10 and -11 chemokines were analysed by reverse transcription–polymerase chain reaction (RT–PCR), followed by densitometry of product bands run on agarose gels containing ethidium bromide, as described.8 The mRNA for E-selectin, VCAM-1 and β-actin was analysed by quantitative real-time PCR using a Quanti-Tect™ probe RT-PCR kit as described previously.8 The VCAM-1 and E-selectin 6-carboxy-fluorescein (FAM)-labelled primers and β-actin VIC-labelled primers were bought as Assay on Demand kits from Applied Biosystems (Warrington, UK). Samples were amplified using the 7900HT Real-Time PCR machine and analysed using the software package SDS 2.2 (Applied Biosystems). Data were expressed as relative expression units to β-actin.
Flow cytometry of endothelial surface receptors
Filters were incubated with non-conjugated antibodies against E-selectin (1.2B6) or VCAM-1 (1.4C3; both Dako, Ely, UK) or with mouse immunoglobulin G1 as negative control for 30 min at 4°, washed and incubated with goat anti-mouse fluorescein isothiocyanate-conjugated secondary antibody (Dako) for 30 min at 4° as previously described.8 All samples were washed and incubated with enzyme-free cell dissociation buffer (Gibco) for 30 min. The EC were retrieved, washed and analysed using a Coulter XL flow cytometer. Data were expressed as median fluorescent intensity.
Statistical analysis
Variation between multiple treatments was evaluated using analysis of variance (anova), followed by Dunnett test for comparison with control or Bonferroni multiple comparison test as appropriate. P < 0·05 was considered as statistically significant.
Results
Migration of neutrophils through co-cultures of EC and fibroblasts
We first investigated whether fibroblasts modified neutrophil migration through endothelium when the two cell types were cultured on opposite sides of 3-μm pore filters, with or without TNF-α treatment. In a static assay, neutrophils adhered to and migrated through EC more efficiently after TNF-α treatment (Fig. 1a,b), but co-culture with fibroblasts had no significant effect on adhesion or migration, with or without endothelial stimulation (Fig. 1a,b). Next, we extended the duration of co-culture to 4 days and re-examined effects on migration after treatment with TNF-α. Under these conditions, migration tended to be increased when EC were cultured with fibroblasts, but this effect was of borderline statistical significance (Fig. 1c; P = 0·06 by anova). Hence, under static conditions, the presence of fibroblasts has only slight effects on neutrophil migration.
Figure 1.
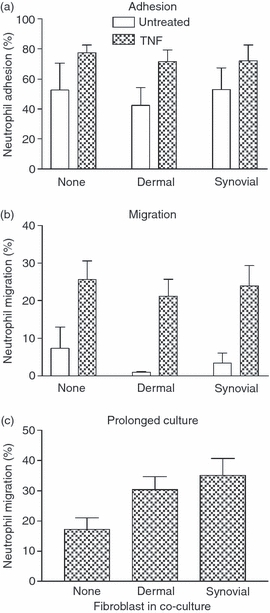
Neutrophil adhesion and transmigration through endothelial cells (EC) cultured with fibroblasts under static conditions. Fibroblasts were cultured with EC for 24 hr and left untreated or stimulated with 100 U/ml tumour necrosis factor-α (TNF) for the last 4 hr. (a) Adhesion and (b) transmigration were analysed 2 hr after the addition of neutrophils and expressed as percentages of neutrophils added. Analysis of variance (anova) showed a significant effect of TNF stimulation on neutrophil adhesion and transmigration (P < 0·05), but no effect of fibroblasts. (c) Neutrophil migration through co-cultures established for 4 days before assay. anova showed an effect of fibroblasts with borderline significance, P = 0·06. Data are mean ± SEM from three or four independent experiments.
Subsequently, we directly observed the effects of fibroblasts on neutrophil recruitment from flow and subsequent migration using a novel filter-based assay.8,25 Synovial fibroblasts induced the capture of flowing neutrophils to otherwise unstimulated EC (353 ± 102 adherent cells/mm2/106 perfused compared with 31 ± 8·2 adherent cells/mm2/106 perfused for unstimulated EC mono-cultures; mean ± SEM; n = 3), in agreement with our previous reports.16,17 The neutrophils were stationary adherent, but rarely underwent transendothelial migration (< 1% of adherent cells transmigrated, data not shown). After TNF-α treatment, co-cultures and EC cultured alone efficiently bound similar numbers of flowing neutrophils (Fig. 2a). Under these conditions, most captured neutrophils were rapidly activated and became stationary adherent, with ∼ 40–50% migrating through the endothelial monolayer after 11 min of washout. The overall proportion of adherent cells transmigrating did not vary significantly between monocultures or co-cultures (Fig. 2b). However, when migrated cells were divided into those above or below the filter, the presence of fibroblasts induced a greater proportion of migrated neutrophils to pass through the filter (Fig. 2c).
Figure 2.
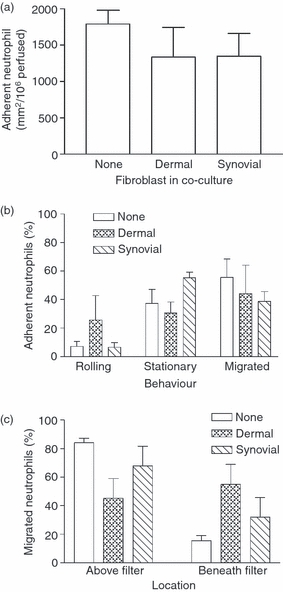
Adhesion and behaviour of flowing neutrophils on cytokine-treated co-cultures. Endothelial cells cultured alone or with fibroblasts for 24 hr were subsequently stimulated with tumour necrosis factor-α (TNF) for 4 hr before perfusion of a 4-min bolus of neutrophils. (a) Number of neutrophils adherent to co-cultures 2 min after washout. (b) Behaviour of adherent neutrophils at 11 min after washout. (c) Location of migrated neutrophils above or below the filter at 11 min expressed as a percentage of total transmigration. In (c), analysis of variance shows a significant effect of culture conditions on the percentage of migrated neutrophils found above or below the filter, P < 0·01. The percentage of migrated neutrophils below the filter tended to be higher for co-cultures with dermal fibroblasts than for monocultures; P = 0·065 by paired t-test. Data are mean ± SEM from three independent experiments.
We also noted that the velocity of cells migrating immediately below the endothelial monolayer tended to be slower in the presence of fibroblasts (6·8 ± 3·7 μm/min with dermal or 6·3 ± 2·1 μm/min with synovial co-cultures) compared with EC alone (9·0 ± 1·2 μm/min; mean ± SEM for three experiments), although this did not reach statistical significance. Overall, fibroblasts had relatively little effect on the migration of neutrophils across the EC, but tended to alter behaviour under the endothelium and promote onward migration.
Migration of lymphocytes through co-cultures of EC and fibroblasts
We examined the ability of fibroblasts to modify the adhesion and migration of lymphocytes through EC with or without TNF-α + IFN-γ. Under static conditions, in the absence of cytokine treatment, PBL and CD4+ or CD8+ T-cell subsets (enumerated separately by flow cytometry) tended to be bound less efficiently to EC co-cultured with fibroblasts compared with EC cultured alone (Fig. 3a). Few lymphocytes migrated through the unstimulated cultures, but again, fewer migrated through co-cultures than endothelial monocultures (Fig. 3b). Adhesion and transmigration were increased and less variable after cytokine treatment. Under these conditions, dermal fibroblasts significantly inhibited adhesion compared with EC cultured alone, and co-culture with either type of fibroblast markedly reduced transmigration (Fig. 3). Similar suppression of migration by fibroblasts was observed when TNF-α alone was used as the stimulus (data not shown).
Figure 3.
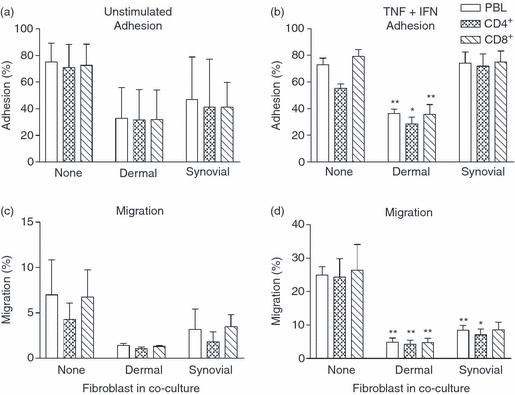
Effect of fibroblasts on the adhesion and migration of T-cell subsets under static conditions. Endothelial cells cultured alone or with fibroblasts for 24 hr and were subsequently left untreated (a, c) or stimulated with tumour necrosis factor-α plus interferon-γ (TNF + IFN) (b, d). Adhesion (a, b) and migration (c, d) were assessed at 24 hr and expressed as a percentage of cells added for peripheral blood lymphocytes (PBL), CD4+ or CD8+ T cells. Data are mean ± SEM from three independent experiments. In (b), analysis of variance (anova) shows a significant effect of fibroblasts on T-cell adhesion, P < 0·01. In (c) and (d), anova shows a significant effect of fibroblasts on migration, P < 0·05. * = P < 0·05 and ** = P < 0·01 compared with None by Dunnett test.
The above effects were evident for both the CD4+ and CD8+ T-cell subsets (Fig. 3). In general, there was enrichment of memory CD4+ T cells in the migrated population, as has been reported previously.7,10,11,27 For example, despite the overall reduction of transmigration, CD45RA− (memory) cells represented 83 ± 7% or 91 ± 4% of CD4+ T cells migrated through dermal or synovial co-cultures respectively, compared with 62 ± 2% in the original PBL population (mean ± SEM; n = 3 in each case; P < 0·01 by Dunnett test). Hence, there was an overall inhibition of the migratory process, rather than the loss of a specific lymphocyte sub-population.
Recruitment of flowing lymphocytes by co-cultures
The inhibition of lymphocyte migration might arise from reduction in migration through the endothelial monolayer and/or through the filter and fibroblast layers, as well as some contribution from impaired adhesion. To evaluate these possibilities, we directly observed recruitment from flow. As expected, few flowing PBL bound to unstimulated EC, and cytokine-treatment greatly increased adhesion to monocultures (Fig. 4a). Surprisingly, co-culture with either dermal or synovial fibroblasts ablated lymphocyte adhesion to EC whether treated with TNF-α + IFN-γ or not (Fig. 4a). Similar loss of binding was observed for co-cultures treated with TNF-α alone (data not shown). We did not detect such dramatic loss of attachment when essentially identical co-cultures were formed on opposite sides of 0·4-μm pore filters,8 suggesting that cell–cell contact may have been a critical determinant of the behaviour of the endothelial monolayer. Direct microscopic observations did not reveal disruption of the endothelial monolayers in co-cultures, whose appearance was identical to those in co-cultures that had supported efficient neutrophil adhesion and migration. Observations of EC or fibroblast mono-cultures did indicate that a few cells of either type could cross the 3-μm pore filters in 24 hr. To test whether prior stabilization of the endothelial monolayer might alter the effect of fibroblasts, EC were cultured on the 3-μm pore filters for 4 days before seeding with fibroblasts. However, the recruitment of flowing PBL was still dramatically reduced by co-culture with dermal or synovial fibroblasts (Fig. 4b).
Figure 4.
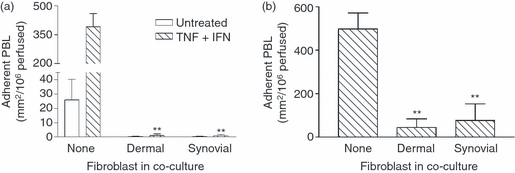
Effect of fibroblasts on lymphocyte recruitment from flow. (a) Fibroblasts were cultured alone for 24 hr, and then with endothelial cells (EC) for 24 hr; co-cultures were then left untreated or stimulated with tumour necrosis factor-α plus interferon-γ (TNF + IFN) for 24 hr. (b) EC were cultured alone for 4 days, then with fibroblasts for 24 hr, and then TNF + IFN were added for 24 hr. Data are mean ± SEM from four independent experiments. In (a) and (b), analysis of variance showed a significant effect of fibroblasts on lymphocyte adhesion (P < 0·01). ** = P < 0·01 compared to Untreated or None by Dunnett test.
Mechanism of inhibition of lymphocyte recruitment – role of soluble mediators
We tested whether soluble mediators released by fibroblasts alone or in co-culture could inhibit lymphocyte recruitment from flow. Conditioned medium from unstimulated fibroblasts or from co-cultures with EC was transferred to a separate endothelial monolayer, and TNF-α + IFN-γ was added for 24 hr. The conditioned media did not reduce the adhesion of flowing lymphocytes compared with standard culture medium (Fig. 5a). Hence, agents released by unstimulated fibroblasts or co-cultures did not inhibit the subsequent response of EC to the cytokines. Alternatively, conditioned medium from cytokine-treated EC cultured alone or with fibroblasts was transferred to a separate endothelial monolayer for 24 hr. Once again, lymphocyte adhesion was similar with these media to that obtained with fresh medium with cytokines added (Fig. 5b). The conditioned medium from cytokine-treated cultures was itself fully stimulatory. It seems, therefore, that suppression of lymphocyte adhesion to co-cultures did not arise from freely diffusing, released soluble mediator(s).
Figure 5.
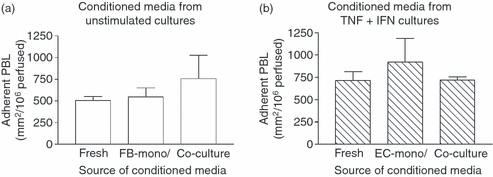
Effect of conditioned media on lymphocyte recruitment to endothelial cells (EC) from flow. Mono- or co-cultures were formed for 24 hr before treatment with or without cytokines for a further 24 hr, after which the conditioned medium was collected. (a) EC were cultured in fresh medium (Fresh) or in conditioned medium from unstimulated fibroblasts cultured alone (FB-mono) or with EC (Co-culture), with tumour necrosis factor-α plus interferon-γ (TNF + IFN) added for 24 hr. (b) Endothelial cells were cultured for 24 hr in fresh medium with TNF + IFN added (Fresh) or in conditioned medium from EC which had been cultured alone (EC-mono) or with fibroblasts (Co-culture) for 24 hr with TNF + IFN. Data are mean ± SEM from three independent experiments.
Mechanism of inhibition of lymphocyte recruitment – expression of adhesion receptors and chemokines by EC
We previously reported that adhesion of flowing lymphocytes to EC that had been treated with TNF-α + IFN-γ was mainly supported through α4β1-integrins binding to VCAM-1 on the EC, with a contribution from E-selectin; stabilization of this attachment required activation of lymphocytes through CXCR3 binding its ligands (CXCL9, CXCL10 and/or CXCL11).8 Using qPCR, we found that co-culture had no significant effect on mRNA levels for E-selectin, and tended to increase mRNA for VCAM-1 for EC treated with TNF-α + IFN-γ (Fig. 6a,b). As judged by flow cytometry, surface expression of E-selectin and VCAM-1 on the EC was not significantly different for cytokine-treated EC cultured alone or with fibroblasts (Fig. 6c,d). The RT-PCR for the CXCR3 ligands CXCL9, CXCL10 and CXCL11 showed their up-regulation in EC by the cytokines, but no difference in levels between EC cultured alone or with fibroblasts (data not shown). Hence, the functional loss observed in co-cultures could not be explained by changes in expression of endothelial adhesion receptors and chemokines identified as active in this model.
Figure 6.
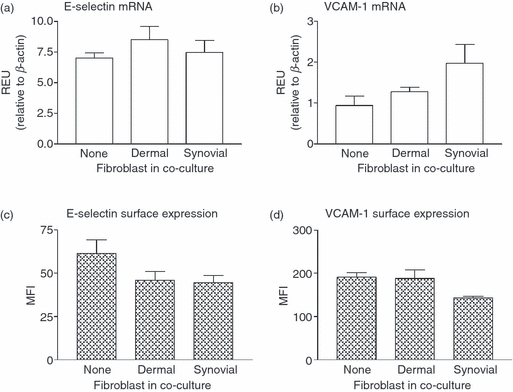
Effect of fibroblasts on the expression of E-selectin or vascular cell adhesion molecule 1 (VCAM-1) by endothelial cells (EC). Co-cultures were formed for 24 hr before treatment with tumour necrosis factor-α plus interferon-γ (TNF + IFN) for a further 24 hr. In (a) and (b), endothelial messenger RNA was isolated from co-cultures treated with TNF + IFN and the gene expression of E-selectin (a) or VCAM-1 (b) was assessed by quantitative polymerase chain reaction. Values were expressed as relative expression units (REU) compared with β-actin. In (c) and (d), EC from cytokine-treated co-cultures were labelled using antibodies against (c) E-selectin or (d) VCAM-1 and surface expression was assessed by flow cytometry (expressed as mean fluorescence intensity; MFI). Data are mean ± SEM from three independent experiments.
Mechanism of inhibition of lymphocyte recruitment – endothelial surface modification
We considered the hypothesis that the effects of close contact with the fibroblasts arose from inhibition of the ability of the EC to present the chemokines necessary to stabilize adhesion and promote subsequent migration. We tried to replace ‘lost’ chemokines by incubating cytokine-treated EC with CXCL12 immediately before perfusion of lymphocytes. The CXCL12 enhanced adhesion to the EC cultured alone, but was unable to recover any of the lymphocyte adhesion to co-cultures (Fig. 7a). This suggested an inability of the EC to bind exogenous CXCL12 in the co-culture system. In general, surface-bound chemokines are presented by glycoaminoglycans (GAGs) which, with the exception of hyaluronan, are associated with a protein backbone. Endothelial cells mainly express the GAG heparan sulphate, along with lower levels of hyaluronan, chrondroitin sulphate and dermatan sulphate.28–31 We therefore investigated whether proteolytic enzymes presented by fibroblasts in close contact with EC might be the cause of loss of lymphocyte binding. Protease inhibitors were added to co-cultures of EC and synovial fibroblasts grown on the 3·0-μm pore filters. Inhibiting MMP activity (with galardin) caused a partial recovery in lymphocyte recruitment to the cytokine-treated co-cultures (Fig. 7b), whereas serine protease inhibitors had no effect. This suggested that loss of MMP-sensitive protein structure(s) on the endothelial surface during co-culture contributed to the reduction in lymphocyte adhesion.
Figure 7.
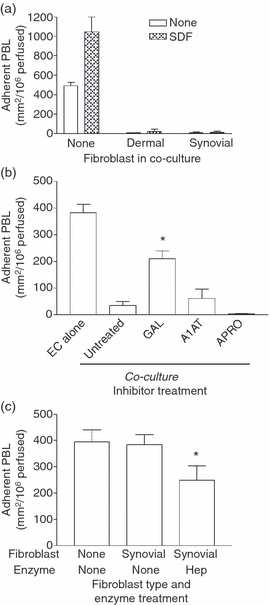
Effects of modifying chemokine presentation on adhesion of flowing lymphocytes to endothelial cells (EC) cultured alone or with fibroblasts. (a) Co-cultures were formed for 24 hr before treatment with tumour necrosis factor-α plus interferon-γ (TNF + IFN) for 24 hr and then 100 ng/ml CXCL12 was added to EC for 30 min before assay. (b) EC were cultured alone or with synovial fibroblasts on either side of 3·0-μm pore filters for 24 hr and treated with TNF + IFN for 24 hr in the presence or absence of 10 μm galardin (GAL), 100 μg/ml α1-antitrypsin (A1AT) or 50 μg/ml aprotinin (APRO). (c) EC were cultured alone (none) or with synovial fibroblasts on either side of 0·4 um pore filters for 24 hr, treated with TNF + IFN for 24 hr and then 10 mU/ml heparinase (HEP) was added for 30 min. Data are the mean ± SEM from two (a) or three (b, c) independent experiments. * = P < 0·05 compared with synovial None by paired t-test.
Returning to the co-cultures on 0·4-μm pore filters, we further tested the concept that loss of GAGs could cause reduction in the otherwise efficient binding of lymphocytes. Lymphocytes efficiently adhered to cytokine-treated EC cultured alone or with synovial fibroblasts on the small-pore filters (Fig. 7c), in agreement with our recent report.8 Indeed, this cytokine-induced adhesion was significantly inhibited when the co-cultures were treated with heparinase (Fig. 7c). Treatment with hyaluronidase did not modify adhesion (data not shown).
Overall, the above findings suggest that impaired presentation of chemokines by GAGs, brought about through proteolytic cleavage of support structures, resulted in loss of binding of lymphocytes when fibroblasts were cultured in close contact with EC. Separation of the cells by 0·4-μm pore filters was sufficient to nullify this phenomenon, which could not be reproduced by soluble mediators released by the various cultures tested.
Migration of lymphocytes through EC cultured with fibroblasts without contact
The question of whether dermal or synovial fibroblasts affect lymphocyte migration in the absence of close contact with EC, could not be answered using the above models. We therefore developed a model in which EC on 24-well, 3-μm pore filters were inserted above fibroblasts on 12-well inserts, leaving a small space between the cells. Under these conditions, fibroblasts increased adhesion of CD4+ or CD8+ T cells to the EC and markedly increased the migration of T cells through EC, with or without treatment with cytokines (Fig. 8c,d).
Figure 8.
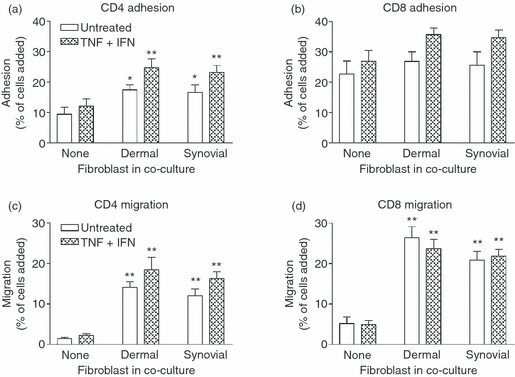
Adhesion and migration of lymphocytes through co-cultures in a two-filter static model. Co-cultures were established by inserting an endothelial-coated 24-well filter into a fibroblast-coated 12-well filter. Cells were cultured together for 24 hr and then with or without tumour necrosis factor-α plus interferon-γ (TNF + IFN) for a further 24 hr. T-cell adhesion (a, b) and migration (c, d) were assessed after 10 min and at 24 hr, respectively, using flow cytometry. Data were expressed as a percentage of cells added. In (a) and (c), analysis of variance (anova) shows a significant effect of fibroblasts on CD4+ T-cell adhesion (P < 0·05) and migration (P < 0·01). In (d), anova shows a significant effect of fibroblasts on CD8+ T-cell migration (P < 0·001). Data are the mean ± SEM from six to nine (untreated) and 12 (TNF + IFN) independent experiments. * = P < 0·05 and ** = P < 0·01 compared with endothelial cells cultured alone (none) with matched cytokine treatment by Dunnett test.
Fibroblasts are known to secrete CXCL12,32–34 and we recently demonstrated a role for this chemokine in the recruitment of flowing lymphocytes to EC cultured with synovial fibroblast.8 We therefore considered whether migration was increased by the presence of this chemokine in the sub-endothelial compartment in co-cultures. However, inhibition of its receptor CXCR4 on lymphocytes did not inhibit the increase in migration induced by co-culture of EC with fibroblasts (Fig. 9a,b). Furthermore neutralization of IL-6 during co-culture had no effect on the increase in T-cell migration induced by fibroblasts (Fig. 9c,d). Consequently, fibroblasts could promote leucocyte migration away from the endothelium, but the mechanism(s) remain uncertain.
Figure 9.
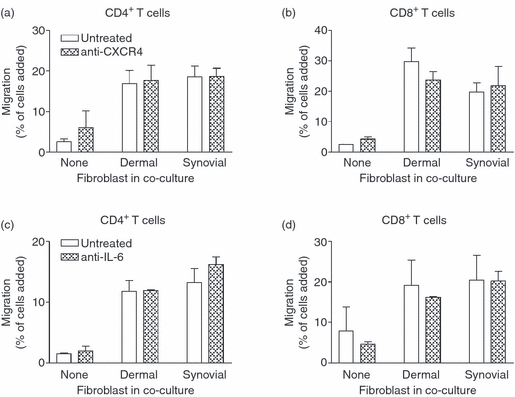
Effects of inhibiting actions of CXCL12 or interleukin-6 (IL-6) on migration of T cells through cytokine-treated co-cultures in a two-filter static model. Co-cultures were established by inserting an endothelial-coated 24-well filter into a fibroblast-coated 12-well filter. Cells were cultured together for 24 hr and then with tumour necrosis factor-α plus interferon-γ (TNF + IFN) for a further 24 hr. In (a) and (b), peripheral blood lymphocytes were untreated or treated with the CXCR4 inhibitor AMD3100 for 30 min before the assay. In (c) and (d), neutralizing antibody against IL-6 was added upon establishment of co-culture and was present throughout cytokine-treatment. CD4+ and CD8+ T-cell migration was assessed at 24 hr by flow cytometry and expressed as a percentage of cells added. Data are the mean ± SEM from two (a–b) or three (c–d) independent experiments.
Discussion
Efficient migration of leucocytes into tissue may require more than a single migratory or chemotactic signal. We have found that TNF-α-treated endothelial cells alone can sequentially present CXC-chemokines and lipid mediators to induce immobilization and transendothelial and onward migration of neutrophils.2,3 On the other hand, although lymphocytes readily adhere to and migrate through cytokine-treated EC, many migrate back and forth across the monolayer and onward migration is much less efficient.7 Here, we set out to investigate whether fibroblasts could provide additional migratory signals for leucocytes, and especially make lymphocyte migration more effective. Previously we found that through soluble mediators, dermal fibroblasts could down-modulate the adhesion of lymphocytes to cytokine-treated EC while fibroblasts from inflamed synovium could induce adhesion themselves.8 Unexpectedly, we found that close contact between fibroblasts and EC cultured on either side of 3-m pore filters ablated lymphocyte capture from flow, and in static systems significantly inhibited their migration through endothelium. In contrast, neutrophil adhesion and migration remained fully effective in essentially identical co-cultures. Indeed, fibroblasts tended to increase the migration of neutrophils away from the sub-endothelial compartment. Inhibition of lymphocyte adhesion was not observed if the cells were cultured on either side of smaller pore (0·4-μm) filters. Moreover, if the EC and fibroblasts were cultured on separate 3-μm pore filters close together, fibroblasts promoted transendothelial and onward migration of lymphocytes through the supporting filter. Hence, stromal cells can powerfully influence the ability of vascular EC to recruit flowing leucocytes and direct their onward migration, with effects dependent on their proximity.
The suppressive effects arising from close contact with fibroblasts could not be reproduced by transferable soluble mediators generated by resting or stimulated mono-cultures or co-cultures, and were similar for both dermal and synovial fibroblasts. Suppression could not be attributed to gross disruption of the endothelial monolayer, which appeared undisturbed and was able to support efficient adhesion and migration of neutrophils. The leucocyte specificity suggested that fibroblasts selectively modified endothelial expression of lymphocyte adhesion receptors and/or chemokine ligands. Previously we demonstrated that cytokine-induced lymphocyte adhesion to endothelium was mediated through VCAM-1 and E-selectin.8 However, here, fibroblasts did not cause loss of endothelial expression of these adhesion receptors at mRNA or protein levels. In addition, we and others showed the importance of CXCR3 ligands in the stabilization of lymphocyte adhesion to endothelium in cytokine-driven models.5,8,35 Here, we found that cytokine-induced up-regulation of the CXCR3-ligands CXCL9, CXCL10 and CXCL11 was not reduced by co-culture with fibroblasts. Our earlier work with inflammatory synovial fibroblasts and EC cultured on opposite sides of 0·4-μm pore filters, indicated that endothelial presentation of fibroblast-derived CXCL12 was required for stable lymphocyte adhesion.8 In the present study, EC cultured in close proximity with these same cells did not support adhesion from flow. The foregoing suggested to us that, if close contact with fibroblasts impaired the ability of EC to present (rather than generate) chemokines, this could explain the phenomena observed.
Chemokines can be measured in culture-conditioned media,36–41 but in our experience it is difficult to detect their surface expression on EC. Pre-loading endothelial monolayers with exogenous chemokines has been a strategy employed to examine their ability to induce lymphocyte migration.6,42,43 For example, incubation of endothelial monolayers with CXCL12 was reported to promote transendothelial migration of lymphocytes in the presence of flow.6 Here, pre-treatment of EC mono-cultures with CXCL12 did enhance the adhesion of flowing lymphocytes, but did not increase lymphocyte adhesion to EC, which had been cultured in close contact with fibroblasts. This showed that we could not re-establish recruitment simply by adding back or substituting a missing chemokine, and supported the concept of impaired ability of the EC to present chemokines.
Surface-bound chemokines are mainly presented by GAGs which are themselves attached to a protein backbone (i.e. proteoglycans; reviewed in refs 31,44,45). For this reason, we considered whether cleavage of GAGs or of their support structures by fibroblasts might explain loss of lymphocyte adhesion. Although released soluble enzymes could not have been responsible for the loss of presentation (judging from the lack of effect of the various culture supernatants), direct contact with fibroblasts (some of which may have reached across and indeed migrated through the 3·0-μm pore filters) could have induced cleavage. There is a precedent for proteolysis of the proteoglycan backbone by members of the MMP or ADAM family influencing leucocyte recruitment in vivo.46 For example, MMP7-induced shedding of epithelial syndecan-1 was reported to generate a chemotactic gradient within the peri-vascular space inducing neutrophil migration into inflamed murine lungs.47 Furthermore, pharmacological inhibition of MMP9 attenuated murine neutrophil and CD4 T-cell migration following hepatic ischaemic–reperfusion injury.48 Here we found that addition of galardin to co-cultures (a broad spectrum inhibitor of MMPs and some ADAM family members) significantly reduced the ability of fibroblasts to inhibit lymphocyte attachment. In contrast, neither α1-antitrypsin nor aprotinin (a serine-protease inhibitor) had any effects on the co-cultures.
We also sought evidence of whether GAGs were necessary for effective lymphocyte adhesion from flow in our ‘undisturbed’ model. Endothelial cells mainly express the GAG heparan sulphate, along with lower levels of hyaluronan, chrondroitin sulphate and dermatan sulphate.28–31 Here, heparinase treatment of cytokine-treated EC cultured with synovial fibroblast without close contact reduced lymphocyte adhesion. Hyaluronidase treatment had no effect. It is notable that in recent studies, we found that heparinase treatment did not inhibit neutrophil adhesion to EC treated with TNF-α.31 Hence, disruption of the glycocalyx mimicked the effect of close contact with fibroblasts, inhibiting lymphocyte but not neutrophil binding. This is consistent with a previous report that the inhibition of interaction of CXCR3 ligands with heparan sulphate suppressed recruitment of CD4 T cells.49
As noted above, the differential effects of fibroblasts on stable adhesion of lymphocytes and neutrophils could arise from the cells different requirements for chemokine presentation. In addition to GAGs, inflammatory chemokines can be presented on the endothelial surface by DARC (Duffy antigen receptor for chemokines) (reviewed in ref. 50), which is insensitive to heparinase treatment.51 Previously we reported that CXCL5 (ENA-78) was presented by DARC to induce neutrophil binding to EC cultured with synovial fibroblasts.17 Overall, it therefore appears that close contact with fibroblasts may cleave structures required for chemokine presentation to lymphocytes but not to neutrophils.
Our original purpose was to investigate whether fibroblasts could actually promote sub-endothelial migration of leucocytes, especially lymphocytes. Recently, we showed that migrating lymphocytes, but not neutrophils, tended to be retained by EC and proposed that a separate signal was required to induce lymphocyte migration into the stroma.7 We also found that different types of fibroblasts, when co-cultured with EC without direct contact, could enhance or inhibit the stable attachment of flowing lymphocytes, depending on the origin of the fibroblasts.8 This left open the question of whether fibroblasts could provide signals(s) to release lymphocytes from the confines of the endothelium or direct their onward migration. Here we used a modified model with EC and fibroblasts cultured on separate 3-μm pore filters one immediately above the other, to investigate this possibility. Indeed, both dermal and synovial fibroblasts promoted T-cell migration through EC and its supporting filter. In a previous report, scleroderma fibroblasts promoted Jurkat migration through an endothelial cell line, through supply of CCL2 (MCP-1).12 A variety of chemokines have been identified as playing roles in lymphocyte migration into inflamed tissues.52–58 Here, the most obvious pro-migratory agent shown to be released by the same fibroblasts in our earlier work, was CXCL12.8,34 However, neutralization of the receptor for this chemokine did not impair fibroblast-induced migration. Nor did neutralization of IL-6 have any effect, although this agent was implicated in the capabilities of the fibroblasts to modulate the earlier lymphocyte adhesion stage in co-cultures.8
The pathophysiological implications of the foregoing depend on the arrangement of tissue in vivo. In immediate post-capillary venules where lymphocyte recruitment is believed to occur in inflammation, the endothelial cells are closely neighboured by an incomplete covering of pericytes. In slightly larger vessels a layer of smooth muscle cells begins to appear. In normal tissue, fibroblasts are likely to be slightly more remote from the EC, where our results suggest they would provide regulatory signals in the migration of neutrophils and especially T cells. However, if tissue is disrupted and fibroblasts come into direct contact with EC, then recruitment of lymphocytes (but not neutrophils) might be suppressed. The effects of pericytes, which are normally closely juxtaposed to EC on migration, are uncertain, although it has been suggested that they modify the basement membrane in a manner that facilitates leucocyte transmigration.59 Several studies have reported the capacity of pericytes to differentiate into various stromal cell types, including fibroblasts, implicating them as a potential source (progenitor) for stromal cells.60–65 It is possible that they might act like fibroblasts in our model, but distinction of the functions of pericyte from fibroblasts is made difficult experimentally by the lack of unique, definitive markers for either (reviewed in ref. 66). Pericyte-specific modulation of leucocyte recruitment appears worthy of further investigation.
Direct contact between fibroblasts and EC may occur in the contexts of dermal tissue injury (wounding) and angiogenesis. Several studies have reported the ability of dermal microvascular endothelial cells to bind and migrate into dermal fibroblast cultures forming tubule-like structures, whereas the fibroblasts themselves enhance and sustain microvessel formation.67–70 Our findings when fibroblasts and EC were brought into close contact tally with the observed early recruitment of neutrophils into wounds without obvious lymphocyte infiltration.71 The same might apply to tissue undergoing neovascularization in chronic inflammatory conditions, or during disorganized growth of vessels in tumours. Under these conditions, a restricted or aberrant form of leucocyte infiltration might prevail until a sufficient remodelling has occurred. However, if there was a failure to establish normal architecture, recruitment might continue to differ from the pattern found in normal resolving inflammatory responses, e.g. the concept of ‘dissonant orchestration’ describing the inability of leucocytes to infiltrate tumours in the accepted sequence.72 Stromal architecture also becomes dramatically disrupted and/or reorganized in chronically inflamed tissues. A good example of this is the invasive migration of synovial fibroblasts into the intimal space neighbouring the vascular supply of the rheumatoid joint.73 The proximity of stromal cells to the local microvasculature may change dynamically during tissue repair, angiogenesis and chronic inflammation, and this may profoundly modulate leucocyte recruitment.
Overall, the current and previous studies8,16,17 suggest that stromal fibroblasts can influence both early and late steps in transendothelial recruitment of leucocytes. For lymphocytes, it is attractive to propose that fibroblasts might provide an additional regulatory step in the migration of T cells, which is not so important for neutrophils. Hence, EC exposed to cytokines are able to capture both types of flowing leucocytes, but can induce efficient onward migration of neutrophils only. Fibroblasts appear able to provide the signal for onward migration of lymphocytes. An exciting possibility worthy of further investigation is that such signal(s), arising from different fibroblasts in different tissues, might be sub-set specific. This would enable the selective migration of discrete populations initially recruited by the less-specific adhesive interaction with the endothelial surface.
Acknowledgments
This work was supported by grants from The Wellcome Trust grant number 077828 and 088630 and the Arthritis Research Council. Umbilical cords were collected with the assistance of the Birmingham Women's Health Care NHS Trust.
Glossary
Abbreviations:
- EC
endothelial cells
- IFN-γ
interferon-γ
- PBL
peripheral blood lymphocytes
- TNF-α
tumour necrosis factor-α
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Luu NT, Rainger GE, Nash GB. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J Immunol. 2000;164:5961–9. doi: 10.4049/jimmunol.164.11.5961. [DOI] [PubMed] [Google Scholar]
- 3.Tull SP, Yates CM, Maskrey BH, et al. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luscinskas FW, Ding H, Lichtman AH. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumour necrosis factor-a-activated vascular endothelium under flow. J Exp Med. 1995;181:1179–86. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–72. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–22. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 7.McGettrick HM, Hunter K, Moss PA, Buckley CD, Rainger GE, Nash GB. Direct observations of the kinetics of migrating T cells suggest active retention by endothelial cells with continual bidirectional migration. J Leukoc Biol. 2009;85:97–107. doi: 10.1189/jlb.0508301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGettrick HM, Smith E, Filer A, et al. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur J Immunol. 2009;39:98–107. doi: 10.1002/eji.200838232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cinamon G, Grabovsky V, Winter E, et al. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001;69:860–6. [PubMed] [Google Scholar]
- 10.Pietschmann P, Cush JJ, Lipsky PE, Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992;149:1170–8. [PubMed] [Google Scholar]
- 11.Brezinschek RI, Lipsky PE, Galea P, Vita R, Oppenheimer-Marks N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J Immunol. 1995;154:3062–77. [PubMed] [Google Scholar]
- 12.Denton CP, Shi-Wen X, Sutton A, Abraham D, Black CM, Pearson JD. Scleroderma fibroblasts promote migration of mononuclear leukocytes across endothelial cell monolayers. Clin Exp Immunol. 1998;114:293–300. doi: 10.1046/j.1365-2249.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mul FP, Zuurbier AE, Janssen H, et al. Sequential migration of neutrophils across monolayers of endothelial and epithelial cells. J Leukoc Biol. 2000;68:529–37. [PubMed] [Google Scholar]
- 14.Rainger GE, Nash GB. Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ Res. 2001;88:615–22. doi: 10.1161/01.res.88.6.615. [DOI] [PubMed] [Google Scholar]
- 15.Edwards S, Lalor PF, Nash GB, Rainger GE, Adams DH. Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology. 2005;41:451–9. doi: 10.1002/hep.20585. [DOI] [PubMed] [Google Scholar]
- 16.Lally F, Smith E, Filer A, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 2005;52:3460–649. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith E, McGettrick HM, Stone MA, et al. Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis Rheum. 2008;58:1968–73. doi: 10.1002/art.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bijuklic K, Jennings P, Kountchev J, et al. Migration of leukocytes across an endothelium–epithelium bilayer as a model of renal interstitial inflammation. Am J Physiol. 2007;293:C486–92. doi: 10.1152/ajpcell.00419.2006. [DOI] [PubMed] [Google Scholar]
- 19.Buttrum SM, Hatton R, Nash GB. Selectin-mediated rolling of neutrophils on immobilized platelets. Blood. 1993;82:1165–74. [PubMed] [Google Scholar]
- 20.Rainger GE, Stone P, Morland CM, Nash GB. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J Immunol Methods. 2001;255:73–82. doi: 10.1016/s0022-1759(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 21.Salmon M, Scheel-Toellner D, Huissoon AP, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–46. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke BM, Usami S, Perry I, Nash GB. A simplified method for culture of endothelial cells and analysis of adhesion of blood cells under conditions of flow. Microvas Res. 1993;45:33–45. doi: 10.1006/mvre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 23.Chappell D, Jacob M, Rehm M, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. Heparinase selectively sheds heparan sulphate from the endothelial glycocalyx. Biol Chem. 2008;389:79–82. doi: 10.1515/BC.2008.005. [DOI] [PubMed] [Google Scholar]
- 24.McGettrick HM, Lord JM, Wang KQ, Rainger GE, Buckley CD, Nash GB. Chemokine- and adhesion-dependent survival of neutrophils after transmigration through cytokine-stimulated endothelium. J Leukoc Biol. 2006;79:779–88. doi: 10.1189/jlb.0605350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravorty SJ, McGettrick HM, Butler LM, Buckley CD, Rainger GE, Nash GB. An in vitro model for analysing neutrophil migration into and away from the sub-endothelial space: roles of flow and CD31. Biorheology. 2006;43:71–82. [PubMed] [Google Scholar]
- 26.Luu NT, Rainger GE, Nash GB. Kinetics of the different steps during neutrophil migration through cultured endothelial monolayers treated with tumour necrosis factor-alpha. J Vas Res. 1999;36:477–85. doi: 10.1159/000025690. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheimer-Marks N, Lipsky PE. Migration of naive and memory T cells. Immunol Today. 1997;18:456–7. doi: 10.1016/s0167-5699(97)82723-5. [DOI] [PubMed] [Google Scholar]
- 28.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–70. [PubMed] [Google Scholar]
- 29.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 30.Szczepanek K, Kieda C, Cichy J. Differential binding of hyaluronan on the surface of tissue-specific endothelial cell lines. Acta Biochim Pol. 2008;55:35–42. [PubMed] [Google Scholar]
- 31.Butler LM, Rainger GE, Nash GB. A role for the endothelial glycosaminoglycan hyaluronan in neutrophil recruitment by endothelial cells cultured for prolonged periods. Exp Cell Res. 2009;315:3433–41. doi: 10.1016/j.yexcr.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA, Zvaifler NJ, Tsukada N, Firestein GS, Kipps TJ. Fibroblast-like synoviocytes support B-cell pseudoemperipolesis via a stromal cell-derived factor-1- and CD106 (VCAM-1)-dependent mechanism. J Clin Invest. 2001;107:305–15. doi: 10.1172/JCI11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol. 2001;166:5749–54. doi: 10.4049/jimmunol.166.9.5749. [DOI] [PubMed] [Google Scholar]
- 34.Bradfield PF, Amft N, Vernon-Wilson E, et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–82. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- 35.Curbishley SM, Eksteen B, Gladue R, Lalor PF, Adams D. CXCR3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol. 2005;167:887–99. doi: 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filer A, Bik M, Parsonage GN, et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 2009;60:1604–14. doi: 10.1002/art.24574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705–14. doi: 10.1053/j.gastro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Koch AE, Kunkel SL, Shah MR, et al. Growth-related gene product alpha. A chemotactic cytokine for neutrophils in rheumatoid arthritis. J Immunol. 1995;155:3660–6. [PubMed] [Google Scholar]
- 39.Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381–5. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 40.Proost P, Verpoest S, Van de BK, et al. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J Leukoc Biol. 2004;75:777–84. doi: 10.1189/jlb.1003524. [DOI] [PubMed] [Google Scholar]
- 41.Ueno A, Yamamura M, Iwahashi M, Okamoto A, Aita T, Ogawa N, Makino H. The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis. Rheumat Int. 2005;25:361–7. doi: 10.1007/s00296-004-0449-x. [DOI] [PubMed] [Google Scholar]
- 42.Shulman Z, Shinder V, Klein E, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–96. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shamri R, Grabovsky V, Gauguet JM, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 44.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–60. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 45.Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–54. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- 46.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–81. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–46. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 48.Khandoga A, Kessler JS, Hanschen M, et al. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol. 2006;79:1295–305. doi: 10.1189/jlb.0805468. [DOI] [PubMed] [Google Scholar]
- 49.Ranjbaran H, Wang Y, Manes TD, et al. Heparin displaces interferon-gamma-inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation. 2006;114:1293–300. doi: 10.1161/CIRCULATIONAHA.106.631457. [DOI] [PubMed] [Google Scholar]
- 50.Colditz IG, Schneider MA, Pruenster M, Rot A. Chemokines at large: in-vivo mechanisms of their transport, presentation and clearance. Thromb Haemost. 2007;97:688–93. [PubMed] [Google Scholar]
- 51.Pruenster M, Mudde L, Bombosi P, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10:101–8. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohan K, Ding Z, Hanly J, Issekutz TB. IFN-gamma-inducible T cell alpha chemoattractant is a potent stimulator of normal human blood T lymphocyte transendothelial migration: differential regulation by IFN-gamma and TNF-alpha. J Immunol. 2002;168:6420–8. doi: 10.4049/jimmunol.168.12.6420. [DOI] [PubMed] [Google Scholar]
- 53.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ransohoff RM, Hamilton TA, Tani M, et al. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 55.Goddard S, Williams A, Morland C, Qin S, Gladue R, Hubscher SG, Adams DH. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72:1957–67. doi: 10.1097/00007890-200112270-00016. [DOI] [PubMed] [Google Scholar]
- 56.Buckley CD, Amft N, Bradfield PF, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–9. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- 57.Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, Garcia-Lazaro FJ. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170:2147–52. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- 58.Burman A, Haworth O, Hardie DL, et al. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693–700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S, Voisin MB, Larbi KY, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiwen X, Rajkumar V, Denton CP, Leask A, Abraham DJ. Pericytes display increased CCN2 expression upon culturing. J Cell Commun Signal. 2009;3:61–4. doi: 10.1007/s12079-009-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–54. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 63.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–24. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 64.Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–23. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 66.Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–6. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 67.Kunz-Schughart LA, Schroeder JA, Wondrak M, van Rey F, Lehle K, Hofstaedter F, Wheatley DN. Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol. 2006;290:C1385–98. doi: 10.1152/ajpcell.00248.2005. [DOI] [PubMed] [Google Scholar]
- 68.Oberringer M, Meins C, Bubel M, Pohlemann T. A new in vitro wound model based on the co-culture of human dermal microvascular endothelial cells and human dermal fibroblasts. Biol Cell. 2007;99:197–207. doi: 10.1042/BC20060116. [DOI] [PubMed] [Google Scholar]
- 69.Sorrell JM, Baber MA, Caplan AI. Human dermal fibroblast subpopulations; differential interactions with vascular endothelial cells in coculture: nonsoluble factors in the extracellular matrix influence interactions. Wound Repair Regen. 2008;16:300–9. doi: 10.1111/j.1524-475X.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Chen B, Lilly B. Fibroblasts potentiate blood vessel formation partially through secreted factor TIMP-1. Angiogenesis. 2008;11:223–34. doi: 10.1007/s10456-008-9102-8. [DOI] [PubMed] [Google Scholar]
- 71.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Carlos TM. Leukocyte recruitment at sites of tumor: dissonant orchestration. J Leukoc Biol. 2001;70:171–84. [PubMed] [Google Scholar]
- 73.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–90. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]


