Abstract
The selective forces that shape and maintain eusocial societies are an enduring puzzle in evolutionary biology. Ordinarily sterile workers can usually reproduce given the right conditions, so the factors regulating reproductive division of labour may provide insight into why eusociality has persisted over evolutionary time. Queen-produced pheromones that affect worker reproduction have been implicated in diverse taxa, including ants, termites, wasps and possibly mole rats, but to date have only been definitively identified in the honeybee. Using the black garden ant Lasius niger, we isolate the first sterility-regulating ant queen pheromone. The pheromone is a cuticular hydrocarbon that comprises the majority of the chemical profile of queens and their eggs, and also affects worker behaviour, by reducing aggression towards objects bearing the pheromone. We further show that the pheromone elicits a strong response in worker antennae and that its production by queens is selectively reduced following an immune challenge. These results suggest that the pheromone has a central role in colony organization and support the hypothesis that worker sterility represents altruistic self-restraint in response to an honest quality signal.
Keywords: social insect, cuticular hydrocarbon, queen signal, Lasius niger, handicap
1. Introduction
Worker sterility is the defining feature of eusociality, and is therefore fundamental to any explanation of its evolutionary origin and maintenance. The degree to which worker sterility is driven by cooperation or conflict (Lehmann & Keller 2006; Ratnieks et al. 2006; Boomsma 2009) and individual- or colony-level selection (Keller 1999; Wilson & Hölldobler 2005; Okasha 2006) remain active areas of research. At the proximate level, the genetic (Grozinger et al. 2003; Nelson et al. 2007; Schwander & Keller 2008; Alaux et al. 2009; Wurm et al. 2010) and developmental (Khila & Abouheif 2008; Roat & Landim 2008; Johnson & Linksvayer 2010) bases of reproductive division of labour have been elucidated with increasing resolution, although the systems that determine when and why individuals relinquish sterility and switch to individual reproduction are less well understood.
Queen-produced pheromones that maintain worker sterility are thought to be taxonomically widespread, as queens, their eggs and queen-derived chemicals have been shown to reduce or eliminate worker reproduction, and because queens typically produce chemicals that are absent or minimally expressed in workers (e.g. Vargo 1992; Peeters et al. 1999; Dietemann et al. 2003; Cuvillier-Hot et al. 2004a; Endler et al. 2004; Monnin 2006; Dengler-Crish & Catania 2007; Korb et al. 2009; Bhadra et al. 2010). However, the honeybee is the only insect in which primer pheromones (i.e. pheromones with a physiological effect) have been definitively identified (Le Conte & Hefetz 2008), meaning that it is difficult to draw general conclusions about the factors regulating sterility. Queen pheromones underpin the proximate and ultimate causes of worker sterility: in the honeybee, they cause changes in worker gene expression (Grozinger et al. 2003; Beggs et al. 2007) and physiology (Kaatz et al. 1992; Beggs et al. 2007) that mediate the transition from indirect to individual reproduction, and they have been postulated to be either a manipulation that is detrimental to workers (‘queen control’) or a signal to which workers are selected to respond (‘queen signal’; Keller & Nonacs 1993; Heinze & d'Ettorre 2009). Queen pheromones are also interesting because they are thought to be central to the colony's ‘social physiology’, the superorganismal analogue of regulatory mechanisms such as hormones (Johnson & Linksvayer 2010). Elucidating the identity, modus operandi and fitness consequences of queen pheromones in additional taxa is therefore likely to produce new insights into social evolution.
Here, we identify a multi-functional queen pheromone from the black garden ant Lasius niger. In a previous study, we found that the cuticular hydrocarbon 3-methylhentriacontane (3-MeC31) was strongly correlated with queen productivity, maturity and likelihood of avoiding execution by workers in colonies with supernumerary queens (Holman et al. 2010). The results of Holman et al. (2010) imply that 3-MeC31 is a signal of queen quality, and verbal models have suggested that the queen pheromones hypothesized to regulate reproductive division of labour are likely to be honest quality signals (Keller & Nonacs 1993; Zahavi & Zahavi 1997; Heinze & d'Ettorre 2009; van Zweden 2010). We therefore developed a novel synthetic pathway for 3-MeC31 in order to test whether it (i) affects worker ovarian activation, (ii) influences worker behaviour and (iii) is detectable by workers. We also quantified queens' chemical profiles after an experimental immune challenge, to assess whether 3-MeC31 could provide information on queen condition to workers. Lastly, we found that 3-MeC31 is abundant on the surface of queen-laid eggs, giving insight into its function and mode of action.
2. Material and methods
(a). Comparison of worker, queen and egg chemical profiles
Cuticular hydrocarbons were extracted from L. niger queens (n = 20) and analysed as previously described (Holman et al. 2010). In short, ant cuticular hydrocarbons were extracted for 10 min in 150 µl pentane; the pentane was allowed to evaporate, and the extract was re-diluted in 60 µl pentane. We then injected 2 µl of extract into the GC-MS using an auto-sampler. Analysis of egg and worker surface hydrocarbons was the same except for the extraction and injection methods; 10 eggs or one worker were placed in a 200 µl glass insert and extracted for 3 or 10 min, respectively, in 20 µl pentane, 2 µl of which was then manually injected into the GC-MS (n = 20). Peak areas were analysed using multivariate statistics (using transformed data as in Holman et al. 2010) and univariate statistics (using proportion data and GLMs).
(b). Synthetic cuticular hydrocarbons
Synthesis of 3-MeC27 and 3-MeC31 is described in the electronic supplementary material. C29 and C31 were purchased from Sigma-Aldrich.
(c). Effects of 3-MeC31 on worker ovarian activation and behaviour
Lasius niger workers were collected from six wild colonies in Copenhagen, Denmark. Collected workers were divided into three equal groups, each of which was given a model queen made from the tip of a glass vial. Every 12 h for 37 days after collection, the model queen was removed, coated with 10 µl of a pentane solution of (i) 0.01 µg µl−1 3-MeC31, (ii) 0.01 µg µl−1 hentriacontane (C31) or (iii) pentane only, and replaced once completely dry (blind, using a labelling code). The alkane C31 was chosen as a control hydrocarbon because it is also a queen-type cuticular hydrocarbon of L. niger (electronic supplementary material, table S1) and has the same chain length, but was previously found to be unrelated to queen productivity, maturity or survival (Holman et al. 2010). Highly purified HPLC-grade pentane (Sigma-Aldrich) was used throughout.
After 37 days, all colonies were frozen for dissection. Ovarian activation was scored on a scale of 1–4: (1) completely empty; (2) one or two very small eggs and/or developing nurse cell material; (3) one to three developing eggs in both ovarioles or large eggs in one ovariole; and (4) well-developed eggs in both ovarioles (blind, using a different labelling code to the behavioural observations). Production of males by workers occurs in natural colonies of L. niger (Fjerdingstad et al. 2002), although oviposition was not observed in our small laboratory colonies.
On days 3–37 of this experiment, we conducted 3 min of behavioural observations (blind to treatment) starting 10 s after the replacement of the model queen, with the aid of Etholog v. 2.2.5 software (Ottoni 2000). We recorded aggression towards the model queen (duration of attack multiplied by number of workers attacking) and the number of aggressive worker–worker interactions. Ovary and behavioural data were analysed with quasi-Poisson GLMMs with colony (and observation day, for the behavioural observations) as a random factor, in order to account for non-normal errors, overdispersion and within-colony similarity.
(d). Electroantennography of synthetic hydrocarbons
We collected workers from a wild colony and used them within 6 h in electroantennography (EAG) trials (protocol adapted from d'Ettorre et al. 2004). The left antennal flagellum was excised (n = 25 workers) and mounted between two pulled glass capillaries containing insect Ringer, which bathed two Ag–AgCl electrodes. The electrode holding the proximal end of the flagellum was connected to a ground wire, while the other was connected via an amplifier to a signal acquisition interface board (IDAC; Syntech, Hilversum, The Netherlands) for signal transfer to a PC. The antenna was placed in a stream of purified, humidified air, and the amplitude of the depolarization response of the antennal neurons was recorded in millivolts (using EAG 2000 software; Syntech) following exposure to six different stimuli: a pentane control, and pentane solutions (all 0.5 µg µl−1) of C29, C31, 3-MeC27 and 3-MeC31, as well as a mixture containing all of these hydrocarbons. These hydrocarbons are all present on the cuticle of queen L. niger (electronic supplementary material, table S1), but all had a non-significant or weak (relative to 3-MeC31) relationship with queen fertility, maturity and survival (Holman et al. 2010).
We placed 10 µl of hydrocarbon solution on a 5 × 15 mm piece of filter paper in a new Pasteur pipette heated to 70°C on a hotplate, and immediately blew a pulse of air through the pipette onto the flagellum. Before starting each run, we blew a single pulse of air onto the antenna to verify that it was responsive. The treatment order was randomized, and the experiment was conducted and analysed blind. Responses were standardized against the control for each antenna by setting the response to the pentane control as 100 and transforming the other treatments accordingly. The data were analysed using a GLMM with Gaussian errors and antenna as a random factor. There was a significant effect of order, such that the antenna tended to display a higher response to stimuli presented later in each replicate, so order was included as a covariate in the model (t = 5.68, n = 25, p < 0.0001).
(e). Effect of immune challenge on production of 3-MeC31 by queens
Lasius niger queens were collected during a mating flight in Copenhagen and allowed to mature and rear workers in the laboratory for 201 days. To administer an immune challenge, we starved queens for 24 h (Moret & Schmid-Hempel 2000) and then pierced their inter-pleural membranes using a sterilized pin coated with either 2.5 µg µl−1 lipopolysaccharide (Sigma-Aldrich) in sterile Ringer, or Ringer alone (blind and randomized). Queens were isolated from their colonies for 24 h then frozen for cuticular hydrocarbon analysis as described above; the peak areas were analysed blind. All statistical tests were performed in R v. 2.8.1 and validated using diagnostic plots.
3. Results
(a). 3-MeC31 is a major component of queen and egg chemical profiles
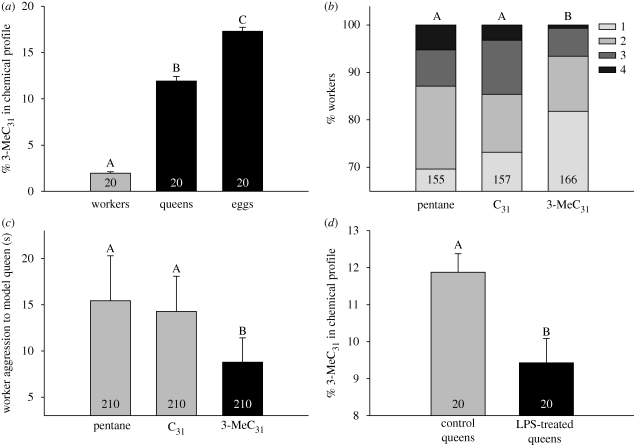
The cuticular hydrocarbon profiles of queens and workers were markedly different (discriminant analysis based on six principal components explaining 85% of the variance: Wilk's λ = 0.03, F6,33 = 155, p < 0.0001), with 3-MeC31 showing the strongest caste specificity (queens had a 6.1 times higher proportion than workers; figure 1a; electronic supplementary material, table S1) and being the most abundant single compound in the queen profile. 3-MeC31 was also the most abundant hydrocarbon on the surface of queen-laid eggs (figure 1a; electronic supplementary material, table S1).
Figure 1.
3-MeC31 is a condition-dependent queen pheromone that affects worker physiology and behaviour in L. niger. (a) The cuticular hydrocarbon profile of queens contains a 6 times higher proportion of 3-MeC31 than that of a worker, while the egg profile has 9 times more; see also electronic supplementary material, table S1. (b) Supplementation of queenless groups of workers with synthetic 3-MeC31 negatively affected ovarian activation relative to controls. Bars show the frequency distributions of ovary activation on a categorical scale from 1 (no activation) to 4 (highest activation). (c) Glass model queens coated with 3-MeC31 were attacked by workers less often than controls. (d) A lipopolysaccharide immune challenge reduced the proportion of 3-MeC31 on the cuticle of queens. (a,c,d) Means ± 1 s.e.; shared letters indicate that two groups are not significantly different. Sample size is shown inside the bars.
(b). Synthetic 3-MeC31 reduces worker ovarian activation and aggressive behaviour
After 37 days of separation from the queen, worker ovarian activation was significantly lower in colony fragments that had been supplemented twice daily with synthetic 3-MeC31 rather than pentane solvent (figure 1b; GLMM: t = 2.76, p = 0.006, n = 478 workers) or the control hydrocarbon C31 (t = 2.27, p = 0.024). Ovarian activation did not differ between C31- and pentane-treated workers (t = 0.64, p = 0.52). This experiment was replicated with workers from six colonies; there was no significant treatment × colony interaction term (F-test comparing models with colony fitted as a fixed factor: F9,260 = 0.95, p = 0.47), showing that the effect of 3-MeC31 was consistent across colonies (electronic supplementary material, figure S1). We therefore conclude that 3-MeC31 is a primer pheromone that negatively affects the activation of worker ovaries.
Workers frequently attacked the glass model queens to which we applied the hydrocarbon solutions. However, models coated with 3-MeC31 were attacked significantly less than those treated with C31 (GLMM: t = 2.27, p = 0.001) or pentane (t = 5.83, p = 0.03). The duration of attack did not differ between the pentane and C31 treatments (t = 1.16, p = 0.25) (figure 1c; n = 630 observations). We also recorded mild aggression among workers in the form of body-jerking threat displays, although the number of aggressive acts did not differ between treatment groups (p > 0.1; n = 630). There was therefore no evidence that the onset of worker ovarian activation was accompanied by increased worker–worker aggression as in some other social insects (e.g. Cuvillier-Hot et al. 2004a; Korb et al. 2009).
(c). 3-MeC31 elicits a strong electrophysiological response in worker antennae
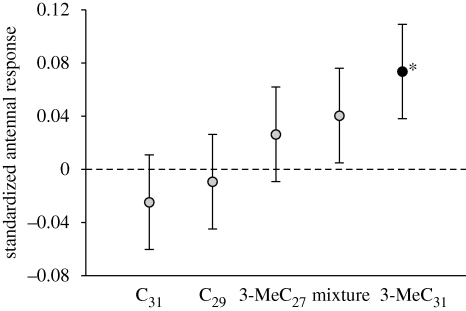
In EAG trials, 3-MeC31 induced a stronger response in excised worker antennae than the pentane control (figure 2; mixed-effects model with antenna as a random factor: t = 2.04, n = 25, p = 0.043), demonstrating that the queen pheromone is detected by worker antennae. None of the other synthetic hydrocarbons tested (C29, C31 and 3-MeC27) induced a significantly greater antennal response than the control (all p > 0.46). The response to 3-MeC31 was significantly higher than that to C29 (t = 2.33, p = 0.048) and C31 (t = 2.75, p = 0.020), and non-significantly higher than that to 3-MeC27 (t = 1.31, p = 0.22) and the mixture of all four hydrocarbons (t = 0.92, p = 0.35).
Figure 2.
The queen pheromone 3-MeC31 elicits a strong response in worker antennae. Response of worker antennae (n = 25) to the five treatment solutions, relative to the pentane control. The plots show the estimated effect ±1 s.e. from contrasts of a mixed model with antenna as a random factor and treatment order as a covariate; a value of zero indicates an antennal response equal to that of the control (pentane only) stimulus. Only 3-MeC31 produced a significantly higher (starred; p < 0.05) electrophysiological response than the control.
(d). Immune challenge reduces the amount of 3-MeC31 present on queen cuticle
The cuticular hydrocarbon profiles of queens subjected to a lipopolysaccharide immune challenge were significantly different from those of controls (discriminant analysis based on eight principal components explaining 86% of the variation; Wilk's λ = 0.60, χ28 = 16.9, p = 0.031), primarily because the mean proportion of the chemical profile composed of 3-MeC31 was 21 per cent lower in challenged queens (figure 1d; ANOVA: t38 = 2.83, p = 0.007). The proportions of all 29 of the other hydrocarbon peaks did not significantly change following immune challenge (all p38 > 0.06).
4. Discussion
These experiments demonstrate that 3-MeC31: (i) is a primer pheromone that negatively affects worker ovarian activation; (ii) is also a releaser pheromone (i.e. one that affects behaviour) that is perceptible to workers and influences aggressive behaviour; (iii) elicits a comparatively strong response in worker antennae, implying the presence of many olfactory receptor neurons sensitive to 3-MeC31, consistent with its function as a pheromone; and (iv) displays condition-dependent expression. We also found that 3-MeC31 is the major component of the chemical profile of queen-laid eggs.
To our knowledge, 3-MeC31 is the first insect primer pheromone to be definitively identified outside of the honeybee (Le Conte & Hefetz 2008), and the first cuticular hydrocarbon demonstrated to affect conspecific reproductive physiology in any species. Primer pheromone activity of queen cuticular hydrocarbons is nevertheless likely to be common throughout the social insects. Differences in the cuticular hydrocarbon profiles of queens and workers have been reported in many species of ants, bees, wasps and termites (e.g. Peeters et al. 1999; Dietemann et al. 2003; Monnin 2006; Sramkova et al. 2008; Liebig et al. 2009; Peeters & Liebig 2009). Moreover, in Camponotus floridanus ants, workers in queenless colonies do not reproduce while queen-laid eggs are present (Endler et al. 2004); the eggs are coated with a hydrocarbon mixture similar to the cuticle of queens, consistent with regulation of worker sterility by one or more hydrocarbons. Similarly, queen corpses reduce the reproductive output of live queens in Solenopsis invicta ants (Vargo 1992), and in the wasps Polistes gallicus and Ropalidia marginata queens apparently prevent subordinate reproduction with chemicals from an abdominal gland (Dapporto et al. 2007) and Dufour's gland (Bhadra et al. 2010), respectively. Interestingly, R. marginata queens had more 3-MeC31 in Dufour's gland than did workers, suggesting that this compound may act as a primer pheromone in distantly related species. Cuticular hydrocarbons have also been shown to regulate reproduction indirectly through their role in ‘worker policing’: illegitimate reproductives are identified by their cuticular hydrocarbons and aggressed by their nest-mates (e.g. Peeters & Liebig 2009; Smith et al. 2009).
In honeybees, queen pheromones are detected via an odorant binding protein in workers' antennae (Wanner et al. 2007), leading to reductions in juvenile hormone titre (Kaatz et al. 1992), dopamine production (Beggs et al. 2007) and dopamine receptor gene expression (Beggs et al. 2007) that cause workers to remain sterile. Available data suggest that the physiological mechanisms by which 3-MeC31 affects worker reproduction in L. niger are probably very similar. Firstly, our results demonstrate that workers perceive 3-MeC31 via their antennae. A recent study of L. niger found a gene (Ln385_5) with worker-biased expression that encodes a homologue of the pheromone-binding protein ASP1 (Graff et al. 2007). This protein is found in the antennal olfactory sensillae of worker and drone honeybees, where it binds to queen pheromone (Danty et al. 1999), so it is possible that Ln385_5 is also involved in the perception of queen pheromones. Secondly, juvenile hormone also regulates ovarian activation in L. niger (Sommer & Hölldobler 1995), and in S. invicta loss of the dominant queen affects the expression of genes that regulate juvenile hormone levels in subordinate queens, causing them to activate their ovaries (Wurm et al. 2010).
Our immune challenge experiment indicates that 3-MeC31 provides information about a queen's immune status or overall condition. This result implies that production of 3-MeC31 is physiologically costly relative to other cuticular hydrocarbons (assuming that pheromone production is always beneficial to queens), and therefore supports the prediction that queen pheromones should only be evolutionary stable when they honestly signal a queen's reproductive potential (Keller & Nonacs 1993), because costly traits act as handicaps that constrain dishonest signalling (Johnstone & Grafen 1993; Zahavi & Zahavi 1997; Heinze & d'Ettorre 2009; van Zweden 2010). Several other lines of evidence suggest that putative social insect queen pheromones are handicaps. Queen-specific cuticular hydrocarbons are typically methylated alkanes or alkenes, which are thought to confer inferior protection against desiccation compared with the hypothetically ancestral compounds, linear alkanes (Monnin 2006; Le Conte & Hefetz 2008). Also, reproductive development in insects is correlated with hormone titres (Heinze & Schrempf 2008) as well as surface chemicals; hormones influence condition and survival, for example through effects on immune function (Rolff & Siva-Jothy 2002) and anti-oxidant activity (Heinze & Schrempf 2008). Therefore, the costs of pheromone synthesis might arise from the physiologically expensive hormone levels required for their production (as suggested for sexual signals; Folstad & Karter 1992), although further biochemical data are required to test this hypothesis. First steps in this direction have been achieved by studies supplementing queens with juvenile hormone analogues, which suppress reproduction; hormone treatment was associated with a reduction in reproductive-like chemicals in queenless ants (Cuvillier-Hot et al. 2004b), but not in honeybees (Malka et al. 2009). Cuticular hydrocarbons associated with reproductive activity can also attract aggression in certain contexts (e.g. when expressed by individuals with relatively low reproductive potential), and thereby incur costs (Peeters & Liebig 2009; Smith et al. 2009).
An alternative to the handicap hypothesis of honest queen pheromones is based on the ‘index’ concept (sensu Maynard Smith & Harper 2003); pheromone production might be inextricably linked to reproductive physiology (e.g. by shared dependence on common biosynthetic pathways), so that dishonest signalling is impossible (Heinze & d'Ettorre 2009; Smith et al. 2009; van Zweden 2010). Our immune challenge data are also consistent with this hypothesis; immune activation might have depressed queens' reproduction, which in turn lowered pheromone production. Future studies will need to investigate the costs, genetics and biochemistry of pheromone production in order to distinguish between these hypotheses.
As well as being abundant on the cuticle of queen L. niger, 3-MeC31 is the most plentiful hydrocarbon on the surface of queen-laid eggs, and is also found on cocoons (Holman et al. 2010). Being present on brood may increase the frequency with which workers encounter 3-MeC31, which has very low volatility; ant brood has been shown to inhibit sexual production (Edwards 1987) and worker oviposition (Endler et al. 2004), suggesting that eggs are a means of distributing queen pheromones. Many L. niger cuticular hydrocarbons, including 3-MeC31, are also present on the nest soil (Lenoir et al. 2009), which could be another mechanism of dispensing the signal. Another function of brood-borne 3-MeC31 may be the regulation of queen productivity via negative feedback. Lasius niger queen productivity was lower in the presence of brood and other queens (Holman et al. 2010), so it is possible that 3-MeC31 affects the reproductive state of queens as well as workers (although the dose–response curve of the two castes would probably be different). The presence of the queen pheromone on eggs may also contribute to ensuring that the pheromone is an honest signal of fertility, because fertile queens will produce more eggs and thereby expose workers to greater quantities of pheromone. Lastly, 3-MeC31 might serve as an egg-marking signal used by workers to decide which eggs to rear (e.g. Endler et al. 2006; van Zweden et al. 2009).
Together with previous work, our results show how condition-dependent queen pheromones could act as parsimonious ‘master signals’ at the centre of the colony's social physiology that quantitatively modulate multiple colony-level traits. If pheromone production declines with the condition of the queen (e.g. when the queen becomes old or ill), worker behaviour associated with the absence of the queen may be initiated before the queen dies. Where the same pheromones are present on brood (Endler et al. 2004; van Zweden et al. 2009), declining brood number may similarly contribute to these worker responses (Edwards 1987; Endler et al. 2004), as well as allowing queens to tune their reproductive rate to the current number of brood as mentioned above. If the queen pheromone also affects worker aggression (as implicated here and in several other ants; Vander Meer & Alonso 2002; Peeters & Liebig 2009; Smith et al. 2009; Moore & Liebig 2010; Wurm et al. 2010), the pheromone could also be used by the colony to decide who should reproduce. In L. niger, colonies are often co-founded by multiple queens, but only one queen survives after the first workers eclose (Sommer & Hölldobler 1995). A queen's likelihood of being spared execution by workers is correlated with the amount of 3-MeC31 on her cuticle, implying that workers use this chemical to selectively kill the least fertile queens (Holman et al. 2010). Queen-like hydrocarbons also facilitate identification and punishment of reproductive workers in the ant Aphaenogaster cockerelli (Smith et al. 2009) and are thought to signal reproductive rank in queenless ants with dominance hierarchies (Peeters & Liebig 2009). A queen-derived cue also modulates worker aggression and consequently adoption of new queens in S. invicta (Vander Meer & Alonso 2002), and chemicals from the queen's sting gland prevent subordinate queens from shedding their wings and becoming reproductive (Vargo 1997). In the honeybee, production of most queen pheromone components is lower in ‘drone-producing’ and virgin queens relative to fully fertile queens (Strauss et al. 2008), resulting in a reduced behavioural response by workers (Kocher et al. 2009), and pheromone quantity or quality deteriorates in old queens (Butler 1957). In bumblebees, developing colonies reach a ‘competition point’ at which workers begin to reproduce, the timing of which is thought to depend on changes in queen-produced pheromone(s) (Alaux et al. 2007).
In the honeybee, a single queen-produced chemical (9-keto-2(E)-decenoic acid) induces near-complete worker sterility in bioassays (Kaatz et al. 1992); several other honeybee pheromones are known, but to our knowledge, there is little evidence that they directly affect worker sterility (although they may have an indirect effect via their positive chemotactic effect on workers; e.g. Hoover et al. 2003). Similarly, in termites, silencing one gene in the colony's queen induces worker behaviour characteristic of recently de-queened colonies (Korb et al. 2009). Furthermore, the chemical profiles of reproductives frequently only differ from sterile workers by a single compound, or a single family of hydrocarbons (reviewed in Monnin 2006). In the present study, we showed that 3-MeC31, but not C31, affects worker sterility, even though both compounds are characteristic of queens; 3-MeC31 was also active in isolation. Based on these data, we suggest that worker sterility might often be regulated by single- rather than multi-component pheromones, and that at present there is insufficient evidence to rule out either of these hypotheses in any species. Single-component queen pheromones imply a mutualistic model of the origin of eusociality characterized by minimal parent–offspring conflict (Boomsma 2009), because in a high-conflict scenario, an evolutionary arms race over reproductive rights is predicted (Keller & Nonacs 1993). This arms race is expected to be characterized by the evolution of resistance to the queen pheromone in subordinates, followed by elaboration of the pheromone (e.g. by adding more component chemicals) and restoration of its manipulative effects (Le Conte & Hefetz 2008; Heinze & d'Ettorre 2009). Under the low-conflict model, workers might instead co-opt a single, arbitrary chemical that honestly indicates the presence of a healthy reproductive as a regulatory mechanism for their self-imposed sterility. Identification of queen primer pheromones in other taxa may reveal universal evolutionary trends and produce unexpected advances in our understanding of the origin and maintenance of eusociality.
Acknowledgements
We are grateful to all members of the Center for Social Evolution, Copenhagen, for a stimulating work environment, and to J. J. Boomsma for comments on the manuscript. This work was supported by the Marie Curie Excellence grant CODICES (MEXT-CT-2004-014202) assigned to P.d'E. and by a Marie Curie Intra-European Fellowship to L.H. (no. 235403; CHEMDOC).
References
- Alaux C., Boutot M., Jaisson P., Hefetz A.2007Reproductive plasticity in bumblebee workers (Bombus terrestris)—reversion from fertility to sterility under queen influence. Behav. Ecol. Sociobiol. 62, 213–222 10.1007/s00265-007-0455-6 (doi:10.1007/s00265-007-0455-6) [DOI] [Google Scholar]
- Alaux C., et al. 2009Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl Acad. Sci. USA 106, 15 400–15 405 10.1073/pnas.0907043106 (doi:10.1073/pnas.0907043106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs K. T., Glendining K. A., Marechal N. M., Vergoz V., Nakamura I., Slessor K. N., Mercer A. R.2007Queen pheromone modulates brain dopamine function in worker honey bees. Proc. Natl Acad. Sci. USA 104, 2460–2464 10.1073/pnas.0608224104 (doi:10.1073/pnas.0608224104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra A., Mitra A., Deshpande S., Chandrasekhar K., Naik D., Hefetz A., Gadagkar R.2010Regulation of reproduction in the primitively eusocial wasp Ropalidia marginata: on the trail of the queen pheromone. J. Chem. Ecol. 36, 424–431 10.1007/s10886-010-9770-x (doi:10.1007/s10886-010-9770-x) [DOI] [PubMed] [Google Scholar]
- Boomsma J. J.2009Lifetime monogamy and the evolution of eusociality. Phil. Trans. R. Soc. B 364, 3191–3207 10.1098/rstb.2009.0101 (doi:10.1098/rstb.2009.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler C.1957The process of queen supersedure in colonies of honeybees (Apis mellifera Linn.). Insect. Soc. 4, 211–223 10.1007/BF02222154 (doi:10.1007/BF02222154) [DOI] [Google Scholar]
- Cuvillier-Hot V., Lenoir A., Crewe R., Malosse C., Peeters C.2004aFertility signalling and reproductive skew in queenless ants. Anim. Behav. 68, 1209–1219 [Google Scholar]
- Cuvillier-Hot V., Lenoir A., Peeters C.2004bReproductive monopoly enforced by sterile police workers in a queenless ant. Behav. Ecol. 15, 970–975 10.1093/beheco/arh072 (doi:10.1093/beheco/arh072) [DOI] [Google Scholar]
- Danty E., et al. 1999Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J. Neurosci. 19, 7468–7475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapporto L., Santini A., Dani F. R., Turillazzi S.2007Workers of a Polistes paper wasp detect the presence of their queen by chemical cues. Chem. Sens. 32, 795–802 10.1093/chemse/bjm047 (doi:10.1093/chemse/bjm047) [DOI] [PubMed] [Google Scholar]
- Dengler-Crish C. M., Catania K. C.2007Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J. Exp. Biol. 210, 4351–4358 10.1242/jeb.009399 (doi:10.1242/jeb.009399) [DOI] [PubMed] [Google Scholar]
- d'Ettorre P., Heinze J., Schulz C., Francke W., Ayasse M.2004Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 207, 1085–1091 10.1242/jeb.00865 (doi:10.1242/jeb.00865) [DOI] [PubMed] [Google Scholar]
- Dietemann V., Peeters C., Liebig J., Thivet V., Hölldobler B.2003Cuticular hydrocarbons mediate discrimination of repoductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl Acad. Sci. USA 100, 10 341–10 346 10.1073/pnas.1834281100 (doi:10.1073/pnas.1834281100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. P.1987Caste regulation in the pharaoh's ant Monomorium pharaonis: the influence of queens on the production of new sexual forms. Physiol. Entomol. 12, 31–39 10.1111/j.1365-3032.1987.tb00721.x (doi:10.1111/j.1365-3032.1987.tb00721.x) [DOI] [Google Scholar]
- Endler A., Liebig J., Schmitt T., Parker J. E., Jones G. R., Schreier P., Holldobler B.2004Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl Acad. Sci. USA 101, 2945–2950 10.1073/pnas.0308447101 (doi:10.1073/pnas.0308447101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A., Liebig J., Hölldobler B.2006Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav. Ecol. Sociobiol. 59, 1–10 [Google Scholar]
- Fjerdingstad E. J., Gertsch P. J., Keller L.2002Why do some social insect queens mate with several males? Testing the sex-ratio manipulation hypothesis in Lasius niger. Evolution 56, 553–562 [DOI] [PubMed] [Google Scholar]
- Folstad I., Karter A. J.1992Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622 10.1086/285346 (doi:10.1086/285346) [DOI] [Google Scholar]
- Graff J., Jemielity S., Parker J. D., Parker K. M., Keller L.2007Differential gene expression between adult queens and workers in the ant Lasius niger. Mol. Ecol. 16, 675–683 10.1111/j.1365-294X.2007.03162.x (doi:10.1111/j.1365-294X.2007.03162.x) [DOI] [PubMed] [Google Scholar]
- Grozinger C. M., Sharabash N. M., Whitfield C. W., Robinson G. E.2003Pheromone-mediated gene expression in the honey bee brain. Proc. Natl Acad. Sci. USA 100, 14 519–14 525 10.1073/pnas.2335884100 (doi:10.1073/pnas.2335884100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze J., d'Ettorre P.2009Honest and dishonest communication in social Hymenoptera. J. Exp. Biol. 212, 1775–1779 10.1242/jeb.015008 (doi:10.1242/jeb.015008) [DOI] [PubMed] [Google Scholar]
- Heinze J., Schrempf A.2008Aging and reproduction in social insects—a mini-review. Gerontology 54, 160–167 10.1159/000122472 (doi:10.1159/000122472) [DOI] [PubMed] [Google Scholar]
- Holman L., Dreier S., d'Ettorre P.2010Selfish strategies and honest signalling: reproductive conflicts in ant queen associations. Proc. R. Soc. B 277, 2007–2015 10.1098/rspb.2009.2311 (doi:10.1098/rspb.2009.2311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover S. E. R., Keeling C. I., Winston M. L., Slessor K. N.2003The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90, 477–480 10.1007/s00114-003-0462-z (doi:10.1007/s00114-003-0462-z) [DOI] [PubMed] [Google Scholar]
- Johnson B. R., Linksvayer T. A.2010Deconstructing the superorganism: social physiology, groundplans, and sociogenomics. Q. Rev. Biol. 85, 57–79 [DOI] [PubMed] [Google Scholar]
- Johnstone R. A., Grafen A.1993Dishonesty and the handicap principle. Anim. Behav. 46, 759–764 10.1006/anbe.1993.1253 (doi:10.1006/anbe.1993.1253) [DOI] [Google Scholar]
- Kaatz H.-H., Hildebrandt H., Engels W.1992Primer effect of queen pheromone on juvenile hormone biosynthesis in adult worker honey bees. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 162, 588–592 [Google Scholar]
- Keller L. (ed.) 1999Levels of selection in evolution. Princeton, NJ: Princeton University Press [Google Scholar]
- Keller L., Nonacs P.1993The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787–794 10.1006/anbe.1993.1092 (doi:10.1006/anbe.1993.1092) [DOI] [Google Scholar]
- Khila A., Abouheif E.2008Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. Proc. Natl Acad. Sci. USA 105, 17 884–17 889 10.1073/pnas.0807351105 (doi:10.1073/pnas.0807351105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher S. D., Richard F.-J., Tarpy D. R., Grozinger C. M.2009Queen reproductive state modulates pheromone production and queen–worker interactions in honeybees. Behav. Ecol. 20, 1007–1014 10.1093/beheco/arp090 (doi:10.1093/beheco/arp090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb J., Weil T., Hoffmann K., Foster K. R., Rehli M.2009A gene necessary for reproductive suppression in termites. Science 324, 758. 10.1126/science.1170660 (doi:10.1126/science.1170660) [DOI] [PubMed] [Google Scholar]
- Le Conte Y., Hefetz A.2008Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 53, 523–542 [DOI] [PubMed] [Google Scholar]
- Lehmann L., Keller L.2006The evolution of cooperation and altruism—a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376 10.1111/j.1420-9101.2006.01119.x (doi:10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- Lenoir A., Depickere S., Devers S., Christides J. P., Detrain C.2009Hydrocarbons in the ant Lasius niger: from the cuticle to the nest and home range marking. J. Chem. Ecol. 35, 913–921 10.1007/s10886-009-9669-6 (doi:10.1007/s10886-009-9669-6) [DOI] [PubMed] [Google Scholar]
- Liebig J., Eliyahu D., Brent C. S.2009Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav. Ecol. Sociobiol. 63, 1799–1807 10.1007/s00265-009-0807-5 (doi:10.1007/s00265-009-0807-5) [DOI] [Google Scholar]
- Malka O., Katzav-Gozansky T., Hefetz A.2009Uncoupling fertility from fertility-associated pheromones in worker honeybees (Apis mellifera). J. Insect Physiol. 55, 205–209 10.1016/j.jinsphys.2008.11.002 (doi:10.1016/j.jinsphys.2008.11.002) [DOI] [PubMed] [Google Scholar]
- Maynard Smith J., Harper D.2003Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- Monnin T.2006Chemical recognition of reproductive status in social insects. Ann. Zool. Fenn. 43, 531–549 [Google Scholar]
- Moore D., Liebig J.2010Mixed messages: fertility signaling interferes with nestmate recognition in the monogynous ant Camponotus floridanus. Behav. Ecol. Sociobiol. 64, 1011–1018 10.1007/s00265-010-0916-1 (doi:10.1007/s00265-010-0916-1) [DOI] [Google Scholar]
- Moret Y., Schmid-Hempel P.2000Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168 10.1126/science.290.5494.1166 (doi:10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Ihle K. E., Fondrk M. K., Page R. E., Amdam G. V.2007The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5, 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasha S.2006Evolution and the levels of selection. Oxford, UK: Oxford University Press [Google Scholar]
- Ottoni E. B.2000EthoLog 2.2: A tool for the transcription and timing of behavior observation sessions. Behavior Research Methods Instruments & Computers 32, 446–449 [DOI] [PubMed] [Google Scholar]
- Peeters C., Liebig J.2009Fertility signaling as a general mechanism of regulating reproductive division of labor in ants. In Organization of insect societies: from genome to socio-complexity (eds Gadau J., Fewell J.), Cambridge, MA: Harvard University Press [Google Scholar]
- Peeters C., Monnin T., Malosse C.1999Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266, 1323–1327 10.1098/rspb.1999.0782 (doi:10.1098/rspb.1999.0782) [DOI] [Google Scholar]
- Ratnieks F. L. W., Foster K. R., Wenseleers T.2006Conflict resolution in insect societies. Annu. Rev. Entomol. 51, 581–608 10.1146/annurev.ento.51.110104.151003 (doi:10.1146/annurev.ento.51.110104.151003) [DOI] [PubMed] [Google Scholar]
- Roat T. C., Landim C. D.2008Temporal and morphological differences in post-embryonic differentiation of the mushroom bodies in the brain of workers, queens, and drones of Apis mellifera (Hymenoptera, Apidae). Micron 39, 1171–1178 10.1016/j.micron.2008.05.004 (doi:10.1016/j.micron.2008.05.004) [DOI] [PubMed] [Google Scholar]
- Rolff J., Siva-Jothy M. T.2002Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc. Natl Acad. Sci. USA 99, 9916–9918 10.1073/pnas.152271999 (doi:10.1073/pnas.152271999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander T., Keller L.2008Genetic compatibility affects queen and worker caste determination. Science 322, 552. 10.1126/science.1162590 (doi:10.1126/science.1162590) [DOI] [PubMed] [Google Scholar]
- Smith A. A., Hölldober B., Liebig J.2009Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr. Biol. 19, 78–81 10.1016/j.cub.2008.11.059 (doi:10.1016/j.cub.2008.11.059) [DOI] [PubMed] [Google Scholar]
- Sommer K., Hölldobler B.1995Colony founding by queen association and determinants of reduction in queen number in the ant Lasius niger. Anim. Behav. 50, 287–294 10.1006/anbe.1995.0244 (doi:10.1006/anbe.1995.0244) [DOI] [Google Scholar]
- Sramkova A., Schulz C., Twele R., Francke W., Ayasse M.2008Fertility signals in the bumblebee Bombus terrestris (Hymenoptera: Apidae). Naturwissenschaften 95, 515–522 10.1007/s00114-008-0353-4 (doi:10.1007/s00114-008-0353-4) [DOI] [PubMed] [Google Scholar]
- Strauss K., Scharpenberg H., Crewe R. M., Glahn F., Foth H., Moritz R. F. A.2008The role of the queen mandibular gland pheromone in honeybees (Apis mellifera): honest signal or suppressive agent? Behav. Ecol. Sociobiol. 62, 1523–1531 10.1007/s00265-008-0581-9 (doi:10.1007/s00265-008-0581-9) [DOI] [Google Scholar]
- Vander Meer R. K., Alonso L. E.2002Queen primer pheromone affects conspecific fire ant (Solenopsis invicta) aggression. Behav. Ecol. Sociobiol. 51, 122–130 [Google Scholar]
- van Zweden J. S.2010The evolution of honest queen pheromones in insect societies. Commun. Integr. Biol. 3, 50–52 10.4161/cib.3.1.9655 (doi:10.4161/cib.3.1.9655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zweden J. S., Heinze J., Boomsma J. J., d'Ettorre P.2009Ant queen egg-marking signals: matching deceptive laboratory simplicity with natural complexity. PLoS ONE 4, e4718. 10.1371/journal.pone.0004718 (doi:10.1371/journal.pone.0004718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo E. L.1992Mutual pheromonal inhibition among queens in polygyne colonies of the fire ant Solenopsis invicta. Behav. Ecol. Sociobiol. 31, 205–210 [Google Scholar]
- Vargo E. L.1997Poison gland of queen fire ants (Solenopsis invicta) is the source of a primer pheromone. Naturwissenschaften 84, 507–510 10.1007/s001140050435 (doi:10.1007/s001140050435) [DOI] [Google Scholar]
- Wanner K. W., Nichols A. S., Walden K. K. O., Brockmann A., Luetje C. W., Robertson H. M.2007A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. Proc. Natl Acad. Sci. USA 104, 14 383–14 388 10.1073/pnas.0705459104 (doi:10.1073/pnas.0705459104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. O., Hölldobler B.2005Eusociality: origin and consequences. Proc. Natl Acad. Sci. USA 102, 13 367–13 371 10.1073/pnas.0505858102 (doi:10.1073/pnas.0505858102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm Y., Wang J., Keller L.2010Changes in reproductive roles are associated with changes in gene expression in fire ant queens. Mol. Ecol. 19, 1200–1211 10.1111/j.1365-294X.2010.04561.x (doi:10.1111/j.1365-294X.2010.04561.x) [DOI] [PubMed] [Google Scholar]
- Zahavi A., Zahavi A.1997The handicap principle: a missing piece of Darwin's puzzle. New York, NY: Oxford University Press [Google Scholar]