Abstract
Interactions involving several parasite species (multi-parasitized hosts) or several host species (multi-host parasites) are the rule in nature. Only a few studies have investigated these realistic, but complex, situations from an evolutionary perspective. Consequently, their impact on the evolution of parasite virulence and transmission remains poorly understood. The mechanisms by which multiple infections may influence virulence and transmission include the dynamics of intrahost competition, mediation by the host immune system and an increase in parasite genetic recombination. Theoretical investigations have yet to be conducted to determine which of these mechanisms are likely to be key factors in the evolution of virulence and transmission. In contrast, the relationship between multi-host parasites and parasite virulence and transmission has seen some theoretical investigation. The key factors in these models are the trade-off between virulence across different host species, variation in host species quality and patterns of transmission. The empirical studies on multi-host parasites suggest that interspecies transmission plays a central role in the evolution of virulence, but as yet no complete picture of the phenomena involved is available. Ultimately, determining how complex host–parasite interactions impact the evolution of host–parasite relationships will require the development of cross-disciplinary studies linking the ecology of quantitative networks with the evolution of virulence.
Keywords: multi-parasitized hosts, multi-host parasites, intrahost competition, immune system, interspecies transmission, epidemiology
1. Introduction
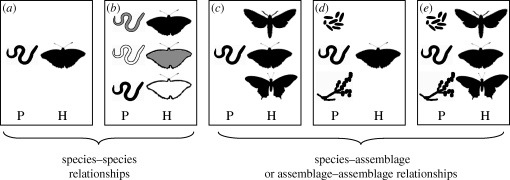
A single-parasite genotype rarely infects only one host genotype and, similarly, a single-host genotype is rarely infected by just one parasite genotype. The same situation is true at the species level, with the majority of parasites having multiple host species and all hosts having multiple parasite species. These facts are not a recent discovery and could be considered as a ‘lieu commun’ for most parasitologists (Combes 2001; Pedersen & Fenton 2007; Poulin 2007). Nevertheless, the attention of evolutionary biologists has only recently focused on this area, mainly owing to theoretical predictions issuing from kin selection and life-history trait theory enabling a conceptual framework for a better understanding of the evolution of virulence (e.g. Frank 1992, 1996; Nowak & May 1994; Alizon & van Baalen 2008). At the intraspecific level, empirical studies have tested and confirmed important predictions of this framework, such as that increased competition between parasite genotypes within hosts increases virulence (e.g. Bell et al. 2006; Ben-Ami et al. 2008). However, counterexamples also exist (e.g. Gower & Webster (2005)), and consequently our understanding of how multiple genotype infections affect the evolution of virulence is now much more nuanced (Alizon 2008). Theoretical and empirical work on the impact of multiple host genotypes on single-parasite genotypes is much more limited. Finally, a few studies have gone beyond looking at just the host or parasite perspective, and have examined interactions between multiple parasite and host genotypes (e.g. Carius et al. 2001; de Roode et al. 2004; Koskella & Lively 2009). However, the vast majority of these studies have focused on single-parasite and single-host species interactions (figure 1a,b), ignoring the broader ecological context of multiple parasite and multiple host species, despite the fact that the evolutionary dynamics of single-species systems are dependent on the environment in which they are situated (reviewed in Wolinska & King (2009)).
Figure 1.
Increasing levels of complexity in the study of host–parasite associations. (a,b) Traditional investigations focus on species–species interactions; (c,d) an increasing number of studies have begun to investigate species–assemblage relationships, but (e) the full complexity (i.e. assemblage–assemblage relationships) is currently unstudied. P and H are parasites or hosts, respectively. This is a fictive illustration of three butterfly species interacting with nematodes, microsporidia and fungi. The different shadings denote different genotypes. For simplicity, the different genotypes of the different host and parasite species are not noted in (c), (d) and (e).
A key aspect of the environment for any given single-species host–parasite system is the presence of other parasite and host species. While ecological studies are increasingly recognizing the importance of such complex, multi-species interactions (e.g. food webs; Lafferty et al. (2008)), this complexity is rarely considered in evolutionary studies. One reason for this may be that evolutionary studies are largely driven by theoretical predictions, and the general perception is that theory for these more complex, but biologically common, relationships is largely lacking. A second reason may be that such studies are simply harder to do than single-species experiments. Despite these stumbling blocks, a few empirical studies have taken into account the role of multiple species interactions in host–parasite relationships, and the number of such studies is increasing with time. Most of the time, these studies look at only one side of the coin, investigating either interactions between multiple parasite species sharing a single-host species (most of the time individual hosts; figure 1d), or a single-parasite species in a community of hosts (figure 1c). In this paper, we review recent advances in the study of multiple species interactions in host–parasite studies, by focusing on the consequences these interactions have on two critical traits of the host–parasite relationship: virulence and parasite transmsission. Since these two traits are linked, we will not treat them separately in our review. Because the evolutionary ecology of multiple host–parasite interactions is in its infancy, we concentrate here on the traditional trade-off approach to virulence. The implications of other aspects of virulence evolution remain unexplored. Our aim is to bring the complexity of host–parasite interactions centre-stage and to inspire future studies to examine the impacts of multiple parasites and multiple host species on the evolution of transmission and virulence. At the very least, we hope that future single-species studies will explicitly acknowledge the impact of interactions among parasite and host species on the interpretation of their results.
2. Definitions
A stumbling point in trying to produce a coherent approach is the terms we use to define this host–parasite complexity. A brief survey of the literature shows that numerous terms, including co-infection, superinfection, mixed infection, concurrent infection, multiple infection, double infection and polyparasitism, have been used to describe the presence of multiple strain or multiple species infections within hosts, sometimes within a single paper. In addition, some of these terms already have a precise meaning (box 1).
Box 1. Proposed terminology and definitions.
In the evolutionary literature, co-infection was originally defined as processes where either multiple parasite strains or species infect a single host and produce transmission stages independently May & Nowak (1995); superinfections were cases where a second parasite strain could infect an already parasitized host and completely prevent the initial parasite strain from producing transmission stages (Nowak & May 1994). These definitions bracket the full range of interactions that parasites sharing a host may have, and thus we suggest that these terms are used only with respect to the original intent of May & Nowak (1995).
We propose to use the following terms under interspecific interaction conditions, to avoid confusion with interactions involving multiple genotypes of a single species.
Multi-parasite hosts: Single host species exploited by several concurrent parasite species, either during their whole life cycle or during a given stage within it, at both the individual and population levels.
These interactions do not include hyperparasitism, where a parasite is itself the host of a parasite (examples in Taylor et al. (1998)).
Multi-host parasites: Single parasite species exploiting several concurrent host species, for either their whole life cycle or a given stage within it, at both the individual and population levels.
This definition is more constrained than heteroxenous parasites, which refers to parasites that use either multiple sequential host species (complex life cycles) or concurrent host species.
In order to distinguish parasite species from strains (which is important, as interspecific interactions are likely to be less predictable than intraspecific interactions), we encourage future studies on interactions between species to use specific definitions. On the parasite side, while ‘polyparasitism’ is used throughout the parasitology community to refer to multiple species infections of a given host, it has not been taken on board by the evolutionary community. We propose to use the terms ‘multi-parasitism’, ‘multi-parasite hosts’ or ‘multi-parasitized hosts’ (box 1), which have the added advantage of mirroring the currently accepted term of ‘multi-host parasites’. On the host side, while studies of parasites that incorporate multiple sequential host species in their life cycle are abundant, here we are specifically talking about parasites that can use concurrent multiple host species for either their whole life cycle or a given stage within it. Thus, we shall refer to these cases as multi-host parasites (box 1).
3. Parasite assemblages: empirical studies, but little theory
(a). Multiple infections may lead to a decrease or an increase in virulence
Parasite interactions within a host may lead to two patterns: exclusion by the most effective competitor or ongoing competitive interaction. At the level of host individuals, there are numerous studies showing that a given parasite species prevents or reduces the infection of their host by other parasite infections (e.g. Ferrari et al. (2009)). At the parasite community level, such interactions have been studied for years, and are looked for using null models of random community structure (e.g. Mouillot et al. (2005)). Such holistic ecological analyses nevertheless do not investigate the consequences for virulence or transmission evolution, and may fail to uncover species-specific interactions nested within a larger community. Most of the time, exclusion occurs through the activation of the host immune system (e.g. Cox 2001; Barton et al. 2007; Cattadori et al. 2007; Graham 2008), or at least the immune system mediates parasite competition (Bradley & Jackson 2008; Bush & Malenke 2008). Direct interactions may also exist, as in the case of bacteria that live on amphibian skin, excluding infections by pathogenic fungi through the production of fungicides (Harris et al. 2009). The result is also an exclusion of these parasites and, consequently, an incomplete knowledge of the parasite's biology would lead to the erroneous view that there is no interaction between the parasites. In fact, some parasites could be seen as protective to their hosts against various types of super-infections, which could lead to the evolution of mutualistic associations (Haine 2008; Fellous & Salvaudon 2009). A priori, the presence of such protective parasites does not modify parasite virulence per se and says nothing about its evolution. Nevertheless, a recent theoretical study Sorrell et al. (2009) suggests that a decrease in virulence could have evolved in parallel with a protective strategy. Silent or latent infections (i.e. those involving few parasite particles or individuals and not inducing pathology) could have evolved as a parasite strategy for survival and transmission, provided such a parasite life-history trait gives protection against other infections, a model supported by empirical data (Barton et al. 2007).
In addition to the case of parasite exclusion, direct competition between species or interactions through the host immune system may either increase or decrease the immediate virulence and transmission of one of the interacting parasites. Immediate increased virulence is illustrated in dual infections by the trematodes Schistosoma mansoni and Echinostoma caproni in their intermediate freshwater snail host. Sandland et al. (2007) observed that early transient interspecific co-infection increases the virulence and transmission potential of E. caproni. Such between-species competition for host exploitation increases virulence and transmission potential, in a similar way to that emerging from competition between strains (Davies et al. 2002). In humans, chronic infections by gastrointestinal helminths may skew the immune response in a way that increases the likelihood of infection success by HIV (Bentwich et al. 1995). HIV, in turn, compromises the immune response and increases the likelihood of infection by several other parasite species, leading to an increase in pathogenicity. In contrast, less virulent parasites can be favoured in the case of competition, as exemplified in the three following case studies that used multiple infections with the fungi Metarhizium. In a leaf-cutting ant species, the less virulent Aspergillus species out-competed Metarhizium and sporulated better in cases of multi-parasitism (Hughes & Boomsma 2004). In the desert locust Schistocerca gregaria, one strain of the avirulent parasite Beauveria bassiana may diminish the virulence caused by Metarhizium and its sporulation, although it should be noted that these two entomopathogenic fungi acted independently, synergistically or antagonistically, depending on environmental conditions and order of infection (Thomas et al. 2003). Finally, while the more virulent Metarhizium are the best competitors during competition between different conspecific strains co-infecting the moth Galleria mellonella, the less virulent strains are the best competitor during competition with another parasite species, the nematode Steinernema feltiae (Staves & Knell in press). In this last case, the differences in competitive abilities according to the type of competitor could be due to differences in trade-offs between the fungus's capacity to produce antagonistic toxins and its capacity to monopolize resources rapidly.
It therefore appears that the outcome of competition between parasite species in terms of the evolution of virulence and transmission exhibits contrasting ecological patterns. From the examples described above, both the dynamics of intrahost competition and mediation by the host immune system appear to play a role in the outcome of competition. Since very few studies have investigated these differences, it is impossible, as yet, to make general predictions for the evolutionary trajectories of virulence and transmission resulting from such interactions. Nevertheless, we speculate that parasites could face a trade-off between being able to cope with the host immune system on one side (either to survive or to manipulate it to avoid secondary infection), and being competitive against secondary-infecting parasite species on the other side. Parasites investing more in the latter may evolve higher virulence in the case of multiple infections because of their ability to monopolize resources, while those investing more in the former strategy may evolve lower virulence.
(b). Multiple infections as triggers of evolutionary changes
Multiple infections may also have surprising effects on the requirements for and mechanisms of evolution itself. A recent study showed that within-host interactions between different strains of a single-parasite species may promote the maintenance of genetic polymorphism in parasite populations: parasite infection success did not consistently increase or decrease in the co-exposure treatments, but depended on the combinations of co-infecting parasite strains (Seppälä et al. 2009). To our knowledge, no study has examined the effect of infections by different parasite species on genetic polymorphism, but, because different strains of a single-parasite species may have different competitive ability against different strains of a given competitor, multiple infections could be responsible for the maintenance of such genetic polymorphism. This phenomenon could provide the material on which selection can act and therefore allow the evolution of virulence.
A very direct way by which virulence can evolve is the creation by recombination of a new parasite ‘strain’ that is more virulent than the parental entities from which it issued. For example, the emergence of new virulent diseases has occurred in cultivated plants via between-species recombination in virus and fungal pathogens (references in Barrett et al. (2009)). Within-host interspecific interactions may favour between-strain recombination in one of the interacting parasites, leading to more virulent or transmissible strains of parasites, as suggested by the study of Escribano et al. (2001). In the moth Spodoptera frugiperda, the interactions between a baculovirus (multiple strains) and a parasitoid are responsible for evolutionary changes in the virus: the most virulent and infective virus genotype, only found in multi-parasitized hosts, appears to be issued from a recombination of the original co-infecting virus strains. The evolution of the virus may therefore be triggered by the competition with the parasitoid (Escribano et al. 2001). We believe that such surprising effects are unlikely to emerge from any a priori theoretical models, and thus warrant the direct study of multi-parasite systems.
(c). Multi-parasitism where transmission modes contrast between parasite species
Changes in virulence may also occur in parasites that are in conflict owing to their transmission modes. Unlike other areas of multi-parasitism, a recent study theoretically investigated the impact of differing transmission mode on the evolution of virulence. Jones et al. (in press) showed that the presence of a vertically transmitted parasite (VTP) may lead to an increase of virulence in horizontally transmitted parasites (HTPs). While it could explain the high degree of virulence of some HTPs, this result stands in contrast to empirical results showing that some VTPs (generally bacteria) can protect their hosts against virulent HTPs (see Haine (2008) for a review). However, while the protective bacteria described so far generally prevent infection by HTP (and therefore probably interact with the host's immune system), the situations analysed by Jones et al. (in press) are when the hosts are equally sensitive to both parasite species. Even in this case, empirical results can contradict the model predictions. Haine et al. (2005) showed that a VT microsporidia can alter the behavioural modification of the host (a gammarid crustacean) induced by an acanthocephalan worm. The transmission of this worm relies on trophic transmission to a final host, and behavioural modification is known to favour this transmission by increasing the predation rate by the final host. This trait is therefore an extreme case of parasite virulence directly favouring transmission. The microsporidia, which is transmitted in the eggs of the gammarid, is able to disrupt the manipulation induced by the worm. This decreases the probability of predation, which is to its own benefit, as the host will have more chance to survive and reproduce, and therefore transmit the microsporidia (Haine et al. 2005). Here, the strength of the selective pressure imposed on the VTP by the HTP may explain why the microsporidia evolved to counteract acanthocephalan virulence. In addition, Jones et al. (in press) predicted that the result of the conflict depends to a large degree on the nature of both host and parasite life-history trade-offs. For example, coevolutionary models show higher virulence in both types of interacting parasites in short-lived hosts (Jones et al. in press).
(d). Multi-parasitism and host populations
So far, we have discussed examples where multiple parasite species exist within single host individuals. However, consequences of multi-parasitized hosts can also be seen at the population level. In host populations, and at an ecological level, the virulence of parasite assemblages may be higher than the impact of each parasite taken separately. For example, the average population fecundity of the crustacean Daphnia magna is negatively associated with overall infection intensity, but also with total endoparasite richness, resulting in a negative impact on host population dynamics, and ultimately in feedback on parasite population dynamics (Decaestecker et al. 2005). However, in a study using several parasitoid species to control populations of the whitefly Bemisia argentifolii, Heinz & Nelson (1996) showed that the quality of interaction between parasite species (i.e. who interacts with who and how) may be more important than the number of these interactions. In addition, even parasitoids showing strong negative interactions did not change the overall virulence on host populations (Bogran et al. 2002). Turning from the host to the parasite perspective, if parasite species exclude each other (see §3a), or if highly virulent parasites wipe out part of the host population, then the effective host population for a given parasite species may be significantly lower than a simple count would suggest (Rutrecht & Brown 2008). Theory suggests that the evolution of virulence and transmission, as well as parasite maintenance within a host population, are strongly related to host population size, with smaller populations selecting for more effective and generalist transmission, and lower virulence (Levin 1996). Consequently, the ecological impacts of multiple infections on host population dynamics need to be determined if we are to achieve a complete understanding of parasite virulence.
4. Host assemblages: from theory to incomplete empirical studies
Multi-host parasites are in a clear majority (e.g. Williams & Jones 1994; Musselman & Press 1995; Cleaveland et al. 2001; Taylor et al. 2001; Pedersen et al. 2005), with recent work suggesting that such generalist parasites may even have evolved from host specialists (Johnson et al. 2009). Consequently, the impact of multiple host backgrounds on parasite transmission and virulence will be key to gaining a true understanding of host–parasite evolution. Unlike the situation of multi-parasitized hosts, a number of attempts have been made at developing a theoretical background for studying multi-host parasites (Regoes et al. 2000; Woolhouse et al. 2001; Gandon 2004). The key factors in these models are the trade-off between virulence in different host species, host quality (as measured by abundance, parasite prevalence and ultimately parasite reproduction) and patterns of transmission (intra- versus interspecific). Here, we review the predictions of these models, and how the results from empirical studies relate to these predictions.
(a). Trade-offs across host species
In the simplest case, we might assume that virulence trades off across host species (i.e. when it is high in host species A, it has to be low in host species B). This is likely to be true for parasites using multi-sequential hosts (e.g. Davies et al. 2001; Gower & Webster 2004), where very different mechanisms are likely to be needed for infecting and reproducing within phylogenetically distant hosts (most such parasites use a vertebrate and an invertebrate in their life cycles). However, how strong such a trade-off is for parasites that share closely related host species, which most multi-host parasites are likely to do (e.g. Davies & Pedersen (2008)), remains unclear. In such a case, parasites are predicted either to evolve into two specialist populations, each with optimal virulence, or to remain as a generalist parasite with lower than optimal virulence in both host species (Regoes et al. 2000). Specialization evolves if the cost of switching hosts is too high, suggesting that frequent transmission among host species is likely to lead to generalist parasites with suboptimal virulence. This prediction is, to some extent, analogous to studies of host specialization, where such specialization is believed to be due either to adaptation to one host species constraining host shifts, or to a lack of opportunities for cross-species transmission (e.g. Tompkins & Clayton (1999)). Interestingly, a study of viruses across multiple plant host species suggested that for generalist parasites, it was those that showed the most specialism (i.e. a skewed distribution of prevalence across host species) that were most successful (Malpica et al. 2006). However, the opposite result has been reported in a field study on avian malaria, where a positive relationship was found between parasite host range and prevalence. This might be explained by an overall higher encounter rate for the parasites with a broader host range compensating for the possible reduced performance of such generalist parasites in each host species (Hellgren et al. 2009). Experimental studies using phages and bacterial populations, where host differences and transmission opportunities can be manipulated, would provide one way to formally test the predictions of the model (Regoes et al. 2000).
(b). Transmission and host quality
Later models have focused more on patterns of transmission and aspects of host quality. These suggest that transmission should evolve in response to host heterogeneity, resulting in higher transmission to higher quality hosts (where quality is measured as parasite reproductive potential within a host, host abundance and parasite prevalence within a host species; Gandon (2004)). In contrast, if populations of different host species are small or undergo significant fluctuations, there may be selection for a generalist transmission strategy, as specialized parasites are more likely to go extinct (Jaenicke & Dombeck 1998).
Predictions for virulence evolution depend on the pattern of transmission and host quality. Under certain conditions, in particular the virulence trade-off, the predictions of the Regoes et al. (2000) model have been confirmed by later models. More broadly, virulence may evolve to be higher or lower in different host species, depending upon transmission and the virulence trade-off across host species (Woolhouse et al. 2001). Specifically, in a situation where hosts differ in quality (which we would suggest is a good starting assumption), and transmission rates allow, parasites should evolve towards optimal virulence in their prime host. This may result in lower or higher virulence in other host species. In particular, if the prime host is more resistant, the parasite should evolve higher virulence, which will only be expressed in other host species (Gandon 2004). This is probably a special case of runaway virulence in hosts that no longer constrain parasite evolution (Woolhouse et al. 2001). While these predictions all make clear intuitive sense, multi-host models can also have more surprising results. Increases in host mortality are generally assumed to select for higher virulence in simple single-species models (Anderson & May 1982). However, in a multi-host system when intraspecific transmission dominates, increases in intrinsic host mortality of the prime host species can result in a switch in relative host abundance, making a second host species of higher quality. If the parasite has lower optimal virulence in this second species (e.g. if the host is less resistant and thus selects for lower virulence), then the increase in intrinsic host mortality will actually lead to an overall decrease in parasite virulence (Gandon 2004).
It is clear that to advance our empirical knowledge, we need studies of transmission rates, virulence across multiple host species and host quality. Unfortunately, such studies are largely lacking from the evolutionary literature, and the studies we have do not reveal a clear or complete picture. Here we focus on three case studies, which vary in their completeness with respect to the models described above, but nevertheless give valuable insight into the forces that might be at work in multi-host parasites.
In laboratory experiments, the generalist nematode parasite Howardula aoronymphium varies in its infection success and growth across Drosophila host species Jaenicke & Dombeck (1998), and exhibits significant variation in virulence across natural host species (Perlman & Jaenicke 2003). Despite this, there is no evidence for specialization across host species (Jaenicke & Dombeck 1998). Potentially high rates of interspecific transmission and fluctuations in relative abundance of host species Jaenicke & Dombeck (1998) have presumably selected for a generalist parasite (Gandon 2004). In this case, variation in virulence is likely to be host-driven and non-adaptive for the parasite.
Similarly, the multi-host hemi-parasitic plant Rhinanthus serotinus exhibits differential fitness and virulence across its hosts Agrostis capillaris and Trifolium pratense, but shows no evidence of specialization (Ahonen et al. 2006). It is unclear which of these hosts is higher quality (only parasite growth, and not relative natural abundance or parasite prevalence, across host species was measured) and thus what pattern of virulence is being expressed. However, the hemi-parasite exhibits genetic variation for performance on its hosts, suggesting that high rates of interspecies transmission are preventing specialization in this system (Gandon 2004).
In contrast, the multi-host microsporidian Nosema bombi appears to exhibit runaway virulence in one host species Otti & Schmid-Hempel (2008) and more adaptive virulence in a second (Rutrecht & Brown 2009). This pattern may match predictions based on host resistance Gandon (2004), but the relationship to host quality is unclear. Quality hosts should be more abundant and enable parasite reproduction, but in this case, the parasite exhibits apparently maladaptive virulence in the most abundant host species in which it is most prevalent (Larsson 2007). Nevertheless, as with Howardula, high interspecific transmission in the field may be maintaining a single-parasite population across host species (Tay et al. 2005).
Testing the prediction that virulence should evolve to optimal levels in high-quality hosts will require laboratory experiments where differential host abundance can be manipulated and parasite virulence can be followed over multiple generations. Potential hosts for such experiments should have short generation times and be able to live in multi-species assemblages under laboratory conditions (e.g. Drosophila, Daphnia, bacterial-phage systems). If the predictions of Gandon Gandon (2004) are correct, experimentally switching host abundance should lead to a similar switch in parasite virulence, matching that seen in single-species cultures of the most abundant host species.
(c). Community-level epidemiology
The models above address the evolution of virulence and host range in parasites interacting with multiple host species. In the context of community ecology and at a more epidemiological level, total transmission can also be directly reduced in mixed-species assemblages. The importance of host community diversity for parasite transmission has been stressed for vector-borne pathogens, and more recently for complex life-cycle parasites (Johnson et al. 2008, 2009). In multi-host systems where interspecific transmission occurs among hosts that are highly variable in their competency, infection of resistant or less suitable hosts results in ‘wasted’ transmission events (i.e. parasite removal), a phenomenon known as a ‘dilution effect’ or a ‘decoy effect’ (references in Hall et al. 2009; Johnson et al. 2009). A negative correlation between community diversity and parasite incidence is therefore expected. However, the net benefit of the dilution effect for the most sensitive host species depends on the trade-off between lower parasite incidence and higher interspecific competition (Johnson et al. 2009; Hall et al. 2009).
From a more epidemiological point of view, a dilution effect can be evidenced by a decrease in disease prevalence when less competent hosts become more abundant. A pluriannual field study on Daphnia species assemblages infected with the fungus Metschnikowia bicuspidata has shown that smaller fungal epidemics are associated with a dominance of less suitable host species at the start of the epidemics (Hall et al. 2009). However, it is the interaction between this dilution effect and resource competition between susceptible hosts and ‘friendly competitors’ that contributes to the termination of fungal epidemics within a season, in addition (or combination) to other ecological factors such as thermal physiology, selective predation, rapid evolution of resistance and increasing algal resources (Hall et al. 2009). How the dilution effect affects the evolution of virulence towards the sensitive and the less suitable coexisting hosts has not been addressed so far, but would need long-term field data and the development of mathematical models incorporating resource competition theory.
(d). Multi-host parasites and transmission
In all of these examples, while we lack data, interspecies transmission appears to be playing a central role in the evolution of the parasite. This is not a novel observation, but it does suggest that a real understanding of multi-host parasites requires the quantitative assessment of transmission routes, either directly or through molecular ecology approaches.
In contrast to a general lack of evolutionary studies, there is clear evidence that the ecological requirements for selection on transmission by multiple hosts are present. Three recent studies (Johnson et al. 2008, 2009; Thieltges et al. 2008) all showed that the presence of multiple hosts had a biologically large and significant impact on both parasite transmission and reproduction.
5. Conclusions and future directions
In this review, we believe we have demonstrated how multi-host parasites and multi-parasite–host assemblages can have dramatic effects on the evolutionary trajectory of individual parasite species. However, this statement is stronger for multi-host interactions than for multi-parasite ones, mainly because most of the studies available so far have investigated the ecological scale in the latter case, and not the evolutionary one. While developing general theory for understanding multi-parasitized hosts may be problematic, both multi-parasitism and multi-host backgrounds need to be studied if we are to understand the evolution of transmission and virulence in parasites. While the field is relatively clear, owing to the relative paucity of current studies, we would suggest that a number of areas are ripe for study (table 1).
Table 1.
Future research directions proposed to improve our knowledge about the impact of complexity of host–parasite relationships on the evolution of virulence.
| level concerned | outstanding questions |
|---|---|
| multi-parasitized hosts | are differences in host exploitation more important than immune mediation (or vice versa) in explaining interaction patterns? |
| could a trade-off between immune manipulation and competitive ability modulate the evolution of virulence in concurrent parasite species? | |
| do multiple infections promote levels of genetic variation in parasite virulence? | |
| multi-host parasites | what are the parasite transmission dynamics between concurrent host species? |
| how does host quality constrain the evolution of transmission and virulence? | |
| what are the consequences of host shifts for the evolution of virulence? | |
| multi-host–multi-parasites | do interactions between multiple infections and multi-host parasites generate higher virulence? |
| can we include ecological networks in the theory of the evolution of virulence? |
The outcome of multi-parasitism on the evolution of virulence and transmission of each individual parasite species critically depends on their respective exploitation strategy in terms of growth or replication and on their respective effect on the immune system of their shared host. More empirical or experimental studies are clearly needed to understand how these parameters vary in single- versus multi-species infections, even if some recent studies suggest that immunity is the most important factor regulating the structure of parasite communities (Bradley & Jackson 2008). In parallel, theoretical investigations are also lacking to discriminate which of these phenomena is the more important to generate general predictions for the evolution of virulence and transmission (or if they act in synergy or in opposite directions). A promising avenue of research could be, as stated earlier, to test the existence of a trade-off in parasites between coping with the host immune system and being competitive against secondary-infecting parasite species, and to investigate the impact on the evolution of virulence. Since it is obvious that interacting with the host immune system is a more constant selective pressure than multiple infections, the evolutionary trajectory of virulence would depend on the prevalence of potential competitors for a given parasite species. Increased virulence could evolve if many parasite species are able to disrupt the host immune system (leading to an increased probability of multiple infections), while decreased virulence would evolve if only a small proportion of parasites are able to manipulate the host immune system to win the competition. Only long-term laboratory experiments with short-generation-time model organisms could address this hypothesis. In addition, asymmetries in virulence are likely to be frequent between parasite species within a host. Only the accumulation of studies will elucidate whether these asymmetries are maintained during multi-parasitism, if the more virulent genotypes are selected from the interaction between parasite species, or if variations in such balances contribute to the maintenance of genetic variation in parasite virulence for each interacting species. Finally, studies of parasite communities also need to be extended to examine their impact on host populations, and especially on how this impact cascades down to affect the individual parasite species within the community (Rutrecht & Brown 2008). This should be particularly ‘easy’ in a number of model systems (e.g. Daphnia and its parasites Ebert (2005)).
On the side of multi-host parasites, we are clearly lacking quantitative studies of transmission routes and dynamics among concurrent host species. These are likely to be key in predicting the evolutionary trajectory of the parasite and whether it will ultimately split into new host-specific parasite species. Such studies should be conducted in the field, documenting and manipulating transmission routes, as well as in the laboratory, where transmission dynamics can be tightly controlled. Results from such work may provide the key to understanding patterns of host specificity across phylogenetic trees. In addition, understanding how transmission routes and virulence are related in multi-host parasites may shed some light on the incipient stages of host shifts, which are increasingly important as the threat of emergent diseases increases. While a better understanding of the ecology of interacting host and parasite communities now allows the prediction of probabilities of host shifts Davies & Pedersen (2008), general predictions for the consequences of these shifts on virulence have yet to be developed.
If we are to understand the rate of evolutionary change in host defence, parasite growth and virulence, and parasite transmission strategies in multi-host–multi-parasite systems, more realism will be gained from field studies or surveys and from their cross-disciplinary analyses. Whether considering multi-parasitized hosts or multi-host parasites, field studies are needed because it is clear from many host–parasite systems that environmental variation mediates host–parasite interactions (either directly by resource-dependent variation in parasite growth and host defence, or indirectly through parasite-mediated competition, and thereby fitness impacts on host). In addition, oppportunities for interspecies transmission in multi-host parasites can be greatly modulated by environmental heterogeneity (Barrett et al. 2009; Hall et al. 2009). Cross-disciplinary approaches, including the tools of epidemiology and animal behaviour, have proved their efficiency in understanding the spread and virulence in a natural multi-host pathogen (e.g. Craft et al. (2008)). Such cross-disciplinary approaches will be necessary to appraise the entire complexity of host–parasite relationships in a given ecosystem (i.e. entire multi-host–multi-parasite systems; figure 1e). This will necessitate broad knowledge of the main host–parasite ecological networks, comparable to the recently increased knowledge of pollinator–plant networks (e.g. Petanidou et al. (2008)), food webs (e.g. Duffy et al. 2007; Johnson et al. 2010) or interspecific behavioural connections (Haydon 2008). Very few studies have attempted to understand this complexity. In a review, Graham et al. (2007) proposed that multi-parasitism may be a source of heterogeneity in both host recovery rate (e.g. inducing variable rates of immune suppression) and parasite(s) transmission. Multi-parasitism therefore has consequences for parasite epidemiology, which, in turn, could facilitate jumps to, and increase the probability of, adapting to new host species. Following this train of thought, the existence of multi-parasitized hosts may act to increase the proportion of multi-host parasites. Since increasing the number of connections in such a multi-host–multi-parasite network may lead to changes in virulence (see examples above), we believe that it could be both useful and urgent to try to link the ecology of quantitative networks to the evolution of virulence, both empirically and theoretically. Empirically, parasites can be integrated as nodes in existing food webs Lafferty et al. (2006), although several technical problems pertaining to how food-web theory accomodates parasites still persist (Lafferty et al. 2008). Furthermore, moving from a map of interactions to a more mechanistic analysis in the study of ecological network is necessary (Ings et al. 2009). The analysis of network configuration and the quantification of interaction strengths have not yet been applied to network incorporating parasites Ings et al. (2009), but should theoretically reveal network-wide patterns of coevolution among host and parasites, and how species move along a generalist–specialist continuum. For multi-host–multi-parasite networks, our current knowledge is far from a full understanding of such complexity, but this is the grail for which we must quest.
The connection between community structure (multi-host–multi-parasite) and parasite incidence (the product of transmission and virulence) has a broad ecological and evolutionary significance. First, at an ecological scale, changes in disease epidemiology and feedback loops on host community structure (due in part to parasite-mediated competition) are expected to occur in multi-host–multi-parasite systems. Second, multi-host–multi-parasite systems also have important implications for practical issues. In crop and animal sciences, biological control, pest outbreaks and invasions are promising tools or increased threats that a sustainable agriculture will have to manage. The role of parasites in biological conservation has been repeatedly stressed (see Smith et al. (2009) for a review); for instance, it is generally acknowledged that generalist pathogens pose the greatest threat to disease-mediated extinction in mammals (Pedersen & Fenton 2007). Lastly, the importance of synergistic pathogenesis between parasites co-infecting humans—as revealed with H1N1 influenza virus promoting infection with Streptococcus pneumoniae (and thereby increasing morbidity and mortality owing to H1N1 infection; Palacios et al. (2009)), and other previous cases (such as HIV pathogenesis)—certainly calls for a more integrated and global analysis of multi-host–multi-parasite systems.
Acknowledgements
T.R. and M.-J.P.-M. were supported by a grant from the French Agence Nationale de la Recherche (ANR; grant BLAN07-3_183300). M.J.F.B. was supported by a Research Frontiers Programme grant from Science Foundation Ireland (SFI; grant 05-RFP-EEB0040).
References
- Ahonen R., Puustinen S., Mutikainen P.2006Host use of a hemiparasitic plant: no trade-offs in performance on different hosts. J. Evol. Biol. 19, 513–521 10.1111/j.1420-9101.2005.01024.x (doi:10.1111/j.1420-9101.2005.01024.x) [DOI] [PubMed] [Google Scholar]
- Alizon S.2008Decreased overall virulence in coinfected hosts leads to the persistence of virulent parasites. Am. Nat. 172, E67–E79 10.1086/588077 (doi:10.1086/588077) [DOI] [PubMed] [Google Scholar]
- Alizon S., van Baalen M.2008Multiple infections, immune dynamics, and the evolution of virulence. Am. Nat. 172, E150–E168 10.1086/590958 (doi:10.1086/590958) [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M.1982Coevolution of hosts and parasites. Parasitology 85, 411–426 10.1017/S0031182000055360 (doi:10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- Barrett L. G., Kniskern J. M., Bodenhausen N. B., Zhang W., Bergelson J.2009Continua of specificity and virulence in plant host–pathogen interactions: causes and consequences. New Phytol. 183, 513–529 10.1111/j.1469-8137.2009.02927.x (doi:10.1111/j.1469-8137.2009.02927.x) [DOI] [PubMed] [Google Scholar]
- Barton E. S., White D. W., Cathelyn J. S., Brett-McClellan K. A., Engle M., Diamond M. S., Miller V. L., Virgin H. W.2007Herpes virus latency confers symbiotic protection from bacterial infection. Nature 447, 326–329 10.1038/nature05762 (doi:10.1038/nature05762) [DOI] [PubMed] [Google Scholar]
- Bell A. S., de Roode J. C., Sim D., Read A. F.2006Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution 60, 358–1371 [PubMed] [Google Scholar]
- Ben-Ami F., Mouton L., Ebert D.2008The effects of multiple infections on the expression and evolution of virulence in a Daphnia–endoparasite system. Evolution 62, 1700–1711 10.1111/j.1558-5646.2008.00391.x (doi:10.1111/j.1558-5646.2008.00391.x) [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Kalinkovich A., Weisman Z.1995Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol. Today 16, 187–191 10.1016/0167-5699(95)80119-7 (doi:10.1016/0167-5699(95)80119-7) [DOI] [PubMed] [Google Scholar]
- Bogran C. E., Heinz K. M., Ciomperlik M. A.2002Interspecific competition among insect parasitoids: field experiments with whiteflies as hosts in cotton. Ecology 83, 653–668 [Google Scholar]
- Bradley J. E., Jackson J. A.2008Measuring immune system variation to help understand host–pathogen community dynamics. Parasitology 135, 807–823 10.1017/S0031182008000322 (doi:10.1017/S0031182008000322) [DOI] [PubMed] [Google Scholar]
- Bush S. E., Malenke J. R.2008Host defence mediates interspecific competition in ectoparasites. J. Anim. Ecol. 77, 558–564 10.1111/j.1365-2656.2007.01353.x (doi:10.1111/j.1365-2656.2007.01353.x) [DOI] [PubMed] [Google Scholar]
- Carius H. J., Little T. J., Ebert D.2001Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145 [DOI] [PubMed] [Google Scholar]
- Cattadori I. M., Albert R., Boag B.2007Variation in host susceptibility and infectiousness generated by co-infection: the myxoma–Trichostrongylus retortaeformis case in wild rabbits. J. R. Soc. Interface 4, 831–840 10.1098/rsif.2007.1075 (doi:10.1098/rsif.2007.1075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaveland S., Laurenson M. K., Taylor L. H.2001Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Phil. Trans. R. Soc. Lond. B 356, 991–999 10.1098/rstb.2001.0889 (doi:10.1098/rstb.2001.0889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes C.2001Parasitism: the ecology and evolution of intimate interactions. Chicago, IL: The University of Chicago Press [Google Scholar]
- Cox F. E. G.2001Concomitant infections, parasites and immune responses. Parasitology 122, S23–S38 10.1017/S003118200001698X (doi:10.1017/S003118200001698X) [DOI] [PubMed] [Google Scholar]
- Craft M. E., Hawthorne P. L., Parker C., Dobson A. P.2008Dynamics of a multihost pathogen in a carnivore community. J. Anim. Ecol. 77, 1257–1264 10.1111/j.1365-2656.2008.01410.x (doi:10.1111/j.1365-2656.2008.01410.x) [DOI] [PubMed] [Google Scholar]
- Davies C. M., Webster J. P., Woolhouse M. E. J.2001Trade-offs in the evolution of virulence in an indirectly transmitted macroparasite. Proc. R. Soc. Lond. B 268, 251–257 10.1098/rspb.2000.1367 (doi:10.1098/rspb.2000.1367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. M., Fairbrother E., Webster J. P.2002Mixed strain schistosome infections of snails and the evolution of parasite virulence. Parasitology 124, 31–38 [DOI] [PubMed] [Google Scholar]
- Davies T. J., Pedersen A. B.2008Phylogeny and geography predict pathogen community similarity in wild primates and humans. Proc. R. Soc. B 275, 1695–1701 10.1098/rspb.2008.0284 (doi:10.1098/rspb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker E., Declerck S., De Meester L., Ebert D.2005Ecological implications of parasites in natural Daphnia populations. Oecologia 144, 382–390 10.1007/s00442-005-0083-7 (doi:10.1007/s00442-005-0083-7) [DOI] [PubMed] [Google Scholar]
- de Roode J. C., Culleton R., Cheesman S. J., Carter R., Read A. F.2004Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc. R. Soc. Lond. B 271, 1073–1080 10.1098/rspb.2004.2695 (doi:10.1098/rspb.2004.2695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. E., Cardinale B. J., France K. E., McIntyre P. B., Thebault E., Loreau M.2007The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538 10.1111/j.1461-0248.2007.01037.x (doi:10.1111/j.1461-0248.2007.01037.x) [DOI] [PubMed] [Google Scholar]
- Ebert D.2005Ecology, epidemiology, and evolution of parasitism in Daphnia. Bethesda, MD: National Library of Medicine (USA), National Center for Biotechnology Information; See http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books [Google Scholar]
- Escribano A., Williams T., Goulson D., Cave R. D., Chapman J. W., Caballero P.2001Consequences of interspecific competition on the virulence and genetic composition of a nucleopolyhedrovirus in Spodoptera frugiperda larvae parasitized by Chelonus insularis. Biocontrol Sci. Technol. 11, 649–662 10.1080/09583150120076193 (doi:10.1080/09583150120076193) [DOI] [Google Scholar]
- Fellous S., Salvaudon L.2009How can your parasites become your allies? Trends Parasitol. 25, 62–66 10.1016/j.pt.2008.11.010 (doi:10.1016/j.pt.2008.11.010) [DOI] [PubMed] [Google Scholar]
- Ferrari N., Cattadori I. M., Rizzoli A., Hudson P. J.2009Heligmosomoides polygyrus reduces infestation of Ixodes ricinus in free-living yellow-necked mice, Apodemus flavicollis. Parasitology 136, 305–316 10.1017/S0031182008005404 (doi:10.1017/S0031182008005404) [DOI] [PubMed] [Google Scholar]
- Frank S. A.1992A kin selection model for the evolution of virulence. Proc. R. Soc. Lond. B 250, 195–197 10.1098/rspb.1992.0149 (doi:10.1098/rspb.1992.0149) [DOI] [PubMed] [Google Scholar]
- Frank S. A.1996Models of parasite virulence. Q. Rev. Biol. 71, 37–78 10.1086/419267 (doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- Gandon S.2004Evolution of multihost parasites. Evolution 58, 455–469 [PubMed] [Google Scholar]
- Gower C. M., Webster J. P.2004Fitness of indirectly transmitted pathogens: restraint and constraint. Evolution 58, 1178–1184 [DOI] [PubMed] [Google Scholar]
- Gower C. M., Webster J. P.2005Intraspecific competition and the evolution of virulence in a parasitic trematode. Evolution 59, 544–553 [PubMed] [Google Scholar]
- Graham A. L.2008Ecological rules governing helminth-microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570 10.1073/pnas.0707221105 (doi:10.1073/pnas.0707221105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. L., Cattadori I. M., Lloyd-Smith J. O., Ferrari M. J., Bjornstad O. N.2007Transmission consequences of coinfection: cytokines writ large? Trends Parasitol. 23, 284–291 10.1016/j.pt.2007.04.005 (doi:10.1016/j.pt.2007.04.005) [DOI] [PubMed] [Google Scholar]
- Haine E. R.2008Symbiont-mediated protection. Proc. R. Soc. B 275, 353–361 10.1098/rspb.2007.1211 (doi:10.1098/rspb.2007.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haine E. R., Boucansaud K., Rigaud T.2005Conflict between parasites with different transmission strategies infecting an amphipod host. Proc. R. Soc. B 272, 2505–2510 10.1098/rspb.2005.3244 (doi:10.1098/rspb.2005.3244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. R., Becker C. R., Simonis J. L., Duffy M. A., Tessier A. J., Caceres C. E.2009Friendly competition: evidence for a dilution effect among competitors in a planktonic host–parasite system. Ecology 90, 791–801 10.1890/08-0838.1 (doi:10.1890/08-0838.1) [DOI] [PubMed] [Google Scholar]
- Harris R. N., et al. 2009Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 3, 818–824 10.1038/ismej.2009.27 (doi:10.1038/ismej.2009.27) [DOI] [PubMed] [Google Scholar]
- Haydon D. T.2008Cross-disciplinary demands of multihost pathogens. J. Anim. Ecol. 77, 1079–1081 10.1111/j.1365-2656.2008.01474.x (doi:10.1111/j.1365-2656.2008.01474.x) [DOI] [PubMed] [Google Scholar]
- Heinz K. M., Nelson J. M.1996Interspecific interactions among natural enemies of Bemisia in an inundative biological control program. Biol. Control 6, 384–393 10.1006/bcon.1996.0049 (doi:10.1006/bcon.1996.0049) [DOI] [Google Scholar]
- Hellgren O., Pérez-Tris J., Bensch S.2009A jack-of-all-trades and still a master of some: prevalence and host range in avian malaria and related blood parasites. Ecology 90, 2840–2849 10.1890/08-1059.1 (doi:10.1890/08-1059.1) [DOI] [PubMed] [Google Scholar]
- Hughes W. O. H., Boomsma J. J.2004Let your enemy do the work: within-host interactions between two fungal parasites of leaf-cutting ants. Proc. R. Soc. Lond. B 271, S104–S106 10.1098/rsbl.2003.0115 (doi:10.1098/rsbl.2003.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings T. C., et al. 2009Ecological networks—beyond food webs. J. Anim. Ecol. 78, 253–269 10.1111/j.1365-2656.2008.01460.x (doi:10.1111/j.1365-2656.2008.01460.x) [DOI] [PubMed] [Google Scholar]
- Jaenicke J., Dombeck I.1998General-purpose genotypes for host species utilization in a nematode parasite of Drosophila. Evolution 52, 832–840 [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Malenke J. R., Clayton D. H.2009Competition promotes the evolution of host generalists in obligate parasites. Proc. R. Soc. B 276, 3921–3926 10.1098/rspb.2009.1174 (doi:10.1098/rspb.2009.1174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. T. J., Hartson R. B., Larson D. J., Sutherland D. R.2008Diversity and disease: community structure drives parasite transmission and host fitness. Ecol. Lett. 11, 1017–1026 10.1111/j.1461-0248.2008.01212.x (doi:10.1111/j.1461-0248.2008.01212.x) [DOI] [PubMed] [Google Scholar]
- Johnson P. T. J., Lund P. J., Hartson R. B., Yoshino T. P.2009Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proc. R. Soc. B 276, 1657–1663 10.1098/rspb.2008.1718 (doi:10.1098/rspb.2008.1718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. T. J., Dobson A., Lafferty K. D., Marcogliese D. J., Memmott J., Orlofske S. A., Poulin R., Thieltges D. W.2010When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 25, 362–371 10.1016/j.tree.2010.01.005 (doi:10.1016/j.tree.2010.01.005) [DOI] [PubMed] [Google Scholar]
- Jones E. O., White A., Boots M.In press The evolutionary implications of conflict between parasites with different transmission modes. Evolution. 10.1111/j.1558-5646.2010.00992.x (doi:10.1111/j.1558-5646.2010.00992.x) [DOI] [PubMed] [Google Scholar]
- Koskella B., Lively C. M.2009Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution 9, 2213–2221 10.1111/j.1558-5646.2009.00711.x (doi:10.1111/j.1558-5646.2009.00711.x) [DOI] [PubMed] [Google Scholar]
- Lafferty K. D., Dobson A. P., Kuris A. M.2006Parasites dominate food web links. Proc. Natl Acad. Sci. USA 103, 11 211–11 216 10.1073/pnas.0604755103 (doi:10.1073/pnas.0604755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. D., et al. 2008Parasites in food webs: the ultimate missing links. Ecol. Lett. 11, 533–546 10.1111/j.1461-0248.2008.01174.x (doi:10.1111/j.1461-0248.2008.01174.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J. I. R.2007Cytological variation and pathogenicity of the bumble bee parasite Nosema bombi (Microspora, Nosematidae). J. Inv. Pathol. 94, 1–11 10.1016/j.jip.2006.07.006 (doi:10.1016/j.jip.2006.07.006) [DOI] [PubMed] [Google Scholar]
- Levin B. R.1996The evolution and maintenance of virulence in microparasites. Emerg. Infect. Dis. 2, 93–102 10.3201/eid0202.960203 (doi:10.3201/eid0202.960203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpica J. M., Sacristán S., Fraile A., García-Arenal F.2006Association and host selectivity in multi-host pathogens. PLoS ONE 1, e41. 10.1371/journal.pone.0000041 (doi:10.1371/journal.pone.0000041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. M., Nowak M. A.1995Coinfection and the evolution of parasite virulence. Proc. R. Soc. Lond. B 261, 209–215 10.1098/rspb.1995.0138 (doi:10.1098/rspb.1995.0138) [DOI] [PubMed] [Google Scholar]
- Mouillot D., Simkova A., Morand S., Poulin R.2005Parasite species coexistence and limiting similarity: a multiscale look at phylogenetic, functional and reproductive distances. Oecologia 146, 269–278 10.1007/s00442-005-0194-1 (doi:10.1007/s00442-005-0194-1) [DOI] [PubMed] [Google Scholar]
- Musselman L. J., Press M. C.1995Introduction to parasitic plants. In Parasitic plants (eds Press M. C., Graves J. D.), pp. 1–13 London, UK: Chapman and Hall [Google Scholar]
- Nowak M. A., May R. M.1994Superinfection and the evolution of parasite virulence. Proc. R. Soc. Lond. B 255, 81–89 10.1098/rspb.1994.0012 (doi:10.1098/rspb.1994.0012) [DOI] [PubMed] [Google Scholar]
- Otti O., Schmid-Hempel P.2008A field experiment on the effect of Nosema bombi in colonies of the bumblebee Bombus terrestris. Ecol. Entomol. 33, 577–582 10.1111/j.1365-2311.2008.00998.x (doi:10.1111/j.1365-2311.2008.00998.x) [DOI] [Google Scholar]
- Palacios G., et al. 2009Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE 4, e8540. 10.1371/journal.pone.00085401-5 (doi:10.1371/journal.pone.00085401-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A. B., Fenton A.2007Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 22, 133–139 10.1016/j.tree.2006.11.005 (doi:10.1016/j.tree.2006.11.005) [DOI] [PubMed] [Google Scholar]
- Pedersen A. B., Altizer S., Poss M., Cunningham A. A., Nunn C. L.2005Patterns of host specificity and transmission among parasites of wild primates. Int. J. Parasitol. 6, 647–657 10.1016/j.ijpara.2005.01.005 (doi:10.1016/j.ijpara.2005.01.005) [DOI] [PubMed] [Google Scholar]
- Perlman S. J., Jaenicke J.2003Infection success in novel hosts: an experimental and phylogenetic study of Drosophila-parasitic nematodes. Evolution 57, 544–557 [DOI] [PubMed] [Google Scholar]
- Petanidou T., Kallimanis A. S., Tzanopoulos J., Sgardelis S. P., Pantis J. D.2008Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization. Ecol. Lett. 11, 564–575 10.1111/j.1461-0248.2008.01170.x (doi:10.1111/j.1461-0248.2008.01170.x) [DOI] [PubMed] [Google Scholar]
- Poulin R.2007Evolutionary ecology of parasites (2nd edn). Princeton, NJ: Princeton University Press [Google Scholar]
- Regoes R. R., Nowak M. A., Bonhoeffer S.2000Evolution of virulence in a heterogeneous host population. Evolution 54, 64–71 [DOI] [PubMed] [Google Scholar]
- Rutrecht S. T., Brown M. J. F.2008The life-history impact and implications of multiple parasites for bumble bee queens. Int. J. Parasitol. 38, 799–808 10.1016/j.ijpara.2007.11.004 (doi:10.1016/j.ijpara.2007.11.004) [DOI] [PubMed] [Google Scholar]
- Rutrecht S. T., Brown M. J. F.2009Differential virulence in a multiple-host parasite of bumble bees: resolving the paradox of parasite survival? Oikos 118, 941–949 10.1111/j.1600-0706.2009.17392.x (doi:10.1111/j.1600-0706.2009.17392.x) [DOI] [Google Scholar]
- Sandland G. J., Rodgers J. K., Minchella D. J.2007Interspecific antagonism and virulence in hosts exposed to two parasite species. J. Invertebr. Pathol. 96, 43–47 10.1016/j.jip.2007.02.005 (doi:10.1016/j.jip.2007.02.005) [DOI] [PubMed] [Google Scholar]
- Seppälä O., Karvonen A., Valtonen E. T., Jokela J.2009Interactions among co-infecting parasite species: a mechanism maintaining genetic variation in parasites? Proc. R. Soc. B 276, 691–697 10.1098/rspb.2008.1229 (doi:10.1098/rspb.2008.1229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. F., Acevedo-Whitehouse K., Pedersen A. B.2009The role of infectious diseases in biological conservation. Anim. Conserv. 12, 1–12 10.1111/j.1469-1795.2008.00228.x (doi:10.1111/j.1469-1795.2008.00228.x) [DOI] [Google Scholar]
- Sorrell I., White A., Pedersen A. B., Hails R. S., Boots M.2009The evolution of covert, silent infection as a parasite strategy. Proc. R. Soc. B 276, 2217–2226 10.1098/rspb.2008.1915 (doi:10.1098/rspb.2008.1915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staves P. A., Knell R. J.In press Virulence and competitiveness: testing the relationship during inter- and intra-specific mixed infections. Evolution. 10.1111/j.1558-5646.2010.00999.x (doi:10.1111/j.1558-5646.2010.00999.x) [DOI] [PubMed] [Google Scholar]
- Tay W. T., O'Mahony E. M., Paxton R. J.2005Complete rRNA gene sequences reveal that the microsporidium Nosema bombi infects diverse bumblebee (Bombus spp.) hosts and contains multiple polymorphic sites. J. Euk. Microbiol. 52, 505–513 10.1111/j.1550-7408.2005.00057.x (doi:10.1111/j.1550-7408.2005.00057.x) [DOI] [PubMed] [Google Scholar]
- Taylor D. R., Jarosz A. M., Lenski R. E., Fulbright D. W.1998The acquisition of hypovirulence in host–pathogen systems with three trophic levels. Am. Nat. 151, 343–355 10.1086/286123 (doi:10.1086/286123) [DOI] [PubMed] [Google Scholar]
- Taylor L. H., Latham S. M., Woolhouse M. E. J.2001Risk factors for human disease emergence. Phil. Trans. R. Soc. Lond. B 356, 983–989 10.1098/rstb.2001.0888 (doi:10.1098/rstb.2001.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieltges D. W., Bordalo M. D., Hernandez A. C., Prinz K., Jensen K. T.2008Ambient fauna impairs parasite transmission in a marine parasite–host system. Parasitology 135, 1111–1116 10.1017/S0031182008004526 (doi:10.1017/S0031182008004526) [DOI] [PubMed] [Google Scholar]
- Thomas M. B., Watson E. L., Valverde-Garcia P.2003Mixed infections and insect–pathogen interactions. Ecol. Lett. 6, 183–188 10.1046/j.1461-0248.2003.00414.x (doi:10.1046/j.1461-0248.2003.00414.x) [DOI] [Google Scholar]
- Tompkins D. M., Clayton D. H.1999Host resources govern the specificity of swiftlet lice: size matters. J. Anim. Ecol. 68, 489–500 10.1046/j.1365-2656.1999.00297.x (doi:10.1046/j.1365-2656.1999.00297.x) [DOI] [Google Scholar]
- Williams H., Jones A.1994Parasitic worms of fish. London, UK: Taylor and Francis [Google Scholar]
- Wolinska J., King K. C.2009Environment can alter selection in host–parasite interactions. Trends Parasitol. 25, 236–244 10.1016/j.pt.2009.02.004 (doi:10.1016/j.pt.2009.02.004) [DOI] [PubMed] [Google Scholar]
- Woolhouse M. E. J., Taylor L. H., Haydon D. T.2001Population biology of multihost pathogens. Science 292, 1109–1112 10.1126/science.1059026 (doi:10.1126/science.1059026) [DOI] [PubMed] [Google Scholar]