Abstract
The problem of achieving widespread immunity to infectious diseases by voluntary vaccination is often presented as a public-goods dilemma, as an individual's vaccination contributes to herd immunity, protecting those who forgo vaccination. The temptation to free-ride brings the equilibrium vaccination level below the social optimum. Here, we present an evolutionary game-theoretic approach to this problem, exploring the roles of individual imitation behaviour and population structure in vaccination. To this end, we integrate an epidemiological process into a simple agent-based model of adaptive learning, where individuals use anecdotal evidence to estimate costs and benefits of vaccination. In our simulations, we focus on parameter values that are realistic for a flu-like infection. Paradoxically, as agents become more adept at imitating successful strategies, the equilibrium level of vaccination falls below the rational individual optimum. In structured populations, the picture is only somewhat more optimistic: vaccination is widespread over a range of low vaccination costs, but coverage plummets after cost exceeds a critical threshold. This result suggests parallels to historical scenarios in which vaccination coverage provided herd immunity for some time, but then rapidly dropped. Our work sheds light on how imitation of peers shapes individual vaccination choices in social networks.
Keywords: vaccination dilemma, peer influence, epidemiology, evolutionary dynamics, mathematical biology
1. Introduction
Pre-emptive vaccination is a fundamental strategy for controlling infectious diseases (CDC 2009, http://www.cdc.gov/vaccines/). While there is vigorous debate about the civil liberties implications of mandatory versus voluntary vaccination policies [1], mounting evidence shows that voluntary vaccination plans fail to protect populations adequately [2–12]. A recent example of this failure is the sharp decline in take-up of the combined measles–mumps–rubella vaccination in Britain soon after administering it to children was made voluntary [13]. Because of declining familiarity with the disease and rising fears of vaccine complications, parents hoped to avoid the alleged vaccination health risk to their own children while implicitly relying on enough other children getting vaccinated to provide herd immunity. The ‘public good’ created by herd immunity gives rise to an enduring social dilemma of voluntary vaccination.
Classical game theory predicts that, when individuals act in their own interests with perfect knowledge of their infection risk, their vaccination decisions converge towards a Nash equilibrium, at which no individuals could be better off by unilaterally changing to a different strategy [5,6]. Although this equilibrium is the result of each individual following her self-interest, it may lead to suboptimal vaccination coverage for the community [3]. The collective result of vaccination decisions determines the level of population immunity and thus the severity of an epidemic strain. With increasing levels of vaccination coverage in the community, even the individuals who are unvaccinated are less likely to become infected; therefore, they have less incentive to get the vaccine. This scenario naturally leads to the ‘free riding’ problem that is commonly observed in public goods studies [14].
Previous studies of vaccinating dynamics have typically combined a game-theoretic model assuming full rationality and complete information with a model of disease transmission in either homogeneously mixed populations [5,6] or random networks [15]. In studies where the assumption of rationality is relaxed, deterministic evolutionary dynamics still recover equilibrium states equivalent to those predicted by models of rational agents [10]. It is worth noting that aggregate population models have been parameterized with empirical data to quantitatively predict vaccinating behaviour in some cases [3,4,10]. Here, we extend this previous work by accounting for decision-makers' social networks and their use of anecdotal information in making vaccination choices. Individuals have incomplete information and tend to rely on salient anecdotes from friends and the media in order to form opinions of disease risk and prevention [16–18]. The rise to prominence in the British media of isolated cases linking the pertussis vaccine and brain damage triggered a sharp decline in coverage in the late 1970s, demonstrating the power of the anecdote [10,19]. Apart from these prominent cases, each person can encounter different anecdotal evidence, depending on her social network [15,20]. Illness of a close friend can impact one's perception of infection risk and the importance of prevention in far more powerful ways than media reports can [18].
Motivated by the above considerations, we propose a simple agent-based model in the spirit of evolutionary game dynamics [21–23] to study the voluntary vaccination dilemma. In order to make precise predictions, we couple the vaccination dynamics with an epidemiological model, in particular the SIR model, which tracks populations of susceptible, infected and resistant/vaccinated individuals over time, within a single season or epidemic. Such models have been used, for example, to design clinical trials of vaccines or to predict whether a vaccination programme will halt an epidemic before it spreads to much of the population [24,25].
Our model captures the strategic interaction between vaccinating and free-riding individuals in the following way. Individuals decide whether to vaccinate during a vaccination campaign, before the seasonal epidemic begins. The epidemiological model then determines whether each susceptible (unvaccinated) individual becomes infected at some point during the season. Once the epidemic ends, individuals can revise their vaccination decision for the next season. Such a model is most appropriate for describing infections such as influenza. Flu vaccines are typically available prior to a predicted outbreak and are effective for only one season owing to mutation of pathogens and waning immunity [7,8].
2. Model and Methods
Consider a well-mixed population of individuals with a voluntary vaccination option. We model the vaccination dynamics as a two-stage game (as illustrated in figure 1). The first stage is a public vaccination campaign, which occurs before any infection. At this stage, each individual decides whether or not to vaccinate. Vaccination incurs a cost, V, to the vaccinated individual. For simplicity, here we assume that vaccination grants perfect immunity from the seasonal infectious disease. (To account for imperfect vaccination, one may rescale the cost of vaccination by its effectiveness and calculate infection risk based on the effective proportion of the population that is vaccinated.) The total cost of vaccination includes the immediate monetary cost, the opportunity cost of time spent to get the vaccine and any perceived or actual adverse health effects. In the second stage, the epidemic strain infects an initial number of individuals I0 and then spreads according to SIR dynamics, with per-day transmission rate r and recovery rate g (see the electronic supplementary material for model details). The epidemic continues until there are no more newly infected individuals (which occurred in under 200 days for all cases simulated). The final size equation [25] gives the infection risk for an infinite population (see the electronic supplementary material for derivations):
| 2.1 |
where R(∞) is the final size of the epidemic (fraction that have been infected at some point in the season), which satisfies R(∞) = (1 − x)(1 − e−R0R(∞)); R0 is the basic reproduction ratio; and x is the fraction of vaccinated individuals.
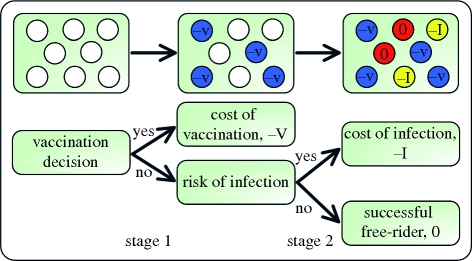
Figure 1.
Schematic of our model. We model the vaccination dilemma as a two-stage game. At stage 1 (vaccination choice), a proportion x of the population decides to vaccinate. Vaccination costs V and provides perfect immunity from the infectious disease. At stage 2 (health outcome), we use the SIR model to simulate the epidemiological process. Each unvaccinated individual faces the risk of infection during the seasonal epidemic outbreak. The cost of infection is I. Those unvaccinated individuals who remain healthy are free-riding off the vaccination efforts of others, and they are indirectly protected by herd immunity.
The infection cost I includes healthcare expenses, lost productivity and the possibility of pain or mortality. After the epidemic, the individuals with the highest payoffs are those who declined vaccination but avoided infection. We call these lucky individuals successful free-riders, as they benefit from others' vaccination efforts. The game dynamics remain unchanged if we rescale the payoffs by defining the relative cost of vaccination c = V/I (0 < c < 1). The values of c appropriate for modelling a particular disease can be estimated from surveys of health opinions, behaviours and outcomes, as done by, e.g. Galvani et al. [3], but in general vaccination cost should be low relative to the cost of infection. The Nash equilibrium of this game can be solved by setting the expected cost of vaccination equal to that of non-vaccination, which implies the mixed strategy
| 2.2 |
This level of vaccination uptake falls short of the social optimum xh = 1 − (1/R0), the level which achieves herd immunity (near-elimination of the risk of contacting an infectious individual) and thereby minimizes the sum of all individuals' costs related to both vaccination and infection (see the electronic supplementary material). The misalignment between individual and group interests leads to a social dilemma.
Here, we relax the assumption of rationality and study this vaccination dilemma from an evolutionary perspective. Each season, an individual adopts a pure strategy, which determines whether or not she vaccinates. At the end of the season, each individual decides whether to change her strategy for the next season, depending on her current payoff. Specifically, individual i randomly chooses individual j from the population as role model. The strategy of a role model with higher payoff is more likely to be imitated. We suppose that the probability that individual i adopts individual j's strategy is given by the Fermi function [26–29]
| 2.3 |
where β denotes the strength of selection (0 < β < ∞).
This updating dynamic diverges from a fully rational model in two ways. First, individuals adjust their strategies retrospectively, in response only to the observed payoff outcomes and not the expected payoffs of strategies. In a population with low vaccination uptake, many non-vaccinators fall ill, but if individual i happens to choose one of the few successful free-riders as a role model, then she will be more likely to imitate the free-rider's strategy. Second, the strength of selection parameter introduces a stochastic element to the model: for small β (weak selection), individuals are less responsive to payoff differences, and an individual with a high payoff may adopt the strategy of a less successful role model. Large values of β (strong selection) diminish this stochastic effect, and individuals reliably switch to (or keep) the strategy with the higher observed payoff, even if the payoff difference is small. Previous work using the same update dynamic has characterized agents with high β as being more rational [27]. This characterization is not appropriate in our context, as higher β only increases an agent's sensitivity to the (perhaps unrepresentative) observed payoff, not the expected payoff.
The model presented here can be conveniently extended to structured populations by restricting the neighbourhood of individuals whom one can infect or imitate. In addition to the well-mixed case, we simulated populations structured as square lattices, Erdős–Rényi random graphs [30] and Barabási–Albert scale-free networks ([31]; see the electronic supplementary material). The initial state consists of equal fractions vaccinators and non-vaccinators, randomly distributed throughout the population. Each two-stage iteration (vaccination strategy updating followed by an epidemic process) updates the frequencies of each strategy. Since we are interested primarily in the effect of population structure on vaccination coverage (rather than on infection risk), we calibrated epidemic parameters to ensure that the infection risk in an unvaccinated population is equal across all population structures ([15]; see the electronic supplementary material). Each simulation was run for 3000 iterations. The long run equilibrium results shown in figures 2–4 represent the average of frequencies over the last 1000 iterations in 100 independent simulations. We present results of simulations that use population sizes between N = 500 and N = 10 000; overall results are robust to varying population size for N as small as 200.
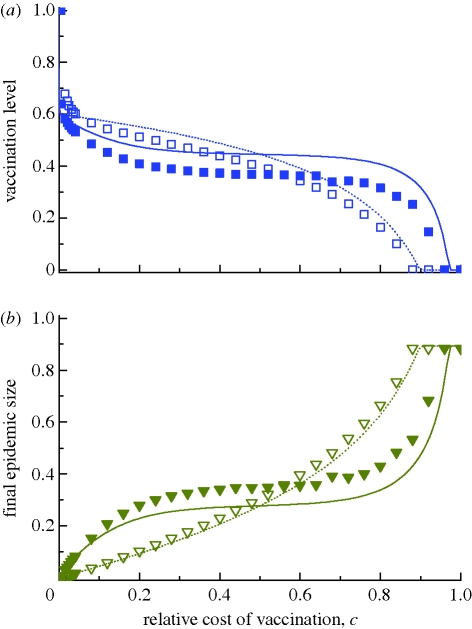
Figure 2.
Vaccination dynamics in well-mixed populations. The fractions (a) vaccinated and (b) infected are shown as functions of the relative cost of vaccination, c, for the intensity of selection β = 1 and 10. The lines are analytical predictions from deterministic equations (see the electronic supplementary material). The deviation between simulation and theory is largely due to stochasticity in disease transmission: holding vaccination constant at some level below the herd immunity threshold (1 − (1/R0) = 0.6), simulated infection risk is smaller than the prediction in equation (2.1) (see electronic supplementary material, figure S1b). Individuals in the simulation respond to this decreased risk by vaccinating less than in the theory, which in turn leads to a larger epidemic versus the theory. Strong selection magnifies individuals' responses, producing larger deviations. For vaccination coverage above the theoretical herd immunity level, the deterministic approximation underestimates infection risk, leading to an opposite deviation at low c. Parameters: population size N = 5000, R0 = 2.5 (realized by setting r = 5/(6N) d−1 person−1 and g = ⅓ d−1), number of infection seeds I0 = 5. (a) Simulations: open squares, β = 1; filled squares, β = 10; theory: dotted line, β = 1; solid line, β = 10. (b) Simulations: open inverted triangles, β = 1; filled inverted triangles, β = 10; theory: dotted line, β = 1; solid line, β = 10.
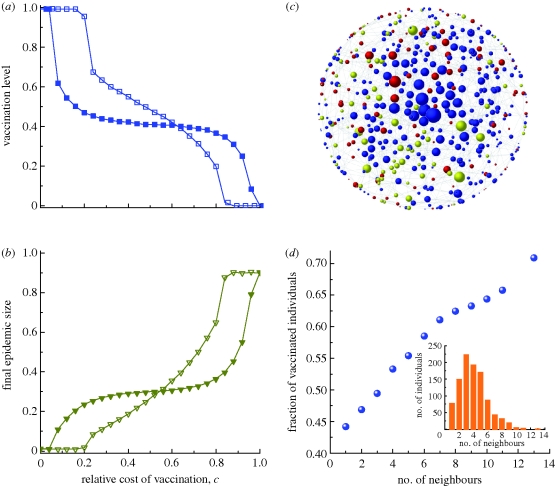
Figure 4.
Vaccination dynamics in random network populations. (a,b) The fractions vaccinated and infected, respectively, as functions of c for the intensity of selection β = 1 and 10. (c) Snapshot of a single simulation on a random network at equilibrium frequencies. The size of a node corresponds to its degree (number of neighbours). Blue nodes are vaccinated, yellow are infected, and red are successful free-riders. (d) The frequency of vaccination on a random network, as a function of the number of neighbours an individual has. The inset in panel (d) shows the degree distribution of the random network. Parameters: (a–d) average degree k̄ = 4, disease transmission rate r = 0.51d−1 person−1, recovery rate g = ⅓ d−1; (a,b,d) N = 1000, I0 = 10; (c) N = 500, I0 = 5; (c,d) c = 0.1, β = 10. The lines in (a) and (b) are visual guides. (a) Open squares with solid line, β = 1; filled squares with solid line, β = 10. (b) Open inverted triangles with solid line, β = 1; filled inverted triangles with solid line, β = 10.
3. Results
In the vaccination game, if all of one's neighbours adopt one strategy, then it is advantageous to adopt the opposite strategy. We therefore always find persistent polymorphisms of vaccinated and unvaccinated individuals for intermediate values of c. Figure 2 plots both the equilibrium frequency of (a) vaccinated and (b) infected individuals for different values of c and β in the well-mixed imitation dynamics. We find qualitative agreement between stochastic simulations and an analytical prediction that uses both the equation for infection risk (2.1) and an infinite-population approximation of the imitation dynamics (described in the electronic supplementary material).
For weak selection (β = 1 in figure 2), the imitation dynamics approximate the rational equilibrium x* given in equation (2.2). One can understand this observation analytically by noting that the strategy update equation (2.3) is roughly linear for small β. First-order approximation of the imitation dynamics closely approximates the replicator dynamics [32–34], which in this game converge to the unique evolutionarily stable strategy—the Nash equilibrium (see the electronic supplementary material). As vaccination falls with increasing c, the final size of the epidemic grows. Above a high cost threshold cH ≈ 0.893, no one chooses vaccination and the epidemic reaches its maximum size.
Strong selection in the imitation dynamics (represented by β = 10 in figure 2) can decrease vaccination uptake below the level predicted by the rational equilibrium. In other words, individuals who carefully attend to peers' health outcomes and reliably copy the behaviour of successful peers will end up attempting to free-ride more than they rationally ‘ought’ to. If, for example, infection is 12 times as costly as vaccination (c = 0.08, a reasonable assumption for influenza, see the electronic supplementary material), then strong selection in our model lowers vaccination coverage by 8 percentage points versus weak selection (figure 2a), which increases the epidemic size from 4 per cent of the population to 15 per cent of the population (figure 2b). With increasing cost of vaccination, the equilibrium vaccination coverage follows a rotated ‘S’ curve, dropping rapidly (slope ≈−(β/2)) from the herd immunity threshold at low values of c, reaching a plateau near 1 − (2 ln 2)/R0 for intermediate values of c, and then dropping rapidly to zero as c grows large. The threshold cH increases with selection strength (figure 2a).
Results are qualitatively similar for any basic reproduction ratio R0> 1 of the infection. Figures S5 and S6 in the electronic supplementary material compare the cases R0 = 2.5 and R0 = 6. The higher value increases infection risk, making the population respond with increased vaccination uptake. Increasing R0 also raises the threshold cH.
Restricting interaction to local neighbourhoods partly ameliorates the free-riding problem, but introduces greater sensitivity to the cost parameter c (figure 3). We consider a population of individuals arranged on a square lattice where each individual has four immediately adjacent neighbours. While the vaccination coverage in well-mixed populations drops from herd immunity levels as soon as c increases above zero, restricted spatial interaction promotes near-universal coverage at a range of positive c, preventing the epidemic. To give a simple operational definition, we say that vaccination ‘prevents the epidemic’ in a structured population if the average final epidemic size is less than twice the size of the initial inoculum. Define as cL the critical vaccination cost below which the epidemic is prevented. For weak selection on the lattice (β = 1 in figure 3), we get cL ≈ 0.022. Above this threshold, the vaccination level drops precipitously, causing an epidemic that is even larger than in the well-mixed case.
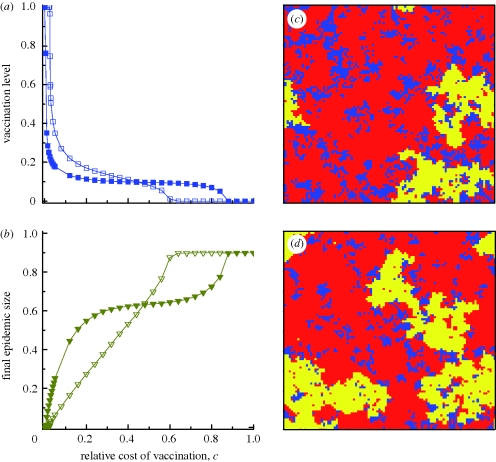
Figure 3.
Vaccination dynamics in lattice populations. (a,b) The fractions vaccinated and infected, respectively, as functions of c for the intensity of selection β = 1 and 10. (c,d) Snapshots of the system at equilibrium frequencies with weak and strong selection, respectively. Blue denotes vaccinated individuals, red successful free-riders and yellow infected individuals. Strong selection breaks apart clusters of vaccinators: 54% of vaccinated individuals' neighbours are also vaccinated in (c), versus only 49% in (d). Parameters: population size N = 100 × 100 with von Neumann neighbourhood, disease transmission rate r = 0.46 d−1 person−1, recovery rate g = ⅓ d−1, number of infection seeds I0 = 10, (c,d) c = 0.08, (c) β = 1, (d) β = 10. The lines in (a) and (b) are visual guides. (a) Open squares with solid line, β = 1; filled squares with solid line, β = 10. (b) Open inverted triangles with solid line, β = 1; filled inverted triangles with solid line, β = 10.
At higher selection strength, the threshold cL is lower, and vaccination coverage is even more sensitive to costs rising above cL (figure 3a). The high-cost threshold cH rises with selection strength, meaning that the transitional region between cL and cH, where vaccinated and unvaccinated individuals coexist, widens with larger β. Holding c constant at a value above cL, increasing the strength of selection leads to more free-riding attempts, breaking apart clusters of vaccinators, thus allowing a larger epidemic to occur (figure 3c versus d).
Most actual populations are heterogeneous in the sense that different individuals may have different numbers of neighbours (i.e. degree; [31]). To account for this feature, we consider vaccination dynamics on Erdős–Rényi random graphs, which have moderate degree heterogeneity; on scale-free networks, which have an even more variable degree distribution, our results are similar (see the electronic supplementary material).
Higher vaccination coverage is typically required to achieve herd immunity in populations with greater degree heterogeneity ([35]; see also figures S2–S4 in the electronic supplementary material). This increased vulnerability to epidemic attacks reduces the temptation to free-ride, actually making it easier for a population of selfish imitators to achieve the high-vaccination threshold required for herd immunity. The threshold cost cL therefore increases versus the lattice case. Vaccination coverage drops after cost exceeds this threshold, although the effect is not quite as extreme as in lattice populations (figure 4a,b). Similarly to lattice populations, increased selection strength increases the size of the intermediate region between cL and cH.
Degree heterogeneity triggers a broad spectrum of individual vaccinating behaviour. Specifically, an individual's vaccination strategy is now influenced by her role in the population, and ‘hubs’ who have many neighbours are most likely to choose to be vaccinated, as they are at greatest risk of infection (figure 4c,d). Hubs that do manage to free-ride successfully become victims of their own success, as their vaccinated neighbours of smaller degree are likely to imitate them and switch strategies, potentially infecting the hubs in the following season.
4. Discussion and conclusion
Our model shows how incomplete information and strong selection (high payoff-sensitivity, parameterized by β) in a population of imitators cause the vaccination coverage to fall well short of the social optimum, and even below the Nash equilibrium. Weak selection in a well-mixed population recapitulates the replicator dynamics, converging to the Nash equilibrium. Strong selection, on the other hand, drives individuals to imitate successful free-riders based on a single observation, even when a rational agent with complete information would realize that attempted free-riding does poorly in expectation. This ‘paradox of imitation’ is a very general phenomenon [36] and may in part explain cases where public vaccination levels are low. In particular, for the range of vaccination cost appropriate to influenza (i.e. c ≈ 0.002 to 0.08, see the electronic supplementary material), the imitation dynamics with strong selection in the well-mixed case falls well short of the rational optimum, leading to over-exploitation of herd immunity and an increase in preventable infections. Our model describes the admittedly extreme case in which each individual observes only one randomly chosen role model each round. Allowing imitators to learn from a somewhat larger group of peers could lessen the sampling error, but would not eliminate it.
This kind of error is reminiscent of, but distinct from, the phenomenon of ‘information cascades’ that generate rationalized conformism or ‘groupthink’ [37,38]. Such cascades may also be obstacles to high vaccination coverage [39]. To explore conformism (or, alternatively, stubbornness) in the context of our model, one might include an additional cost τ of switching strategy in the thermal updating rule [29,40]; that is, f(ΔP) = 1/(1 + exp(−β(ΔP + τ))). A large negative (positive) τ would then represent the tendency to copy one's peers (stick with the current strategy), regardless of payoff comparisons. Previous studies have shown in detail how this sort of payoff-neglecting imitation can lead to widespread conformism and adoption of sub-optimal strategies [37,38].
It is widely known that population structure can promote the evolution of cooperative behaviour [41–47]. We have shown, however, that population structure is a ‘double-edged sword’ for public health: it can promote high levels of voluntary vaccination and herd immunity, but small increases in the cost beyond a certain threshold cL cause vaccination to plummet—and infections to rise—more dramatically than in well-mixed populations. For example, the random network population under strong selection (β = 10) can prevent the epidemic completely for costs up to c = 0.04, but 11 per cent of the population become infected at cost c = 0.08. In the well-mixed population, the epidemic grows gradually, from 8 to 15 per cent, over the same cost range. This threshold effect is robust to changes in population structure and exists in lattice (figure 3a,b) and scale-free network (electronic supplementary material, figure S7a,b) populations as well.
In social networks, individuals' degrees vary greatly, and highly-connected individuals (hubs) can spread disease to a large number of peers if infected. The vaccination of hubs can play a vital role in containing infections [35], and public health programmes often try to promote herd immunity by allocating vaccinations preferentially to these hubs [48]. Physicians who are hubs in a disease-transmission network, for instance, have high rates of vaccine uptake [49]. Our model shows that even individuals with incomplete information can self-organize to achieve this pro-social outcome (figure 4). Since hubs generally face greater infection risk than small-degree individuals do, they have increased incentive to vaccinate; hubs' self-interest is therefore relatively well-aligned with overall welfare.
Recent work with a detailed model designed to mimic a smallpox outbreak on a random network [15] reaches a complementary conclusion about the fragility of high-coverage equilibria: voluntary vaccination can contain a disease in low-degree networks, but as the average degree increases, the system reaches a critical threshold past which it behaves like a well-mixed population and the epidemic spreads. This work focused on vaccination decisions made during the course of an epidemic in response to local disease prevalence, as opposed to season-by-season updating of pre-emptive vaccination decisions. Taken together, our current work and this previous result demonstrate how local disease transmission and decision-making based on local context change the character of vaccination dynamics. Voluntary vaccination can be a viable policy for achieving high coverage and eradicating disease, but the final outcome is sensitive to small changes in (actual or perceived) vaccination cost and in the social network. This sensitivity may in part explain how anecdotal evidence of vaccine-related health risks has been able to trigger steep declines in coverage and loss of population immunity [10,13,19]. Policy levers that subsidize vaccination can take advantage of these threshold effects to promote disease containment and eradication.
Achieving socially optimal coverage through voluntary vaccination is a problem of cooperation with limited information and uncertainty about outcomes. The problem is similar to public goods games studied by economists [50], as herd immunity provides a communal benefit. Individuals' use of salient anecdotes to cope with uncertainty, however, is not a typically studied feature of public goods games. Two sources of uncertainty face an individual deciding whether to vaccinate: uncertainty about contracting the infection if unvaccinated, and uncertainty regarding adverse reactions to the vaccine itself. Our current work focuses on the former uncertainty, treating the vaccine cost as a fixed quantity, which is a summary of all expected costs. It may also be instructive to treat vaccine cost as a random variable, as a way of explicitly modelling public fears concerning vaccine safety. These fears often have a tremendous impact on vaccine take-up and public health [19,51].
Acknowledgements
We thank two anonymous referees for their constructive and insightful comments. We thank Alison Hill for helpful advice about the Gillespie algorithm for simulating epidemiological processes on graphs. We are grateful for support from the John Templeton Foundation, the National Science Foundation/National Institute of Health joint programme in mathematical biology (NIH grant no. R01GM078986), the Bill and Melinda Gates Foundation (Grand Challenges grant 37874), an NSF Graduate Research Fellowship, China Scholarship Council, NSFC (grant nos. 10972002, 60736022 and 60674050) and J. Epstein.
References
- 1.Colgrave J.2006State of immunity: the politics of vaccination in twentieth-century America. Berkeley, CA: University of California Press [Google Scholar]
- 2.Fine P., Clarkson J.1986Individual versus public priorities in the determination of optimal vaccination polices. Am. J. Epidemiol. 124, 1012–1020 [DOI] [PubMed] [Google Scholar]
- 3.Galvani A. P., Reluga T. C., Chapman G. B.2007Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proc. Natl Acad. Sci. USA 104, 5692–5697 10.1073/pnas.0606774104 (doi:10.1073/pnas.0606774104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu S., Chapman G. B., Galvani A. P.2008Integrating epidemiology, psychology, and economics to achieve HPV vaccination targets. Proc. Natl Acad. Sci. USA 105, 19 018–19 023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauch C. T., Galvani A. P., Earn D. J. D.2003Group interest versus self interest in smallpox vaccination policy. Proc. Natl Acad. Sci. USA 100, 10 564–10 567 10.1073/pnas.1731324100 (doi:10.1073/pnas.1731324100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauch C. T., Earn D. J. D.2004Vaccination and the theory of games. Proc. Natl Acad. Sci. USA 101, 13 391–13 394 10.1073/pnas.0403823101 (doi:10.1073/pnas.0403823101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardavas R., Breban R., Blower S.2007Can influenza epidemics be prevented by voluntary vaccination? PLoS Comp. Biol. 3, e85. 10.1371/journal.pcbi.0030085 (doi:10.1371/journal.pcbi.0030085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breban R., Vardavas R., Blower S, 2007Mean-field analysis of an inductive reasoning game: application to influenza vaccination. Phys. Rev. E 76, 031127. 10.1103/PhysRevE.76.031127 (doi:10.1103/PhysRevE.76.031127) [DOI] [PubMed] [Google Scholar]
- 9.van Boven M., Klinkenberg D., Pen I., Weissing F. J., Heesterbeek H.2008Self-interest versus group-interest in antiviral control. PLoS ONE 3, e1558. 10.1371/journal.pone.0001558 (doi:10.1371/journal.pone.0001558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauch C. T.2005Imitation dynamics predict vaccinating behaviour. Proc. R. Soc. B 272, 1669–1675 10.1098/rspb.2005.3153 (doi:10.1098/rspb.2005.3153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cojocaru M. G.2008Dynamic equilibria of group vaccination strategies in a heterogeneous population. J. Glob. Optim. 40, 51–63 10.1007/s10898-007-9204-7 (doi:10.1007/s10898-007-9204-7) [DOI] [Google Scholar]
- 12.Reluga T. C., Bauch C. T., Galvani A. P.2006Evolving public perceptions and stability in vaccine uptake. Math. Biosci. 204, 185–198 10.1016/j.mbs.2006.08.015 (doi:10.1016/j.mbs.2006.08.015) [DOI] [PubMed] [Google Scholar]
- 13.Jansen V. A., Stollenwerk N., Jensen H. J., Ramsay M. E., Edmunds W.J., Rhodes C. J.2003Measles outbreaks in a population with declining vaccine uptake. Science 301, 804. 10.1126/science.1086726 (doi:10.1126/science.1086726) [DOI] [PubMed] [Google Scholar]
- 14.Hardin G.1968The tragedy of the commons. Science 162, 1243–1248 10.1126/science.162.3859.1243 (doi:10.1126/science.162.3859.1243) [DOI] [PubMed] [Google Scholar]
- 15.Perisic A., Bauch C. T.2008Social contact networks and disease eradicability under voluntary vaccination. PLoS Comp. Biol. 5, e1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tversky A., Kahneman D.1973Availability: a heuristic for judging frequency and probability. Cogn. Psychol. 5, 207–232 10.1016/0010-0285(73)90033-9 (doi:10.1016/0010-0285(73)90033-9) [DOI] [Google Scholar]
- 17.Johnson E. J., Tversky A.1983Affect, generalization, and the perception of risk. J. Pers. Soc. Psychol. 45, 20–31 10.1037/0022-3514.45.1.20 (doi:10.1037/0022-3514.45.1.20) [DOI] [Google Scholar]
- 18.Palekar R., Pettifor A., Behets F., MacPhail C.2008Association between knowing someone who died of AIDS and behavior change among South African youth. AIDS Behav. 12, 903–912 10.1007/s10461-007-9325-5 (doi:10.1007/s10461-007-9325-5) [DOI] [PubMed] [Google Scholar]
- 19.Nicoll A., Elliman D., Ross E.1998MMR vaccination and autism 1998: déjà vu–pertussis and brain damage 1974? Br. Med. J. 316, 715–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eames K. T. D.2009Networks of influence and infection: parental choices and childhood disease. J. R. Soc. Interface 6, 811–814 10.1098/rsif.2009.0085 (doi:10.1098/rsif.2009.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maynard-Smith J.1982Evolution and the theory of games. Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Nowak M. A., Sigmund K.2004Evolutionary dynamics of biological games. Science 303, 793–799 10.1126/science.1093411 (doi:10.1126/science.1093411) [DOI] [PubMed] [Google Scholar]
- 23.Nowak M. A.2006Evolutionary dynamics: exploring the equations of life. Cambridge, MA: Harvard University Belknap Press [Google Scholar]
- 24.Levin B. R., Lipsitch M., Bonhoeffer S.1999Population biology, evolution, and infectious disease: convergence and synthesis. Science 283, 806–809 10.1126/science.283.5403.806 (doi:10.1126/science.283.5403.806) [DOI] [PubMed] [Google Scholar]
- 25.Diekmann O., Heesterbeek J. A. P.2000Mathematical epidemiology of infectious diseases: model building, analysis, and interpretation. Chichester, UK: Wiley [Google Scholar]
- 26.Blume L. E.1993The statistical mechanics of strategic interaction. Games Econ. Behav. 5, 387–424 10.1006/game.1993.1023 (doi:10.1006/game.1993.1023) [DOI] [Google Scholar]
- 27.Szabó G., Tőke C.1998Evolutionary prisoner's dilemma game on a square lattice. Phys. Rev. E 58, 69. 10.1103/PhysRevE.58.69 (doi:10.1103/PhysRevE.58.69) [DOI] [Google Scholar]
- 28.Traulsen A., Pacheco J. M., Nowak M. A.2007Pairwise comparison and selection temperature in evolutionary game dynamics. J. Theor. Biol. 246, 522–529 10.1016/j.jtbi.2007.01.002 (doi:10.1016/j.jtbi.2007.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traulsen A., Semmann D., Sommerfeld R. D., Krambeck H.-J., Milinski M.2010Human strategy updating in evolutionary games. Proc. Natl Acad. Sci. USA 107, 2962–2966 10.1073/pnas.0912515107 (doi:10.1073/pnas.0912515107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdős P., Rényi A.1959On random graphs. Publ. Math. Debrecen 6, 290–297 [Google Scholar]
- 31.Barabási A. L., Albert R.1999Emergence of scaling in random networks. Science 286, 509–512 10.1126/science.286.5439.509 (doi:10.1126/science.286.5439.509) [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer J., Sigmund K.1998Evolutionary games and population dynamics. Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Schuster P., Sigmund K.1983Replicator dynamics. J. Theor. Biol. 100, 533–538 10.1016/0022-5193(83)90445-9 (doi:10.1016/0022-5193(83)90445-9) [DOI] [Google Scholar]
- 34.Taylor P. D., Jonker L. B.1978Evolutionarily stable strategies and game dynamics. Math. Biosci. 40, 145–156 10.1016/0025-5564(78)90077-9 (doi:10.1016/0025-5564(78)90077-9) [DOI] [Google Scholar]
- 35.Pastor-Satorras R., Vespignani A.2002Immunization of complex networks. Phys. Rev. E 65, 036104. 10.1103/PhysRevE.65.036104 (doi:10.1103/PhysRevE.65.036104) [DOI] [PubMed] [Google Scholar]
- 36.Schlag K. H.1998Why imitate, and if so, how? A boundedly rational approach to multi-armed bandits. J. Econ. Theory 78, 130–156 10.1006/jeth.1997.2347 (doi:10.1006/jeth.1997.2347) [DOI] [Google Scholar]
- 37.Banerjee A. V.1992A simple model of herd behavior. Q. J. Econ. 100, 992–1026 [Google Scholar]
- 38.Bikhchandani S., Hirshleifer D., Welch I.1992A theory of fads, fashion, custom, and cultural change as informational cascades. J. Pol. Econ. 107, 797–817 [Google Scholar]
- 39.Barton A. M.2009Application of cascade theory to online systems: a study of email and Google cascades. Minn. J.L. Sci. Tech. 10, 473–502 [Google Scholar]
- 40.Szabó G., Hauert C.2002Phase transitions and volunteering in spatial public goods games. Phys. Rev. Lett. 89, 11810. 10.1103/PhysRevLett.89.118101 (doi:10.1103/PhysRevLett.89.118101) [DOI] [PubMed] [Google Scholar]
- 41.Hauert C., Doebeli M.2004Spatial structure often inhibits the evolution of cooperation in the Snowdrift game. Nature 428, 643–646 10.1038/nature02360 (doi:10.1038/nature02360) [DOI] [PubMed] [Google Scholar]
- 42.Nowak M. A., May R. M.1992Evolutionary games and spatial chaos. Nature 359, 826–829 10.1038/359826a0 (doi:10.1038/359826a0) [DOI] [Google Scholar]
- 43.Nowak M. A.2006Five rules for the evolution of cooperation. Science 314, 1560–1563 10.1126/science.1133755 (doi:10.1126/science.1133755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak M. A., Tarnita C. E., Antal T.2010Evolutionary dynamics in structured populations. Phil. Trans. R. Soc. B 365, 19–30 10.1098/rstb.2009.0215 (doi:10.1098/rstb.2009.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtsuki H., Hauert C., Lieberman E., Nowak M. A.2006A simple rule for the evolution of cooperation on graphs and social networks. Nature 441, 502–505 10.1038/nature04605 (doi:10.1038/nature04605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarnita C. E., Antal T., Ohtsuki H., Nowak M. A.2009Evolutionary dynamics in set structured populations. Proc. Natl Acad. Sci. USA 106, 8601–8604 10.1073/pnas.0903019106 (doi:10.1073/pnas.0903019106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarnita C. E., Ohtsuki H., Antal T., Fu F., Nowak M. A.2009Strategy selection in structured populations. J. Theor. Biol. 259, 570–581 10.1016/j.jtbi.2009.03.035 (doi:10.1016/j.jtbi.2009.03.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bansal S., Pourbohloul B., Meyers L. A.2006A comparative analysis of influenza vaccination programs. PLoS Med. 3, e387. 10.1371/journal.pmed.0030387 (doi:10.1371/journal.pmed.0030387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capolongo M. J., DiBonaventura M. D., Chapman G. B.2006Physician vaccinate thyself: why influenza vaccination rates are higher among clinicians than among nonclinicians. Ann. Behav. Med. 31, 288 296. 10.1207/s15324796abm3103_11 (doi:10.1207/s15324796abm3103_11) [DOI] [PubMed] [Google Scholar]
- 50.Palfrey T. R., Rosenthal H.1984Participation and the provision of discrete public goods: a strategic analysis. J. Pub. Econ. 24, 171–193 10.1016/0047-2727(84)90023-9 (doi:10.1016/0047-2727(84)90023-9) [DOI] [Google Scholar]
- 51.Donald A., Muthu V.2002MMR links with autism and inflammatory bowel disease. Clin. Evid. 7, 331–340 [PubMed] [Google Scholar]