Abstract
Each autumn billions of songbirds migrate between the temperate zone and tropics, but little is known about how events on the breeding grounds affect migration to the tropics. Here, we use light level geolocators to track the autumn migration of wood thrushes Hylocichla mustelina and test for the first time if late moult and poor physiological condition prior to migration delays arrival on the winter territory. Late nesting thrushes postponed feather moult, and birds with less advanced moult in August were significantly farther north on 10 October while en route to the tropics. Individuals in relatively poor energetic condition in August (high β-Hydroxybutyrate, low triglyceride, narrow feather growth bars) passed into the tropics significantly later in October. However, late moult and poor pre-migratory condition did not result in late arrival on the winter territory because stopover duration was highly variable late in migration. Although carry-over effects from the winter territory to spring migration may be strong in migratory songbirds, our study suggests that high reproductive effort late in the season does not impose time constraints that delay winter territory acquisition.
Keywords: migration, geolocator, neotropical migrant, plasma metabolite, moult
1. Introduction
Carry-over effects between different stages of the life cycle are important for understanding the evolution of life-history traits but are difficult to study in migratory animals where seasonal interactions often occur across different continents. In nearctic–neotropical migratory songbirds, carry-over effects from the non-breeding ‘wintering’ season to the breeding season have been well documented using stable carbon isotopes to infer tropical habitat quality occupied prior to spring migration [1]. Habitat quality in the tropics affects an individual's body condition and consequently the timing of spring migration, arrival time on the breeding territory, future breeding success and annual survival [2–4]. However, few studies have examined how breeding season events carry-over into autumn migration [5] because the key variables, reproductive effort and timing do not have isotopic signatures that can be measured on the wintering grounds. Only recently has it become possible to track small songbirds over thousands of kilometres [6].
The short breeding season of migratory birds creates time and energetic trade-offs between reproductive effort, feather moult and migration. Nesting late in the breeding season can increase annual reproductive success but may also delay moult because of the costs of overlapping parental care and moult [7–9]. One of the most energetically demanding periods in a songbird's annual cycle is the post-breeding moult of flight and body feathers that can increase energy expenditure by over 30 per cent [10] and impose energetic constraints on pre-migratory fattening [11]. Despite the importance of the moult period in avian life cycles, it is rarely addressed in studies of carry-over effects. Norris et al. [5] found that male American redstarts with high reproduction effort late in the season were more likely to moult tail feathers during migration, resulting in reduced carotenoid content of the feather.
A possible carry-over effect of late breeding and moult is delayed migration and arrival on the winter territory. In many species, individuals complete moult, or nearly so, prior to the initiation of autumn migration [12,13] so birds are expected to face trade-offs between timing of nesting, moult and migration. Competition for high-quality territories in the tropics is intense [14–16] and if late breeding delays arrival on the winter territory this could constrain reproductive effort of migratory songbirds [8,9]. Reproductive effort and late nesting may also reduce post-breeding energetic condition, [17–19] which in turn could result in a slower migration [20,21]. Stopover site studies assess short-term migration decisions and have shown that poor energetic condition and stopover habitat quality delay resumption of migration [21–23].
Many migratory birds follow an overall time-minimization strategy [24] where migration behaviour can be viewed as a series of optimal refueling stops such that birds should travel as fast as possible given their energetic condition, stopover habitat quality and weather conditions. Under a time-minimization model, late departure and poor physiological condition prior to departure should be hard to overcome and result in later arrival on the winter territory. Here, we use light level geolocators to track the autumn migration of wood thrushes Hylocichla mustelina and test for the first time in a migratory songbird if late moult and poor physiological condition prior to migration delays migration and arrival on the winter territory.
2. Material and methods
(a). Study site and species
We studied a breeding population of 40–50 pairs of wood thrushes in a 150 ha forest at the Hemlock Hill Biological Research Area in Crawford County, PA, USA (41.8° N, 79.9° W). Birds (47–55 g) were captured using mist nets and banded with a numbered U.S. Geological Service aluminum band and a unique colour-band combination. We aged birds as first-time breeders (second year) or older (after second year) based on plumage characteristics [25]. Most birds were fitted with a 1.6 g radio-transmitter (Model BD-2, 14 week battery; Holohil, Inc.) in late May or early June to obtain complete nesting histories and to aid in capturing adults during the moult period in August, at which point the radio-transmitter was replaced with a geolocator (see below). Almost half (49%) of females attempted a second brood after fledging young from their first nest and average number of young fledged per female was 2.7 ± 0.30 (range 0–10, n = 39). Nesting completion date was defined as the day of fledging, or day of nest loss, of an individual's last nesting attempt of the season and could only be accurately determined if an individual had been radio-tagged owing to high, concealed nests and occasional territory switching between nesting attempts.
(b). Geolocator deployment
We used archival light level geolocators to reconstruct the timing and route of migration of individual wood thrushes [6]. In 2007 and 2008, 47 adult wood thrushes (23 females, 24 males; 18 second year, 29 after second year) were fitted with an Mk14S geolocator (1.6 g, British Antarctic Survey) using a leg-loop backpack harness. Most geolocators (35 of 47) were deployed in August, but 12 were deployed in 25 June–29 July on nesting birds. We retrieved geolocators in May or June of subsequent years from 12 different males and two females. Two of these birds were tracked in multiple years but only the first was used for analysis. We observed but could not capture four other returning geolocator birds (three males, one female). The annual return rate for geolocator birds (16 of 24 males, 66% males; three of 23 females, 13%) was not lower than for non-geolocator birds (11 of 26 males, 42%; four of 35 females, 11%).
Latitude and longitude are inferred from day length and sunrise times, respectively. Day-to-day error was estimated by the standard deviation while a bird was on its territory (electronic supplementary material; breeding: 230 km latitude, 105 km longitude; wintering: 390 km latitude, 140 km longitude). Movements away from the breeding site, and from one stopover location to another, were defined as long distance movements (greater than 250 km latitude, greater than 150 km longitude) that were consistent with autumn migration. We used the term ‘regional’ stopover to describe birds that interrupted migration for one or more days, though birds could have moved short distances within a given region.
While in the tropics, wood thrushes defend feeding territories from conspecifics and remain on their winter territory from late autumn until early spring [26]. The birds in our study were initially sampled on their breeding territory, had already spent at least one winter in the tropics, and were probably returning to the same winter territory occupied the year before. Arrival date on the winter territory was defined as when the latitude and longitude ceased to shift in a direction consistent with autumn migration, fluctuated around a narrow range of values consistent with a stationary bird, and remained similar until the onset of northward migration in spring. During presumed stopovers and while stationary during winter, location was determined by calculating average latitude and longitude during the period.
(c). Energetic condition during moult
We captured wood thrushes from 9 to 24 August to determine the extent of moult and obtain a blood sample to assess physiological condition. Most birds (17 of 20) were captured after their nesting completion date (22 ± 2.6 days later, range 6–43); two of three birds sampled 0–5 days prior to fledging their final brood had begun moult. All birds were sampled 06.00–11.00 EST by taking a 100 µl blood sample via brachial venipuncture within 3 min of capture.
Blood plasma metabolites have been widely used with migratory birds to assess individual energetic state [17,22,23,27]. β-Hydroxybutyrate levels increase during fasting and mass loss, while triglyceride levels increase during feeding and fat deposition. Plasma metabolites were assayed on a microplate spectrophotometer in 400 µl flat-bottom microplates. All samples were diluted 1 : 2 in 0.9 per cent NaCl saline solution to bring concentrations within the set of standards that produced the standard curve. Triglyceride was measured in a single endpoint assay (Triglyceride Reagent T2449; [28]) and β-hydroxybutyrate was measured by kinetic endpoint assay (R-Biopharm, Marshall, MI, USA; [22]). All samples were run in duplicate. Samples where both assays yielded a concentration that fell outside of the standard curve were excluded.
We quantified the extent of flight feather moult by scoring the nine innermost primaries, six secondaries, three tertials and six rectrices from the left side of all birds. We assigned each of the 24 feathers a moult score of 0–5 based on its stage of growth (0: old feather; 1: missing feather or pin; 2: less than 1/4 grown; 3: 1/4 to 1/2 grown; 4: 1/2–3/4 grown; 5: 3/4 grown to full feather). The average moult score of geolocator birds was 11.0 ± 2.4 (range 0–26; maximum possible 120) and nine of 10 individuals had no visible fat. Full flight feather moult in wood thrushes lasts an average of 38 days [13].
We obtained moult score and blood metabolite data for 20 individuals and 10 subsequently returned with geolocators. Four geolocator birds were not sampled during moult in August but we retrieved a tail feather after migration. We removed the third rectrix (tail feather) from returning birds to measure its rate of feather growth during the previous moult using daily growth bars; narrow growth bars indicate the feather grew slowly and thus a lower nutritional state of the bird [29]. We measured growth bar width by taking digital black and white images using a gel documentation system (Alpha-Innotech, San Leandro, CA, USA; [30]). A digital ruler was positioned at the centre of the most visible dark band in the image proximate to the base of the feather and the cumulative length of nine growth bars distal to it were measured. Growth bar width was the total length divided by the number of growth bars; each feather was measured three times and averaged.
3. Results
The date on which individuals first entered the tropics (23.4° N), about 2000 km south of the breeding site, spanned three weeks (9 October–1 November) and estimated arrival date on the winter territory was even more variable (14 October–6 December; figures 1 and 2). Autumn migration routes were similar as all birds remained east (less than 86° W) as they moved south through the USA, with 10 of 14 birds continuing south through Florida and Cuba while four crossed the Gulf of Mexico from Florida to Mexico. Most individuals (13 of 14) over-wintered in Honduras or Nicaragua, and flew an average of 3460 km (±126) to their winter territory.
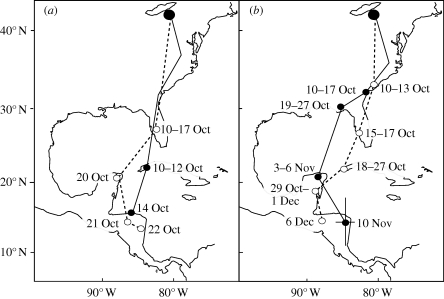
Figure 1.
Autumn migration of four male wood thrushes illustrating (a) two birds (solid versus dashed line) that crossed into the tropics (23.4° N) relatively early and arrived on the winter territory by 14 and 22 October, respectively, and (b) two relatively slow birds that were still north of 32° N on 10 October and arrived on the winter territory 10 November and 6 December, respectively. The daily location error, based on average standard deviation for individuals on the wintering grounds, is shown for one individual with error bars. Lines connect daily positions, but do not necessarily reflect actual flight paths.
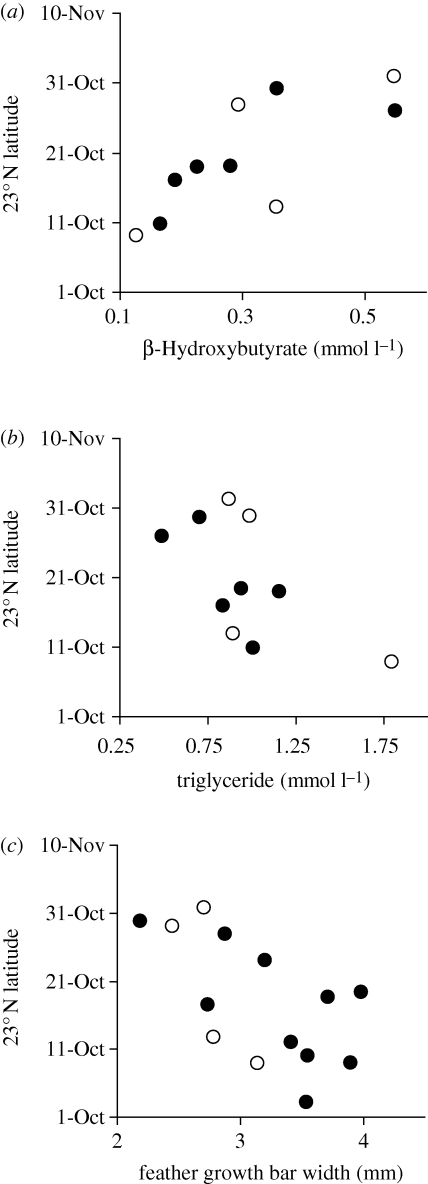
Figure 2.
Relationship between moult score in August and (a) completion date of the individual's last nest, (b) latitude on 10 October while migrating, and (c) date of arrival on winter territory. Open symbols are 1-year-old breeders, closed symbols are 2 years or older. (d) Arrival date on the winter territory versus estimated number of stopover days after 10 October, defined as individuals remaining in a region for one or more days after arrival. One individual that arrived its winter territory less than a week after 10 October was not included. (a) rs, −0.89; p, <0.001. (b) rs, −0.64; p, 0.047. (c) rs, −0.15; p, 0.67. (d) rs, 0.95; p, <0.001.
Nesting completion date spanned almost two months (2 July–24 August) and wood thrushes that nested late into the season produced more fledglings (Spearman rank correlation, rs = 0.62, n = 23, p = 0.002). Moult score in August was negatively correlated with nesting completion date (figure 2a) and individuals in an advanced stage of moult in mid-August were those that had finished nesting in early July. The departure date for autumn migration was unknown because latitude cannot be inferred from sunrise and sunset times within two weeks of the autumn equinox in September, and all our birds left the breeding site (41.8° N) during this period. By 10 October geolocator birds ranged in latitude from 16 to 36° N and birds that were farther north had lower moult scores when captured six to eight weeks earlier (figure 2b). Late moulting birds also tended to cross into the tropics later (rs = −0.52, n = 10, p = 0.13) but contrary to prediction did not arrive significantly later on their winter territory (figure 2c).
Individuals varied greatly in their pace of autumn migration after 10 October (110 ± 15.8 km d−1, range 42–212 km d−1). Seven of 14 birds had a regional stopover greater than 10 days long, and two of these birds stopped in the tropics greater than three weeks. For example, one male was in southern Florida 15–17 October, stopped in western Cuba for about 9 days and then stopped in northern Belize for about four weeks before completing migration (figure 1b). Early migrants were not more likely to spend more time on stopovers, as latitude on 10 October was not correlated with subsequent number of stopover days (rs = 0.11, n = 13, p = 0.73). Arrival date on the winter territory was determined largely by the cumulative number of stopover days (figure 2d) and was only weakly associated with an individual's latitude on 10 October (rs = 0.37, n = 14, p = 0.19).
Relatively poor post-breeding energetic condition was significantly correlated with later migration. Thushes with relatively high β-hydroxybutyrate and low triglyceride in August entered the tropics significantly later in October (figure 3a,b). As expected, plasma β-hydroxybutyrate concentration of individuals was negatively correlated with triglyceride concentration (rs = −0.51, n = 33, p = 0.003). Slow growth of tail feathers (narrow bars) was also strongly associated with later migration into the tropics (figure 3c). Post-breeding energetic condition showed similar, but in some cases non-significant, patterns with latitude on 10 October (β-hydroxybutyrate: rs = 0.64, p = 0.05; triglyceride: rs = −0.43, p = 0.21; growth bar: rs = 0.42, p = 0.14).
Figure 3.
Timing of migration into the tropics was correlated with an individual's post-breeding energetic condition in August as measured by (a) plasma β-hydroxybutyrate, (b) triglyceride and (c) growth bar width of the rectrix. Open symbols are 1-year-old breeders, closed symbols are 2 years or older. (a) rs, −0.78; p, 0.008. (b) rs, −0.64; p, 0.05. (c) rs, −0.62; p, 0.02.
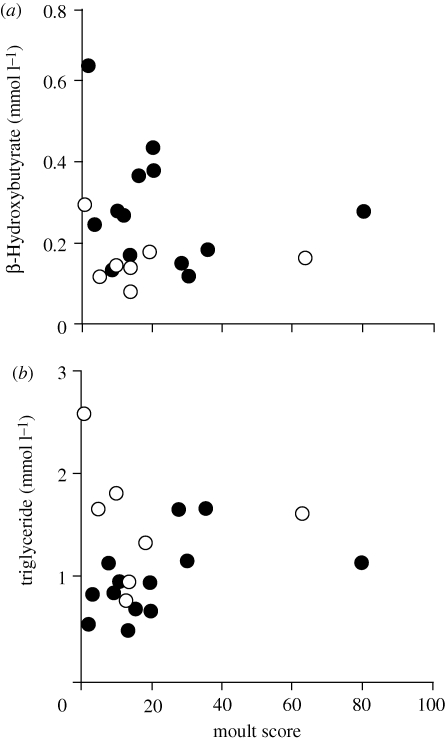
The relationship between blood metabolites and timing of migration could occur if metabolite profiles change with progression of moult; so we tested if high moult score was associated lower β-hydroxybutyrate and higher triglyceride. We also tested the prediction that high reproductive effort and late breeding reduce energetic condition during moult. We used a general linear model with metabolite concentration (log transformed) as the dependent variable, nest completion date, annual reproductive success (total number of fledglings) and moult score as covariates and age class as a random factor. For β-hydroxybutyrate the overall model was significant (figure 4a; F1,15 = 16.43, n = 21, p = 0.002; reproductive success: p = 0.61; last nest date: p = 0.31; moult score: p = 0.44; age class: p = 0.02). For triglyceride the overall model was not significant (figure 4b; whole model: F1,15 = 0.96, n = 21, p = 0.35; reproductive success: p = 0.74; last nest date: p = 0.21; moult score: p = 0.12; age class: p = 0.035). There was a significant age class effect and first-time breeders tended to have lower β-hydroxybutyrate and higher triglyceride than older birds.
Figure 4.
Relationship between post-breeding blood plasma metabolite concentration and moult score for thrushes captured during August (n = 20). Open symbols are 1-year-old breeders, closed symbols are 2 years or older.
4. Discussion
We tested for the first time if late nesting and moult imposes carry-over effects on timing of autumn migration and arrival on the winter territory. Late nesting wood thrushes had higher annual reproductive success and delayed moult and in early October, while en route to the tropics, were farther north suggesting that they probably departed the breeding grounds later. Despite this initial delay in autumn migration, late moulting birds did not arrive later on their winter territories.
Long-distance migration of birds is divided into alternating phases of stopover and flight, and overall migration speed is thought to be driven by fuel deposition rate at stopover sites [24,31]. Late breeding thrushes could, in theory, have mitigated time constraints imposed by late moult and migration departure by increasing fuel deposition rates and shortening stopover duration. However, birds that were farther north in early October did not subsequently increase their migration speed. Several individuals had long (greater than 14 days) stopovers on autumn migration, prior to or after entering the tropics, which were probably longer than necessary to simply refuel [20]. Mortality during migration can be high [32] and prolonged stopovers could benefit individuals by reducing short-term mortality risks via improved flight performance, lower predation or reduced physiological costs of endurance flights [33,34].
Strong seasonal carry-over effects occur during spring migration in American redstarts (Setophaga ruticilla) because poor winter territory quality delays spring migration [1] and late arrival on the breeding grounds reduces reproductive success [2,4]. For species that defend individual winter territories, rapid autumn migration and early arrival at the destination could be under strong selection if early-arriving individuals can better compete for high-quality winter territories that, in turn, enhance survival and body condition [3,16,26]. Norris et al. [5] found that male redstarts with high late season reproductive effort began migration prior to completing moult. We also found carry-over effects of late moult into early migration, but the prolonged stopovers are not consistent with strong selection for early arrival on the winter territory. It is not known if later arrival of experienced birds, as demonstrated here, compromises an individual's ability to re-claim its former winter territory or reduces survival [26].
Post-breeding energetic condition, while still on the breeding grounds, was strongly correlated with the timing of entry into the tropics two months later, and 2000 km away. Energetic condition affects day-to-day decisions of migrants to stay versus fly once the trip is underway [21–23] but why would post-breeding condition be related to a bird's position two months later? β-Hydroxybutyrate typically decreases, and triglyceride increases, as moult progresses and onset of migration approaches [17]. Thus individuals with low β-hydroxybutyrate and high triglyceride in mid-August could simply be those who finished nesting early and were in a more advanced state of moult. However, variation in post-breeding plasma metabolite concentration in wood thrushes was not significantly related to nest completion date or stage of moult. Although parental effort can have energetic carry-over effects on the moult period [18,19], post-breeding energetic condition was also not related to prior number of young fledged. Consumption of high-protein food is an important determinant of energetic condition and fat storage in birds with a flexible diet [35] so habitat quality at the moulting site may have an important influence on post-breeding energetic condition and hence timing of migration.
However, given the prolonged autumn migration of wood thrush it is difficult to view their overall autumn migration speed, and timing of entry into the tropics, as constrained by post-breeding energetic condition two months earlier. We suggest the possibility that some individuals prepare to migrate more rapidly than others by investing more heavily in fat storage during the early stages of moult. Geolocator tracking now allows carry-over effect hypotheses for autumn migration to be tested with experimental manipulation of timing of breeding, reproductive effort and energetic condition. The selective advantages of fast versus slow migration remain to be determined and will require monitoring physiological condition, territory acquisition and survival of geolocator birds after they arrive on the wintering grounds.
Acknowledgements
We thank C. Guglielmo for use of his physiology laboratory at the University of Western Ontario. Field assistance was provided by M. Brady, R. Kresnik, T. Piraino, and C. Stanley and many volunteers. Funding was from the Natural Sciences and Engineering Research Council of Canada, the National Geographic Society, Molson Foundation and proceeds from Silence of the Songbirds (Stutchbury 2007, Walker & Co.).
References
- 1.Marra P. P., Hobson K. A., Holmes R. T.1998Linking winter and summer events in a migratory bird by using stable carbon isotopes. Science 282, 1884–1886 10.1126/science.282.5395.1884 (doi:10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 2.Norris D. R., Marra P. P., Kyser T. K., Sherry T. W., Ratcliffe L. M.2004aTropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. R. Soc. Lond. B 271, 59–64 10.1098/rspb.2003.2569 (doi:10.1098/rspb.2003.2569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelier F., Holberton R. L., Marra P. P.2009Does stress response predict return rate in a migratory bird species? A study of American redstarts and their non-breeding habitat. Proc. R. Soc. B 276, 3545–3551 10.1098/rspb.2009.0868 (doi:10.1098/rspb.2009.0868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reudink M. W., Marra P. P., Kyser T. K., Boag P. T., Langin K. M., Ratcliffe L. M.2009Non-breeding season events influence sexual selection in a long-distance migratory bird. Proc. R. Soc. B 276, 1619–1626 10.1098/rspb.2008.1452 (doi:10.1098/rspb.2008.1452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris D. R., Marra P. P., Mongomerie R., Kyser T. K., Ratcliffe L. M.2004bReproductive effort, moulting latitude, and feather color in a migratory songbird. Science 306, 2249–2250 10.1126/science.1103542 (doi:10.1126/science.1103542) [DOI] [PubMed] [Google Scholar]
- 6.Stutchbury B. J. M., Tarof S. A., Done T., Gow E., Kramer P., Tautin J., Fox J. W., Afanasyev V.2009Tracking long-distance songbird migration using geolocators. Science 323, 896. 10.1126/science.1166664 (doi:10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 7.Siikamaki P., Hovi M., Ratti O.1994A trade-off between current reproduction and moult in the pied flycatcher—an experiment. Funct. Ecol. 8, 587–593 10.2307/2389919 (doi:10.2307/2389919) [DOI] [Google Scholar]
- 8.Evans Ogden L. J., Stutchbury B. J. M.1996Constraints on double brooding in a neotropical migrant, the hooded warbler. Condor 98, 736–744 10.2307/1369855 (doi:10.2307/1369855) [DOI] [Google Scholar]
- 9.Mulvihill R. S., Latta S. C., Newell F. L.2009Temporal constraints on the incidence of double brooding in the Lousiana waterthrush. Condor 111, 341–348 10.1525/cond.2009.080037 (doi:10.1525/cond.2009.080037) [DOI] [Google Scholar]
- 10.Cyr N. E., Wikelski M., Romero L. M.2008Increased energy expenditure but decreased stress responsiveness during moult. Physiol. Biochem. Zool. 81, 452–462 10.1086/589547 (doi:10.1086/589547) [DOI] [PubMed] [Google Scholar]
- 11.Bonier F., Martin P. R., Jensen J. P., Butler L. K., Ramenofsky M., Wingfield J. C.2007Pre-migratory life history stages of juvenile arctic birds: costs, constraints, and trade-offs. Ecology 88, 2729–2735 10.1890/07-0696.1 (doi:10.1890/07-0696.1) [DOI] [PubMed] [Google Scholar]
- 12.Holmgren N., Hedenström A.1995The scheduling of molt in migratory birds. Evol. Ecol. 9, 354–368 10.1007/BF01237759 (doi:10.1007/BF01237759) [DOI] [Google Scholar]
- 13.Vega Rivera J. H., McShea W. J., Rappole J. H., Haas C.1998Pattern and chronology of prebasic molt for the wood thrush and its relation to reproduction and migration departure. Wilson Bull. 110, 384–392 [Google Scholar]
- 14.Marra P. P.2000The role of behavioral dominance in structuring patterns of habitat occupancy in a migrant bird during the nonbreeding season. Behav. Ecol. 11, 299–308 10.1093/beheco/11.3.299 (doi:10.1093/beheco/11.3.299) [DOI] [Google Scholar]
- 15.Johnson M. D., Sherry T. W., Holmes R. T., Marra P. P.2006Assessing habitat quality for a migratory songbird wintering in natural and agricultural habitats. Conserv. Biol. 20, 1433–1444 10.111/j.1523-1739.2006.00490.X (doi:10.111/j.1523-1739.2006.00490.X) [DOI] [PubMed] [Google Scholar]
- 16.Brown D. R., Sherry T. W.2008Alternative strategies of space use and response to resource change in a wintering migrant songbird. Behav. Ecol. 19, 1314–1325 10.1093/beheco/arn073 (doi:10.1093/beheco/arn073) [DOI] [Google Scholar]
- 17.Jenni-Eiermann S., Jenni L.1996Metabolic differences between the post-breeding, molting and migratory periods in feeding and fasting passerine birds. Funct. Ecol. 10, 62–72 10.2307/2390263 (doi:10.2307/2390263) [DOI] [Google Scholar]
- 18.Hõrak P., Jenni-Eiermann S., Ots I.1999Do great tits (Parus major) starve to reproduce? Oecologia 119, 293–299 [DOI] [PubMed] [Google Scholar]
- 19.Kern M., Bacon W., Long D., Cowie R. J.2005Blood metabolite and corticosterone levels in breeding adult pied flycatchers. Condor 107, 665–677 10.1650/0010-5422(2005)107[0665:BMACLI]2.0.CO;2 (doi:10.1650/0010-5422(2005)107[0665:BMACLI]2.0.CO;2) [DOI] [Google Scholar]
- 20.Yong W., Moore F.1997Spring stopover of intercontinental migratory thrushes along the northern coast of the Gulf of Mexico. Auk 114, 263–278 [Google Scholar]
- 21.Goymann W., Spina F., Ferri A., Fusani L.2010Body fat influences departure from stopover sites in migratory birds: evidence from whole-island telemetry. Biol. Lett. 10.1098/rsbl.2009.1028 (doi:10.1098/rsbl.2009.1028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglielmo C. G., Cerasale D. J., Eldermire C.2005A field validation of plasma metabolite profiling to assess refueling performance of migratory birds. Physiol. Biochem. Zool. 78, 116–125 10.1086/425198 (doi:10.1086/425198) [DOI] [PubMed] [Google Scholar]
- 23.Fusani L., Cardinale M., Carere C., Goymann W.2009Stopover decision during migration: physiological conditions predict nocturnal restlessness in wild passerines. Biol. Lett. 5, 302–305 10.1098/rsbl.2008.0755 (doi:10.1098/rsbl.2008.0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedenström A.2008Adaptations to migration in birds: behavioural strategies, morphology and scaling effects. Phil. Trans. R. Soc. B 363, 287–299 10.1098/rstb.2007.2140 (doi:10.1098/rstb.2007.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyle P., Howell S. N. G., DeSante R. P., Yunick R. P., Gustafson M.1997Identification guide to North American passerines. Bolinas, CA: Slate Creek Press [Google Scholar]
- 26.Rappole J. H., Ramos M. A., Winker K.1989Wintering wood thrush movements and mortality in southern Veracruz. Auk 106, 402–410 [Google Scholar]
- 27.Jenni-Eiermann S., Jenni L.1994Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the garden warbler. Auk 112, 888–899 [Google Scholar]
- 28.Guglielmo C. G., O'Hara P. D., Williams T. D.2002Extrinsic and intrinsic sources of variation in plasma lipid metabolites of free-living western sandpipers (Calidris mauri). Auk 119, 437–445 10.1642/0004-8038(2002)119[0437:EAISOV]2.0.CO;2 (doi:10.1642/0004-8038(2002)119[0437:EAISOV]2.0.CO;2) [DOI] [Google Scholar]
- 29.Grubb T. C., Jr1989Ptilochronology: feather growth bars as indicators of nutritional status. Auk 106, 314–320 [Google Scholar]
- 30.Shawkey M. D., Beck M. L., Hill G. E.2003Use of a gel documentation system to measure feather growth bars. J. Field Orn. 74, 125–128 [Google Scholar]
- 31.Schaub M., Jenni L.2001Variation of fuelling rates among sites, days and individuals in migrating passerine birds. Funct. Ecol. 15, 584–594 10.1046/j.0269-8463.2001.00568.x (doi:10.1046/j.0269-8463.2001.00568.x) [DOI] [Google Scholar]
- 32.Sillett T. S., Holmes R. T.2002Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71, 296–308 10.1046/j.1365-2656.2002.00599.x (doi:10.1046/j.1365-2656.2002.00599.x) [DOI] [Google Scholar]
- 33.Schwilch R., Grattarola A., Spina F., Jenni L.2002Protein loss during long-distance migratory flight in passerine birds: adaptation and constraint. J. Exp. Biol. 205, 687–695 [DOI] [PubMed] [Google Scholar]
- 34.Owen J. C., Moore F. R.2006Seasonal differences in immunological condition of three species of thrushes. Condor 108, 389–398 10.1650/0010-5422(2006)108[389:SDIICO]2.0.CO;2 (doi:10.1650/0010-5422(2006)108[389:SDIICO]2.0.CO;2) [DOI] [Google Scholar]
- 35.Smith S. B., McWilliams S. R., Guglielmo C. G.2007Effect of diet composition on plasma metabolite profiles in a migratory songbird. Condor 109, 48–58 10.1650/0010-5422(2007)109[48:EODCOP]2.0.CO;2 (doi:10.1650/0010-5422(2007)109[48:EODCOP]2.0.CO;2) [DOI] [Google Scholar]