Abstract
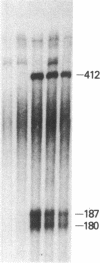
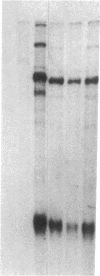
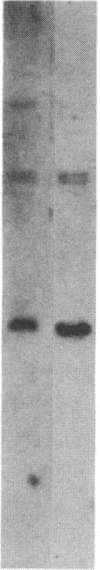
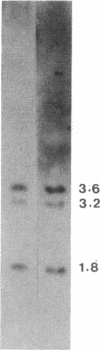
We examined the expression of the alpha subunit of the stimulatory GTP-binding protein (Gs) in fibroblasts of subjects with pseudohypoparathyroidism (PHP) type Ia by transfer blot hybridization and S1 nuclease analyses. Six subjects with PHP type Ia showed decreased steady-state content of Gs alpha mRNA. S1 nuclease analysis indicates that both long and short forms of Gs alpha mRNA are decreased, with no apparent change in the ratio of long to short forms in PHP compared with normal individuals. It appears likely that in some cases of PHP type Ia the genetic lesion affects the maintenance of mRNA levels for all forms of the Gs alpha subunit.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita Y., Saito T., Yajima Y., Sakuma S. The stimulatory and inhibitory guanine nucleotide-binding proteins of adenylate cyclase in erythrocytes from patients with pseudohypoparathyroidism type I. J Clin Endocrinol Metab. 1985 Dec;61(6):1012–1017. doi: 10.1210/jcem-61-6-1012. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Beiderman B., Steinberg F., Brothers V. M. Three adenylate cyclase phenotypes in S49 lymphoma cells produced by mutations of one gene. Mol Pharmacol. 1982 Jul;22(1):204–210. [PubMed] [Google Scholar]
- Bourne H. R., Kaslow H. R., Brickman A. S., Farfel Z. Fibroblast defect in pseudohypoparathyroidism, type I: reduced activity of receptor-cyclase coupling protein. J Clin Endocrinol Metab. 1981 Sep;53(3):636–640. doi: 10.1210/jcem-53-3-636. [DOI] [PubMed] [Google Scholar]
- Bray P., Carter A., Simons C., Guo V., Puckett C., Kamholz J., Spiegel A., Nirenberg M. Human cDNA clones for four species of G alpha s signal transduction protein. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8893–8897. doi: 10.1073/pnas.83.23.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase L. R., Melson G. L., Aurbach G. D. Pseudohypoparathyroidism: defective excretion of 3',5'-AMP in response to parathyroid hormone. J Clin Invest. 1969 Oct;48(10):1832–1844. doi: 10.1172/JCI106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Downs R. W., Sekura R. D., Levine M. A., Spiegel A. M. The inhibitory adenylate cyclase coupling protein in pseudohypoparathyroidism. J Clin Endocrinol Metab. 1985 Aug;61(2):351–354. doi: 10.1210/jcem-61-2-351. [DOI] [PubMed] [Google Scholar]
- Drezner M., Neelon F. A., Lebovitz H. E. Pseudohypoparathyroidism type II: a possible defect in the reception of the cyclic AMP signal. N Engl J Med. 1973 Nov 15;289(20):1056–1060. doi: 10.1056/NEJM197311152892003. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Downs R. W., Jr, Moses A. M., Breslau N. A., Marx S. J., Lasker R. D., Rizzoli R. E., Aurbach G. D., Spiegel A. M. Resistance to multiple hormones in patients with pseudohypoparathyroidism. Association with deficient activity of guanine nucleotide regulatory protein. Am J Med. 1983 Apr;74(4):545–556. doi: 10.1016/0002-9343(83)91008-2. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Downs R. W., Jr, Singer M., Marx S. J., Aurbach G. D., Spiegel A. M. Deficient activity of guanine nucleotide regulatory protein in erythrocytes from patients with pseudohypoparathyroidism. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1319–1324. doi: 10.1016/0006-291x(80)90563-x. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Eil C., Downs R. W., Jr, Spiegel A. M. Deficient guanine nucleotide regulatory unit activity in cultured fibroblast membranes from patients with pseudohypoparathyroidism type I. a cause of impaired synthesis of 3',5'-cyclic AMP by intact and broken cells. J Clin Invest. 1983 Jul;72(1):316–324. doi: 10.1172/JCI110971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R., Codina J., Crozat A., Kidd V., Woo S. L., Birnbaumer L. Identification by molecular cloning of two forms of the alpha-subunit of the human liver stimulatory (GS) regulatory component of adenylyl cyclase. FEBS Lett. 1986 Sep 29;206(1):36–42. doi: 10.1016/0014-5793(86)81336-9. [DOI] [PubMed] [Google Scholar]
- Naya-Vigne J., Johnson G. L., Bourne H. R., Coffino P. Complementation analysis of hormone-sensitive adenylate cyclase. Nature. 1978 Apr 20;272(5655):720–722. doi: 10.1038/272720a0. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]
- Schleifer L. S., Garrison J. C., Sternweis P. C., Northup J. K., Gilman A. G. The regulatory component of adenylate cyclase from uncoupled S49 lymphoma cells differs in charge from the wild type protein. J Biol Chem. 1980 Apr 10;255(7):2641–2644. [PubMed] [Google Scholar]
- Seiler-Tuyns A., Birnstiel M. L. Structure and expression in L-cells of a cloned H4 histone gene of the mouse. J Mol Biol. 1981 Oct 5;151(4):607–625. doi: 10.1016/0022-2836(81)90426-5. [DOI] [PubMed] [Google Scholar]
- Silverman S. J., Rose M., Botstein D., Fink G. R. Regulation of HIS4-lacZ fusions in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1212–1219. doi: 10.1128/mcb.2.10.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dop C., Bourne H. R. Pseudohypoparathyroidism. Annu Rev Med. 1983;34:259–266. doi: 10.1146/annurev.me.34.020183.001355. [DOI] [PubMed] [Google Scholar]