Abstract
Whales are unique among vertebrates because of the enormous oil reserves held in their soft tissue and bone. These ‘biofuel’ stores have been used by humans from prehistoric times to more recent industrial-scale whaling. Deep-sea biologists have now discovered that the oily bones of dead whales on the seabed are also used by specialist and generalist scavenging communities, including many unique organisms recently described as new to science. In the context of both cetacean and deep-sea invertebrate biology, we review scientific knowledge on the oil content of bone from several of the great whale species: Balaenoptera musculus, Balaenoptera physalus, Balaenoptera borealis, Megaptera novaeangliae, Eschrichtius robustus, Physeter macrocephalus and the striped dolphin, Stenella coeruleoalba. We show that data collected by scientists over 50 years ago during the heyday of industrial whaling explain several interesting phenomena with regard to the decay of whale remains. Variations in the lipid content of bones from different parts of a whale correspond closely with recently observed differences in the taphonomy of deep-sea whale carcasses and observed biases in the frequency of whale bones at archaeological sites.
Keywords: bioerosion, Osedax, whale-fall, marine mammals
1. Introduction
Humans have hunted whales for centuries, obtaining food and a wide range of commodities from their carcasses. In the nineteenth century, one commodity in particular spurred whaling into a booming industry, namely whale oil. Initially obtained for domestic and street lighting, its uses diversified to include lubricants, margarine and even the manufacture of explosives. In this review, we reveal how forgotten data from this whaling industry can shed new light on life in the deep sea and past human civilizations.
As whale fisheries expanded in the twentieth century, and bone ‘biofuel’ became a major industry, biologists started to speculate as to how animals in the food-poor deep sea might benefit from the bounty of sunken whale carcasses (e.g. [1,2]). Only in recent decades have these questions been answered with the discovery that whale carcasses arriving on the deep-sea floor are hot spots for biological diversity, the food they provide equivalent to 2000 years of normal background detritus per square metre [3]. This food bonanza quickly attracts scavengers and opportunists that feed on the abundant labile organic matter.
Following the removal of soft tissues (which can take up to 2 years), the exposed whale skeletons can support specialized communities for several decades [4,5]. The surprising longevity of this habitat appears to be a result of the high lipid content of the whale skeleton, which is broken down by bacteria to provide a continual flux of sulphides into the surrounding sea water and sediment [6,7]. The sulphides fuel the chemoautotrophic symbionts of the fauna living on or around it, including specialist bivalve molluscs and vestimentiferan tubeworms. These organisms have close relatives in hydrothermal vent and cold-seep chemosynthetic communities, and there is good evidence to suggest that whale carcasses have acted as dispersal and evolutionary stepping stones between these habitats [8,9]. Thus, the chemosynthetic fauna and the time that a skeleton will be able to sustain them may be dependant on its lipid content.
Some members of these ‘whale-fall’ communities use the whale bones directly for food. The sipunculan worm Phascolosoma saprophagicum is known to feed on lipids in the bones [10], while osteopeltid limpets [11] graze bacteria that grow on the bone tissue. Members of the polychaete worm genus Osedax degrade the bone matrix to obtain nutrition, aided by endosymbiotic heterotrophic bacteria [12,13].
By supporting such trophically diverse fauna in a globally distributed habitat, whale carcasses may have played important roles in maintaining deep-sea diversity [14,15] and facilitating adaptive radiations of sulphophilic fauna in the deep-sea environment. The long-lasting skeletons serve as a primary source of energy for these communities; yet, information regarding their organic content is poorly appreciated. The disparate data cited in the recent literature have appeared conflicting and are certainly not comprehensive (table 1). For example, Glover et al. [16] bemoan ‘the current poor knowledge both of marine vertebrate taphonomy and perhaps crucially, the food quality of the bones themselves’. Indeed, many of the estimates seem to be based on anecdotal evidence from whalers.
Table 1.
Data on the percentage composition of cetacean bone.
| species | skeletal element | values % |
reference | |||
|---|---|---|---|---|---|---|
| water | ash | protein | lipid | |||
| bone tissue | ||||||
| P. macrocephalus | vertebrae | 26 | 34 | 40a | [17] | |
| B. physalus | ribs | 32 | 31 | 36a | [17] | |
| B. physalus | fin bone | 7.3 | — | 28a | [53] | |
| B. physalus | tympanic bulla | 4.38 | 92.50 | 3.09a | [54] | |
| B. physalus | tympanic bulla | 4.4 | — | 8.7a | [53] | |
| B. physalus | tympanic bulla | 5.3 | — | 9.3a | [53] | |
| Tursiops sp. | petrosal bulla | 1.70 | 95.98 | 1.00a | [54] | |
| Tursiops and Delphinus sp. | — | 41.5 | 30 | 25 | 3.3 | [25] |
| bone organ | ||||||
| P. macrocephalus | vertebrae | 70 | 10 | 4 | 9 | [17] |
| B. physalus | ribs | 40 | 13 | 13 | 27 | [17] |
| Balaenoptera sp. | vertebra | — | — | — | ≤65b | [6] |
| Balaenoptera sp. | vertebrae | — | — | — | 44 | [5] |
aThis value is for the ‘total organic fraction’.
bHighest value measured in the centre of the bone. See text for explanation of bone tissue and bone organ.
There is, however, a detailed set of data available in the literature on the composition of whale bones, most of which dates from the middle of the twentieth century. It is hardly surprising that these data should exist considering the importance of the whaling industry in the nineteenth and twentieth centuries, but with the decline of this industry, much of the data have been forgotten or omitted from modern reference databases.
This review paper will cover the available literature concerning the composition of large whale skeletons, particularly the oil content of their bones. By synthesizing the wide range of data, it is possible to identify and explain patterns in the composition of skeletal elements. We compare these patterns with previous observations from whale falls to generate novel hypotheses that link bone oil content to whale skeletal taphonomy, whale-fall community structure and deep-sea biology. We also highlight the utility of these data for analysing the habits of ancient whaling populations that may have used bones as fuel.
2. What are whale bones made of?
(a). Data from the whale fisheries
It is important to begin by recognizing a distinction between two types of bone measurements that occur in the literature (table 1). First, there are those that describe the composition of bone tissue itself, i.e. the solid structural material composed of bone cells. Second, there are those authors who treat the bone as a complete organ, including in their measurements the marrow and interstitial material found among the bone matrix. Tont et al. [17] describe this distinction as an ‘obvious prerequisite for intercomparison of results by different workers’, yet subsequent authors have failed to make this clear. The majority of detailed measurements found in the literature are those that treat the bone as an organ, because it was not the bone tissue itself that was of interest to the whale fishermen, but the oil held within them.
One of the first substantial attempts to quantify the oil content of different skeletal elements was by Heyerdahl [18] who measured the composition of whale bones at whaling stations in the Southern Ocean. Feltmann et al. [19] aimed to build on this and carried out a more detailed analysis of the oil content alone, though in fewer individuals (table 2). The two datasets are the only ones that provide information from the prized blue whales and were the first attempts to quantify the enormous biofuel reserves of these giant creatures.
Table 2.
Lipid content of various bones for blue (Balaenoptera musculus) and fin whales (B. physalus) [19].
| species | fin | blue | blue | blue | blue | blue | blue |
|---|---|---|---|---|---|---|---|
| length (m) | 17.7 | 23.8 | 26.5 | 27.1 | 25.6 | 23.8 | 24.7 |
| sex | M | M | F | F | F | M | F |
| pregnant | — | — | yes | no | no | — | no |
| time dead (h) | 7 | 11 | 26 | 52 | 3 | 15 | 3 |
| skeletal element | |||||||
| lower jaw | 73.4 | 84.2 | |||||
| rostrum of skull | 65.4 | 51.4 | 81.6 | 67.5 | |||
| scapula | 69.3 | ||||||
| rib | 32.0 | ||||||
| thoracic vertebra | 3.4 | 20.2 | 7.2 | 24.0 | |||
| lumbar vertebra (ant.) | 67.0 | 18.4 | 49.2 | 27.3 | 54.0 | ||
| lumbar vertebra (post.) | 60.6 | ||||||
| caudal vertebra | 52.0 | 53.9 | 62.3 | 38.5 | |||
| humerus | 64 | ||||||
| radius and ulna (cancellous bone) | 69 | 63 | |||||
| metacarpal bones | 60 | ||||||
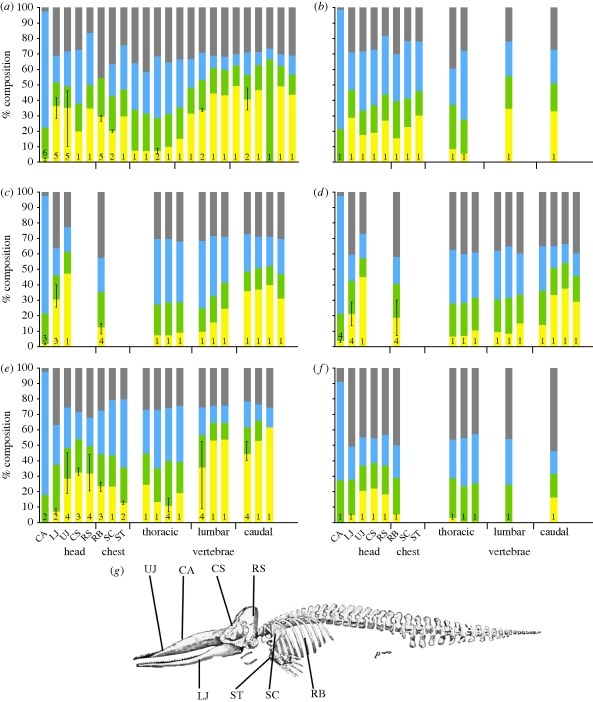
The two most systematic approaches to measuring the composition of whale skeletons are provided by Watanabe & Suzuki [20,21] and Kharkof [22], working aboard the industrial factory ships. The data from these two sources, working independently of one another, have been combined in figure 1a–e to produce composition profiles for the skeletons of the fin whale (Balaenoptera physalus), sei whale (Balaenoptera borealis), humpback whale (Megaptera novaeangliae), grey whale (Eschrichtius robustus) and the sperm whale (Physeter macrocephalus). Both sampled a similar range of skeletal elements, facilitating combination of the data. The authors do not detail the methods used, but the concordance of data strongly suggests that they were obtained in similar ways. Measurements from the vertebral column were consistently taken from four specific vertebrae by Watanabe & Suzuki [20,21], while Kharkof [22] divided the spine into equal sections and sampled each section. In figure 1, the data of both authors have been combined by assigning measurements from specific vertebrae to the corresponding sections of Kharkof [22]. Thus, when measurements of cervical vertebrae have been taken, they are combined with those of the first thoracic segment. A small amount of data (not figured) on the bone composition of the beaked whale (Berardius bairdii) and the killer whale (Orcinus orca) are also provided by Kharkof [22].
Figure 1.
Skeletal composition profiles for several species showing the per cent composition (lipid, yellow; protein, green; water, blue; ash, grey) of different skeletal elements. The number of individuals sampled is indicated at the base of each bar. Error bars show maximum and minimum values of lipid content in the datasets. (a) fin whale, B. physalus; (b) sei whale, B. borealis; (c), humpback whale, M. novaeangliae; (d) grey whale, E. robustus; (e) sperm whale, P. macrocephalus; (f) striped dolphin, S. coeruleoalba. (g) Diagram of a sperm whale skeleton illustrating the approximate sample locations. CA, cartilage; LJ, lower jaw; UJ, upper jaw; CS, central skull; RS, rear skull; RB, rib; SC, scapula; ST, sternum.
A comparable range of skeletal parts was surveyed by Honda et al. [23,24] in the striped dolphin (Stenella coeruleoalba) (figure 1f). Dolphins were not hunted for their oil, with the exception of that found in the lower jaw [25], because of their small size.
(b). The distribution of lipids in whale skeletons
Data from these whaling sources reveal marked differences in the composition of bones from different parts of the whale skeleton (figure 1). Even bones of a similar structure such as vertebrae show 30–40% differences in lipid content between different parts of the spine. There are also clearly taxonomic differences in the composition of bones (figure 1), but general trends are apparent across the datasets outlined above.
In the great whales (figure 1a–e), the lower jaw contains approximately 30 per cent lipid in the rorquals, dropping to only 20 per cent in the grey whale and even lower in the odontocete sperm whale (7.2%). From the tip of the upper jaw, there is an increase in lipid content towards the rear of the skull in every species. Kharkof [22] details this posteriorly progressive increase in lipid content by sampling the front (approx. 23%), middle (approx. 32%) and back (approx. 47%) of the jaw bones; however, he did not measure the skull bones. The anomalously low oil content for the fin whale upper jaw and skull bones comes from a single specimen measured by Watanabe & Suzuki [21], which showed an overall lower lipid content than other species.
Proceeding down the vertebral column, the lipid content decreases in the cervical vertebrae and continues to decrease in the thoracic vertebrae, which is usually less than 10 per cent. The lipid content then increases greatly in the lumbar vertebrae and caudal vertebrae. In the fin, sei and sperm whales, this increase up to a level of approximately 50 per cent lipid occurs sharply in the lumbar vertebrae, while in the humpback and grey whales, the increase is more gradual, with the caudal vertebrae only reaching 40 per cent lipid content.
The bones of the chest region (scapula, sternum and ribs) are generally intermediate between the skull and the thoracic vertebrae in lipid content, ranging from 15 to 30 per cent lipid in most cases. The ribs of the humpback and grey whale appear to be lower in lipid content (approx. 10%), but this may be owing to high variation between specimens (figure 1d).
The values of lipid content of dolphin bones are markedly lower than those for larger cetaceans (average 9%, maximum 22%), but still show a similar pattern. It is difficult to know whether the lower values are owing to the different sampling methods used [23] or because there is a disproportionate decrease in the lipid content of bone with decreasing size of the species (figure 2). Measurements were taken from a wide age range of specimens and show that lipid content of bones sharply increases after birth and continues to do so until adult maturity (8 years for the dolphin). This trait is probably ubiquitous among cetaceans [19].
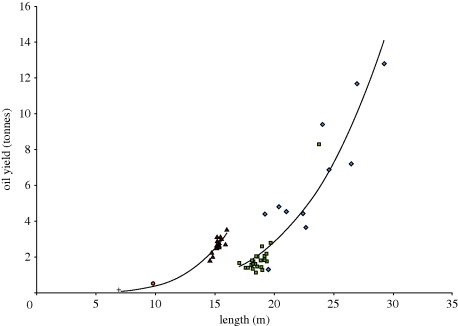
Figure 2.
Oil yield of skeletons for several whale species: Balaenoptera musculus (blue, diamonds), B. physalus (fin, squares), P. macrocephalus (sperm, triangles), B. bairdii (bottlenose, circles) and O. orca (killer, plus symbols). The regression line for odontocete species is defined by the equation y = 9.28 × 10−6 x4.61 (r2 = 0.91; p > 0.001) and that for the rorquls is given by y = 9.25 × 10−6 x4.22 (r2 = 0.87; p > 0.001), where y is oil yield in tonnes and x is whale length in metres. Data of oil yield from flensed carcasses were obtained from Heyerdahl [18] and Tomlin [29]. These values were multiplied by 0.45 to account for oil from the meat on the skeleton, according to Lund [55].
(c). Ontogenetic and physiological changes in whale bone composition
Almost every worker who has looked at whale bone composition has noted the substantial increase in oil content from the thoracic vertebrae to the caudal vertebrae. Heyerdahl [18] first observed that those vertebrae which were ‘blood stained’ had contained lower levels of oil and attributed this to puncturing of the bone during harpooning. Feltmann et al. [19] pointed out that this blood staining was in fact red bone marrow, sites of blood cell production in mammals, and it was the marrow type that determined oil content of the bones. Conversely, those bones filled with yellow fatty marrow were the ones with high oil content. Ohe [26] showed that the distribution of red bone marrow coincided exactly with those parts of the skeleton that had a low oil content. In young whales, even the caudal vertebrae contain red marrow, but this is gradually replaced by yellow marrow as the whale ages, starting in the caudal vertebrae and progressing cranially, reflected by the data of Honda et al. [23,24]. Thus, the low oil content of a vertebra reported by Tont et al. [17] is almost certainly because it was filled with red marrow, indicated by the footnote explaining that it contained ‘a large amount of blood’. This also accounts for the large variability in the lipid content of lumbar vertebrae shown in figure 1 and table 2. The lumbar vertebrae of younger whales may still contain red marrow, while those of older specimens will be filled with fatty yellow marrow.
Intuitively, the structure of the bone will also affect its composition. Bones that are primarily spongy in structure will have a greater capacity to contain oil-rich marrow than bones with higher tissue densities. The density of bone tissue decreases from the outer cortical layer of each bone to the inner cancellous bone [27,28]. The effect that this can have on the measurement of bone composition is illustrated by Heyerdahl [18] who measured oil content in samples taken from the outer part and inner part of a vertebra and found that they differed by 17 per cent. Consequently, the values of lipid content provided for the ‘spongosia’ of limb bones [19] and the centre of a vertebra [6] cannot be considered representative of the entire bone.
Seasonal changes in the physiology and feeding of the whales will affect their fat stores, especially in the mysticetes. This is evident in the records of whales captured during different seasons, where the oil productivity per whale may increase by up to 30 per cent [29]. The oil obtained from whale carcasses varied after the blubber had been removed (figure 2), suggesting that the bones and flesh were also affected by these seasonal changes. Furthermore, ‘it has long been known from the whaling practice that pregnant females are much fatter than barren or lactating ones’ [29], a fact demonstrated quantitatively by Lockyer [30]. Many of the record yields of oil have come from large pregnant females. Feltmann et al. [19] measured 84 per cent oil content for the mandible of a blue whale, which seems anomalously high even within their dataset, and may be because this particular whale was pregnant (table 2).
Post-mortem changes may further complicate the measurement of bone composition. Feltmann et al. [19] measured a substantial decrease (approx. 50% in some bones) in the fat content of bones for a whale that had been dead for 52 h in comparison to a nearly identical one that had been dead for only 3 h (table 2). This is especially true of the thoracic and lumbar vertebra where, ‘all the bone marrow has disappeared; the meshes of the spongy bone (were) empty’ [19]. The decrease in fat content is matched by a proportional increase in the percentage of fatty acids in the bone, suggesting decomposition of the large-molecule fats to smaller fatty acids.
3. Whale bones as biofuel
These data have been unnoticed for half a century, their obscurity resulting in few authors considering their potential relevance. This previously unrecognized heterogeneity in whale bone lipid content corresponds to several features of whale-fall communities and fossil whale-bone assemblages, suggesting a modern significance.
(a). Do oily bones make better homes in the deep sea? The oil-gradient hypothesis
Whale carcasses represent the largest, most nutritious food parcels reaching the deep-sea floor and as such create their own unique habitats [31]. Following the exhaustion of soft tissues by mobile scavengers (e.g. hagfish, crabs), a chemosynthesis-based community develops in which the primary source of sulphides is from the bacterial degradation of lipids in the bones [32]. It follows that abundance of sulphophilic organisms in late-stage whale-fall communities may be expected to correspond to the changes in oil throughout the whale skeleton over time (oil-gradient hypothesis). In older whale-fall communities, the bulk of the biomass would be expected to be located on the skull and posterior vertebrae—the most oil-rich and thus, sulphide-rich bones. Since the differences in bone composition have not been previously recognized, little attention has been paid to the distribution of fauna on the whale skeletons reported in the literature. However, sulphur-oxidizing bacterial mats, which form the basis of the grazing food chain on whale falls, may provide a useful proxy to the sulphide output of the bones (e.g. [7]).
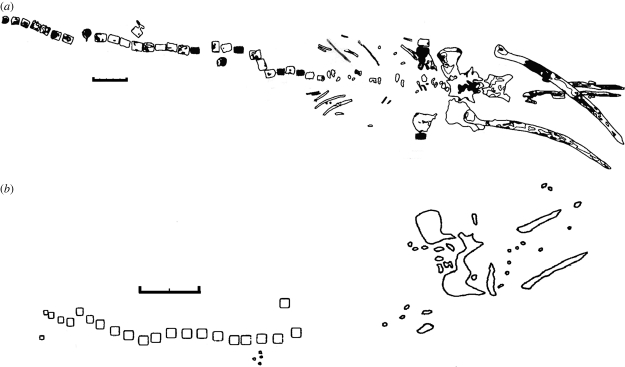
The first whale fall discovered and one of the oldest to be studied is known from the Santa Catalina Basin off California [8]. On this skeleton, which was estimated to be 44 years old at the time of sampling [5], mats of ‘sulphur bacteria’ were evident on most of the bones, ‘except that they were largely absent from the buried or highly degraded ribs and vertebrae of the thoracic region’ [33]. In addition, regions of the skull and caudal vertebrae seem to be the most covered in bacterial mats (figure 3). This pattern of bacterial mat coverage strongly mirrors areas of high lipid content in the skeleton and suggests that these are areas where large quantities of sulphide are being generated. The pattern may be confounded by differential degradation of the thoracic region (see below), with the assumption that oils will be more readily lost from degraded bones.
Figure 3.
Drawings of whale skeletons discovered on the sea bed. (a) Santa Catalina whale fall (Balaenoptera musculus or B. physalus), with black shading indicating areas of bacterial mat growth, modified after Bennett et al. [33]. Skull, rib, lumbar and caudal bones were visible at the time of the study. (b) Torishima seamount whale fall (Balaenoptera edeni), modified after Naganuma et al. [38]. Skull, lumbar and caudal bones were present when discovered. Scale bars, (a,b) 1 m.
At early-stage whale falls, where the sediment-produced sulphides still dominate, this pattern is not so clear. For example, bacterial mats on a 7-year-old skeleton were more abundant on thoracic rather than caudal vertebrae, although the skull consistently displayed the highest abundance [7]. In this case, the rate of sulphide production in the sediments was approximately one order of magnitude higher than on bones, but the authors state, ‘this ratio likely changed over the years in favour of the bones, since we can assume that biomass in the sediments is consumed faster than in bones’ [7].
Smith & Baco [3] noted that the fauna found on skeletons of juvenile whales does not seem to be as reliant on chemoautotrophy as on larger skeletons. This is to be expected since juvenile carcasses will contain more red marrow [26] and thus lower lipid content overall [23]. The lower lipid content of the juvenile skeletons would also reduce the time that whale-fall fauna could be sustained [3].
The persistence of whale-fall communities over decades is one of their most remarkable features. We propose that these late-stage sulphophilic communities will follow a predictable pattern of distribution and abundance based on the distribution of lipids in the whale skeletons presented in this review. Identifying these patterns may help in estimating the age of naturally occurring whale falls. This initial evidence supports the oil-gradient hypothesis outlined above, but it remains to be tested at other late-stage whale-fall sites. More detailed reporting of whale-fall communities will help to establish the fidelity of fauna to oil-rich bones over time.
(b). Bone oil prevents bone spoil: the oil-protection hypothesis
Whale falls provide a useful insight into the processes that affect their fossilization [34]. Understanding these processes (i.e. the study of taphonomy) is vital to gauging the fidelity of the fossil record and help palaeontologists reconstruct the evolutionary history of cetaceans. Whale-fall taphonomy may also be informative for the study of extinct marine reptiles. Plesiosaurids had a similar bone structure to that of modern whales [35] and may even have had a high enough lipid content to support similar sulphophilic fauna [36].
The data reviewed here may help to explain the degradation patterns observed at both modern and fossil whale skeletons. Articulated fossil skeletons have shown an increased level of degradation of thoracic vertebrae compared with lumbar and caudal ones. Dominici et al. [37] remark of fossil skeletons that ‘their [the vertebrae] cortical bone layer is corroded… increasingly so as the chest region is approached. Thoracic vertebrae are lacking’. This pattern has also been noted at several naturally occurring whale falls (figure 3 [33,38]), including a recently discovered skeleton [39]. The degree of degradation corresponds strikingly with those bones that have a low oil content.
At skeletons where rapid bone degradation by macrofauna is not seen (see below), long-term degradation of bone may be caused by micro-organisms [34,40,41]. There is evidence to suggest that microbial bioerosion is negatively correlated to the oil content of the bone. Deming et al. [6] showed that bacterial density was greatest on the surface of a whale-fall vertebra and decreased by four orders of magnitude to the centre of the bone, while the lipid content of the bone showed the exact opposite trend. Studies of bones from recent and fossil whale falls indicate that micro-bioerosion is coincidentally limited to the edges of bones [34,41–43]. In a study specifically looking at the bioerosion of bird bones in the marine environment, Davis [44] observed, ‘bones which contained large amounts of fat (marrow) were more resistant to bioerosion’. This evidence strongly points to a negative relationship between micro-bioerosion and oil content of the bone. It may be that the bioeroding organisms in the sea water are excluded from the bones by the hydrophobic oils, or that the breakdown of the lipids creates an environment that is not conducive to bioeroding micro-organisms (e.g. high sulphide levels).
In contrast to the slow degradation that occurs in some skeletons, others may be rapidly degraded, in part by Osedax worms that bore into the bones at high densities [45]. The taphonomic significance of these worms for the fossil record of whales has only recently been recognized [46–48]. It may be expected that Osedax would be most abundant on bones with the highest organic content, since the bone appears to be their primary source of nutrition. As yet there is insufficient evidence to show that these worms prefer certain bones to others, although the data shown here may aid in determining ecological differences between different species.
Many of the rapidly degraded skeletons were those of experimentally implanted juveniles, which have much lower concentrations of lipids in their bones for reasons discussed above. Smith & Baco [3] observed that ‘juvenile skeletons appeared to decompose much more rapidly that than those of adult whales, releasing lipid reservoirs relatively quickly’. While Osedax worms undoubtedly played a large role in the degradation of juvenile carcasses [45], their rapid degradation may also be explained by their low oil content. Additionally, long-lasting whale skeletons such as the Santa Catalina Basin skeleton also support Osedax sp. (A. G. Glover 2005, personal observation).
(c). A burning desire for whale bones: the oil utility index
Whales have played a central part in native North American cultures since prehistoric times. Examination of the bone assemblages found at archaeological sites in this region has yielded valuable information about how these cultures used whales. The size of hunted whale carcasses necessitated that they were butchered and the valuable parts transported back to settlements.
Savelle [49] constructed utility indices for various bones found at prehistoric Thule Eskimo sites in the Canadian Arctic to try and explain variations in the frequency of certain bones at these sites. He considered the utility of each element in terms of its meat content (food value) and in terms of its architectural utility (e.g. house building) and found that architectural utility better explained the composition of the whale bone assemblages than meat utility [49].
In an analysis of 2500-year-old Nuu'chah'nulth whale bone assemblages from the Pacific Northwest, Monks [50] also found that meat utility did not explain patterns in bone frequency. Instead, he proposed that bones were collected primarily for their oil content, to be used as fuel. To show this, he devised an ‘oil utility index’ based on the oil content of various skeletal elements. This was calculated by grouping bones into four body regions and applying complicated rough conversion factors to each, which were derived from general whale texts. Using these data, he showed that bones containing the most oil were the most likely to be transported to settlements [50].
The data presented in our review provide a superior measure with which the hypothesis of Monks [50] can be tested. In his analysis, it is the grouping of the bones that is significant, since the data are ranked. Comparison with the data in figure 1 reveals that it is inappropriate to group all of the bones of the head together in terms of oil content. Similarly, the grouping of the caudal, thoracic vertebrae and scapula together under a single oil grade hides significant variation.
Whale bones may be used for fuel by cutting them to liberate the oil or burned directly (reviewed in Heizer [51]). Hence, the oil utility of whale bones has also been proposed to account for butchering and burning of whale bones at other sites [52]. Such evidence can provide valuable insights into the behaviour and society of ancient civilizations, but needs to be based on accurate data. This review provides a foundation for further analysis of other whale-bone bearing archaeological sites.
4. Conclusions
Analysis of the composition of large whale skeletons from several mysticete and odontocete species shows a recurring pattern where the lipid content of the skeleton is concentrated in the skull and caudal vertebrae, and the thoracic vertebrae contain much less lipid. Such high lipid content bones support dense sulphophilic microbial mats at whale falls, and we suggest that late-stage whale-fall communities in the deep sea will correspond to these bones with their high energy availability. This skeletal lipid gradient also corresponds to areas of low micro-bioerosion and so may have played a significant taphonomic role in the preservation of marine vertebrate carcasses. The differences in lipid content between different bones revealed in this review may also explain the selection of certain bones by ancient whale-hunting peoples for use as fuel.
Acknowledgements
We would like to thank the library staff of the Natural History Museum, London, for their help in gathering data sources and for their services in preserving this information, without which this review would not have been possible. We are grateful to Lenka Neal and Maria Vorontsova, who generously helped with translation of Russian texts and to Thomas Dahlgren for his aid in translating Norwegian sources. The manuscript was improved with the help of constructive comments from two anonymous reviewers. N.D.H. is supported by a CASE award from the Natural Environment Research Council, UK.
References
- 1.Krogh A.1934Conditions of life at great depths in the ocean. Ecol. Monogr. 4, 430–439 10.2307/1961649 (doi:10.2307/1961649) [DOI] [Google Scholar]
- 2.Bruun A. T.1956The abyssal fauna: its ecology, distribution and origin. Nature 177, 1105–1108 10.1038/1771105a0 (doi:10.1038/1771105a0) [DOI] [Google Scholar]
- 3.Smith C., Baco A.2003Ecology of whale falls at the deep-sea floor. In Oceanography and marine biology, vol. 41 (eds Gibson R. N., Atkinson R. J. A.), pp. 311–354 London, UK: Taylor & Francis [Google Scholar]
- 4.Baco A., Smith C.2003High species richness in deep-sea chemoautotrophic whale skeleton communities. Mar. Ecol Prog. Ser. 260, 109–114 10.3354/meps260109 (doi:10.3354/meps260109) [DOI] [Google Scholar]
- 5.Schuller D., Kadko D., Smith C.2004Use of 210pb/226ra disequilibria in the dating of deep-sea whale falls. Earth Planet. Sci. Lett. 218, 277–289 10.1016/S0012-821X(03)00690-3 (doi:10.1016/S0012-821X(03)00690-3) [DOI] [Google Scholar]
- 6.Deming J., Reysenbach A., Macko S., Smith C.1997Evidence for the microbial basis of a chemoautotrophic invertebrate community at a whale fall on the deep seafloor: bone-colonizing bacteria and invertebrate endosymbionts. Microsc. Res. Tech. 37, 162–170 (doi:10.1002/(SICI)1097-0029(19970415)37:2<162::AID-JEMT4>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 7.Treude T., Smith C., Wenzhöfer F., Carney E., Bernardino A., Hannides A., Krüger M., Boetius A.2009Biogeochemical processes at a deep-sea whale fall: sulfate reduction, sulfide efflux and methanogenesis. Mar. Ecol. Prog. Ser. 382, 1–21 10.3354/meps07972 (doi:10.3354/meps07972) [DOI] [Google Scholar]
- 8.Smith C., Kukert H., Wheatcroft R., Jumars P., Deming J.1989Vent fauna on whale remains. Nature 341, 27–28 10.1038/341027a0 (doi:10.1038/341027a0) [DOI] [Google Scholar]
- 9.Distel D., Baco A., Chuang E., Morrill W., Cavanaugh C., Smith C.2000Marine ecology: do mussels take wooden steps to deep-sea vents? Nature 403, 725–726 10.1038/35001667 (doi:10.1038/35001667) [DOI] [PubMed] [Google Scholar]
- 10.Gibbs P. E.1987A new species of Phascolosoma (Sipuncula) associated with a decaying whale's skull trawled at 880m depth in the southwest Pacific. NZ J. Zool. 14, 135–137 [Google Scholar]
- 11.Marshall B. A.1987Osteopeltidae (Mollusca: Gastropoda): a new family of limpets associated with whale bone in the deep sea. J. Molluscan Stud. 53, 21–127 [Google Scholar]
- 12.Rouse G., Goffredi S., Vrijenhoek R.2004Osedax: bone-eating marine worms with dwarf males. Science 305, 668–671 10.1126/science.1098650 (doi:10.1126/science.1098650) [DOI] [PubMed] [Google Scholar]
- 13.Goffredi S. K., Orphan V. J., Rouse G. W., Jahnke L., Embaye T., Turk K., Lee R., Vrijenhoek R. C.2005Evolutionary innovation: a bone-eating marine symbiosis. Environ. Microbiol. 7, 1369–1378 10.1111/j.1462-2920.2005.00824.x (doi:10.1111/j.1462-2920.2005.00824.x) [DOI] [PubMed] [Google Scholar]
- 14.Smith C. R.1994Tempo and mode in deep-sea benthic ecology: punctuated equilibrium revisited. Palaios 9, 3–13 10.2307/3515074 (doi:10.2307/3515074) [DOI] [Google Scholar]
- 15.Butman C. A., Carlton J. T., Palumbi S. R.1995Whaling effects on deep-sea biodiversity. Conserv. Biol. 9, 462–464 10.1046/j.1523-1739.1995.9020462.x (doi:10.1046/j.1523-1739.1995.9020462.x) [DOI] [Google Scholar]
- 16.Glover A. G., Kemp K. M., Smith C. R., Dahlgren T. G.2008On the role of bone-eating worms in the degradation of marine vertebrate remains. Proc. R. Soc. B 275, 1959–1961 10.1098/rspb.2008.0177 (doi:10.1098/rspb.2008.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tont S., Pearcy W., Arnold J.1977Bone structure of some marine vertebrates. Mar. Biol. 39, 191–196 10.1007/BF00387004 (doi:10.1007/BF00387004) [DOI] [Google Scholar]
- 18.Heyerdahl E. F.1932The whaling industry: a technical–chemical investigation, vol. 7, pp. 1–160 Sandefjord, Norway: Commander Chr. Christensen's Whaling Museum [Google Scholar]
- 19.Feltmann C. F., Slijper E. J., Vervoort W.1948Preliminary researches on the fat-content of meat and bone of blue and fin whales. Proc. R. Neth. Acad. Arts Sci. 51, 604–615 [Google Scholar]
- 20.Watanabe H., Suzuki K.1950Chemical composition of various parts of sperm whale. Bull. Jpn Soc. Fish. 15, 735–740 [Google Scholar]
- 21.Watanabe H., Suzuki K.1950Chemical composition of various parts of whale. Bull. Jpn Soc. Fish. 15, 741–743 [Google Scholar]
- 22.Kharkof J.1940On the weight and chemical composition of whales. Trans. Inst. Mar. Fish. Oceanogr. USSR 15, 3–50 [Google Scholar]
- 23.Honda K., Fujise Y., Tatsukawa R., Miyazaki N.1984Composition of chemical-components in bone of striped dolphin, Stenella-coeruleoalba: distribution characteristics of major inorganic and organic-components in various bones, and their age-related-changes. Agr. Biol. Chem. Tokyo 48, 409–418 [Google Scholar]
- 24.Honda K., Fujise Y., Itano K., Tatsukawa R.1984Composition of chemical-components in bone of striped dolphin, Stenella-coeruleoalba: distribution characteristics of heavy-metals in various bones. Agr. Biol. Chem. Tokyo 48, 677–683 [Google Scholar]
- 25.Lantz A. W., Gunasekera C.1955Commercial utilization of dolphins and porpoises in Ceylon. Bull. Fish. Res. Sta. Ceylon 3, 1–14 [Google Scholar]
- 26.Ohe T.1950Distribution of the red marrow in bones of the fin whale. Sci. Rep. Whale Res. Inst. Tokyo 3, 17–22 [Google Scholar]
- 27.Felts W. J. L., Spurrell F. A.1965Structural orientation and density in cetacean humeri. Am. J. Anat. 116, 171–203 10.1002/aja.1001160109 (doi:10.1002/aja.1001160109) [DOI] [PubMed] [Google Scholar]
- 28.Felts W. J. L., Spurrell F. A.1966Some structural and developmental characteristics of cetacean (Odontocete) radii. A study of adaptive osteogenesis. Am. J. Anat. 118, 103–134 10.1002/aja.1001180107 (doi:10.1002/aja.1001180107) [DOI] [PubMed] [Google Scholar]
- 29.Tomlin A. G.1967Mammals of the U.S.S.R. and adjacent countries: Cetacea. (transl. from the Russian). Jerusalem, Israel: Israel Program for Scientific Translations [Google Scholar]
- 30.Lockyer C.1986Body fat condition in northeast Atlantic fin whales, Balaenoptera physalus, and its relationship with reproduction and food resource. Can. J. Fish. Aquat. Sci. 43, 142–147 10.1139/f86-015 (doi:10.1139/f86-015) [DOI] [Google Scholar]
- 31.Smith C. R.2006Bigger is better: the role of whales as detritus in marine ecosystems. In Whales, whaling and ocean ecosystems (eds Estes J. A., DeMaster D. P., Doak D. F., Williams T. M., Brownell R. L.), pp. 286–302 London, UK: University of California Press [Google Scholar]
- 32.Smith C. R.1992Whale falls: chemosynthesis on the deep-sea floor. Oceanus 36, 74–78 [Google Scholar]
- 33.Bennett B., Smith C., Glaser B., Maybaum H.1994Faunal community structure of a chemoautotrophic assemblage on whale bones in the deep northeast Pacific Ocean. Mar. Ecol Prog. Ser. 108, 205–223 10.3354/meps108205 (doi:10.3354/meps108205) [DOI] [Google Scholar]
- 34.Allison P., Smith C., Kukert H., Deming J., Bennett B.1991Deep-water taphonomy of vertebrate carcasses: a whale skeleton in the bathyal Santa-Catalina basin. Paleobiology 17, 78–89 [Google Scholar]
- 35.Wiffen J., DeBuffrenil V., DeRicqles A., Mazin J.1995Ontogenetic evolution of bone structure in Late Cretaceous Plesiosauria from New Zealand. Geobios 28, 625–640 10.1016/S0016-6995(95)80216-9 (doi:10.1016/S0016-6995(95)80216-9) [DOI] [Google Scholar]
- 36.Kaim A., Kobayashi Y., Echizenya H., Jenkins R. G., Tanabe K.2008Chemosynthesis-based associations on Cretaceous plesiosaurid carcasses. Acta Palaeontol. Pol. 53, 97–104 10.4202/app.2008.0106 (doi:10.4202/app.2008.0106) [DOI] [Google Scholar]
- 37.Dominici S., Cioppi E., Danise S., Betocchi U., Gallai G., Tangocci F., Valleri G., Monechi S.2009Mediterranean fossil whale falls and the adaptation of mollusks to extreme habitats. Geology 37, 815–818 10.1130/G30073A.1 (doi:10.1130/G30073A.1) [DOI] [Google Scholar]
- 38.Naganuma T., Wada H., Fujioka K.1996Biological community and sediment fatty acids associated with the deep-sea whale skeleton at the Torishima Seamount. J. Oceanogr. 52, 1–15 10.1007/BF02236529 (doi:10.1007/BF02236529) [DOI] [Google Scholar]
- 39.Lundsten L., Paull C. K., Schlining K. L., McGann M., Ussler W.2010Biological characterization of a whale-fall near Vancouver Island, British Columbia, Canada. Deep-Sea Res. I 57, 918–922 [Google Scholar]
- 40.Trueman N. C., Martill D. M.2002The long-term survival of bone: the role of bioerosion. Archaeometry 44, 371–382 10.1111/1475-4754.t01-1-00070 (doi:10.1111/1475-4754.t01-1-00070) [DOI] [Google Scholar]
- 41.Shapiro R. S., Spangler E.2009Bacterial fossil record in whale-falls: petrographic evidence of microbial sulfate reduction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 274, 196–203 10.1016/j.palaeo.2009.02.006 (doi:10.1016/j.palaeo.2009.02.006) [DOI] [Google Scholar]
- 42.Amano K., Little C. T. S.2005Miocene whale-fall community from Hokkaido, northern Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 215, 345–356 10.1016/j.palaeo.2004.10.003 (doi:10.1016/j.palaeo.2004.10.003) [DOI] [Google Scholar]
- 43.Amano K., Little C. T. S., Inoue K.2007A new Miocene whale-fall community from Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 247, 236–242 10.1016/j.palaeo.2006.10.017 (doi:10.1016/j.palaeo.2006.10.017) [DOI] [Google Scholar]
- 44.Davis P.1997The bioerosion of bird bones. Int. J. Osteoarchaeol. 7, 388–401 (doi:10.1002/(SICI)1099-1212(199707/08)7:4<388::AID-OA357>3.0.CO;2-H) [DOI] [Google Scholar]
- 45.Braby C. E., Rouse G. W., Johnson S. B., Jones W. J., Vrijenhoek R. C.2007Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey Bay, California. Deep-Sea Res. I 54, 1773–1791 10.1016/j.dsr.2007.05.014 (doi:10.1016/j.dsr.2007.05.014) [DOI] [Google Scholar]
- 46.Higgs N. D., Glover A. G., Dahlgren T. G., Little C. T. S.In press Using computed-tomography to document borings by Osedax mucofloris in whale bone. Cah. Biol. Mar. [Google Scholar]
- 47.Kiel S., Goedert J. L., Kahl W. A., Rouse G. W.2010Fossil traces of the bone-eating worm Osedax in Early Oligocene whale bones. Proc. Natl Acad. Sci. USA 107, 8656–8659 10.1073/pnas.1002014107 (doi:10.1073/pnas.1002014107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Little C. T. S.2010The prolific afterlife of whales. Sci. Am. 302, 64–69 [DOI] [PubMed] [Google Scholar]
- 49.Savelle J.1997The role of architectural utility in the formation of zooarchaeological whale bone assemblages. J. Archaeol. Sci. 24, 869–885 10.1006/jasc.1996.0167 (doi:10.1006/jasc.1996.0167) [DOI] [Google Scholar]
- 50.Monks G. G.2002An oil utility index for whale bones. In The exploitation and cultural importance of sea mammals (ed. Monks G. G.), pp. 138–153 Oxford, UK: Oxbow books [Google Scholar]
- 51.Heizer R. F.1963Fuel in primitive society. J. R. Anthropol. Inst. Great Brit. Ireland 93, 186–194 10.2307/2844241 (doi:10.2307/2844241) [DOI] [Google Scholar]
- 52.Volkmer de Castilho P.2008Utilization of cetaceans in shell mounds from the southern coast of Brazil. Quaternary Int. 180, 107–114 [Google Scholar]
- 53.Mkukuma L., Skakle J., Gibson I., Imrie C., Aspden R., Hukins D.2004Effect of the proportion of organic material in bone on thermal decomposition of bone mineral: an investigation of a variety of bones from different species using thermogravimetric analysis coupled to mass spectrometry, high-temperature x-ray diffraction, and Fourier transform infrared spectroscopy. Calc. Tissue Int. 75, 321–328 10.1007/s00223-004-0199-5 (doi:10.1007/s00223-004-0199-5) [DOI] [PubMed] [Google Scholar]
- 54.Lees S., Escoubes M.1987Vapor pressure isotherms, composition and density of hyperdense bones of horse, whale and porpoise. Connect. Tissue Res. 16, 305–322 10.3109/03008208709005617 (doi:10.3109/03008208709005617) [DOI] [PubMed] [Google Scholar]
- 55.Lund J.1952Whale oil and its processing. Norw. Whaling Gaz. 41, 66–70 [Google Scholar]