Abstract
Bluefin tuna have a unique physiology. Elevated metabolic rates coupled with heat exchangers enable bluefin tunas to conserve heat in their locomotory muscle, viscera, eyes and brain, yet their hearts operate at ambient water temperature. This arrangement of a warm fish with a cold heart is unique among vertebrates and can result in a reduction in cardiac function in the cold despite the elevated metabolic demands of endothermic tissues. In this study, we used laser scanning confocal microscopy and electron microscopy to investigate how acute and chronic temperature change affects tuna cardiac function. We examined the temporal and spatial properties of the intracellular Ca2+ transient (Δ[Ca2+]i) in Pacific bluefin tuna (Thunnus orientalis) ventricular myocytes at the acclimation temperatures of 14°C and 24°C and at a common test temperature of 19°C. Acute (less than 5 min) warming and cooling accelerated and slowed the kinetics of Δ[Ca2+]i, indicating that temperature change limits cardiac myocyte performance. Importantly, we show that thermal acclimation offered partial compensation for these direct effects of temperature. Prolonged cold exposure (more than four weeks) increased the amplitude and kinetics of Δ[Ca2+]i by increasing intracellular Ca2+ cycling through the sarcoplasmic reticulum (SR). These functional findings are supported by electron microscopy, which revealed a greater volume fraction of ventricular SR in cold-acclimated tuna myocytes. The results indicate that SR function is crucial to the performance of the bluefin tuna heart in the cold. We suggest that SR Ca2+ cycling is the malleable unit of cellular Ca2+ flux, offering a mechanism for thermal plasticity in fish hearts. These findings have implications beyond endothermic fish and may help to delineate the key steps required to protect vertebrate cardiac function in the cold.
Keywords: calcium transient, thermal acclimation, cardiomyocyte, sarcoplasmic reticulum, mitochondria
1. Introduction
Pacific bluefin tuna, Thunnus orientalis, range across the entire north Pacific Ocean as juveniles. Electronic tagging studies show juvenile Pacific bluefin sojourn into the cool productive waters of the California Current late in year one and occupy this region for several years [1]. While in the eastern Pacific, the bluefin move annually along the North American coast between Baja and California [2], where they experience sea surface temperatures between 11°C and 24°C [1]. Juvenile bluefin tuna can dive to depths of 500 m in search of prey, encountering colder temperatures (5–6°C) and experiencing acute temperature changes of more than 10°C [2]. These data indicate that Pacific bluefin tuna are capable of expansive migrations into northern latitudes and have a thermal tolerance that ranges from 5°C to 24°C.
A unique endothermic physiology contributes to the expansive thermal niche of bluefin tunas. Bluefins have elevated metabolic rates, defend a thermal neutral zone [3] and conserve metabolic heat with vascular counter-current heat exchangers located in viscera, brain, eyes and muscles (e.g. [4]). The tuna heart is not supplied with a circulatory counter-current heat exchanger and receives coronary blood from the gills at ambient water temperature [5]. Thus, bluefin tunas have the unique physiological arrangement of a heart at ambient water temperature supplying blood to warm endothermic tissues. The thermal sensitivity of the tuna heart may therefore limit the niche occupied by tunas [6]. Indeed, cooling causes bradycardia and an associated reduction in cardiac output in bluefin and yellowfin (T. albacares) tunas [7,8]. However, the Pacific bluefin tuna heart generates greater contractile force and maintains cardiac rhythm at colder temperatures than its tropical sister taxa [7,9]. Understanding cardiac thermal sensitivity in the tunas may help to delineate the key steps that protect cardiac function in the cold. We hypothesize that cold tolerance in bluefin tunas is directly related to increased use of the sarcoplasmic reticulum (SR) during excitation–contraction (e–c) coupling in the cardiac myocytes that make up the heart.
Cardiac e–c coupling underlies myocyte contraction and relaxation by controlling the cycling of cellular Ca2+. The general schema for e–c coupling in fishes differs from that of mammals primarily with regard to the SR (see [10] for a review of e–c coupling in the fish heart). Most fishes do not use the internal Ca2+ stores of the SR for contraction and relaxation, rather, Ca2+ is cycled back and forth across the myocyte (sarcolemmal) membrane with each beat [10]. However, isolated tissue studies have revealed that elevated cardiac performance in fishes is associated with increased use of SR Ca2+ cycling [9,11–16]. Biochemical studies with isolated SR vesicles clearly show that bluefin tuna have more SR Ca2+ ATPase (SERCA) than their warmer sister taxa [17,18] and structural studies demonstrate that an extensive SR is present in bluefin tuna hearts [19]. However, to date, only one study [20] has directly investigated the physiological role of SR Ca2+ during e–c coupling in fish heart, and this study did not examine the effect of temperature.
Temperature affects both the rate and strength of contraction and relaxation of cardiac myocytes. Temperature has direct (i.e. Q10) effects on the ion channels and pumps that underlie the cardiac action potential [21,22] and that cycle Ca2+ during e–c coupling (e.g. ICa: [22,23,24]; ryanodine receptor: [25–27]; SERCA: [17,18,26]; Na+–Ca2+ exchanger: [28]). In an attempt to compensate for the direct effects of temperature on e–c coupling pathways, some animals adjust their phenotype during chronic temperature exposure. For example, in freshwater fishes, cold acclimation (CA) can induce ventricular hypertrophy [29], modify K+ channel conductance and reduce action potential duration, which enables higher heart rates [21,30]. Importantly, SR function can also increase in the cold. Isolated tissue studies show that CA increases the ryanodine-sensitivity of cardiac isometric force production in rainbow trout, Oncorhynchus mykiss [12,14,31]. Biochemical studies indicate that CA increases the activity of the SERCA pump in rainbow trout [11] and morphometric analysis has indicated increased volume of SR following CA in perch (Perca fluviatilis [32]).
In this study, we use laser-scanning confocal microscopy to investigate the effect of acute and chronic temperature change on the temporal and spatial properties of the intracellular Ca2+ transient (Δ[Ca2+]i) in Pacific bluefin tuna ventricular myocytes. We show, to our knowledge, the first physiological evidence for Ca2+-induced Ca2+-release [33] during e–c coupling in the tuna heart underscoring the importance of the SR for tuna cardiac performance. We show that acute (less than 5 min) cooling limits cellular Ca2+ cycling but that chronic cold (greater than 4 weeks) invokes a remodelling of cellular Ca2+ flux that offers partial compensation. We provide, to our knowledge, the first functional evidence that enhanced SR Ca2+ cycling underlies the cardiac remodelling associated with cold-tolerance in an endothermic fish. We support these functional findings with electron microscopy and stereology results indicating cold-induced increases in SR volume.
2. Material and methods
(a). Fish origin and care
Pacific bluefin tuna, T. orientalis (mean mass: 11.1 ± 0.1 kg, mean fork length: 84.5 ± 2.4 cm, n = 10, males and females) were captured from the wild and held as previously described [22]. Fish were acclimated for at least four weeks and up to eight weeks in two identical 109 m3 circular tanks; one tank at 24°C (warm acclimated, WA) and one at 14°C (CA) under a 12 L : 12 D photoperiod.
(b). Myocyte isolation
Myocytes from bluefin tuna were isolated as described previously [22,34] and explained briefly in the electronic supplementary material.
(c). Confocal imaging
For confocal imaging of Δ[Ca2+]i, ventricular myocytes were loaded with 4 µM Fluo-4-AM for 20 min at room temperature. Myocytes were washed by dilution, allowed 30 min for de-esterification and then transferred to a temperature regulated recording chamber on the stage of an Olympus Fluoview confocal microscope. The extracellular solution perfusing the myocytes was precisely controlled with an in-line temperature controller. Myocytes were perfused at their respective acclimation temperatures (14°C and 24°C), and at a common test temperature of 19°C. Temperature changes were accomplished within 2 min. To investigate the role of SR, cells were bathed with solutions containing both the SR Ca2+ release channel inhibitor ryanodine (5 µM) and the SR Ca2+ ATPase inhibitor thapsigargin (2 µM). Myocytes were stimulated to contract via platinum plate electrodes (50–100 V, 5–15 ms pulses). Ca2+ was imaged by exciting Fluo-4 at 488 nm and detecting emitted fluorescence at wavelengths greater than 505 nm. All line scan images are presented as the original raw fluorescence (F) signal. Background fluorescence (Fo) was measured in each cell in a region that did not have localized or transient fluorescent elevation. F/Fo was converted to Δ[Ca2+]i in accordance with Cheng et al. [35] and Loughrey et al. [36] and corrected for temperature (see [37]). See supplementary material for full details of solutions, confocal set-up and fluorescence calibrations.
(d). Electron microscopy and stereology
Detailed methodology for fixation and stereology of bluefin tuna heart has been described previously [20]. A brief description is provided in the electronic supplementary material.
3. Results
(a). Effect of acute temperature change on cellular Ca2+ cycling
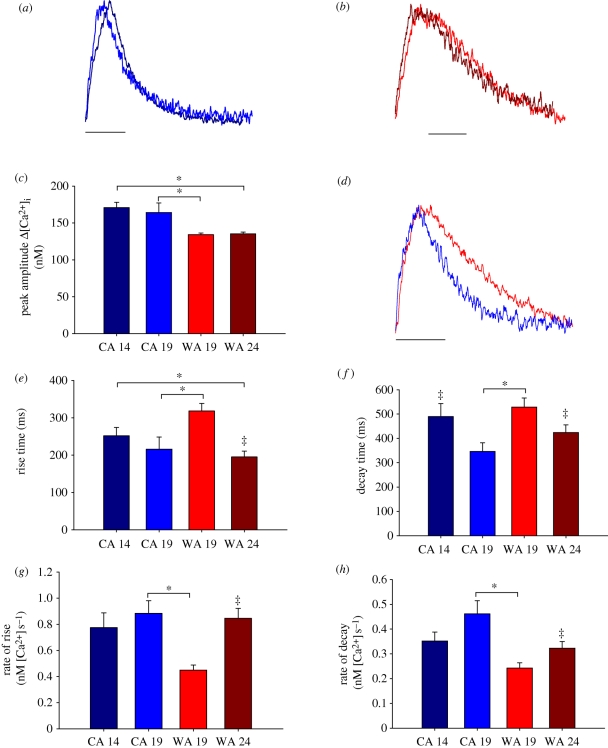
Acute warming accelerated the temporal properties of Δ[Ca2+]i and acute cooling slowed the temporal properties of Δ[Ca2+]i as illustrated in figure 1. The kinetic responses are emphasized in figure 1a (acute warming of CA myocytes from 14°C to 19°C) and figure 1b (acute cooling of WA myocytes from 24°C to 19°C), which shows the time courses for Δ[Ca2+]i at each temperature with the amplitudes normalized. Rapid warming and cooling by 5°C did not affect the amplitude of Δ[Ca2+]i in either WA or CA myocytes (figure 1c).
Figure 1.
Temporal properties of Δ[Ca2+]i measured transversely across the cell from CA myocytes at their acclimation temperature of 14°C (CA 14, black line) and at the common temperature of 19°C (CA 19, blue line), and from WA myocytes at their acclimation temperature of 24°C (WA 24, brown line) and at the common temperature of 19°C (WA 19, red line). (a) The effects of acute warming on CA myocyte Δ[Ca2+]i kinetics when the time course amplitude of a CA myocyte at 14°C and 19°C are normalized. (b) The effect of acute cooling on WA myocyte Δ[Ca2+]i kinetics when the time course amplitude of a WA myocyte at 14°C and 19°C are normalized. (c) The effects of acute and chronic temperature change on the peak amplitude of Δ[Ca2+]i. (d) The time course of a WA (red line) and CA (blue line) myocyte with the amplitudes normalized at 19°C to emphasize the effect of thermal acclimation on Δ[Ca2+]i kinetics. (e,g) The mean kinetics of the rise and (f,h) the mean kinetics of the fall of Δ[Ca2+]i in response to acute and chronic temperature change. Asterisks (*) indicate significant effect of acclimation; double-dagger (‡) indicates significant effect of acute temperature change (Student's t-test or Mann–Whitney rank sum test, p < 0.05; n = 6–16 cells from four to five animals at each acclimation temperature). Scale bars, (a,b,d) 500 ms.
(b). Effect of thermal acclimation on cellular Ca2+ cycling
When tested at their respective acclimation temperature, CA myocytes exhibited larger Δ[Ca2+]i (figure 1c) and slower rise times (figure 1e) than WA myocytes. However, decay kinetics and the rate of rise were unchanged between WA myocytes tested at 24°C and CA myocytes tested at 14°C, indicating remodelling of cellular Ca2+ flux pathways in response to thermal acclimation.
Remodelling of ventricular myocytes with thermal acclimation is best revealed by comparing the temporal properties of cellular Ca2+ cycling in WA and CA myocytes at a common test temperature. Figure 1 shows mean data for Δ[Ca2+]i peak amplitude (figure 1c) and kinetics (figure 1e–h) across the width of CA and WA myocytes at the common temperature of 19°C. CA increased the amplitude of Δ[Ca2+]i and accelerated the kinetics of contraction and relaxation when compared with WA. These kinetic differences are illustrated in figure 1d, where Δ[Ca2+]i amplitudes are normalized. The increased rate of Ca2+ cycling after CA is consistent with an upregulation of SR function in the cold.
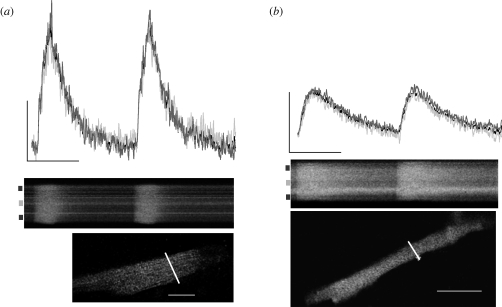
Remodelling of cellular Ca2+ flux in response to thermal acclimation could involve spatial changes in the time course of the transient across the width of the myocyte. This was investigated by monitoring Ca2+ flux in the cell centre and cell periphery as well as across the whole width of the cell in WA and CA myocytes at 19°C. Figure 2 shows the time course and corresponding raw line scan image of cellular Ca2+ flux across the width of a CA (figure 2a) and WA (figure 2b) myocyte at 19°C. There was a uniform rise in the Ca2+ wavefront across the width of the cell in both acclimation groups as indicated by the line scan images and the unchanged time course of the transients at cell periphery when compared with cell centre. This suggests that Ca2+ is rapidly propagated from the cell periphery to the cell centre in both acclimation groups at 19°C indicating Ca2+-induced Ca2+-release during e–c coupling. Thermal acclimation does not alter spatial characteristics of the Ca2+ transient when SR function is intact.
Figure 2.
Spatial and temporal cellular Ca2+ flux in ventricular myocytes from thermally acclimated Pacific bluefin tuna. Representative time courses (top) and corresponding raw line scan images (below) show temporal and spatial characteristics of Ca2+ flux in a (a) CA and (b) WA myocyte. Markings on left side of line scan images show regions (cell centre, light grey lines; cell periphery, dark grey lines) for spatial characterization (see electronic supplementary methods for details). The time course of the Ca2+ transient across the whole width of the cell is given by the black time course line, which is partially obscured by the overlapping time courses at the cell centre and periphery. Images below the line scans show the corresponding myocyte loaded with Fluo-4 AM. The white line indicates position of scan. The white scale bar is 20 µm in both images. Time course scale is 100 nM [Ca2+] by 1 s. Line scans are 2500 lines × 512 pixels.
(c). The role of SR in CA myocytes
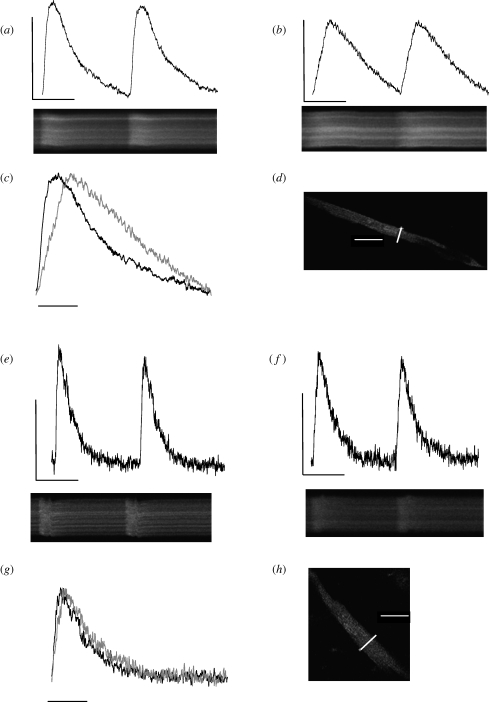
The increased rate of Ca2+ cycling after CA is illustrated in figures 1 and 2, and is consistent with an upregulation of SR function in the cold [11,38]. To investigate this possibility, the SR was inhibited in CA myocytes at 14°C, which caused a slowing of the rise and fall of Δ[Ca2+]i and a decline in amplitude. Effects on kinetics are emphasized in figure 3c, where the amplitude of the transients before and after SR inhibition has been normalized. These temporal differences are supported by spatial differences in the rise of the Ca2+ wavefront after SR inhibition. The Ca2+ wavefront rose faster (p < 0.05, Mann–Whitney rank sum test; n = 11 cells) in the cell periphery (381 ± 33 ms) than the cell centre (501 ± 50 ms) after SR inhibition. This is evidenced visually by the more v-shaped rise in the Ca2+ wavefront in the raw line scan image in figure 3b when compared with the uniform rise in the Ca2+ wavefront across the width of the cell in figure 3a.
Figure 3.
The effect of SR inhibition on cellular Ca2+ flux in bluefin tuna ventricular myocytes. (a–d) Data from a CA myocyte tested at its acclimation temperature of 14°C. Figure shows the transient time course (top) and the corresponding raw line scan image (bottom) under control conditions (a) and after SR inhibition (b) for the myocyte loaded with Fluo-4 AM shown in (d). The effects of SR inhibition on Ca2+ cycling kinetics at 14°C are emphasized when the amplitudes are normalized (c). (e–h) Data from a WA myocyte tested at its acclimation temperature of 24°C. (e) Transient time course (top) and the corresponding raw line scan image (bottom) under control conditions and after SR inhibition (f) for the myocyte loaded with Fluo-4 AM shown in (h). (g) The effect of SR inhibition on kinetics as the amplitudes are normalized. The white line in (d,h) indicates position of scan; the white scale bar is 50 µm. Time course scale in is 100 nM [Ca2+] by 1 s. Line scans are 2500 lines × 512 pixels. Scale bars, (c,g) 500 ms.
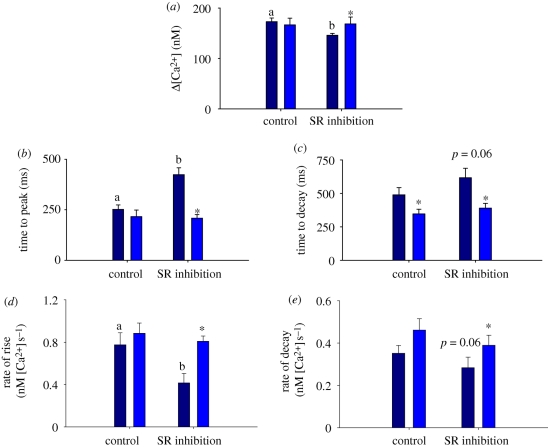
Mean data are provided in figure 4 and show how SR inhibition reduces the peak amplitude of Δ[Ca2+]i, and slows the kinetics of contraction and relaxation in CA myocytes at 14°C. There were no significant effects of SR inhibition on the amplitude or kinetics of Δ[Ca2+]i after acute warming (figure 4), indicating that acute warming negates the need for increased Ca2+ cycling through the SR and suggests that sarcolemmal Ca2+ is sufficient for activation of these myocytes. Indeed, acute warming from 14°C to 19°C (under control conditions with SR function intact) tended to accelerate the kinetics of Δ[Ca2+]i as indicated by the normalized recordings in figure 1a.
Figure 4.
The effect of SR inhibition and acute warming on the temporal properties of Δ[Ca2+]i in CA ventricular myocytes from the Pacific bluefin tuna. (a) At 14°C, SR inhibition decreased the peak amplitude and slowed the kinetics of Δ[Ca2+]i (compare dark blue bars; dissimilar letters indicate significant effect of SR inhibition). (b–e) When CA myocytes were acutely warmed to 19°C, SR inhibition had no effect on Δ[Ca2+]i (compare light blue bars; asterisks indicate significant effect of acute warming); p < 0.05; Student's t-test or Mann–Whitney rank sum test. Data are means ± s.e.m., n = 6–16 cells from four to five animals at each acclimation temperature.
(d). The role of SR in WA myocytes
Inhibiting the SR in WA myocytes at 24°C slowed the kinetics of Δ[Ca2+]i rise as shown in figure 3e–h, but did not affect the amplitude or decay. Mean data are given in the electronic supplementary material. These results lend further support to the idea that warm temperatures reduce the need for Ca2+ cycling through the SR in bluefin tuna ventricular myocytes when contraction frequency is constant at 0.5 Hz.
(e). Effects of thermal acclimation on morphology
Electron microscopy revealed that the abundance of SR profiles was higher in CA when compared with WA ventricular myocytes (figure 5) as quantified by morphometric analysis using stereological techniques (table 1). CA increased overall SR surface area to volume ratio by approximately 7 per cent, and SR volume relative to total volume by approximately 42 per cent, or relative to cytosolic volume by approximately 38 per cent (table 1). CA also increased mitochondrial content (table 1) and appeared to increase glycogen droplets (figure 5).
Figure 5.
Comparison of longitudinal thin sections of (a) WA and (b) CA Pacific bluefin tuna ventricular myocytes. Two forms of sarcoplasmic reticulum (SR) are evident. Free SR (fSR) is the Ca2+ pumping SR domain and is situated around the myofibrils or in narrow gaps between the myofilaments (arrows). Junctional SR (jSR) is also apparent and appears in the form of wide cisternae in association with the sarcolemma, forming peripheral couplings associated with Ca2+ release (arrowheads). Both fSR and jSR are combined to provide mean SR data in table 1. The jSR cisternae contain dense aggregates of the calcium-binding protein calsequestrin, which is often located at the opposite side of the surface membrane. CA cells also had a greater mitochondrial density and appeared to contain more glycogen droplets than WA cells. Stereological quantifications are provided in table 1 (scale bar, 1 µm).
Table 1.
Volume and surface measurements of Pacific bluefin tuna ventricular myocytes. Stereological data of SR volume and surface area of cold acclimated (CA) and warm acclimated (WA) myocytes. (All values are expressed as mean ± s.d. from n = 62 cells from each animal with n = 4 (CA) and n = 5 (WA) animals. SR, sarcoplasmic reticulum; M, mitochondria; Cyto, cytoplasm. Symbols indicate significant differences between CA and WA with Student's t-test at *p = 3.36 × 10−5; **p = 3.97 × 10−6; ***p = 0.02 and ****p = 0.005.)
| SR |
mitochondria |
||||
|---|---|---|---|---|---|
| volume fraction | SR-vol/cyto-vol | surface area (SSR/Vtot) | volume fraction | m-vol/cyto-vol | |
| (VSR/Vtot) | (VSR/VCyto) | (mm2 mm−3) | (VM/Vtot) | (VM/VCyto) | |
| CA | 0.037 ± 0.01* | 0.05 ± 0.01** | 0.16 ± 0.06*** | 0.28 ± 0.14**** | 0.49 ± 0.3**** |
| WA | 0.026 ± 0.01 | 0.036 ± 0.01 | 0.15 ± 0.03 | 0.24 ± 0.09 | 0.31 ± 0.2 |
Remodelling of SR membranes and cellular mitochondrial volume was associated with morphological changes in the myocyte. The mean width measured using confocal microscopy across the single isolated ventricular myocyte (at the centre point of cell length) showed that CA myocytes (15.5 ± 1.45 µm, n = 31) were wider than (p < 0.001, Mann-Whitney Rank sum test) WA myocytes (8.45 ± 0.73 µm, n = 34). We did not observe an effect of CA on the ratio of whole heart (ventricle, atrium and bulbus arteriosus) mass to body mass (CA, 0.37 ± 0.02%; WA, 0.37 ± 0.01%).
4. Discussion
In bluefin tuna, thermal gradients of approximately 10°C are common between ambient sea surface and internal body temperatures, and can increase to approximately 20°C when diving into cool waters [1,2,39]. Thus, the bluefin tuna heart must supply the high oxygen demand of warm tissues while functioning across a wide range of temperatures. In this study, confocal imaging of live contracting ventricular myocytes has be employed to test the hypothesis that cold tolerance in bluefin tuna is directly related to SR use during e–c coupling. We provide structural and functional evidence for robust SR involvement in e–c coupling in the bluefin tuna heart and suggest that this arrangement is important in preserving heart function when diving into cooler waters. We then show that chronic cooling induced remodelling of cellular Ca2+ flux through further enhancement of SR function consistent with thermal compensation. We suggest that this remodelling is important for robust cardiac function of tuna in cold northerly latitudes and that it may be a conserved trait for cardio-protection from the cold across vertebrate species (see below [23,38,40,41]).
(a). Bluefin tuna e–c coupling
Bluefin tuna hearts are dependent upon SR Ca2+ cycling during e–c coupling. We found a homogeneous rise in the Ca2+ wavefront across the width of the bluefin tuna myocyte, irrespective of temperature. This indicates that voltage-dependent Ca2+ entry triggers Ca2+ release from peripheral junctional SR that subsequently induces Ca2+ release in more central regions of the cell through propagated Ca2+-induced Ca2+-release (i.e. [33]). Our functional model is supported by morphological evidence for Ca2+-induced Ca2+-release in bluefin tuna myocytes [19], recent electrophysiological studies [21,34], and biochemical studies on isolated SR vesicles [17,18].
In mammalian myocytes, the homogeneous rise in the Ca2+ wavefront is attributed to the T-tubular system which couples the SR to the sarcolemma throughout the entire cell volume [42]. Bluefin tuna ventricular myocytes lack T-tubules [19] similar to all other non-mammalian ventricular myocytes. We suggest that the rapid propagation of the Ca2+ signal between neighbouring ryanodine receptors in the bluefin heart gives rise to this homogeneous Ca2+ wavefront in the absence of T-tubules (e.g. figure 2). The small physical dimensions (shallow depth < 10 µm, narrow width < 20 µm) of the myocytes will also play a role in the spatio-temporal characteristics of the bluefin Ca2+ transient. However, in rainbow trout myocytes, which have similar dimensions, the Ca2+ transient wavefront is not homogeneous, presumably owing to less robust coupling between SR Ca2+ release units [20].
(b). The role of SR in thermal remodelling
We show here that prolonged cold exposure increases the amplitude and accelerates the kinetics of Δ[Ca2+]i, thereby offering partial compensation for the direct effects of cold. These physiological responses are consistent with a cold-induced increase in SR function. Increased SR function in the cold has been documented in eurythermal rainbow trout [12,14,31], cold stenothermal burbot [28,43], and hibernating [23,38,41] and torpid [40] mammals, suggesting that enhanced SR function may be a conserved trait for cardioprotection in the cold across vertebrates.
Our study shows that CA increases both the rate of rise and the rate of decay of the Ca2+ transient indicating thermal compensation in both SR Ca2+ uptake and SR Ca2+ release mechanisms. An increase in SERCA function could account for both of these responses and previous biochemical studies have indicated that SERCA function is the feature of e–c coupling that distinguishes the Pacific bluefin tuna from other scombrids [18,19]. However, our study does not distinguish the precise mechanism for increased SR function after CA. A number of mechanisms could be involved including: (i) greater amount of SR; (ii) greater activity of SERCA; (iii) greater activity of ryanodine receptors; (iv) changes in SR luminal Ca2+; or (v) changes in accessory proteins (i.e. phospholamban, triadin, junctin, FKBPs) regulating SR Ca2+ cycling.
Our structural data clearly show increased free and junctional SR after CA in the bluefin ventricle, which is in line with earlier studies on CA perch [32]. This alone could account for the increased rate and amplitude of Δ[Ca2+]i in the bluefin, however SR vesicles from rainbow trout have demonstrated increased maximal Ca2+ uptake velocity after CA [11], suggesting that enzyme activity could also be enhanced. By contrast, no effect of temperature acclimation has yet been observed for the expression or subcellular localization of the ryanodine receptor in trout [44] or carp [45]. This may indicate that the ryanodine receptor plays less of a role than SERCA in the thermal remodelling response in fishes. To date, there have, to our knowledge, been no studies investigating the effect of thermal acclimation on SR accessory proteins in fishes. However, recently, Korajoki & Vornanen [46] showed expression of the SR luminal Ca2+ buffer, calsequestrin, does not vary with acclimation in the rainbow trout heart. Detailed biophysical studies combined with molecular biological approaches are required to discern the precise molecular mechanism for enhanced SR function in the CA bluefin heart.
In the hearts of most ectotherms, action potential shape is significantly altered by thermal acclimation to avoid temperature-induced disturbances in electrical excitability [31]. Interestingly, previous work with Pacific bluefin tuna showed no change in action potential duration with thermal acclimation [22]. Similarly, ventricular myocytes from Siberian hamsters (Phodopus sungorus) showing daily bouts of torpor (from 37 to approximately 15°C) exhibit an increased amplitude of Δ[Ca2+]i and an acceleration of rise and fall kinetics, with no change in action potential duration, when compared with non-torpid littermates [40]. This may suggest that animals that experience rapid changes in temperature on a daily cycle (e.g. diving tuna or torpid mammals) do not compensate seasonally via action potential excitability, but rather via SR Ca2+ flux mechanisms.
(c). Acute effects of temperature on Δ[Ca2+]i
Electronic tagging of juvenile Pacific bluefin tuna show that they experience rapid temperature changes of 5–10°C daily as they dive through the stratified water column [1,2]. In isolated ventricular myocytes, a 5°C acute temperature change altered the kinetics of Δ[Ca2+]i, consistent with changes in force development in isolated strips of tuna cardiac muscle [9,16]. In vivo and in situ studies indicate that bluefin tuna exhibit a robust bradycardia in response to rapid cooling [7]. We hypothesize that it is the combined effect of acute temperature change on the inotropic and chronotropic output of the heart and the challenges of meeting the oxygen demands of the warm body that induce myocyte remodelling during prolonged cold exposure.
(d). Effect of thermal acclimation on heart morphology
In addition to the effect of CA on SR content, we observed increased mitochondrial density in bluefin tuna ventricular myocytes after CA. Adjustments in mitochondrial properties and capacities are crucial for acclimation to the cold in some fishes as are increases in cardiac glycogen storage [47]. These data suggest that remodelling in cellular energetics may accompany the remodelling in cellular Ca2+ flux in the bluefin tuna heart. The increase we observed in myocyte width with CA is consistent with the increase in cell capacitance (a measure of cell surface area) reported previously for CA bluefin tuna myocytes [21].
Acknowledgements
All procedures used in these experiments were in accordance with Stanford University institutional animal use protocols and adhered to the United Kingdom Home Office Animals Scientific Procedures Act of 1986 for Schedule 1 killing of fish.
This study was supported by a grant from the National Oceanic and Atmospheric Administration to B.A.B., and the Monterey Bay Aquarium Foundation. We thank the staff of the Tuna Research and Conservation Center and the Monterey Bay Aquarium and the Captains and crew of the F/V Shogun for their help collecting and maintaining the bluefin. We are indebted to the owners of Mariculture del Norte and the Mexican Government for their cooperation and generosity. We are grateful to Prof. Clara Franzini-Armstrong for the use of her Philips 410 Electron Microscope.
References
- 1.Boustany A. M., Matteson R., Castleton M., Farwell C., Block B. A.In press Movements of pacific bluefin tuna (Thunnus orientalis) in the Eastern North Pacific revealed with archival tags. Prog. Ocean. [Google Scholar]
- 2.Kitagawa T. B., Boustany A. M., Farwell C., Williams T. D., Casatleton M. R., Block B. A.2007Horizontal and vertical movements of juvenile bluefin tuna (Thunnus orientalis) in relation to seasons and oceanographic conditions in the eastern Pacific Ocean. Fish. Oceanogr. 16, 409–421 10.1111/j.1365-2419.2007.00441.x (doi:10.1111/j.1365-2419.2007.00441.x) [DOI] [Google Scholar]
- 3.Blank J. M., Morrissette J. M., Farwell C. J., Price M., Schallert R. J., Block B. A.2007Temperature effects on metabolic rate of juvenile Pacific bluefin tuna Thunnus orientalis. J. Exp. Biol. 210, 4254–4261 10.1242/jeb.005835 (doi:10.1242/jeb.005835) [DOI] [PubMed] [Google Scholar]
- 4.Linthicum D. S., Carey F. G.1972Regulation of brain and eye temperatures by the bluefin tuna. Comp. Biochem. Physiol. A 43, 425–433 10.1016/0300-9629(72)90201-0 (doi:10.1016/0300-9629(72)90201-0) [DOI] [PubMed] [Google Scholar]
- 5.Brill R. W., Bushnell P. G.2001The cardiovascular system of tunas. In Tunas: physiology, ecology and evolution (eds Hoar W. S., Randall D. J., Farrell A. P.), pp. 79–120 San Diego, CA: Academic Press [Google Scholar]
- 6.Brill R. W.1987On the standard metabolic rates of tropical tunas; including the effect of body size and acute temperature-change. Fish. Bull. 85, 25–35 [Google Scholar]
- 7.Blank J. M., Morrissette J. M., Landeira-Fernandez A. M., Blackwell S. B., Williams T. D., Block B. A.2004In situ cardiac performance of Pacific bluefin tuna hearts in response to acute temperature change. J. Exp. Biol. 207, 881–890 10.1242/jeb.00820 (doi:10.1242/jeb.00820) [DOI] [PubMed] [Google Scholar]
- 8.Korsmeyer K. E., Lai N. C., Shadwick R. E., Graham J. B.1997Heart rate and stroke volume contribution to cardiac output in swimming yellowfin tuna: response to exercise and temperature. J. Exp. Biol. 200, 1975–1986 [DOI] [PubMed] [Google Scholar]
- 9.Galli G. L., Shiels H. A., Brill R. W.2009Temperature sensitivity of cardiac function in pelagic fishes with different vertical mobilities: yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus), mahimahi (Coryphaena hippurus), and swordfish (Xiphias gladius). Physiol. Biochem. Zool. 82, 280–290 10.1086/597484 (doi:10.1086/597484) [DOI] [PubMed] [Google Scholar]
- 10.Vornanen M., Shiels H. A., Farrell A. P.2002Plasticity of excitation–contraction coupling in fish cardiac myocytes. Comp. Biochem. Physiol. A 132, 827–846 [DOI] [PubMed] [Google Scholar]
- 11.Aho E., Vornanen M.1998CaATPase activity and Ca uptake by sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J. Exp. Biol. 201, 525–532 [DOI] [PubMed] [Google Scholar]
- 12.Aho E., Vornanen M.1999Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout (Oncorhynchus mykiss): effects of thermal acclimation. J. Exp. Biol. 202, 2663–2677 [DOI] [PubMed] [Google Scholar]
- 13.Keen J. E., Farrell A. P., Tibbits G. F., Brill R. W.1992Cardiac physiology in tunas. 2. Effect of ryanodine, calcium, and adrenaline on force frequency relationships in atrial strips from skipjack tuna, Katsuwonus pelamis. Can. J. Zool. 70, 1211–1217 10.1139/z92-168 (doi:10.1139/z92-168) [DOI] [Google Scholar]
- 14.Keen J. E., Vianzon D. M., Farrell A. P., Tibbits G. F.1994Effect of temperature and temperature-acclimation on the ryanodine sensitivity of the trout myocardium. J. Comp. Physiol. B 164, 438–443 10.1007/BF00714580 (doi:10.1007/BF00714580) [DOI] [Google Scholar]
- 15.Rocha M. L., Rantin F. T., Kalinin A. L.2007Importance of the sarcoplasmic reticulum and adrenergic stimulation on the cardiac contractility of the neotropical teleost Synbranchus marmoratus under different thermal conditions. J. Comp. Physiol. B 177, 713–721 10.1007/s00360-007-0166-3 (doi:10.1007/s00360-007-0166-3) [DOI] [PubMed] [Google Scholar]
- 16.Shiels H. A., Freund E. V., Farrell A. P., Block B. A.1999The sarcoplasmic reticulum plays a major role in isometric contraction in atrial muscle of yellowfin tuna. J. Exp. Biol. 202, 881–890 [DOI] [PubMed] [Google Scholar]
- 17.Castilho P. C., Landeira-Fernandez A. M., Morrissette J., Block B. A.2007Elevated CaATPase (SERCA2) activity in tuna hearts: comparative aspects of temperature dependence. Comp. Biochem. Physiol. A 148, 124–132 [DOI] [PubMed] [Google Scholar]
- 18.Landeira-Fernandez A. M., Morrissette J. M., Blank J. M., Block B. A.2004Temperature dependence of the Ca2+-ATPase (SERCA2) in the ventricles of tuna and mackerel. Am. J. Physiol. 286, R398–R404 [DOI] [PubMed] [Google Scholar]
- 19.Di Maio A., Block B. A.2008Ultrastructure of the sarcoplasmic reticulum in cardiac myocytes from Pacific bluefin tuna. Cell Tissue Res. 334, 121–134 10.1007/s00441-008-0669-6 (doi:10.1007/s00441-008-0669-6) [DOI] [PubMed] [Google Scholar]
- 20.Shiels H. A., White E.2005Temporal and spatial properties of cellular Ca flux in trout ventricular myocytes. Am. J. Physiol. 288, R1756–R1766 [DOI] [PubMed] [Google Scholar]
- 21.Galli G. L., Lipnick M. S., Block B. A.2009Effect of thermal acclimation on action potentials and sarcolemmal K+ channels from Pacific bluefin tuna cardiomyocytes. Am. J. Physiol. 297, R502–R509 [DOI] [PubMed] [Google Scholar]
- 22.Shiels H. A., Vornanen M., Farrell A. P.2000Temperature-dependence of L-type Ca channel current in atrial myocytes from rainbow trout. J. Exp. Biol. 203, 2771–2780 [DOI] [PubMed] [Google Scholar]
- 23.Herve J. C., Yamaoka K., Twist V. W., Powell T., Ellory J. C., Wang L. C.1992Temperature dependence of electrophysiological properties of guinea pig and ground squirrel myocytes. Am. J. Physiol. 263, 177–184 [DOI] [PubMed] [Google Scholar]
- 24.Puglisi J. L., Yuan W. L., Bassani J. W. M., Bers D. M.1999Ca influx through Ca channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circ. Res. 85, E7–E16 [DOI] [PubMed] [Google Scholar]
- 25.Hove-Madsen L., Llach A., Tort L.2001The function of the sarcoplasmic reticulum is not inhibited by low temperatures in trout atrial myocytes. Am. J. Physiol. 281, R1902–R1906 [DOI] [PubMed] [Google Scholar]
- 26.Shiels H. A., Vornanen M., Farrell A. P.2002Temperature dependence of cardiac sarcoplasmic reticulum function in rainbow trout myocytes. J. Exp. Biol. 205, 3631–3639 [DOI] [PubMed] [Google Scholar]
- 27.Sitsapesan R., Montgomery R. A. P., Macleod K. T., Williams A. J.1991Sheep cardiac sarcoplasmic-reticulum calcium-release channels: modification of conductance and gating by temperature. J. Physiol. 434, 469–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiels H. A., Paajanen V., Vornanen M.2006Sarcolemmal ion currents and sarcoplasmic reticulum Ca2+ content in ventricular myocytes from the cold stenothermic fish, the burbot (Lota lota). J. Exp. Biol. 209, 3091–3100 10.1242/jeb.02321 (doi:10.1242/jeb.02321) [DOI] [PubMed] [Google Scholar]
- 29.Graham M. S., Farrell A. P.1989The effect of temperature-acclimation and adrenaline on the performance of a perfused trout heart. Physiol. Zool. 62, 38–61 [Google Scholar]
- 30.Haverinen J., Vornanen M.2009Responses of action potential and K+ currents to temperature acclimation in fish hearts: phylogeny or thermal preferences? Physiol. Biochem. Zool. 82, 468–482 10.1086/590223 (doi:10.1086/590223) [DOI] [PubMed] [Google Scholar]
- 31.Shiels H. A., Farrell A. P.1997The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J. Exp. Biol. 200, 1607–1621 [DOI] [PubMed] [Google Scholar]
- 32.Bowler K., Tirri R.1990Temperature dependence of the heart isolated from the cold or warm acclimated perch (Perca fluviatilis). Comp. Biochem. Physiol. A 96, 177–180 10.1016/0300-9629(90)90061-V (doi:10.1016/0300-9629(90)90061-V) [DOI] [Google Scholar]
- 33.Fabiato A.1983Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 245, 1–14 [DOI] [PubMed] [Google Scholar]
- 34.Shiels H. A., Blank J. M., Farrell A. P., Block B. A.2004Electrophysiological properties of the L-type Ca2+ current in cardiomyocytes from bluefin tuna and Pacific mackerel. Am. J. Physiol. 286, R659–R668 [DOI] [PubMed] [Google Scholar]
- 35.Cheng H., Lederer W. J., Cannell M. B.1993Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science 262, 740–744 10.1126/science.8235594 (doi:10.1126/science.8235594) [DOI] [PubMed] [Google Scholar]
- 36.Loughrey C. M., MacEachern K. E., Cooper J., Smith G. L.2003Measurement of the dissociation constant of Fluo-3 for Ca2+ in isolated rabbit cardiomyocytes using Ca2+ wave characteristics. Cell Calcium 34, 1–9 10.1016/S0143-4160(03)00012-5 (doi:10.1016/S0143-4160(03)00012-5) [DOI] [PubMed] [Google Scholar]
- 37.Woodruff M. L., Sampath A. P., Matthews H. R., Krasnoperova N. V., Lem J., Fain G. L.2002Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542, 843–854 10.1113/jphysiol.2001.013987 (doi:10.1113/jphysiol.2001.013987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belke D. D., Milner R. E., Wang L. C.1991Seasonal variations in the rate and capacity of cardiac SR calcium accumulation in a hibernating species. Cryobiology 28, 354–363 10.1016/0011-2240(91)90042-M (doi:10.1016/0011-2240(91)90042-M) [DOI] [PubMed] [Google Scholar]
- 39.Walli A., Teo S. T. H., Boustany A., Farwell C. J., Williams T., Dewar H., Prince E., Block B. A.2009Seasonal movements, aggregations and diving behavior of Atlantic bluefin tuna (Thunnus thynnus) revealed with archival tags. PLoS ONE 4, e6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dibb K. M., Hagarty C. L., Loudon A. S., Trafford A. W.2005Photoperiod-dependent modulation of cardiac excitation contraction coupling in the Siberian hamster. Am. J. Physiol. 288, R607–R614 [DOI] [PubMed] [Google Scholar]
- 41.Yatani A., Kim S. J., Kudej R. K., Wang Q., Depre C., Irie K., Kranias E. G., Vatner S. F., Vatner D. E.2004Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am. J. Physiol. 286, H2219–H2228 [DOI] [PubMed] [Google Scholar]
- 42.Wier W. G., Balke C. W.1999Ca2+ release mechanisms, Ca2+ sparks, and local control of excitation–contraction coupling in normal heart muscle. Circ. Res. 85, 770–776 [DOI] [PubMed] [Google Scholar]
- 43.Tiitu V., Vornanen M.2002Regulation of cardiac contractility in a stenothermal fish, the burbot (Lota lota). J. Exp. Biol. 205, 1597–1606 [DOI] [PubMed] [Google Scholar]
- 44.Birkedal R., Christopher J., Thistlethwaite A., Shiels H. A.2009Temperature acclimation has no effect on ryanodine receptor expression or subcellular localization in rainbow trout heart. J. Comp. Physiol. B 179, 961–969 10.1007/s00360-009-0377-x (doi:10.1007/s00360-009-0377-x) [DOI] [PubMed] [Google Scholar]
- 45.Tiitu V., Vornanen M.2003Ryanodine and dihydropyridine receptor binding in ventricular cardiac muscle of fish with different temperature preferences. J. Comp. Physiol. B 173, 285–291 10.1007/s00360-003-0334-z (doi:10.1007/s00360-003-0334-z) [DOI] [PubMed] [Google Scholar]
- 46.Korajoki H., Vornanen M.2009Expression of calsequestrin in atrial and ventricular muscle of thermally acclimated rainbow trout. J. Exp. Biol. 212, 3403–3414 10.1242/jeb.031617 (doi:10.1242/jeb.031617) [DOI] [PubMed] [Google Scholar]
- 47.Driedzic W. R., Gesser H.1994Energy-metabolism and contractility in ectothermic vertebrate hearts: hypoxia, acidosis, and low-temperature. Physiol. Rev. 74, 221–258 [DOI] [PubMed] [Google Scholar]