Abstract
As environments change, animals update their internal representations of the external world. New information about the environment is learned and retained whereas outdated information is disregarded or forgotten. Retroactive interference (RI) occurs when the retrieval of previously learned information is less available owing to the acquisition of recently acquired information. Even though RI is thought to be a major cause of forgetting, its functional significance is still under debate. We find that natural allelic variants of the Drosophila melanogaster foraging gene known to affect rover and sitter behaviour differ in RI. More specifically, rovers who were previously shown to experience greater environmental heterogeneity while foraging display RI whereas sitters do not. Rover responses are biased towards more recent learning events. These results provide an ecological context to investigate the function of forgetting via RI and a suitable genetic model organism to address the evolutionary relevance of cognitive tasks.
Keywords: Drosophila, environmental variation, memory, retroactive interference
1. Introduction
Animals need to obtain and store new information and also forget older information. Forgetting results when information decays with time or by interference [1–5]. Interference happens when information learned at one point in time conflicts with information learned at another point in time. Retroactive interference (RI) occurs when the retrieval of previously learned information is hindered by more recently acquired information. Proactive interference (PI) occurs when older information inhibits the potential acquisition of new memory [6]. RI has been measured experimentally in mammals and social insects [7]; however, the functional significance of RI in animal behaviour is under debate [8,9]. While some authors suggest that RI is a constraint on cognition [10–12] others propose that RI is an adaptive process facilitating memory updating under appropriate conditions [13,14]. An existing memory is updated when new information is substituted for less current information. Updating memory can be more efficient than forming entirely new memories. Such cognitive flexibility is thought to be critical for organisms living in changing environments [15,16]. Therefore, linking cognitive flexibility and environmental change should provide a glimpse into the evolution of cognition.
The Drosophila melanogaster foraging gene (for) encodes a cGMP-dependent protein kinase (PKG). Natural variation in for gives rise to the rover and sitter behavioural variants [17] known to differ in a suite of behavioural and metabolic traits including food-related movement patterns as well as learning and memory in larval and adult stages [18]. Adult flies with a rover allele (forR) have higher PKG activities in their heads than those homozygous for sitter alleles (fors). Rover and sitter allelic frequencies are affected by environmental heterogeneity [19], are density dependent [20] and are subject to frequency-dependent selection at the larval stage [21], suggesting that rover and sitter foraging variants are subject to selection in nature.
The number and distribution of food patches available to D. melanogaster varies in time and space according to, for example, the type and maturity of the fruit and the extent of fermentation it has undergone [22]. Larvae and adult rovers leave food patches more readily than sitters [19,23,24] and they differ in their post-feeding search behaviour. Rovers transition from local search (high turning rate and low patch leaving) to ranging (high turning rate and high patch leaving) more rapidly than sitters. Rovers also visit more and farther patches than sitters and tend not to revisit previous patches [23–27]. These behavioural differences strongly suggest that rovers experience greater environmental heterogeneity over time while foraging compared with sitters [26].
Fundamental properties of memory such as interference probably function adaptively to reflect characteristics of an animal's world. More specifically, the temporal resolution of memory should reflect the probable usefulness of the information that is remembered. As rovers experience greater temporal heterogeneity in nature, we expect rovers to place less value on older information compared with sitters. Based on this, we predicted that rovers will perform better than sitters in response to their most recent learning experience. Here, we compared rovers and sitters for passive memory decay and for performance in two separate interference paradigms.
2. Material and methods
(a). Fly strains
The rover (forR) and sitter (fors) natural allelic variants of the second chromosome foraging (for) gene were used along with the fors2 strain, a sitter mutant generated on a rover genetic background. As mentioned above, for encodes PKG. forR rovers have significantly higher PKG enzyme activities in adult heads than do fors and fors2 sitters [17]. The percentage differences in PKG enzyme activity in adult rover and sitter heads is approximately 10 per cent [17]; in well-fed rover and sitter larvae it is 40 per cent [28]. The precise DNA polymorphism in for that is responsible for the natural rover and sitter behavioural differences is not known. fors2 controls for genetic background differences between the rover (forR) and sitter (fors) strains. X and third chromosomes from the natural rover variant were substituted into the natural sitter variant and mutant sitter strain. Flies were cultured on a standard cornmeal medium at 12 L : 12 D. Flies were bred, trained and tested at 25°C and never anesthetized.
(b). Learning assays
We used a Pavlovian olfactory conditioning paradigm where flies learned to associate one or more odours with a mechanical shock in all experiments [29]. Previous work [30,31] showed that variation in performance in this conditioning protocol could also be generalized to other aversive reinforcements. This classical conditioning paradigm allows us to control the amount of shock and odours received by the flies and to dissect the memory dynamics.
Both training and testing were performed on mixed-sex groups of about 100 adult flies aged 3–5 days post-ecolosion. The basic training protocol consisted of a single training cycle divided into four steps.
— Flies were exposed to a first odour CS+ (CS: conditioned stimulus) for 1 min along with mechanical shock, 1 s every 5 s.
— Flies received a 1 min rest period without odour or shock.
— Flies were exposed to a second odour CS− for 1 min without shock.
— Flies were given another 1 min rest period.
Training A+ /B− indicates that, in the same training cycle, A was used as the odour associated with the shock whereas B was the odour not associated with the shock. A, B, C and D can be any of the odours described below except when specified. Previous experiments, where the CS− was administrated first and the CS+ second, showed that the three Drosophila strains did not differ in their ‘susceptibility’ to the event sequence (see the electronic supplementary material, figure S1).
Four moderately repulsive odours were used: 3-octanol (SIGMA CAS number: 589-98-0; 0.6 ml l−1 of paraffin), 4-methylcyclohexanol (SIGMA CAS number: 589-91-3; 0.6 ml l−1 of paraffin), isoamyl acetate (SIGMA CAS number: 123-92-2; 0.25 ml l−1 of paraffin) and ethyl acetate (SIGMA CAS number: 141-78-6; 0.25 ml l−1 of paraffin).
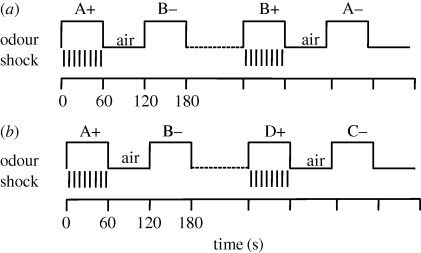
We used this paradigm to measure passive memory decay and to test the flies in two separate interference paradigms: a reversal learning paradigm (figure 1a) and a multiple association learning paradigm (figure 1b). Each consisted of two training sessions. In the reversal paradigm, flies were first trained A+ /B− and then trained with the reciprocal B+ /A−. In the multiple associations learning paradigm flies were first trained with one odour pair A+ /B− and then trained with another odour pair D+ /C−.
Figure 1.
The time course of (a) reversal and (b) interference paradigms. Flies were exposed to one odour (A+ ) and simultaneously subject to mechanical shocks. After a 60 s pause, during which they received clean air, they were exposed to another odour (B−) without shock and then again to a 60 s pause. 0, 20, 40 or 60 min after (a) or immediately after (b) flies were exposed to a second conditioning cycle using either the same odour pair but reversed ((a), B+ , A−) or using a different odour pair ((b), D+ , C−). Flies were immediately tested.
(c). Passive memory decay
For passive memory decay assessment, the odours 3-octanol and 4-methylcyclohexanol were used. Flies received one training cycle and were then tested for their memory scores at different time points (0, 20, 40 and 80 min). For each test, flies were transferred to the choice point of a T-maze and exposed to two converging currents of air (one carrying octanol and the other methylcyclohexanol). They were allowed to choose between the two odours for 60 s. The number of flies in each arm of the maze after 60 s was used to calculate the proportion of flies choosing (i.e. moving towards) octanol; flies that remained in the entry chamber of the T-maze were excluded from this calculation.
For the analysis, a unit of replication consisted of two samples of 50 flies. One sample was trained to avoid octanol and the other to avoid methylcyclohexanol; a single value memory index was calculated as the difference in the proportion of flies choosing octanol between these two samples. Scores range from −1 (all flies went towards the odour given with the shock) to 1 (all flies avoided the odour given with the shock). A score of 0 indicates no response to training. For statistical comparison of the learning scores (but not for graphical representation of the data) all proportions were arcsine-square-root-transformed before the analyses [32]. Memory scores were analysed using ANOVA including a random block factor, time between training and test as covariate and strain as factors.
(d). Reversal learning assay
For the reversal paradigm, 3-octanol and 4-methylcyclohexanol were used. The flies received a first training cycle A+ /B−. Then they received a second training cycle of the reverse, B+ /A−. The second training was carried out at different time points: 0, 20, 40 or 80 min after the first training cycle. Flies were tested immediately following the second training cycle. In half of the replicates ‘A’ was 3-octanol, in the other half ‘A’ was 4-methylcyclohexanol. This was to control for any potential odour bias. Reversal memory scores were calculated as the difference in the proportion of flies choosing octanol when trained to avoid methylcyclohexanol minus the proportion of flies choosing octanol when trained to avoid octanol in the second reverse training. Memory scores were analysed using ANOVA including a random block factor with time between two consecutive training trials as a covariate and strain as fixed factor.
(e). Multiple associations learning assay
All four odours were used in the multiple associations learning assay. Flies received a first training cycle A+ /B− with either the odour pair octanol–methylcyclohexanol or the odour pair isoamyl acetate–ethyl acetate. Results from the reversal learning assay (see below) indicated that the highest level of interference was observed when reversal was performed immediately after the first training. For this reason, following the first training, flies were immediately given a second training cycle C+ /D− with a different odour pairing. All reciprocal combinations were performed (see table 1). Testing immediately followed the second training trial. Controls were simultaneously performed where only a single training using one odour pair was used.
Table 1.
Interference paradigm. Representation of all odour combinations performed for the two conditioning cycles. (For graphical representation, we averaged the memory scores over all experimental or control combinations. OCT: 3-Octanol; MCH: 4-methylcyclohexanol; EA: ethyl-acetate; IAA: isoamyl acetate.)
| first training |
second training |
||||
|---|---|---|---|---|---|
| odour paired with shock | odour not paired with shock | odour paired with shock | odour not paired with shock | test | |
| OCT | MCH | IAA | EA | OCT versus MCH | test of first training |
| OCT | MCH | EA | IAA | OCT versus MCH | |
| MCH | OCT | IAA | EA | OCT versus MCH | |
| MCH | OCT | EA | IAA | OCT versus MCH | |
| IAA | EA | OCT | MCH | IAA versus EA | |
| IAA | EA | MCH | OCT | IAA versus EA | |
| EA | IAA | OCT | MCH | IAA versus EA | |
| EA | IAA | MCH | OCT | IAA versus EA | |
| OCT | MCH | IAA | EA | IAA versus EA | test of second training |
| OCT | MCH | EA | IAA | IAA versus EA | |
| MCH | OCT | IAA | EA | IAA versus EA | |
| MCH | OCT | EA | IAA | IAA versus EA | |
| IAA | EA | OCT | MCH | OCT versus MCH | |
| IAA | EA | MCH | OCT | OCT versus MCH | |
| EA | IAA | OCT | MCH | OCT versus MCH | |
| EA | IAA | MCH | OCT | OCT versus MCH | |
| IAA | EA | IAA versus EA | control | ||
| EA | IAA | IAA versus EA | |||
| OCT | MCH | OCT versus MCH | |||
| MCH | OCT | OCT versus MCH | |||
Flies were tested by presenting either the first A versus B or the second C versus D odour pair. The proportion of flies choosing octanol or ethyl-acetate (depending on the test performed and the odour pair associated) was measured. Memory scores for the first or second association were then calculated as the difference in the proportion of individuals choosing octanol or ethyl-acetate when not trained to avoid this odour and when they were trained to avoid it. Memory scores were analysed using ANOVA including a random block factor, treatment (control, first training, and second training) and strain as fixed factor.
We controlled for the potential effect of a second learning experience versus the presentation of another odour pair or the presentation of mechanical shock alone. Flies were first trained with an odour pair (octanol–methylcyclohexanol or the odour pair isoamyl acetate–ethyl acetate) and then subjected to a second ‘pseudoconditioning’ cycle either without any mechanical shock but the presentation of another odour pair, or without any odour presentation but with a mechanical shock. The timing of odour presentation or mechanical shock was always maintained.
Further controls were used to disentangle the effect of the CS+ position (first or second) and the CS− position. We also trained flies A+ /B−, then C+ /D− and tested A versus D and B versus C and compared the results with the tests A versus B and C versus D.
We also tested whether repeated training trials would affect interference level in a multiple association assay. Flies were first subjected to one training cycle A+ /C− and then to another cycle D+ /C− immediately after. This procedure was repeated three times without rest intervals. As a control, flies were trained three consecutive times to associate a single odour with shock by being trained either on A + /C− or D+ /C−.
3. Results
(a). Passive memory decay
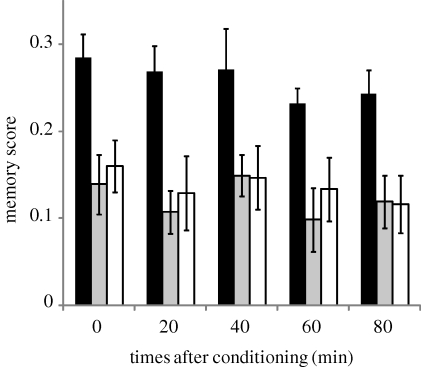
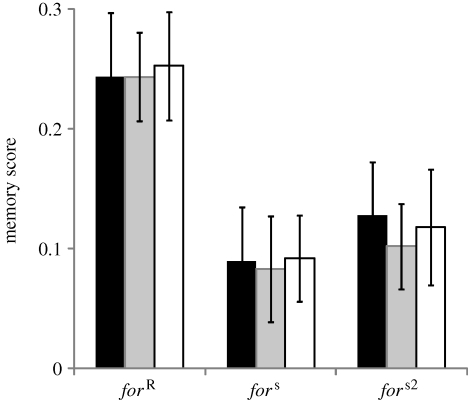
Following a single training event, rovers and sitters did not show any significant reductions in memory over 80 min (figure 2; ANOVA time effect, forR: F1,51 = 1.9, p = 0.17; fors: F1,51 = 0.3, p = 0.56; fors2: F1,51 = 1.9, p = 0.17) and, despite the overall strong difference between rover and sitter memory scores, no difference in the slope of the memory scores were observed (ANOVA line: F2,177 = 10.6, p < 10−3; time: F1,177 = 3.6, p = 0.06; line × time: F2,177 = 0.32, p = 0.73).
Figure 2.
Passive memory decay of the for strains after a single conditioning cycle. Error bars represent standard error of the mean memory score (±s.e.m.; n = 13 for each bar; black bars, forR; grey bars, fors; white bars, fors2).
(b). Reversal learning assay
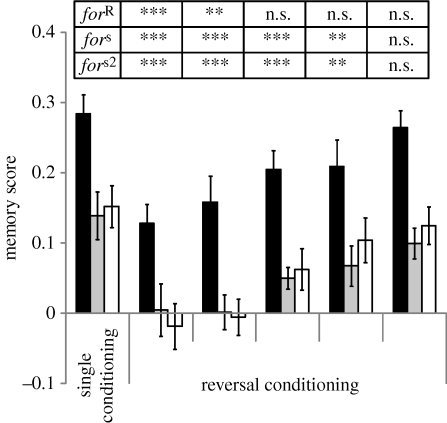
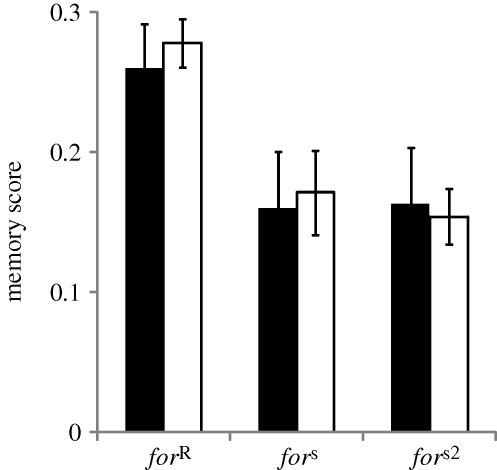
We observed a strong effect of the first training on the reversed second training especially when the time between the two training events was brief. As the time between the first and the reversal training increased, both rovers and sitters showed improved reversal memory scores and this improvement did not differ among lines (figure 3; ANOVA, line: F2,177 = 12.4, p < 10−3; time: F1,177 = 27.6, p < 10−3; line × time: F2,177 = 0.35, p = 0.70). This suggests that PI affects both rovers and sitters and that this effect vanished over the time between the first and second training.
Figure 3.
Reversal learning in the for strains over time (min). Flies were trained once and either tested immediately after (single conditioning) or subjected to reversal conditioning at different time points (reversal conditioning) before being immediately tested. Top table indicates comparison of memory scores between single conditioning and each reversal conditioning for each strain (two-sided Dunnett test with single conditioning as control category: ***p < 0.01, **0.01 < p < 0.05, n.s. p > 0.05). Error bars represent standard error of the mean memory score (±s.e.m.; n = 13 for each bar; black bars, forR; grey bars, fors; white bars, fors2).
(c). Multiple associations learning assay
Since interference between training events was greatest at short intervals, we exposed the flies to a multiple association learning assay which involved an A+ /B− training cycle followed immediately by a C+ /D− training cycle. We then tested memory for the first or second association.
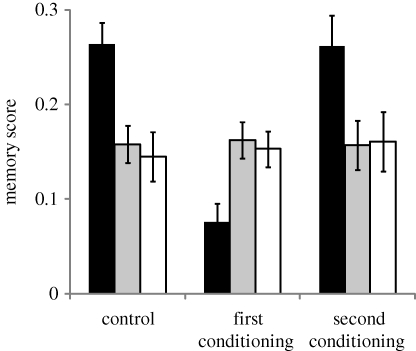
In this assay, rovers and sitters did not show any PI; i.e. the response to the second trained odour-pair was not impaired by the first. Their performance on the second trained odour-pair did not differ from controls that had no prior training (figure 4; ANOVA treatment: F1,165 = 0.01, p = 0.9; line: F2,165 = 16.6, p < 10−3; treatment × line: F2,165 = 0.18, p = 0.8). However, performance tests of the first trained odour-pair supported the hypothesis that rovers but not sitters display RI. That is, rovers learning a new association decreased their response on the first training (figure 4, first-second training comparison; ANOVA, F1,49 = 9.96, p < 0.001). By contrast, sitters showed no difference in response when asked to recall either the first or second training event (fors: F1,49 = 0.28, p = 0.75; fors2: F1,49 = 0.11, p = 0.85) indicating that sitters do not show RI. Replacing the second training trial by either the aversive mechanical shock stimulus only or a second odour-pair alone did not affect the performance on the first training for the rover and sitter strains (figure 5). This suggests that the observed rover RI effect is specific to learning new information and is not a function of non-learning-based stimuli (the conditioned or unconditioned stimulus alone).
Figure 4.
Multiple association learning assay. Memory scores for the first or the second trained odour-pair in the retroactive interference experiment. Rovers (forR) place significantly more emphasis on their most recent trained odour-pair. By contrast, sitters (fors) and sitter mutants (fors2), remember their first and second trained odour-pair similarly. Control flies did not receive a second odour-pair conditioning. The control represents flies trained only once and tested at the same time as flies trained twice. Error bars represent ±s.e.m. (n = 20 for each bar; black bars, forR; grey bars, fors; white bars, fors2).
Figure 5.
Controls for the multiple association learning assay. Replacing the second training trial by either the aversive mechanical shock stimulus only (grey bars) or a second odour-pairing alone (white bars) did not affect the performance on the first training for both rover and sitter strains (treatment, fors2: F2,32 = 0.11, p = 0.89; fors: F2,32 = 0.55, p = 0.58; forR: F2,32 = 0.29, p = 0.75). Error bars represent ±s.e.m. (n = 12 for each bar; black bars, control; grey bars, shock only; white bars, odour only).
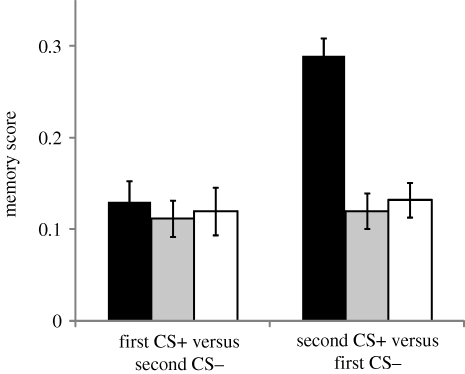
Interestingly, when the flies were tested for ‘first CS+ versus second CS−’ or ‘second CS+ versus first CS−’ we found that the position of the CS + , but not of the CS−, had the strongest effect on rover RI (figure 6). Rover memory score was lower when presented ‘first CS+ versus second CS−’ compared with ‘second CS+ versus first CS−’ but sitter memory scores were not affected. Thus, the effect of the CS− in interference seems to be relatively low.
Figure 6.
Controls for the multiple association learning assay. Memory score when presenting to the flies either the first CS+ versus second CS−, or the second CS+ versus first CS− after a two association learning assay. Error bars represent ±s.e.m. (n = 12 for each bar; black bars, forR; grey bars, fors; white bars, fors2).
We then asked whether, when repeatedly trained for two different CS+, RI induces or not a decrease of the learning scores. Rovers showed no difference in the responses to an odour whether learned individually (A+ C−) or simultaneously with another odour (A+ C− D+ C−) (figure 7; ANOVA comparison control-multiple training: forR: F1,38 = 0.19, p = 0.60; fors: F1,38 = 0.06, p = 0.8; fors2: F1,38 = 0.06, p = 0.80). This suggests that the RI effect observed in rovers resulted from learning two associations separated in time. However, the number of repeated presentations of different CS+ affects RI. When these two associations were repeatedly learned the RI effect vanished, suggesting no memory constraint.
Figure 7.
Controls for the multiple association learning assay. The simultaneous conditioning experiment shows that rovers (forR), sitters (fors) and sitter mutants (fors2) were able to perform similarly in single and double conditioning trials. The memory scores do not differ even when flies were required to learn more information in the double conditioning. Error bars represent ±s.e.m. (n = 20 for each bar; black bars, single training; white bars, double training).
4. Discussion
Behavioural-ecologists and psychologists have only recently investigated the adaptive significance of learning by studying the relationships between learning, memory and the natural environment (reviewed in Shettleworth [33]). Here, we present evidence of genetic variation in RI associated with different foraging behaviours. Rovers show better learning ability compared with sitters [30,31]. However, within 80 min we could not detect any passive memory decay in any of the strains. The reversal assay showed that PI affected rover and sitters similarly and was strongest when reversal was carried out immediately after learning. This diminished gradually when the time between the two training sessions was increased. In the multiple association assay rovers and sitters showed no detectable PI even if the second training was performed immediately after the first one. This is in accordance with previous studies [34,35] which show that the degree of interference may depend on the type of task and on the similarity between the tasks. However, rovers showed strong RI compared with sitters. Interestingly this RI effect disappeared when the two associations were repeatedly learned, which supports the idea that RI is not a memory loss of the first association [36,37].
This research provides new insight into the potential relationships between animal cognition and adaptation to environmental conditions. Rovers showed better learning scores after a single training, and their response was more accurately predicted by their more recent experience. By contrast, sitters accumulated experience over time and appeared to treat these experiences similarly. Little is known about the mechanisms underlying RI. Vertebrate studies suggest the involvement of serotonin [38,39]; it is unclear whether serotonin plays a role in insect learning ([40–43]; but see [44]). Interestingly, PKG is thought to modulate serotonin (5-hydroxytryptamine; 5-HT) transporter activity [43]. Shuai et al. [45] found that a small G protein Rac-dependent contributes both to passive memory decay and RI level and suggested that both forms of forgetting share a common mechanism. However, in the present study although we did find differences in RI between rovers and sitters, we did not observe any difference in passive memory decay or PI, suggesting that RI may be independently regulated. The paradigms developed here using the Drosophila genetic model facilitate research into the mechanisms responsible for these types of cognitive flexibility.
From an adaptive perspective, the relationship between learning speed and retention has been the source of intensive debate [46–48]. The connectionist theory predicts that high learning rate is associated with high RI [49]. In the present study, we found that rovers which have higher learning abilities [30,31] also show increased RI, suggesting a trade-off between acquisition and retention. In vertebrates, support for this trade-off has been mixed [50–53].
Comparisons among closely related species have shown relationships between variation in learning abilities and, searching strategies [54] or environmental diversity [55]. These studies suggest that memory retention is likely to be beneficial when an organism frequently encounters similar environmental conditions [56–58]. However, when the probability of re-encountering similar conditions is low, information should be ignored or learning transitory. Interestingly, when the probability of encountering a similar resource is unpredictable (high environmental heterogeneity), natural selection in favour of active updating processes such as RI may be more beneficial than the evolution of specific passive memory decay.
The adaptive significance of rover and sitter learning phenotypes may be related to their differences in foraging behaviour. Rovers move more rapidly through the foraging environment, encountering new food patches more frequently than sitters and therefore experience greater environmental heterogeneity. This has led to an increased bias in rovers to respond to more recent learning events for which the reliability is the strongest. In this case, RI may serve as a gating mechanism to prevent accumulation of non-reliable information. However, RI may not be beneficial when individuals frequently meet similar non-contradictory environmental conditions. In this case, storing all information may be the best strategy. Interestingly, when rovers were repetitively trained to avoid one odour and then another, they did not significantly differ in their subsequent avoidance of each odour (figure 7). This suggests that RI does not occur when multiple environmental associations are commonly encountered. We suggest that the adaptive significance of RI may be observed under strong environmental heterogeneity when learned information is only temporarily valid.
Our results suggest that for plays a critical role in modulating learned responses to environmental heterogeneity while foraging. Questions regarding phenomena such as interference [6,36], adaptive forgetting and retrieval inhibition [15] can now be subjected to the powerful genetic tools of Drosophila as well as examined from an evolutionary perspective using the for model.
Acknowledgements
We thank C. Moreno for laboratory assistance and J.G. Burns, S. Douglas, B. Hughson, T. Preat, C. Rankin, C. Riedl, S. Shettleworth and E. Snell-Rood for useful comments on previous versions of the manuscript. The work was supported by an ATIP grant from the Life Sciences Division of the Center National de la Recherche Scientifique and from the European Research Council under the European Community's Seventh Framework Program (FP7/2007-2013) /ERC Grant agreement no. 209 540 to F.M., and a National Sciences and Engineering Research (NSERC) grant to M.B.S. and an NSERC fellowship to C.J.R.
References
- 1.Bouton M. E.2007Learning and behaviour: a contemporary synthesis, p. 150 Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Mensink G. J., Raaijmakers J. G. W.1988A model for interference and forgetting. Psychol. Rev. 95, 434–445 10.1037/0033-295X.95.4.434 (doi:10.1037/0033-295X.95.4.434) [DOI] [Google Scholar]
- 3.Waugh N. C., Norman D. A.1965Primary memory. Psychol. Rev. 72, 89–104 10.1037/h0021797 (doi:10.1037/h0021797) [DOI] [PubMed] [Google Scholar]
- 4.Wixted J. T.2004The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 55, 235–269 10.1146/annurev.psych.55.090902.141555 (doi:10.1146/annurev.psych.55.090902.141555) [DOI] [PubMed] [Google Scholar]
- 5.Jonides J., Lewis R. L., Nee D. E., Lustig C. A., Berman M. G., Moore K. S.2008The mind and brain of short-term memory. Annu. Rev. Psychol. 59, 193–224 10.1146/annurev.psych.59.103006.093615 (doi:10.1146/annurev.psych.59.103006.093615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouton M. E.1993Context, time and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 114, 80–99 10.1037/0033-2909.114.1.80 (doi:10.1037/0033-2909.114.1.80) [DOI] [PubMed] [Google Scholar]
- 7.Anderson M. C.2003Rethinking interference theory: executive control and the mechanisms of forgetting. J. Mem. Lang. 49, 415–445 10.1016/j.jml.2003.08.006 (doi:10.1016/j.jml.2003.08.006) [DOI] [Google Scholar]
- 8.Barrouillet P., Camos V.2009Interference: unique source of forgetting in working memory? Trends Cogn. Sci. 13, 145–146 10.1016/j.tics.2009.01.002 (doi:10.1016/j.tics.2009.01.002) [DOI] [PubMed] [Google Scholar]
- 9.Lewandowsky S., Oberauer K., Brown G. D.2009No temporal decay in verbal short-term memory. Trends Cogn. Sci. 13, 120–126 10.1016/j.tics.2008.12.003 (doi:10.1016/j.tics.2008.12.003) [DOI] [PubMed] [Google Scholar]
- 10.Krushke J. K.2001Toward a unified model of attention in associative learning. J. Math. Psychol. 45, 812–863 [Google Scholar]
- 11.Laughlin K., Mendl M.2004Costs of acquiring and forgetting information affect spatial memory and its susceptibility to interference. Anim. Behav. 68, 97–103 10.1016/j.anbehav.2003.10.019 (doi:10.1016/j.anbehav.2003.10.019) [DOI] [PubMed] [Google Scholar]
- 12.Ratcliffe R.1990Connectionist models of recognition memory: constraints imposed by learning and forgetting functions. Psychol. Rev. 97, 285–308 [DOI] [PubMed] [Google Scholar]
- 13.Altmann E. M., Gray W. D.2002Forgetting to remember: the functional relationship of decay and interference. Psychol. Sci. 13, 27–33 10.1111/1467-9280.00405 (doi:10.1111/1467-9280.00405) [DOI] [PubMed] [Google Scholar]
- 14.Anderson J. R., Milson R.1989Human memory: an adaptive perspective. Psychol. Rev. 96, 703–719 10.1037/0033-295X.96.4.703 (doi:10.1037/0033-295X.96.4.703) [DOI] [Google Scholar]
- 15.Kraemer P. J., Golding J. M.1997Adaptive forgetting in animals. Psychon. Bull. Rev. 4, 480–491 [Google Scholar]
- 16.Anderson J. R., Schooler L. J.1991Reflections of the environment in memory. Psychol. Sci. 2, 396–408 10.1111/j.1467-9280.1991.tb00174.x (doi:10.1111/j.1467-9280.1991.tb00174.x) [DOI] [Google Scholar]
- 17.Osborne K. A., Robichon A., Burgess E., Butland S., Shaw R. A., Coulthard A., Pereira H. S., Greenspan R. J., Sokolowski M. B.1997Natural behaviour polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 277, 834–836 [DOI] [PubMed] [Google Scholar]
- 18.Reaume C. J., Sokolowski M. B.2009cGMP-dependent protein kinase as a modifier of behaviour. In cGMP: generators, effects and therapeutic implications (eds Schmidt H. H. W., Hofmann F., Stasch J. P.), pp. 423–443 Berlin, Germany: Springer; [DOI] [PubMed] [Google Scholar]
- 19.Sokolowski M. B.1986Ecological genetics and behaviour of Drosophila melanogaster larvae in nature. Anim. Behav. 34, 403–408 10.1016/S0003-3472(86)80109-9 (doi:10.1016/S0003-3472(86)80109-9) [DOI] [Google Scholar]
- 20.Sokolowski M. B., Pereira H. S., Hughes K.1997Evolution of foraging behaviour in Drosophila by density-dependent selection. Proc. Natl Acad. Sci. USA 94, 7373–7377 10.1073/pnas.94.14.7373 (doi:10.1073/pnas.94.14.7373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick M. J., Feder E., Rowe L., Sokolowski M. B.2007Maintaining a behaviour polymorphism by frequency-dependent selection on a single gene. Nature 447, 210–212 10.1038/nature05764 (doi:10.1038/nature05764) [DOI] [PubMed] [Google Scholar]
- 22.Reaume C. J., Sokolowksi M. B.2006Natural history and ecology of Drosophila melanogaster. Curr. Biol. 16, R623–R628 10.1016/j.cub.2006.07.042 (doi:10.1016/j.cub.2006.07.042) [DOI] [PubMed] [Google Scholar]
- 23.Nagle K. J., Bell W. J.1987Genetic-control of the search tactic of Drosophila melanogaster: an ethometric analysis of rover sitter traits in adult flies. Behav. Gen. 17, 386–408 [DOI] [PubMed] [Google Scholar]
- 24.Pereira H. S., Sokolowski M. B.1993Mutations in the larval foraging gene affect adult locomotory behaviour after feeding in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 90, 5044–5046 10.1073/pnas.90.11.5044 (doi:10.1073/pnas.90.11.5044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell W. J., Tortorici C.1987Genetic and non-genetic control of search duration in adults of two morphs of Drosophila melanogaster. J. Insect Physiol. 33, 51–54 10.1016/0022-1910(87)90103-X (doi:10.1016/0022-1910(87)90103-X) [DOI] [Google Scholar]
- 26.Stamps J. A., Buechner M., Alezander K., Davis J., Zuniga N.2005Genotypic differences in space use and movement patterns in Drosophila melanogaster. Anim. Behav. 70, 609–618 10.1016/j.anbehav.2004.11.018 (doi:10.1016/j.anbehav.2004.11.018) [DOI] [Google Scholar]
- 27.Tortorici C., Bell W. J.1988Search orientation in adult Drosophila melanogaster: responses of rovers and sitters to resource dispersion in a food patch. J. Insect Behav. 1, 209–224 10.1007/BF01052239 (doi:10.1007/BF01052239) [DOI] [Google Scholar]
- 28.Kaun K. R., Riedl C. A. L., Charkaborty-Chatterjee M., Belay A. T., Douglas S., Gibbs A. G., Sokolowski M. B.2007Natural polymorphism in a cGMP-dependent protein kinase affects food intake and absorption in Drosophila. J. Exp. Biol. 210, 3547–3558 10.1242/jeb.006924 (doi:10.1242/jeb.006924) [DOI] [PubMed] [Google Scholar]
- 29.Mery F., Kawecki T. J.2005A cost of long-term memory in Drosophila. Science 308, 1148. 10.1126/science.1111331 (doi:10.1126/science.1111331) [DOI] [PubMed] [Google Scholar]
- 30.Mery F., Belay A. T., So A. K.-C., Sokolowski M. B., Kawecki T. J.2007Natural polymorphism affecting learning and memory in Drosophila. Proc. Natl Acad. Sci. USA 104, 13 051–13 055 10.1073/pnas.0702923104 (doi:10.1073/pnas.0702923104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mery F., Pont J., Preat T., Kawecki T. J.2007Experimental evolution of olfactory memory in Drosophila melanogaster. Physiol. Biochem. Zool. 80, 399–405 10.1086/518014 (doi:10.1086/518014) [DOI] [PubMed] [Google Scholar]
- 32.Sokal R. R., Rohlf F. J.1995Biometry: the principles and practice of statistics in biological research, p. 887, 3rd edn New York, NY: WH Freeman [Google Scholar]
- 33.Shettleworth S. J.1998Cognition, evolution, and behaviour, p. 688 New York, NY: Oxford University Press [Google Scholar]
- 34.Chittka L., Thomson J. D.1997Sensory-motor learning and its relevance for task specialization in bumble bees. Behav. Ecol. Sociobiol. 41, 385–398 10.1007/s002650050400 (doi:10.1007/s002650050400) [DOI] [Google Scholar]
- 35.Laverty T. M.1994Costs to foraging bumble bees of switching plant species. Can. J. Zool. 72, 43–47 10.1139/z94-007 (doi:10.1139/z94-007) [DOI] [Google Scholar]
- 36.Cheng K., Wignall A. E.2006Honeybees (Apis mellifera) holding on to memories: response competition causes retroactive interference effects. Anim. Cogn. 9, 141–150 10.1007/s10071-005-0012-5 (doi:10.1007/s10071-005-0012-5) [DOI] [PubMed] [Google Scholar]
- 37.Chittka L.1998Sensory-motor learning in bumble bees: long term retention and reversal training. J. Exp. Biol. 201, 515–524 [Google Scholar]
- 38.Clarke H. F., Dalley J. W., Crofts H. S., Robbins T. W., Roberts A. C.2004Cognitive inflexibility after prefrontal serotonin depletion. Science 304, 878–880 10.1126/science.1094987 (doi:10.1126/science.1094987) [DOI] [PubMed] [Google Scholar]
- 39.Vallender E. J., Lynch L., Novak M. A., Miller G. M.2009Polymorphisms in the 3′ UTR of the serotonin transporter are associated with cognitive flexibility in rhesus macaques. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsh J.1989Molecular genetics of dopa decarboxylase and biogenic amines in Drosophila. Dev. Genet. 10, 232–238 10.1002/dvg.1020100312 (doi:10.1002/dvg.1020100312) [DOI] [PubMed] [Google Scholar]
- 41.Menzel R., Heyne A., Kinzel C., Gerber B., Fiala A.1999Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav. Neurosci. 113, 744–754 10.1037/0735-7044.113.4.744 (doi:10.1037/0735-7044.113.4.744) [DOI] [PubMed] [Google Scholar]
- 42.Sitaraman D., Zars M., LaFerriere H., Chen Y.-C., Sable-Smith A., Kitamoto T., Rottinghaus G. E., Zars T.2008Serotonin is necessary for place memory in Drosophila. Proc. Natl Acad. Sci. USA 105, 5579–5584 10.1073/pnas.0710168105 (doi:10.1073/pnas.0710168105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu C.-B., Carneiro A. M., Dostmann W. R., Hewlett W. A., Blakely R. D.2004p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 280, 15 649–15 658 10.1074/jbc.M410858200 (doi:10.1074/jbc.M410858200) [DOI] [PubMed] [Google Scholar]
- 44.Yarali A., Krischke M., Michels B., Saumweber T., Mueller M. J., Gerber B.2009Genetic distortion of the balance between punishment and relief learning in Drosophila. J. Neurogenet. 23, 235–247 10.1080/01677060802441372 (doi:10.1080/01677060802441372) [DOI] [PubMed] [Google Scholar]
- 45.Shuai Y., Lu B., Hu Y., Wang L., Sun K., Zhong Y.2010Forgetting is regulated through Rac activity in Drosophila. Cell 140, 579–589 10.1016/j.cell.2009.12.044 (doi:10.1016/j.cell.2009.12.044) [DOI] [PubMed] [Google Scholar]
- 46.Chittka L., Reinhold H.1999Towards an individual based approach to insect learning. In Neurobiology report (eds Elsner N., Eysel U.), p. 257 Goettingen, Germany: Thieme Verlag [Google Scholar]
- 47.Karpicke J. D., Roediger H. L.2008The critical importance of retrieval for learning. Science 319, 966–968 10.1126/science.1152408 (doi:10.1126/science.1152408) [DOI] [PubMed] [Google Scholar]
- 48.McGeoch J. A.1942The psychology of human learning. New York, NY: Longmans, Green [Google Scholar]
- 49.Schneider W.1993Varieties of working memory as seen in biology and in connectionist/control architectures. Mem. Cognit. 21, 184–192 [DOI] [PubMed] [Google Scholar]
- 50.Kyllonen P. C., Tirre W. C.1988Individual differences in associative learning and forgetting. Intelligence 12, 393–421 10.1016/0160-2896(88)90004-9 (doi:10.1016/0160-2896(88)90004-9) [DOI] [Google Scholar]
- 51.MacDonald S. W. S., Stigsdotter-Neely A., Derwinger A., Backman L.2006Rate of acquisition, adult age, and basic cognitive abilities predict forgetting: new views on a classic problem. J. Exp. Psychol. 135, 368–390 10.1037/0096-3445.135.3.368 (doi:10.1037/0096-3445.135.3.368) [DOI] [PubMed] [Google Scholar]
- 52.Slamecka N. J., McElree B.1983Normal forgetting of verbal lists as a function of their degree of learning. J. Exp. Psychol. Learn. Mem. Cogn. 9, 384–397 [Google Scholar]
- 53.Worden B. D., Skemp A. K., Papaj D. R.2005Learning in two contexts: the effects of interference and body size in bumblebees. J. Exp. Biol. 208, 2045–2053 10.1242/jeb.01582 (doi:10.1242/jeb.01582) [DOI] [PubMed] [Google Scholar]
- 54.Smid H. M., Wang G., Bukovinszky T., Steidle J. L. M., Bleeker M. A. K., Van Loon J. J. A., Vet L. E. M.2007Species-specific acquisition and consolidation of long-term memory in parasitic wasps. Proc. R. Soc. B 274, 1539–1546 10.1098/rspb.2007.0305 (doi:10.1098/rspb.2007.0305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odling-Smee L. C., Boughman J. W., Braithwaite V. A.2008Sympatric species of threespine stickleback differ in their performance in a spatial learning task. Behav. Ecol. Sociobiol. 62, 1935–1945 10.1007/s00265-008-0625-1 (doi:10.1007/s00265-008-0625-1) [DOI] [Google Scholar]
- 56.Dukas R.1998Evolutionary ecology of learning. In Cognitive ecology: the evolutionary ecology of information processing and decision making (ed. Dukas R.), pp. 129–174 Chicago, IL: University of Chicago Press; [DOI] [PubMed] [Google Scholar]
- 57.McNamara J. M., Houston A. I.1987Memory and the efficient use of information. J. Theor. Biol. 125, 385–395 10.1016/S0022-5193(87)80209-6 (doi:10.1016/S0022-5193(87)80209-6) [DOI] [PubMed] [Google Scholar]
- 58.Snell-Rood E. C., Papaj D. R.2009Patterns of phenotypic plasticity in common and rare environments: a study of host use and color learning in the cabbage white butterfly, Pieris rapae. Am. Nat. 173, 615–631 10.1086/597609 (doi:10.1086/597609) [DOI] [PubMed] [Google Scholar]