Abstract
I explore the relationship between metabolism and personality by establishing how selection acts on metabolic rate and risk-taking in the context of a trade-off between energy and predation. Using a simple time budget model, I show that a high resting metabolic rate is not necessarily associated with a high daily energy expenditure. The metabolic rate that minimizes the time spent foraging does not maximize the net gain rate while foraging, and it is not always advantageous for animals to have a higher metabolic rate when food availability is high. A model based on minimizing the ratio of mortality rate to net gain rate is used to determine how a willingness to take risks should be correlated with metabolic rate. My results establish that it is not always advantageous for animals to take greater risks when metabolic rate is high. When foraging intensity and metabolic rate coevolve, I show that in a particular case different combinations of foraging intensity and metabolic rate can have equal fitness.
Keywords: energy budget, foraging, metabolic rate, risk-taking, time minimization, trade-off between energy and predation
1. Introduction
A personality trait must be stable over time and consistent across different contexts (Dall et al. 2004; Sih et al. 2004; Dingemanse & Réale 2005; Biro & Stamps 2008; Sih & Bell 2008). There is a variety of explanations for the maintenance of different personalities in a population, e.g. Stamps (2007), Wolf et al. (2007, 2008), Sih & Bell (2008), McNamara et al. (2009), Dingemanse & Wolf (2010) and Wolf & Weissing (2010). These explanations are not my current concern. Motivated by Careau et al. (2008) and various empirical studies, I will focus on selection acting on metabolism and behaviour and the associated implications for personality.
Much of the work on personality has investigated traits such as tendency to explore, aggressiveness or level of activity (e.g. Réale & Festa-Bianchet 2003; Wilson & Stevens 2005; Johnson & Sih 2007; Pintor et al. 2008; Brodin 2009; Farwell & McLaughlin 2009). Stamps (2007) points out that these traits can be seen as controlling the relationships between energetic gains and mortality. Biro & Stamps (2008) show that in some cases the level of danger is positively correlated with the energetic gain. One possible reason for such a correlation is that high activity levels increase encounters with food but also make the forager more conspicuous to predators. Given this correlation, theoretical work on the trade-off between energetic gain and predation risk provides a framework for exploring the action of natural selection on these traits. Whereas the trade-off between energetic gain and predation is often analysed in terms of optimal behaviour (e.g. Abrams 1982; McNamara & Houston 1986; Brown 1988; Houston & McNamara 1989), I extend the analysis to include optimal physiology.
Basal metabolic rate (BMR; Hulbert & Else 2004) (or standard metabolic rate (SMR) in ectotherms, Hulbert & Else (2000)) captures the idea of a minimum rate of energy expenditure. BMR is defined as the rate of energy expenditure of an animal that is resting without any energetic costs associated with digestion, growth, reproduction or thermoregulation. Resting metabolic rate (RMR) is less restrictive in that it does not require that there are no digestive costs (Speakman 2000). Following Careau et al. (2008), I will focus on RMR and will usually refer to it as ‘metabolic rate’. Although I am concerned with metabolic rate, this rate is a consequence of various aspects of morphology and physiology, and hence will be associated with many effects. Drent & Daan (1980) proposed that animals are limited in the rate of energy expenditure that they can sustain and that this rate is proportional to BMR. This idea has been very influential, but the existence of a limit of the form envisaged by Drent & Daan has not been established (Speakman & Krol 2005). A less-specific view is that RMR could be linked to metabolic rate while active, ability to catch food or to escape from predators. These effects can be complex. For example, in juvenile salmon (Salmo salar), high SMR is associated with a high energy cost of processing a meal but a short-lived increase in the rate of energy expenditure (Millidine et al. 2009). The approach that I adopt provides a fairly general way to explore possible trade-offs.
I take metabolic rate to be a reasonably stable trait that can be favoured by natural selection. In support of this view, there is an evidence that metabolic rate is heritable and consistent (Versteegh et al. 2008; Tieleman et al. 2009a,b). Note, however, that although measurements of metabolic rate are repeatable in some contexts, some forms of experience will change the metabolic rate (Wiersma et al. 2005; McKechnie 2008; Duarte et al. 2010). For example, Wiersma et al. (2005) found that starlings (Sturnus vulgaris) have a lower metabolic rate when feeding conditions are poor.
Careau et al. (2008) review a range of issues concerning metabolic rate, behaviour and personality. One possibility is that personality might influence measurements of metabolic rate. I do not consider this idea. Instead, I explore the effects of selection on metabolic rate and behaviour. Careau et al. (2008) point out that various correlations can be expected. Using schematic models, I obtain conditions for correlations to occur. If variation in a trait is maintained over evolutionary time, this analysis indicates whether selection on another trait will produce a correlation between the traits. This corresponds to what Wolf & Weissing (2010) call ‘non-evolved differences in states’. As they point out, alternative personalities do not need to have equal fitness in this case. I also look at a model in which behaviour and metabolic rate coevolve and show that different combinations of behaviour and metabolic rate can have equal fitness.
2. Metabolic rate and daily energy expenditure
Careau et al. (2008) draw attention to the fact that there is not always a strong correlation between BMR and daily energy expenditure (DEE). I now use a simple deterministic model of time and energy budgets (Houston 1993, 2009; Houston et al. 1996; Gorman et al. 1998; Speakman 2000) to investigate this issue, ignoring the distinction between BMR and RMR. Let m be the RMR and g(m) the gross rate of gain while foraging. This rate depends on m. For example, a higher metabolic rate might improve an animal's ability to detect or catch prey. The rate of energy expenditure while foraging is mf (m). This rate is likely to increase with m. (Notation is summarized in table 1.) In this section, I assume that individual members of a population differ in their metabolic rate and explore the consequences for DEE. The outcome is not straightforward because an increase in metabolic rate increases the rate of expenditure while foraging but also increases the rate of gain. All else being equal, an increase in gain decreases the time spent foraging.
Table 1.
Symbols and their meaning.
| symbol | meaning |
|---|---|
| m | resting metabolic rate |
| a | parameter that influences intake rate |
| g | gross rate of gain |
| t | time spent foraging |
| γ | net rate of gain |
| μ | rate of death as a result of predation |
| mf | rate of energy expenditure while foraging |
| σ | sustained metabolic scope |
| V | value of animal's life |
| θ | marginal rate of substitution of predation for energy |
| u | foraging intensity |
During a total time T, the animal either forages or rests. The time spent foraging is t, so the time spent resting is T – t. If the animal is in energy balance (energy gained equals energy spent) then
and so
| 2.1 |
The equation for t can be used to explore the correlation between BMR and DEE. BMR will be similar to m. Because of energy balance, energy expenditure over the period T is tg(m). Thus, if T = 24 h,
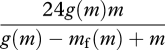 |
2.2 |
is the animal's DEE, so this expenditure (often expressed as kJ d−1 and referred to as the field metabolic rate; see Nagy et al. (1999) and Nagy (2005) for reviews) emerges from assumptions about metabolism and energy balance.
It follows that across individuals DEE increases with m if
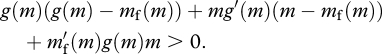 |
If the metabolic rate while foraging is proportional to RMR, i.e. mf = βm, the condition becomes
Whether this condition is satisfied depends on several factors, including β and m. This means that the condition might hold for some ranges of m but not for others. Note that if the condition is not satisfied, DEE decreases with m.
The analysis is simpler in the case of sustained metabolic scope σ, i.e. the ratio of the rate of energy expenditure that an organism can maintain without losing mass to RMR (Peterson et al. 1990; Hammond & Diamond 1997), i.e. σ = DEE/24 m. Thus, from equation (2.2),
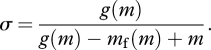 |
Sustained scope increases with RMR (dσ/dm > 0), if
3. Selection on behaviour and metabolic rate
Careau et al. (2008) raise the general issue of the implications of metabolic rate for personality. Part of their analysis looks at the interaction between metabolic rate and behaviour in different environments.
As an introduction to selection acting on metabolic rate, I extend the time budget model by allowing the gross rate of gain g to depend on both metabolic rate m and a parameter a that influences intake. The parameter could be environmental (e.g. food availability) or morphological (e.g. beak size in a bird, muscles of a predator). If it is morphological, it may have an effect on the rate of energy expenditure, in which case mf would depend on a as well as m.
Assume that it is optimal to minimize the time spent foraging (see Schoener (1971)). This would be reasonable if the animal is exposed to predators while foraging but is safe while resting. Dividing the top and bottom of equation (2.1) by m, it can be seen that natural selection should act on metabolic rate so as to maximize
This is the net rate of gain divided by the metabolic rate. If mf = βm, then this currency simplifies to
and the optimal value m* of m maximizes g(a,m)/m. The optimal solution satisfies the marginal value condition
By implicit differentiation with respect to a:
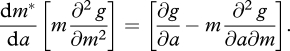 |
To be a maximum, 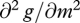 must be negative, so m* increases with a if and only if
must be negative, so m* increases with a if and only if
This condition is based on how the effect of metabolic rate m on gross rate of gain g depends on food availability a. The mixed partial derivative 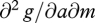 gives the slope of gain as a function of a as metabolic rate increases. For example, if g(a,m) = ams, then
gives the slope of gain as a function of a as metabolic rate increases. For example, if g(a,m) = ams, then 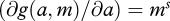 and
and 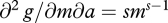 . Thus,
. Thus, 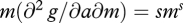 and m* should increase with a if s >1.
and m* should increase with a if s >1.
This analysis is based on natural selection tuning metabolic rate to long-term food availability. The evolved metabolic rate has effects across contexts because it will influence the rate at which energy is spent while active. (In this particular case, the rate of expenditure while foraging is proportional to m.)
In many circumstances, animals have options that differ in energetic gain and the risk of predation (Lima 1998). If high energetic gain is associated with high predation risk, then the optimal decision depends on the benefit of gaining energy and the cost of being killed. The simple time budget model does not capture this possibility. There have been many theoretical treatments of optimal behaviour in these circumstances. The approach suggested by Gilliam (1982) is based on an animal having to grow to a critical size before it can reproduce. The animal's net rate of gain is γ and its rate of mortality (often taken to be the result of predation) is μ. Both of these can depend on size and behaviour. The time to reach the critical size is proportional to 1/γ and so the probability of reaching the critical size increases as μ/γ decreases. For further discussion and examples, see Werner & Gilliam (1984), Houston et al. (1993), Houston (1998) and Brown & Kotler (2004).
For example, assume that
where auh(m) is the gross rate of energy intake and mf(m) is the rate of energy expenditure. The gross rate of intake depends on a parameter a that can represent the availability of food and on the animal's foraging intensity u, which can be though of as the proportion of time that the animal spends foraging (cf. Houston et al. 1993). The function h(m) represents the effect of metabolic rate on intake, with ah(m) being energy intake rate if u = 1.
The final component of the model is the rate of mortality, which I take to be μ = ku2, where k is a positive constant. The idea behind this assumption is that predation is an increasing and accelerating function of foraging intensity.
I now assume that animals differ in their metabolic rate m and that selection will result in each animal adopting the best behaviour for its value of m. This means that the optimal value u* of u minimizes
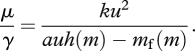 |
From the condition ∂/∂u = 0 it follows that
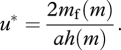 |
For u* to increase with m, 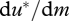 must be positive, which is equivalent to
must be positive, which is equivalent to
or
As a simple example, let
Then
so u* is positively correlated with m if y > x and negatively correlated with m if y < x. If the animal adopts its optimal behaviour, then μ/γ is proportional to my −2x. Because it is advantageous to decrease μ/γ, there is selection to increase m if y < 2x and to decrease m if y > 2x.
(a). Coevolution and equal fitness
I now allow both foraging behaviour and metabolism to be optimized. McNamara & Houston (1994) assume that γ = au − m. Let μ = u2/m. This function decreases with m to represent the advantage provided by an increased metabolic rate in terms of escaping from predators. If natural selection can act on both foraging intensity and metabolic rate, then the outcome is given by solutions of the equations
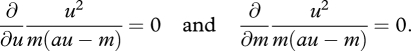 |
It follows that 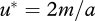 , and the payoff if u* is adopted is
, and the payoff if u* is adopted is 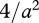 for any feasible value of m. In other words, in an environment with a particular level of food availability a, there is a valley in (u, m) space along which μ/γ is constant, so that many combinations of behaviour u and metabolic rate m have the same fitness.
for any feasible value of m. In other words, in an environment with a particular level of food availability a, there is a valley in (u, m) space along which μ/γ is constant, so that many combinations of behaviour u and metabolic rate m have the same fitness.
4. Discussion
Accounts of the evolution of personality address two questions:
Why is behaviour consistent across conditions?
What maintains different types in a population?
One answer to question (i) is that metabolic rate is fixed and influences rate of expenditure during all activities and hence acts to support consistency across contexts. Question (ii) might then be answered by appeal to non-evolved differences in states, as discussed by Wolf & Weissing (2010).
The time budget model explores the consequences of individuals in a population having different values of RMR, m. If an increase in m increases both the rate of expenditure while foraging and the rate of gain then animals with a higher metabolic rate have a higher DEE only if a particular condition holds. If the condition does not hold, then animals with a higher metabolic rate have a lower DEE. Because the condition depends on m, it may hold for some values of m and not for others so that DEE is not a monotonic function of m. This point is relevant to other conditions that I obtain.
If selection on metabolism acts so as to minimize the time spent foraging, then selection does not result in the metabolic rate that maximizes the net rate of gain. Instead, the net rate of gain divided by the metabolic rate should be maximized. In the notation that I have used, this currency is
This currency is not efficiency, which is
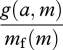 |
(Houston 1987; McNamara & Houston 1997). It is also not the same as the form of efficiency that should be maximized if an animal is subject to energetic constraints. This form is
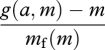 |
(Hedenström & Alerstam 1995; Houston 1995; McNamara & Houston 1997).
Some personality differences are associated with differences in the extent to which animals are prepared to risk their life (e.g. Stamps 2007). Such differences in risk-taking can be understood in terms of a trade-off between energetic gain and predation risk. Houston & McNamara (1989) show that instantaneous foraging decisions involving the trade-off between energetic gain and the risk of predation should maximize
where V is the reproductive value, γ is the net rate of energetic gain and μ is the rate of mortality. V depends on the animal's state (e.g. size, energy reserves), and γ and μ depend on its state and behaviour. This currency has been used in a variety of contexts (Sih 1992; Moody et al. 1996; Welton & Houston 2001). When reproductive value is high, an animal's life is valuable, and it should be less inclined to take risks (McNamara & Houston 1986; Houston & McNamara 1988; McNamara 1990; Clark 1994). This is called the asset-protection principle by Clark (1994) and is used in the context of personality differences by Wolf et al. (2007); for further discussion, see Luttbeg & Sih (2010). Note that risktaking in this context refers to actions that put the animal's life in danger, and not actions that have variable outcomes (McNamara & Houston 1987, 1992a).
An idea of the correlations that can be generated by selection acting on metabolic rate or behaviour can be obtained by investigating how the optimal foraging intensity u* depends on various parameters (cf. Stamps 2007; Careau et al. 2008). McNamara & Houston (1994) address this question by establishing how the optimal decision is influenced by a change in the environment. The answer depends on whether the change is long term or short term. Using the currency W, the optimal-foraging intensity satisfies
where 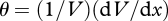 is the marginal rate of substitution of predation for energy. Either it or its reciprocal is used to characterize the energy–predation trade-off, e.g. Caraco (1979), Brown (1988) and Houston & McNamara (1999). If an environmental change lasts only for a short time, θ is constant. A long-term change means that V and hence θ will change. McNamara & Houston (1994) show that the effect of a short-term change depends on how the change influences the animal's options. For example, an increase in foraging intensity in response to an increase in food availability is likely if the increase has a stronger effect on good options than on poor ones.
is the marginal rate of substitution of predation for energy. Either it or its reciprocal is used to characterize the energy–predation trade-off, e.g. Caraco (1979), Brown (1988) and Houston & McNamara (1999). If an environmental change lasts only for a short time, θ is constant. A long-term change means that V and hence θ will change. McNamara & Houston (1994) show that the effect of a short-term change depends on how the change influences the animal's options. For example, an increase in foraging intensity in response to an increase in food availability is likely if the increase has a stronger effect on good options than on poor ones.
Careau et al. (2008) and Stamps (2007) use examples involving the optimal activity level in a particular environment. It is important to remember that the optimal activity level depends not only on the environment but also on the animal's state. State-dependent models of activity level or foraging effort are considered by Mangel & Clark (1986), Houston et al. (1988), Houston & McNamara (1993) and Luttbeg & Sih (2010). The currency W incorporates state, but it only provides a snapshot at a particular state. A full account would be based on finding the optimal state-dependent strategy. This is similar to the point that McNamara & Houston (1992b) make about analysing clutch size. They argue that instead of looking for the optimal clutch size, it is necessary to look for the optimal strategy, i.e. way for clutch size to depend on circumstances.
I have used Gilliam's currency to investigate selection on metabolic rate m and behaviour u. The analysis could apply to permanent differences in environments or long-term changes; see McNamara & Houston (1994). Using a simple example of how intake rate and predation rate depend on m and u, I have shown that optimal behaviour may either increase or decrease with m. In other words, if animals differ in metabolic rate and selection means that each animal adopts the best behaviour for its metabolic rate, the correlation between metabolic rate and behaviour can be positive or negative.
It is tempting to view an organism's morphology and physiology as fixed and its behaviour as plastic. Such a view is not correct; morphology and physiology can change with circumstances; see Piersma & Lindstrom (1997), Piersma & Drent (2003) and McKechnie (2008) for reviews. Previous work has shown that it can be advantageous for small birds to allow their body temperature to drop in response to environmental conditions (Clark & Dukas 2000; Pravosudov & Lucas 2000; Welton et al. 2002). This change in metabolism is based on the trade-off between energy and predation—the reduction in temperature saves energy but increases the risk of being killed by a predator. The energy versus predation trade-off can also be used to explain the change in behaviour that results when an animal detects a predator (McNamara et al. 2005). Future work could explore the general conditions for metabolic rate to change in response to changes in the environment. Such an analysis would need to include the cost of changing metabolic rate (cf. DeWitt et al. 1998).
I have not looked at question (ii) in detail, but the example based on Gilliam's currency shows that combinations of foraging ability and metabolic rate can be equivalent. This is a stronger result than that of Mangel & Stamps (2001), who showed that a range of life-history strategies could have similar rather than equal fitness. If fitness is not exactly equal, then it is necessary to consider the strength of selection (Sih 1982; McNamara & Houston 1986; Houston 2000). Combinations of behaviour and metabolism that result in equal fitness might occur in other contexts. For example, in models involving a probability of finding a food item (e.g. Iwasa et al. 1981; McNamara & Houston 1985), a trade-off between detection and metabolic rate could result in various values of metabolic rate being equally successful. It is important to note that both models based on Gilliam's currency make particular assumptions about how behaviour and metabolic rate influence net rate of gain and mortality rate. Further theoretical work should explore the generality of the results I have presented. This could involve establishing general qualitative trends and computing solutions in particular cases.
Acknowledgements
I thank John McNamara, Alexander Houston, the editors (Denis Réale, Niels Dingemanse, Anahita Kazem and Jonathan Wright) and two anonymous referees for comments on previous versions of this manuscript.
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Abrams P. A.1982Functional responses of optimal foragers. Am. Nat. 120, 382–390 10.1086/283996 (doi:10.1086/283996) [DOI] [Google Scholar]
- Biro P. A., Stamps J. A.2008Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Brodin T.2009Behavioral syndrome over the boundaries of life—carryovers from larvae to adult damselfly. Behav. Ecol. 20, 30–37 10.1093/beheco/arn111 (doi:10.1093/beheco/arn111) [DOI] [Google Scholar]
- Brown J. S.1988Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47 10.1007/BF00395696 (doi:10.1007/BF00395696) [DOI] [Google Scholar]
- Brown J. S., Kotler B. P.2004Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 7, 999–1014 10.1111/j.1461-0248.2004.00661.x (doi:10.1111/j.1461-0248.2004.00661.x) [DOI] [Google Scholar]
- Caraco T.1979Time budgeting and group size: theory. Ecology 60, 611–617 10.2307/1936081 (doi:10.2307/1936081) [DOI] [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Réale D.2008Energy metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Clark C. W.1994Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159–170 10.1093/beheco/5.2.159 (doi:10.1093/beheco/5.2.159) [DOI] [Google Scholar]
- Clark C. W., Dukas R.2000Winter survival strategies for small birds: managing energy expenditure through hypothermia. Evol. Ecol. Res. 2, 473–491 [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- DeWitt T. J., Sih A., Wilson D. S.1998Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 10.1016/S0169-5347(97)01274-3 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Réale D.2005Natural selection and animal personality. Behaviour 142, 1159–1184 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- Dingemanse N. J., Wolf M.2010Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drent R. H., Daan S.1980The prudent parent: energetic adjustments in avian breeding. Ardea 68, 225–252 [Google Scholar]
- Duarte L. C., Vaanholt L. M., Sinclair R. E., Gamo Y., Speakman J. R.2010Limits to sustained energy intake XII: is the poor relation between resting metabolic rate and reproductive performance because resting metabolism is not a repeatable trait? J. Exp. Biol. 213, 278–287 10.1242/jeb.037069 (doi:10.1242/jeb.037069) [DOI] [PubMed] [Google Scholar]
- Farwell M., McLaughlin R. L.2009Alternative foraging tactics and risk taking in brook charr (Salvelinus fontinalis). Behav. Ecol. 20, 913–921 10.1093/beheco/arp059 (doi:10.1093/beheco/arp059) [DOI] [Google Scholar]
- Gilliam J. F. Foraging under mortality risk in size-structured populations. Michigan State University,; MI, USA: 1982. PhD thesis, [Google Scholar]
- Gorman M. L., Mills M. G., Raath J. P., Speakman J. R.1998High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391, 479–481 10.1038/35131 (doi:10.1038/35131) [DOI] [Google Scholar]
- Hammond K. A., Diamond J.1997Maximal sustained energy budgets in humans and animals. Nature 386, 457–462 10.1038/386457a0 (doi:10.1038/386457a0) [DOI] [PubMed] [Google Scholar]
- Hedenström A., Alerstam T.1995Optimal flight speed of birds. Phil. Trans. R. Soc. Lond. B 348, 471–487 10.1098/rstb.1995.0082 (doi:10.1098/rstb.1995.0082) [DOI] [Google Scholar]
- Houston A. I.1987Optimal foraging by parent birds feeding dependent young. J. Theor. Biol. 124, 251–274 10.1016/S0022-5193(87)80115-7 (doi:10.1016/S0022-5193(87)80115-7) [DOI] [Google Scholar]
- Houston A. I.1993The efficiency of mass loss in breeding birds. Proc. R. Soc. Lond. B 254, 221–225 10.1098/rspb.1993.0149 (doi:10.1098/rspb.1993.0149) [DOI] [Google Scholar]
- Houston A. I.1995Energetic constraints and foraging efficiency. Behav. Ecol. 6, 393–396 10.1093/beheco/6.4.393 (doi:10.1093/beheco/6.4.393) [DOI] [Google Scholar]
- Houston A. I.1998Models of optimal avian migration: state, time and predation. J. Avian Biol. 29, 395–404 10.2307/3677158 (doi:10.2307/3677158) [DOI] [Google Scholar]
- Houston A. I.2000The strength of selection in the context of migration speed. Proc. R. Soc. Lond. B 267, 2393–2395 10.1098/rspb.2000.1296 (doi:10.1098/rspb.2000.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston A. I.2009Flying in the face of nature. Behav. Process. 80, 295–305 10.1016/j.beproc.2008.12.007 (doi:10.1016/j.beproc.2008.12.007) [DOI] [PubMed] [Google Scholar]
- Houston A. I., McNamara J. M.1988A framework for the functional analysis of behaviour. Behav. Brain Sci. 11, 117–163 10.1017/S0140525X00053061 (doi:10.1017/S0140525X00053061) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1989The value of food: effects of open and closed economies. Anim. Behav. 37, 546–562 10.1016/0003-3472(89)90034-1 (doi:10.1016/0003-3472(89)90034-1) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1993A theoretical investigation of the fat reserves and mortality levels of small birds in winter. Ornis Scand. 24, 205–219 10.2307/3676736 (doi:10.2307/3676736) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1999Models of adaptive behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- Houston A., Clark C., McNamara J., Mangel M.1988Dynamic models in behavioural and evolutionary ecology. Nature 332, 29–34 10.1038/332029a0 (doi:10.1038/332029a0) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M., Hutchinson J. M. C.1993General results concerning the trade-off between gaining energy and avoiding predation. Phil. Trans. R. Soc. Lond. B 341, 375–397 10.1098/rstb.1993.0123 (doi:10.1098/rstb.1993.0123) [DOI] [Google Scholar]
- Houston A. I., Thompson W. A., Gaston A. J.1996The use of a time and energy budget model of a parent bird to investigate limits to fledging mass in the thick-billed murre. Funct. Ecol. 10, 432–439 10.2307/2389935 (doi:10.2307/2389935) [DOI] [Google Scholar]
- Hulbert A. J., Else P. L.2000Mechanisms underlying the cost of living in animals. Annu. Rev. Physiol. 62, 207–235 10.1146/annurev.physiol.62.1.207 (doi:10.1146/annurev.physiol.62.1.207) [DOI] [PubMed] [Google Scholar]
- Hulbert A. J., Else P. L.2004Basal metabolic rate: history, composition, regulation, and usefulness. Physiol. Biochem. Zool. 77, 869–876 10.1086/422768 (doi:10.1086/422768) [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Higashi M., Yamamura N.1981Prey distribution as a factor determining the choice of optimal foraging strategy. Am. Nat. 117, 710–723 10.1086/283754 (doi:10.1086/283754) [DOI] [Google Scholar]
- Johnson J. C., Sih A.2007Fear, food, sex and parental care: a syndrome of boldness in the fishing spider, Dolomedes triton. Anim. Behav. 74, 1131–1138 10.1016/j.anbehav.2007.02.006 (doi:10.1016/j.anbehav.2007.02.006) [DOI] [Google Scholar]
- Lima S. L.1998Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. Stress Behav. 27, 215–290 10.1016/S0065-3454(08)60366-6 (doi:10.1016/S0065-3454(08)60366-6) [DOI] [Google Scholar]
- Luttbeg B., Sih A.2010Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990 10.1098/rstb.2010.0207 (doi:10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel M., Clark C. W.1986Towards a unified foraging theory. Ecology 67, 1127–1138 10.2307/1938669 (doi:10.2307/1938669) [DOI] [Google Scholar]
- Mangel M., Stamps J.2001Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol. Ecol. Res. 3, 583–593 [Google Scholar]
- McKechnie A. E.2008Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J. Comp. Physiol. B Biochem. System. Environ. Physiol. 178, 235–247 10.1007/s00360-007-0218-8 (doi:10.1007/s00360-007-0218-8) [DOI] [PubMed] [Google Scholar]
- McNamara J. M.1990The policy which maximizes long-term survival of an animal faced with the risks of starvation and predation. Adv. Appl. Probability 22, 295–308 10.2307/1427537 (doi:10.2307/1427537) [DOI] [Google Scholar]
- McNamara J., Houston A.1985A simple model of information use in the exploitation of patchily distributed food. Anim. Behav. 33, 553–560 10.1016/S0003-3472(85)80078-6 (doi:10.1016/S0003-3472(85)80078-6) [DOI] [Google Scholar]
- McNamara J. M., Houston A. I.1986The common currency for behavioral decisions. Am. Nat. 127, 358–378 10.1086/284489 (doi:10.1086/284489) [DOI] [Google Scholar]
- McNamara J. M., Houston A. I.1987A general framework for understanding the effects of variability and interruptions on foraging behaviour. Acta Biotheor. 36, 3–22 10.1007/BF00159228 (doi:10.1007/BF00159228) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Houston A. I.1992aRisk-sensitive foraging—a review of the theory. Bull. Math. Biol. 54, 355–378 [Google Scholar]
- McNamara J. M., Houston A. I.1992bState-dependent life-history theory and its implications for optimal clutch size. Evol. Ecol. 6, 170–185 10.1007/BF02270710 (doi:10.1007/BF02270710) [DOI] [Google Scholar]
- McNamara J. M., Houston A. I.1994The effect of a change in foraging options on intake rate and predation rate. Am. Nat. 144, 978–1000 10.1086/285721 (doi:10.1086/285721) [DOI] [Google Scholar]
- McNamara J. M., Houston A. I.1997Currencies for foraging based on energetic gain. Am. Nat. 150, 603–617 10.1086/286084 (doi:10.1086/286084) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Barta Z., Houston A. I., Race P.2005A theoretical investigation of the effect of predators on foraging behaviour and energy reserves. Proc. R. Soc. B 272, 929–934 10.1098/rspb.2004.3037 (doi:10.1098/rspb.2004.3037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. M., Stephens P. A., Dall S. R. X., Houston A. I.2009Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B 276, 605–613 10.1098/rspb.2008.1182 (doi:10.1098/rspb.2008.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millidine K. J., Armstrong J. D., Metcalfe N. B.2009Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proc. R. Soc. B 276, 2103–2108 10.1098/rspb.2009.0080 (doi:10.1098/rspb.2009.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody A. L., Houston A. I., McNamara J. M.1996Ideal free distributions under predation risk. Behav. Ecol. Sociobiol. 38, 131–143 10.1007/s002650050225 (doi:10.1007/s002650050225) [DOI] [Google Scholar]
- Nagy K. A.2005Field metabolic rate and body size. J. Exp. Biol. 208, 1621–1625 10.1242/jeb.01553 (doi:10.1242/jeb.01553) [DOI] [PubMed] [Google Scholar]
- Nagy K. A., Girard I. A., Brown T. K.1999Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 19, 247–277 10.1146/annurev.nutr.19.1.247 (doi:10.1146/annurev.nutr.19.1.247) [DOI] [PubMed] [Google Scholar]
- Peterson C. C., Nagy K. A., Diamond J.1990Sustained metabolic scope. Proc. Natl Acad. Sci. USA 87, 2324–2328 10.1073/pnas.87.6.2324 (doi:10.1073/pnas.87.6.2324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma T., Drent J.2003Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233 10.1016/S0169-5347(03)00036-3 (doi:10.1016/S0169-5347(03)00036-3) [DOI] [Google Scholar]
- Piersma T., Lindstrom A.1997Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol. Evol. 12, 134–138 10.1016/S0169-5347(97)01003-3 (doi:10.1016/S0169-5347(97)01003-3) [DOI] [PubMed] [Google Scholar]
- Pintor L. M., Sih A., Bauer M. L.2008Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117, 1629–1636 10.1111/j.1600-0706.2008.16578.x (doi:10.1111/j.1600-0706.2008.16578.x) [DOI] [Google Scholar]
- Pravosudov V. V., Lucas J. R.2000The costs of being cool: a dynamic model of nocturnal hypothermia by small food-caching birds in winter. J. Avian Biol. 31, 463–472 10.1034/j.1600-048X.2000.310405.x (doi:10.1034/j.1600-048X.2000.310405.x) [DOI] [Google Scholar]
- Réale D., Festa-Bianchet M.2003Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65, 463–470 10.1006/anbe.2003.2100 (doi:10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- Schoener T. W.1971Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404 10.1146/annurev.es.02.110171.002101 (doi:10.1146/annurev.es.02.110171.002101) [DOI] [Google Scholar]
- Sih A.1982Optimal patch use: variation in selective pressure for efficient foraging. Am. Nat. 120, 666–685 10.1086/284019 (doi:10.1086/284019) [DOI] [Google Scholar]
- Sih A.1992Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 139, 1052–1069 10.1086/285372 (doi:10.1086/285372) [DOI] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Adv. Study Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 [DOI] [PubMed] [Google Scholar]
- Speakman J. R.2000The cost of living: field metabolic rates of small mammals. Adv. Ecol. Res. 30, 177–297 10.1016/S0065-2504(08)60019-7 (doi:10.1016/S0065-2504(08)60019-7) [DOI] [Google Scholar]
- Speakman J. R., Krol E.2005Limits to sustained energy intake IX: a review of hypotheses. J. Comp. Physiol. B Biochem. System. Environ. Physiol. 175, 375–394 10.1007/s00360-005-0013-3 (doi:10.1007/s00360-005-0013-3) [DOI] [PubMed] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Tieleman B. I., Versteegh M. A., Fries A., Helm B., Dingemanse N. J., Gibbs H. L., Williams J. B.2009aGenetic modulation of energy metabolism in birds through mitochondrial function. Proc. R. Soc. B 276, 1685–1693 10.1098/rspb.2008.1946 (doi:10.1098/rspb.2008.1946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman B. I., Versteegh M. A., Helm B., Dingemanse N. J.2009bQuantitative genetics parameters show partial independent evolutionary potential for body mass and metabolism in stonechats from different populations. J. Zool. 279, 129–136 10.1111/j.1469-7998.2009.00597.x (doi:10.1111/j.1469-7998.2009.00597.x) [DOI] [Google Scholar]
- Versteegh M. A., Heim B., Dingemanse N. J., Tieleman B. I.2008Repeatability and individual correlates of basal metabolic rate and total evaporative water loss in birds: a case study in European stonechats. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150, 452–457 10.1016/j.cbpa.2008.05.006 (doi:10.1016/j.cbpa.2008.05.006) [DOI] [PubMed] [Google Scholar]
- Welton N. J., Houston A. I.2001A theoretical investigation into the direct and indirect effects of state on the risk of predation. J. Theor. Biol. 213, 275–297 10.1006/jtbi.2001.2419 (doi:10.1006/jtbi.2001.2419) [DOI] [PubMed] [Google Scholar]
- Welton N. J., Houston A. I., Ekman J., McNamara J. M.2002A dynamic model of hypothermia as an adaptive response by small birds to winter conditions. Acta Biotheor. 50, 39–56 10.1023/A:1014761227478 (doi:10.1023/A:1014761227478) [DOI] [PubMed] [Google Scholar]
- Werner E. E., Gilliam J. F.1984The ontogenetic niche and species interactions in size structured populations. Annu. Rev. Ecol. Syst. 15, 393–425 10.1146/annurev.es.15.110184.002141 (doi:10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- Wiersma P., Salomons H. M., Verhulst S.2005Metabolic adjustments to increasing foraging costs of starlings in a closed economy. J. Exp. Biol. 208, 4099–4108 10.1242/jeb.01855 (doi:10.1242/jeb.01855) [DOI] [PubMed] [Google Scholar]
- Wilson A. D. M., Stevens E. D.2005Consistency in context-specific measures of shyness and boldness in rainbow trout, Oncorhynchus mykiss. Ethology 111, 849–862 10.1111/j.1439-0310.2005.01110.x (doi:10.1111/j.1439-0310.2005.01110.x) [DOI] [Google Scholar]
- Wolf M., Weissing F. J.2010An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J.2008Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]


