Abstract
The ecological factors responsible for the evolution of individual differences in animal personality (consistent individual differences in the same behaviour across time and contexts) are currently the subject of intense debate. A limited number of ecological factors have been investigated to date, with most attention focusing on the roles of resource competition and predation. We suggest here that parasitism may play a potentially important, but largely overlooked, role in the evolution of animal personalities. We identify two major routes by which parasites might influence the evolution of animal personality. First, because the risk of acquiring parasites can be influenced by an individual's behavioural type, local parasite regimes may impose selection on personality traits and behavioural syndromes (correlations between personality traits). Second, because parasite infections have consequences for aspects of host ‘state’, parasites might induce the evolution of individual differences in certain types of host behaviour in populations with endemic infections. Also, because infection often leads to specific changes in axes of personality, parasite infections have the potential to decouple behavioural syndromes. Host–parasite systems therefore provide researchers with valuable tools to study personality variation and behavioural syndromes from a proximate and ultimate perspective.
Keywords: personality, behavioural syndrome, parasitism, phenotypic plasticity, correlational selection, population differentiation
1. Introduction
In common with other phenotypic characteristics, behavioural traits show considerable inter-individual and heritable variation (Stirling et al. 2002; Réale et al. 2007; Bell et al. 2009). Yet, despite their widespread existence, individual differences in behaviour have typically been treated as ‘noise’ by behavioural and evolutionary ecologists and effectively disregarded, though there have been some notable exceptions to this (e.g. Huntingford 1976; Wilson et al. 1993). There is, however, increasing evidence that the way in which individuals behave can be consistent and predictable over time and/or across contextually different situations, suggesting the existence of important variation in underlying behavioural ‘type’, or personality (Réale et al. 2007). In spiders, for instance, certain individuals are consistently more aggressive than others, and these differences are maintained in the context of both competition and mating (Johnson & Sih 2005). Furthermore, distinct personality traits can be correlated within populations, and these correlations—which are emergent properties of populations, not individuals—are often referred to as behavioural syndromes (Sih et al. 2004b; Bell 2007; Réale et al. 2007). For example, the standard personality traits ‘activity’, ‘aggressiveness’, ‘boldness’ and ‘exploration’ are structured in an aggressiveness–boldness syndrome in many—though not all—populations of stickleback fish (Huntingford 1976; Bell 2005; Dingemanse et al. 2007).
There is currently great interest in the evolutionary origins and ecological consequences of animal personalities (Réale et al. 2007) and behavioural syndromes (Sih et al. 2004b; Bell 2007). (N.B. For the sake of clarity, we follow the approach of Biro & Stamps (2008) and make a distinction between animal personalities and behavioural syndromes; however, we recognize that others have used both terms to refer to the same phenomenon). New empirical studies have substantially increased our understanding of the adaptive nature of this variation by documenting how selection acts on behavioural traits in the wild (Dingemanse & Réale 2005; Smith & Blumstein 2008), and by revealing the ecological conditions favouring behavioural syndromes (Bell & Sih 2007; Dingemanse et al. 2007). Fluctuating selection acting on behavioural traits has, for instance, now been documented in wild populations of birds (Dingemanse et al. 2004; Quinn et al. 2009), lizards (Cote et al. 2008), squirrels (Boon et al. 2007) and ungulates (Réale & Festa-Bianchet 2003), and is suggested to help maintain variation in animal personality in the wild. Similarly, comparative approaches have revealed that behavioural syndromes do not always exist (Bell 2005), and that the existence of syndromes can be predicted on the basis of population differences in ecological factors (Dingemanse et al. 2007), suggesting that they evolve in response to selection (Bell & Sih 2007). Theoreticians have also begun to explore the types of conditions that might favour the evolution of animal personalities and behavioural syndromes (see Dingemanse & Wolf 2010 for a recent review).
2. Parasitism as an ecological factor influencing behaviour, personality and syndromes
Yet, despite this increasing interest in individual variation in behaviour, the ecological factors that shape variation in personality and behavioural syndromes remain relatively unknown. Attention has focused largely on the roles of resource competition (Dingemanse et al. 2004; Cote et al. 2008) and predation regimes (Bell & Sih 2007; Dingemanse et al. 2007), with other ecological factors being largely ignored. In this paper, we outline why parasites might play a key role in shaping behaviour, the evolution of divergent individual personalities and population-level variation in behavioural syndromes.
Parasitism was originally suggested as being a factor of potential importance in an influential descriptive study by Wilson et al. (1993), in which the exploration behaviour of wild individual pumpkinseed sunfish Lepomis gibbosus covaried with the level of infection with two species of trematode. Fish caught in traps (i.e. the more ‘exploratory’ fish) harboured significantly higher levels of infection with ‘blackspot’ (Neascus sp.), and significantly lower levels of infection with ‘white grub’ (Posthodiplostomum minimum) when compared with the population at large. One interpretation of these results is that natural variation in behaviour differentially exposes individuals to different types of parasites. However, because parasite infections often change the behaviour of host organisms—including their patterns of habitat use, risk taking and ‘trappability’ (Moore 2002; see also our table 1)—separating cause from effect in correlational studies such as this is challenging (Wilson et al. 1993). In the subsequent development of personality research, until very recently, the potential importance of parasitism has largely been ignored, despite an increasing recognition of the importance of parasitism in ecosystem processes (Lafferty & Morris 1996; Lafferty et al. 2006, 2008; Kuris et al. 2008), and the fact that patterns of host behaviour and parasite infections can interact in a variety of ways (Moore 2002). There is, however, a growing recognition that parasites may be important in shaping the evolution of personality in animals (Coats et al. 2010; Poulin 2010).
Table 1.
Selected examples of personality traits (‘temperament’ traits, following terminology outlined by Réale et al. (2007)) that have been demonstrated to differ between parasitized and non-parasitized individuals within a population or species.
| temperament trait | tests | response of parasitized individuals | host | parasite | reference |
|---|---|---|---|---|---|
| boldnessa | predator presentation | approached model predator more readily | stickleback | Schistocephalus solidus (Cestoda) | Giles (1983) |
| approached predator odour more readily | rat | Toxoplasma gondii (Protozoa) | Berdoy et al. (2000) | ||
| approached predator odour more readily | mouse | Eimeria vermiformis (Protozoa) | Kavaliers & Colwell (1995) | ||
| more likely to be found close to a predator | isopod | Acanthocephalus dirus (Acanthocephala) | Hechtel et al. (1993) | ||
| no fear of predator odour | mouse | Heligmosomoides polygyrus (Nematoda) | Kavaliers et al. (1997) | ||
| enhanced predator avoidance response | stickleback | Glugea anomala (Microsporidia) | Milinski (1985) | ||
| predator stimulus | reduced escape responses | stickleback | S. solidus (Cestoda) | Barber et al. (2004) | |
| reduced escape responses | ant | Dilepid cestode (Cestoda) | Plateaux (1972) | ||
| returned to food more readily after attack | stickleback | S. solidus (Cestoda) | Giles (1987) | ||
| trappability | more likely to be trapped | pumpkinseed sunfish | Neascus spp. (Trematoda) | Wilson et al. (1993) | |
| less likely to be trapped | pumpkinseed sunfish | Posthodiplostomum minimum (Trematoda) | Wilson et al. (1993) | ||
| more likely to be shot by human hunters | moose | Echinococcus granulosus (Cestoda) | Rau & Caron (1979) | ||
| more likely to be trapped | Rat | T. gondii (Protozoa) | Webster et al. (1994) | ||
| other | more likely to take risks while driving | human | T. gondii (Protozoa) | Flegr et al. (2002) | |
| exploration | open field test | increased exploration of novel objects | rat | T. gondii (Protozoa) | Webster et al. (1994) |
| increased exploration of novel environment | rat | T. gondii (Protozoa) | Berdoy et al. (1995) | ||
| activityb | cage activity test | increased conspicuous behaviours | killifish | Euhaplorchis californensis (Trematoda) | Lafferty & Morris (1996) |
| reduced activity | copepod | Diphyllobothrium spp. (Cestoda) | Pasternak et al. (1995) | ||
| open field test | increased activity | rat | T. gondii (Protozoa) | Webster (1994) | |
| sociabilityc,d | separation test | reduced tendency to join conspecific groups | stickleback | S. solidus (Cestoda) | Barber et al. (1995) |
| reduced tendency to join conspecific groups | killifish | Crassiaphiala bulboglossa (Trematoda) | Krause & Godin (1994) | ||
| aggressiveness | social interactions/dyadic encounters | infected individuals less aggressive | red grouse | Trichostrongylus tenuis (Nematoda) | Fox & Hudson (2001) |
| infected individuals less aggressive | mice | Taenia crassiceps (Cestoda) | Gourbal et al. (2002) | ||
| infected individuals more aggressive | leaf beetle | Chrysomelobia labidomerae (Acarina) | Abbot & Dill (2001) |
aMore than 20 host–parasite systems in Moore 2002, table 3.4.
bMore than 120 examples in Moore 2002, table 3.7.
cMore than 30 examples in Moore 2002, table 4.2.
dAltered tendency to join groups may alternatively be interpreted as altered antipredator behaviour, rather than an example of altered sociability.
Here, we propose that (i) parasites can shape the behaviour of individuals in host populations, (ii) parasites can act as selective agents on personality, and (iii) parasites can play a role in the evolution of behavioural syndromes. We propose three reasons why parasitism is likely to be of importance in the context of animal personality research.
— An individual's behaviour has implications for the level of parasite exposure it experiences (Hart 1990), so variation in behaviour generates the potential for differential exposure. Because infections invariably impact negatively on components of host fitness (Bush et al. 2001), we suggest that common, debilitating parasites might play an important direct role as agents of selection on animal behaviour. Because the risk of infection can vary substantially over time and space, this could generate inter- and intrapopulation variation in the amount of individual variance in behaviour. Furthermore, the specific composition of local parasite communities could have implications for the evolution of behavioural syndromes.
— Parasites may also play an important indirect role in the evolution of individuality, as a consequence of their impacts on host condition. Recent theoretical modelling approaches suggest that adaptive variation in personality can arise as a result of life-history trade-offs when there is individual variation in reproductive value (Wolf et al. 2007), or as a consequence of (stochastic) variation in initial ‘state’ (e.g. energy reserves) across individuals (Dall et al. 2004; McElreath & Strimling 2006; Stamps 2007; Dingemanse & Wolf 2010). Because parasites rely on host nutrition to fuel their growth and development, infections routinely alter both the reproductive potential and the state of hosts, and so they potentially induce personality in their host.
— Many parasites are also known to alter aspects of host behaviour in ways that are expected to increase the efficiency of transmission, a process that appears to be especially common in parasites transmitted through food webs, i.e. ‘parasite-increased trophic transmission’, sensu Lafferty (1999). Behaviours that are commonly changed in parasitized animals are often those identified as key personality axes in behavioural studies. We therefore suggest that examining the effects of experimentally induced parasite infections on behavioural syndromes may yield information on the proximate mechanisms that link such behaviours at a phenotypic level. For example, if infections can ‘decouple’ normally correlated behaviours (syndromes), this may provide evidence that behavioural traits can be controlled independently at a physiological level, potentially providing researchers with experimental tools to study the consequences of coupled versus uncoupled behaviours. Furthermore, if syndromes have evolved as adaptations to local selective environments, then their decoupling by parasites may have ecological consequences, for example, in terms of altering the susceptibility of infected individuals to predators. Syndromes themselves, rather than their constituent behaviours, may therefore be the targets of manipulation by parasites (Poulin 2010).
3. Parasites as direct selective agents on the evolution of behaviour, personality and behavioural syndromes
Parasite transmission strategies exploit a diverse range of host behaviours, including social, sexual and foraging behaviours. Hence, individual variation in any aspect of behaviour has the potential to generate differential exposure to infections among a host population. In humans, for example, sexual promiscuity is a significant risk factor for a wide range of sexually transmitted diseases (Vandeperre et al. 1987; Gertig et al. 1997; van de Laar et al. 1998), whereas in antelope and penguins, the extent to which individuals engage in allogrooming largely dictates their ectoparasite load, with unpaired individuals and territorial males respectively (neither of which engage in allogrooming) developing higher parasite burdens (Brooke 1985; Mooring & Hart 1995). Individual variation in behaviour can also influence the types, as well as the number, of parasites that infect hosts. This is perhaps best exemplified by parasitological studies of sympatric morphs of polymorphic species that differ in their foraging behaviour and habitat use. In studies of polymorphic arctic char Salvelinus alpinus and three-spined stickleback Gasterosteus aculeatus, separate sympatric morphs typically develop divergent parasite faunas, reflecting morph-specific differences in foraging ecology and distribution (Dörücü et al. 1995; Knudsen et al. 2003; MacColl 2009).
The concept of harm caused to the host organism is central to most definitions of parasitism (Bush et al. 2001; Poulin 2007); parasites can impair host health and fitness through a wide variety of mechanisms. However, evolutionary processes may either increase or decrease the virulence of parasites (Anderson & May 1982; Poulin 2007), so the severity of their effects on hosts varies considerably. Yet, even if infectious agents have low virulence, host survival may be impacted if parasite acquisition leads to the mounting of costly immune responses, increased susceptibility to secondary infections or reduced nutritional status. Reproductive output may also be reduced if infections affect host sexual development, reproductive behaviour and their attractiveness as mates (Read 1990).
Parasite infections therefore have considerable potential to impact the fitness of host animals. Consequently, avoiding infection by certain parasites may have similar fitness pay-offs as avoiding predators, and where parasites present a significant threat, animals have evolved a wide range of behavioural strategies that provide a ‘first line of defence’ against infection (Hart 1990, 1992, 1997). As with antipredator responses, behaviours that protect against parasites impose other costs, so may only be expected to evolve when parasites pose a threat that outweighs the costs of the behaviour (Lafferty 1992). Furthermore, since the selection pressure imposed on hosts by parasites depends on the number and types of parasites present and can vary as a result of interactions with ecological factors, including food availability (Barber 2005), the level of environmental stress (Lafferty & Kuris 1999) and the coevolutionary history of hosts and parasites (Frank 1996; Morand et al. 1996), population variation in the fitness consequences of such behaviours is expected. Hence, behavioural traits that increase encounter rates with a parasite should only be suppressed in host populations where that parasite poses a threat. Similarly, parasite avoidance behaviours are unlikely to evolve in host populations that are not under significant threat of infection (Cruz & Wiley 1989; Moskat et al. 2002; Cruz et al. 2008).
(a). Behaviour and the risk of parasite infection
Although variation in almost any behavioural trait has the potential to generate individual differences in parasite exposure, behaviours commonly accepted as major axes of personality may be particularly important. For example, variation among individuals in the extent to which they approach novel entities in their environment (i.e. neophilia) may determine whether they are exposed to previously un-encountered sources of infection, predisposing more exploratory individuals to infection with novel parasites and generating a cost of exploration. Similarly, individual variation in social behaviour (e.g. Pike et al. 2008; Croft et al. 2009; Dingemanse et al. 2009) may influence the level of exposure to directly transmitted parasites, with less social individuals acquiring fewer parasites.
One way in which behavioural types often vary is in terms of their activity levels and propensity to explore their habitats, e.g. squirrels: Boon et al. (2008); tits: van Overveld & Mattysen (2010); killifish: Fraser et al. (2001). Because the risk of infection by parasites that are transmitted via mobile free-living stages or vectors is habitat dependent, animals that range more widely (i.e. more active or exploratory individuals) may therefore be exposed to a wider range of parasites. Behavioural type can also influence fine-scale positioning within animal social networks (Pike et al. 2008; Croft et al. 2009; Sih et al. 2009; Krause et al. 2010), generating links between behavioural type and the frequency and intensity of social contact, which—in turn—is likely to affect the level of exposure to socially transmitted parasites (Godfrey et al. 2009; Madden et al. 2009; Perkins et al. 2009). In cats, the level of infection with feline immunodeficiency virus and feline leukaemia virus—transmitted through aggressive and social contact, respectively—covaries in predictably opposite directions with host aggressiveness (Pontier et al. 1998). Hence, there are a variety of mechanisms by which individual differences in personality are likely to influence parasite exposure in nature.
Yet, although studies have regularly examined, for example, the propensity of individuals to feed on or avoid parasitized prey items (e.g. Urdal et al. 1995; Wedekind & Milinski 1996; Mazzi & Bakker 2003), we are aware of no such studies that have related this to individual behavioural propensities (personality). Recent studies examining ‘reciprocal effects’ in host–parasite interactions, however, provide support for the potential of pre-existing phenotypic host variation to influence infection susceptibility. Whereas phenotypic differences between infected and non-infected members of a host species are traditionally considered to reflect either causes or consequences of infection, reciprocal effects studies typically use statistical path analysis to investigate the possibility that both could be important, even in relation to a single host trait (Blanchet et al. 2009b). For example, in a recent study of rainbow smelt Osmerus mordax, pre-exposure fish growth rate positively influenced the level of infection with the trophically acquired endoparasite Proteocephalus tetrastomus, whereas parasite mass negatively impacted fish growth after infection (Blanchet et al. 2009a). Other field studies have also employed path analysis to reveal directional links between personality traits and infection levels. For example, the positive relationship between tick load and boldness among chipmunks Tamias sibiricus in an introduced population in France was better explained by a model in which activity–exploration predicted tick load than by a model invoking post-infection personality change among infected animals (Boyer et al. 2010). However, there is a general lack of studies that specifically examine the susceptibility, in terms of both exposure and resistance, to infection of different behavioural types. Experimental studies are now required that explicitly examine the potential of personality traits to influence infection susceptibility, and to test the hypothesis that such behaviours may also interact in a reciprocal fashion with infection status.
There are also reasons to expect that behavioural types may vary in their resistance to invading parasites, as well as in their likelihood of encountering them. For instance, positive correlations between hiding time and immune responses have been documented in some populations of field crickets Gryllus integer (Kortet et al. 2007). The mechanisms linking variation in personality traits to immunocompetence are generally poorly understood. However, behavioural types often vary in their metabolic rate, which is typically higher among bolder individuals (Careau et al. 2008), so the compromised anti-parasite defences of bolder individuals could arise as a consequence of immune/metabolic trade-offs (Sheldon & Verhulst 1996; Lochmillar & Deerenberg 2000). If behavioural type and the ability to withstand parasite infections covary, this potentially generates different pay-offs to behavioural types, and the solutions to the behaviour/immune response trade-off will vary between habitats that differ in their inherent risk of parasitism.
(b). Implications for the evolution of behavioural syndromes
A major explanatory hypothesis for the existence of behavioural syndromes—phenotypic correlations between discrete personality traits within populations—is that the fitness pay-offs of certain combinations of such traits exceed others (Sih et al. 2004a,b; Dingemanse & Réale 2005; Bell & Sih 2007). For example, ecological circumstances might dictate that both exploratory/aggressive and non-exploratory/non-aggressive individuals have greater fitness than those exhibiting alternative combinations of trait values. Furthermore, ecological variation between habitats might mean that the particular combinations of traits that generate maximum success differ between populations, generating different phenotypic correlations (or a lack of them) in different populations. For example, in a study of three-spined stickleback populations from the island of Anglesey, Wales, UK, significant positive correlations between aggression, boldness and exploration were only observed in populations that had coevolved with predatory fish (Dingemanse et al. 2007); where predation pressure was relaxed, correlations were significantly weaker or even negative (Dingemanse et al. 2007, 2010a).
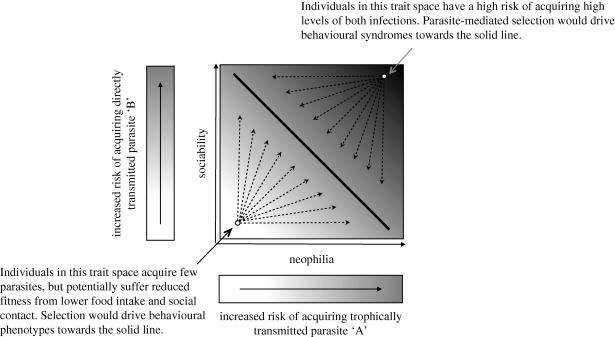
If different personality traits are associated with the risk of acquiring infections, we propose that local parasite communities could, in principle, select for particular personality trait combinations. Consider a host population, with a parasite community dominated by two species (A and B), where the probability of infection with each (pA and pB) is associated with a separate discrete personality trait. Hosts in this population may be able to reproduce successfully if they harbour high loads of either one of the parasites or intermediate loads of both, but not if they develop high loads of both parasites. This scenario is not unrealistic, as co-infections are often more debilitating than predicted by knowledge of their individual effects (Petney & Andrews 1998). The threat imposed by each parasite would, in such a scenario, impose separate selection on unrelated behaviours, and individuals exhibiting suboptimal combinations of behaviours would be selected against (see figure 1 for a graphical explanation). Such a situation could explain why there might be correlational selection on multiple—apparently unrelated—behavioural traits, and potentially provides a mechanism by which syndromes could evolve. Even in situations where multiple parasites do not impose separate selection on different behaviours, parasites could potentially interact with other environmental stressors, such as predators, to structure behavioural syndromes among host populations.
Figure 1.
Graphical illustration of how parasites could influence the evolution of behavioural syndromes. In this example, one parasite (‘A’, an introduced endoparasite) is acquired through the predation of an introduced (and hence novel) prey item, whereas another parasite (‘B’, an established ectoparasite) is acquired directly, through social contact with conspecifics. Hence, neophilic individuals may have a higher probability of exposure to parasite A, since they are more likely to approach the previously unencountered prey item, whereas more sociable individuals would be more exposed to parasite B. It is assumed that neophilia also has benefits, such as discovering new feeding opportunities, and that sociality has benefits. In this population, acquiring heavy loads of either species of parasite, or intermediate loads of each, is manageable, but acquiring high loads of both is fatal as a result of synergistic effects of infection. The diagonal line shows the syndrome that is likely to evolve in the host population because of parasite-induced correlational survival selection.
Just as the level and type of threat posed by predators varies considerably across prey populations, and has led to the evolution of divergent antipredator morphology and behaviour (Reimchen 1994), so does the threat of parasitism across populations of host species. Typically, both the risk of acquiring parasites and the associated fitness consequences of infections vary considerably between populations, and also temporally within them. One of the major hypotheses proposed to explain population differences in personality traits and behavioural syndromes is that differences in local selective regimes promote divergent behavioural types and also select for different optimal combinations of personality traits (Bell 2005; Bell & Sih 2007; Dingemanse et al. 2007). Parasites thus have the potential to impose fluctuating selection pressure in much the same way as predators or food availability, and potentially maintain variation in personality within and between populations.
4. Indirect effects on personality evolution: parasite infections that alter host state
Ecologists are used to considering the role of predators in shaping the behavioural strategies and morphology of prey organisms, but may be far less likely to consider that parasites might play a similar role. As outlined above, because infections can impose severe fitness costs on hosts, behaviours that limit contact with parasites—or reduce levels of infection—should evolve in an analogous fashion to antipredator behaviours. However, parasites could also influence the evolution of host personality in a less obvious way. Unlike predators, parasites typically do not kill their victims, at least not directly. Instead, parasites more commonly impact host state, defined by Houston & Macnamara (1999) (Reimchen 1994) as ‘aspects of an organism…or aspects of an organism's environment… [that] may constrain its possible actions’. State variables typically include factors that describe the individual or its extended phenotype, such as its energy reserves, body size or reproductive potential. State variables may be highly labile (such as energetic status, or the levels of circulating hormones) or less amenable to rapid change (such as body size or morphology). Differences in internal state play an important role in determining the efficacy of behaviour, limiting the actions that can be performed, and influencing the costs and benefits of alternate behaviours (Dall et al. 2004; McElreath & Strimling 2006). Consistent individual differences in behavioural traits can therefore arise, at least in part, as a result of variation in state variables (Dingemanse & Wolf 2010; Wolf & Weissing 2010), especially in cases where state and behaviour feed back positively (see below).
Because of their energetic, immunostimulatory and other myriad effects, parasites have the potential to change host state profoundly. Infection status may therefore influence the fitness consequences of different behavioural types. Parasites might thus potentially act as selective agents on host behaviours that influence the success of the parasitized host, i.e. the ‘mixed phenotype’, sensu Dawkins (1982). If some host personality traits are more likely to increase the survival and fitness of hosts harbouring state-altering infections, then—if there is a high probability of infection within a host population—selection should favour behavioural types that are ‘compatible’ with the infected state. For example, individuals that are aggressive in competition over resources may be better suited to survive and reproduce after acquiring a nutritionally demanding parasite. Conversely, hosts acquiring parasites that interfere with cryptic morphology—and hence increase visibility to predators—may be more likely to survive if they are of a personality type that is predisposed to spending more time hiding.
(a). Feedback processes, behavioural consistency and parasites
Much recent discussion on the evolution of animal personalities has focused on the types of feedback mechanisms that might generate consistent differences between individuals (Sih & Bell 2008; Dingemanse & Wolf 2010; Wolf & Weissing 2010), and these arguments have important implications for considering the role of parasites and their effects on host state. Wolf et al. (2007) proposed a model to explain the evolution of consistent individual behaviour (personality), based on the principle of asset protection—the idea that individuals with the most to lose should behave most cautiously and those with least to lose should be the most reckless (Clark 1994). However, as pointed out by McElreath et al. (2007), such processes potentially generate a negative feedback loop that erodes initial state differences between individuals; initially asset-poor individuals (that survive) would become asset-rich and hence become more cautious. This poses a problem for the evolution of behavioural consistency. On the other hand, positive feedback loops may be more likely to lead to the evolution of consistent behaviour since they can serve to reinforce and stabilize even minor initial variation in state or behaviour. For example, Wolf et al. (2008) showed how positive feedback loops can reduce the costs of responsiveness (an animal's propensity to adjust behaviour in the face of environmental change), leading to responsiveness becoming less costly to those individuals that have previously been responsive.
Parasites might therefore select for adaptive personalities only in particular situations, namely where state and behaviour feed back positively on each other. We consider a situation where internal energy state drives boldness in the context of foraging, and where energetically demanding parasites are acquired in food in a probabilistic manner. On acquiring a parasite, an initially bold forager experiences an elevated energetic demand, which reinforces bold foraging behaviour and increases food intake, leading to the acquisition of further parasites. In order to prosper in the face of infection, food intake may need to be maximized to sustain parasite energetic needs and permit host growth and maturation, and such a scenario may select for a bold host population. Importantly, however, the dynamics of feedback loops will depend critically on the specific biology of the host–parasite system in question. We therefore advocate research examining the various feedback loops involved between parasite infections, state and behaviour in a wide range of model host–parasite systems.
5. Behavioural changes in parasitized hosts: relevance to personality studies
Variation in host behaviour associated with parasite load is widely recorded (Moore 2002) and experimental infection studies have confirmed causation in an increasing number of systems (Barber et al. 2004). Many of the behaviours that are reportedly affected by parasite infection are key traits studied in personality research (e.g. boldness, exploration, activity and sociability); in table 1, we provide a selective review of studies where these traits have been associated with parasite status. There are traditionally three ways of interpreting the evolutionary basis of infection-associated changes in host behaviour (Poulin 1994; Thomas et al. 2005). First, host behaviour may be altered as a result of unavoidable, evolutionarily neutral side effects of infection (i.e. ‘sickness’ effects) that are adaptive to neither parasite nor host. Second, behaviour changes may arise as adaptive, facultative responses of hosts that serve to reduce infection loads or mitigate the effects of parasites (Hart 1997). So we may see patterns of grooming behaviour, the visiting of cleaning stations or even self-medication (Huffman 1997) change after an animal becomes infected. Third, host behaviour may change after infection in a manner that benefits the parasite, i.e. the behaviour change may constitute an adaptive manipulation of the host by the parasite, which accrues fitness benefits as a result. In the case of trophically transmitted parasites, aspects of antipredator behaviour are often affected, and this intuitively facilitates transmission to susceptible predators (Lafferty & Morris 1996). Such manipulation is predicted among indirectly transmitted parasites because of the absolute requirement of predation for parasite reproduction, and the many observations of altered antipredator behaviour are consistent with the hypothesis that behaviour changes facilitate successful completion of the life cycle (see Moore 2002 for a review). Trophically transmitted parasites should also benefit by enhancing the antipredator behaviour of host organisms during developmental (pre-infective) periods when they are not competent to infect subsequent hosts, as should parasites that die if their host is ingested, and examples of each of these phenomena have been reported (Milinski 1985; Tierney et al. 1993).
(a). Behavioural syndromes as targets for behaviour-manipulating parasites
To maximize their fitness value, behavioural changes that are brought about by parasites are predicted to be rather specific, influencing behavioural traits that facilitate transmission while leaving others intact. For example, recent studies of rats infected with the cat-transmitted protozoan parasite Toxoplasma gondii have demonstrated that previously documented ‘fearlessness’ (Berdoy et al. 1995, 2000) turns out to be highly specific to the investigation of pheromones emanating from cat urine (and no other tested stimuli). The particular behaviours affected by infection are similarly discrete. Vyas et al. (2007) quantified multiple behaviours of experimentally parasitized male Long-Evans rats and showed that, whereas infection converted the normal aversion to cat odour into mild attraction, infection status was not associated with changes in activity, fear conditioning, neophobia towards novel food or novel scents, nor social transmission of food preference.
Parasites may therefore have specific effects on a single personality axis without affecting others and thus have the potential to decouple behavioural syndromes in an infected population, for example if they affect boldness but not aggression. Identifying host–parasite systems where this is the case may provide useful tools for gaining insight into the stability of behavioural syndromes and potentially for gaining a greater understanding of their physiological and/or evolutionary basis. Furthermore, as has been pointed out by Poulin (2010), if behavioural syndromes evolve as adaptive responses to local environmental conditions, then the correlation between host behaviours—rather than the individual behaviours themselves—may be the target of disruption by manipulative parasites. Similarly, parasites could manipulate hosts in a manner that forces correlations between previously unconnected behaviours, if this leads to increased parasite fitness. Such forms of ‘syndrome manipulation’ may be particularly relevant to trophically transmitted parasites, since certain trait combinations may be particularly sensitive to predation. A recent study of amphipods infected with two species of trematode parasite has been the first that we are aware of to examine parasite manipulation of hosts in a behavioural syndromes framework (Coats et al. 2010). In that study, infected amphipods showed stronger, rather than weaker, behavioural correlations compared with those of uninfected hosts, possibly suggesting that infections led to previously uncorrelated behaviours becoming linked within individuals. However, because the amphipods studied were wild-caught, behavioural correlations among captured infected individuals may have arisen as a result of differential post-infection mortality of amphipods based on pre-existing trait combinations that were differently compatible with the infected state (i.e. as discussed in §2). Experimental infection studies are now required to test directly the impact of parasites on behavioural correlations and their consequences for ecosystem level processes, including trophic dynamics.
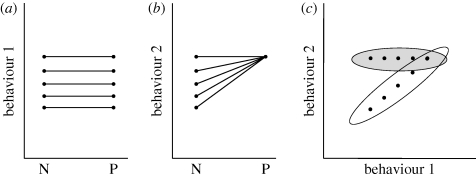
The potential of parasites to differentially affect suites of normally correlated behaviours also has implications for studies that seek to identify population variation in behavioural syndromes. Whereas among non-parasitized populations, behaviours such as boldness and aggression may correlate, among parasitized populations there may be no correlation, because of the fact that all parasitized individuals (which exhibit the full range of aggression phenotypes) score highly for boldness (see figure 2 for a graphical explanation).
Figure 2.
Graphical illustration of how infection status might generate intraspecific variation in behavioural syndromes, if parasites selectively alter behaviour. (a) A ‘behavioural reaction norm’ plot (Dingemanse et al. 2010b) with two environmental conditions (N, no parasites, and P, with parasites) on the x-axis and values of a behavioural trait on the y-axis, where each individual is represented by two points connected by a line. The parallel lines indicate that this behaviour is unaffected by parasite infection. (b) the reaction norm plot for a second behaviour, where behavioural phenotypes of individuals differ under condition ‘N’ but are all the same in condition ‘P’ (i.e. all show high values when parasitized). (c) The expected correlation between the two behaviours (1 versus 2), with the two ellipses depicting the predicted phenotypic correlations for the parasitized (shaded) and non-parasitized (open) states.
There is considerable research interest in systems where host behaviour is adaptively manipulated by parasites, and in many cases research effort focuses on investigating the mechanistic basis of behavioural manipulation (Adamo 2002; Coats et al. 2010). For example, freshwater gammarids infected with acanthocephalan parasites have been experimentally demonstrated to exhibit altered responses to predator stimuli, and evidence is accruing for the mechanistic basis of this behavioural modulation; parasites appear to alter host behaviour via modifications of host serotonergic pathways (Helluy & Holmes 1990; Tain et al. 2006, 2007). Studies such as these, which elucidate the physiological mechanisms parasites use to mediate host behaviour, provide potential opportunities to investigate the ‘constraint’ versus ‘adaptive’ hypotheses for the existence of behavioural syndromes.
6. Prospects for future research
In this paper, we identify a number of ways in which parasites potentially influence the evolution of behaviour, personality and behavioural syndromes in animal populations. Our intention is to stimulate targeted research to investigate the importance of parasitism as an ecological factor influencing the evolution of individuality in animals. We make the following suggestions for future studies that are likely to yield important information regarding the interactions of parasites and personality, and outline possible approaches that could be taken.
— Does behavioural type have implications for infection susceptibility? Studies should use experimental infection systems to separate effects on parasite exposure from parasite resistance, and should employ both immunological assays and controlled exposure experiments to investigate the parasite exposure risk and the immunocompetence of different behavioural types.
— Does individual variation in basal metabolic rate (BMR; and other physiological variables related to behavioural type) affect parasite resistance? Experimental manipulation of BMR or any other physiological trait that is linked to behavioural type could be undertaken to determine the impact on an animal's ability to withstand experimental parasite infections.
— Are some personalities more compatible than others with the infected state? To what extent does an individual's pre-existing behavioural type influence its fitness in the face of parasite infection? Do personality × infection status (P × I) interactions influence the success of the parasitized host? Studies should quantify reliable fitness correlates of experimentally parasitized hosts that differ in personality, to test the hypothesis that parasite selective environments may have important implications for the evolution of behaviour.
— Can parasites decouple otherwise robust behavioural syndromes? One potentially fruitful approach would be to quantify syndrome structure among individuals before and after experimentally infecting them with a parasite known to affect host behaviour. Detailed investigations of the physiological mechanisms underlying specific behaviour changes in parasitized hosts could give insights into the constraint and adaptive hypotheses for the existence of behavioural syndromes. Studies could also focus on the extent to which decoupling of normally adaptive syndromes by parasites (or the linking together of previously uncorrelated behaviours) is used as a strategy for enhanced transmission, by examining the ecological consequences of changes in syndrome structure.
— Can multiple parasites select for correlated behaviours and hence potentially generate adaptive syndromes in natural populations? One approach to tackling this question would be to experimentally manipulate the presence of two parasite species that infect a host species through different routes and hence whose acquisition is linked to separate behaviours; the levels of infection with each parasite could then be quantified and linked to the individual's behavioural phenotype, to determine whether some behavioural trait combinations lead to less debilitating infection levels.
— How do parasites and other selective agents (e.g. predators) interact to generate selection on personality and behavioural syndromes in the wild? Parasites represent just one of a suite of selective agents in natural ecosystems, and future work should address the implications of such ecosystem complexity for personality and behavioural syndromes in host populations. Quantifying the consequences of personality variation for the risk of predation and parasitism, for example, would provide a first step to understanding how these factors might interact to select behavioural phenotypes among host populations. Furthermore, because natural ecosystems typically include a suite of predators that vary in their suitability as hosts for particular parasites, there is a need to understand how the composition of predator communities alters selection on parasite manipulation of hosts. In recent years, there has been a growing focus on the role of parasites in ecosystem dynamics (Lafferty et al. 2006, 2008; Kuris et al. 2008), and there is now an urgent requirement to integrate studies of host personality within this framework.
Acknowledgements
We are very grateful to Robert Poulin for bringing in press and newly published material to our attention and to one other referee for constructive comments on our manuscript. We thank the editorial team for providing constructive feedback on the manuscript. I.B. is supported by research funding from the UK Natural Environment Research Council (NE/F019440/1). N.J.D. is supported by the Max Planck Society (MPG).
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Abbot P., Dill L. M.2001Sexually transmitted parasites and sexual selection in the milkweed leaf beetle, Labidomera clivicollis. Oikos 92, 91–100 10.1034/j.1600-0706.2001.920111.x (doi:10.1034/j.1600-0706.2001.920111.x) [DOI] [Google Scholar]
- Adamo S. A.2002Modulating the modulators: parasites, neuromodulators and host behavioural change. Brain Behav. Evol. 60, 370–377 10.1159/000067790 (doi:10.1159/000067790) [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M.1982Coevolution of hosts and parasites. Parasitology 85, 411–426 10.1017/S0031182000055360 (doi:10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- Barber I.2005Parasites grow larger in faster growing fish hosts. Int. J. Parasitol. 35, 137–143 10.1016/j.ijpara.2004.11.010 (doi:10.1016/j.ijpara.2004.11.010) [DOI] [PubMed] [Google Scholar]
- Barber I., Huntingford F. A., Crompton D. W. T.1995The effect of hunger and cestode parasitism on the shoaling decisions of small fresh-water fish. J. Fish Biol. 47, 524–536 10.1111/j.1095-8649.1995.tb01919.x (doi:10.1111/j.1095-8649.1995.tb01919.x) [DOI] [Google Scholar]
- Barber I., Walker P., Svensson P. A.2004Behavioural responses to simulated avian predation in female three spined sticklebacks: the effect of experimental Schistocephalus solidus infections. Behaviour 141, 1425–1440 10.1163/1568539042948231 (doi:10.1163/1568539042948231) [DOI] [Google Scholar]
- Bell A. M.2005Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M.2007Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761 10.1098/rspb.2006.0199 (doi:10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M., Sih A.2007Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Hankison S. J., Laskowski K. L.2009The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783 10.1016/j.anbehav.2008.12.022 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoy M., Webster J. P., MacDonald D. W.1995The manipulation of rat behaviour by Toxoplasma gondii. Mammalia 59, 605–613 10.1515/mamm.1995.59.4.605 (doi:10.1515/mamm.1995.59.4.605) [DOI] [Google Scholar]
- Berdoy M., Webster J. P., Macdonald D. W.2000Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B. 267, 1591–1594 10.1098/rspb.2000.1182 (doi:10.1098/rspb.2000.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P. A., Stamps J. A.2008Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Blanchet S., Mejean L., Bourque J. F., Lek S., Thomas F., Marcogliese D. J., Dodson J. J., Loot G.2009aWhy do parasitized hosts look different? Resolving the ‘chicken–egg’ dilemma. Oecologia 160, 37–47 10.1007/s00442-008-1272-y (doi:10.1007/s00442-008-1272-y) [DOI] [PubMed] [Google Scholar]
- Blanchet S., Thomas F., Loot G.2009bReciprocal effects between host phenotype and pathogens: new insights from an old problem. Trends Parasitol. 25, 364–369 10.1016/j.pt.2009.05.005 (doi:10.1016/j.pt.2009.05.005) [DOI] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2007The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecol. Lett. 10, 1094–1104 10.1111/j.1461-0248.2007.01106.x (doi:10.1111/j.1461-0248.2007.01106.x) [DOI] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2008Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117, 1321–1328 10.1111/j.0030-1299.2008.16567.x (doi:10.1111/j.0030-1299.2008.16567.x) [DOI] [Google Scholar]
- Boyer N., Réale D., Marmet J., Pisanu B., Chapuis L.2010Personality, space use and tick load in an introduced population of Siberian chipmunks Tanias sibiricus. J. Anim. Ecol. 79, 538–547 10.1111/j.1365-2656.2010.01659.x (doi:10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- Brooke M. D.1985The effect of allopreening on tick burdens of molting eudyptid penguins. Auk 102, 893–895 [Google Scholar]
- Bush A. O., Fernández J. C., Esch G. W., Seed J. R.2001Parasitism: the diversity and ecology of animal parasites. Cambridge, UK: Cambridge University Press [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Réale D.2008Energy metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Clark C. W.1994Antipredator behavior and the asset protection principle. Behav. Ecol. 5, 159–170 10.1093/beheco/5.2.159 (doi:10.1093/beheco/5.2.159) [DOI] [Google Scholar]
- Coats J., Poulin R., Nakagawa S.2010The consequences of parasitic infections for host behavioural correlations and repeatability. Behaviour 147, 367–382 10.1163/000579509X12574307194101 (doi:10.1163/000579509X12574307194101) [DOI] [Google Scholar]
- Cote J., Dreiss A., Clobert J.2008Social personality trait and fitness. Proc. R. Soc. B. 275, 2851–2858 10.1098/rspb.2008.0783 (doi:10.1098/rspb.2008.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D. P., Krause J., Darden S. K., Ramnarine I. W., Faria J. J., James R.2009Behavioural trait assortment in a social network: patterns and implications. Behav. Ecol. Sociobiol. 63, 1495–1503 10.1007/s00265-009-0802-x (doi:10.1007/s00265-009-0802-x) [DOI] [Google Scholar]
- Cruz A., Wiley J. W.1989The decline of an adaptation in the absence of a presumed selection pressure. Evolution 43, 55–62 10.2307/2409163 (doi:10.2307/2409163) [DOI] [PubMed] [Google Scholar]
- Cruz A., Prather J. W., Wiley J. W., Weaver P. F.2008Egg rejection behavior in a population exposed to parasitism: Village Weavers on Hispaniola. Behav. Ecol. 19, 398–403 10.1093/beheco/arm147 (doi:10.1093/beheco/arm147) [DOI] [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- Dawkins R.1982The extended phenotype. Oxford, UK: Oxford University Press [Google Scholar]
- Dingemanse N. J., Réale D.2005Natural selection and animal personality. Behaviour 142, 1159–1184 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- Dingemanse N. J., Wolf M.2010Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M.2004Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Van der Plas F., Wright J., Réale D., Schrama M., Roff D. A., Van der Zee E., Barber I.2009Individual experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc. R. Soc. B 276, 1285–1293 10.1098/rspb.2008.1555 (doi:10.1098/rspb.2008.1555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Dochtermann N. A., Wright J.2010aA method for exploring the structure of behavioural syndromes to allow formal comparison within and between datasets. Anim. Behav. 79, 439–450 10.1016/j.anbehav.2009.11.024 (doi:10.1016/j.anbehav.2009.11.024) [DOI] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010bBehavioural reaction norms: where animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Dörücü M., Adams C. E., Huntingford F. A., Crompton D. W. T.1995How fish–helminth associations arise: an example from Arctic Charr in Loch Rannoch. J. Fish Biol. 47, 1038–1043 10.1111/j.1095-8649.1995.tb06027.x (doi:10.1111/j.1095-8649.1995.tb06027.x) [DOI] [Google Scholar]
- Flegr J., Havlicek J., Kodym P., Maly M., Smahel Z.2002Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case–control study. BMC Infect. Dis. 2, 11. 10.1186/1471-2334-2-11 (doi:10.1186/1471-2334-2-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Hudson P. J.2001Parasites reduce territorial behaviour in red grouse (Lagopus lagopus scoticus). Ecol. Lett. 4, 139–143 10.1046/j.1461-0248.2001.00207.x (doi:10.1046/j.1461-0248.2001.00207.x) [DOI] [Google Scholar]
- Frank S. A.1996Models of parasite virulence. Q. Rev. Biol. 71, 37–78 10.1086/419267 (doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- Fraser D. F., Gilliam J. F., Daley M. J., Le A. N., Skalski G. T.2001Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135 10.1086/321307 (doi:10.1086/321307) [DOI] [PubMed] [Google Scholar]
- Gertig D. M., Kapiga S. H., Shao J. F., Hunter D. J.1997Risk factors for sexually transmitted diseases among women attending family planning clinics in Dar-es-Salaam, Tanzania. Genitourinary Med. 73, 39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N.1983Behavioral effects of the parasite Schistocephalus solidus (Cestoda) on an intermediate host, the 3-spined stickleback, Gasterosteus aculeatus L. Anim. Behav. 31, 1192–1194 10.1016/S0003-3472(83)80025-6 (doi:10.1016/S0003-3472(83)80025-6) [DOI] [Google Scholar]
- Giles N.1987Predation risk and reduced foraging activity in fish—experiments with parasitized and non-parasitized 3-spined sticklebacks, Gasterosteus aculeatus L. J. Fish Biol. 31, 37–44 10.1111/j.1095-8649.1987.tb05212.x (doi:10.1111/j.1095-8649.1987.tb05212.x) [DOI] [Google Scholar]
- Godfrey S. S., Bull C. M., James R., Murray K.2009Network structure and parasite transmission in a group living lizard, the gidgee skink, Egernia stokesii. Behav. Ecol. Sociobiol. 63, 1045–1056 10.1007/s00265-009-0730-9 (doi:10.1007/s00265-009-0730-9) [DOI] [Google Scholar]
- Gourbal B. E. F., Lacroix A., Gabrion C.2002Behavioural dominance and Taenia crassiceps parasitism in BALB/c male mice. Parasitol. Res. 88, 912–917 [DOI] [PubMed] [Google Scholar]
- Hart B. L.1990Behavioral adaptations to pathogens and parasites—5 strategies. Neurosci. Biobehav. Rev. 14, 273–294 10.1016/S0149-7634(05)80038-7 (doi:10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- Hart B. L.1992Behavioral adaptations to parasites—an ethological approach. J. Parasitol. 78, 256–265 10.2307/3283472 (doi:10.2307/3283472) [DOI] [PubMed] [Google Scholar]
- Hart B. L.1997Behavioural defence. In Host–parasite evolution: general principle and avian models (eds Clayton D. H., Moore J.), pp. 59–77 Oxford, UK: Oxford University Press [Google Scholar]
- Hechtel L. J., Johnson C. L., Juliano S. A.1993Modification of antipredator behavior of Caecidotea intermedius by its parasite Acanthocephalus dirus. Ecology 74, 710–713 10.2307/1940798 (doi:10.2307/1940798) [DOI] [Google Scholar]
- Helluy S., Holmes J. C.1990Serotonin, octopamine, and the clinging behavior induced by the parasite Polymorphus paradoxus (Acanthocephala) in Gammarus lacustris (Crustacea). Can. J. Zool. 68, 1214–1220 10.1139/z90-181 (doi:10.1139/z90-181) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1999Models of adaptive behaviour: an approach based on state. Cambridge, UK: Cambridge University Press [Google Scholar]
- Huffman M. A.1997Current evidence for self-medication in primates: a multidisciplinary perspective. Yearbook Phys. Anthropol. 40, 171–200 10.1002/(SICI)1096-8644(1997) (doi:10.1002/(SICI)1096-8644(1997)) [DOI] [Google Scholar]
- Huntingford F. A.1976Relationship between anti-predator behavior and aggression among conspecifics in 3-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 24, 245–260 10.1016/S0003-3472(76)80034-6 (doi:10.1016/S0003-3472(76)80034-6) [DOI] [Google Scholar]
- Johnson J. C., Sih A.2005Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav. Ecol. Sociobiol. 58, 390–396 10.1007/s00265-005-0943-5 (doi:10.1007/s00265-005-0943-5) [DOI] [Google Scholar]
- Kavaliers M., Colwell D. D.1995Decreased predator avoidance in parasitized mice—neuromodulatory correlates. Parasitology 111, 257–263 10.1017/S0031182000081816 (doi:10.1017/S0031182000081816) [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Colwell D. D., PerrotSinal T. S.1997Opioid and non-opioid NMDA-mediated predator-induced analgesia in mice and the effects of parasitic infection. Brain Res. 766, 11–18 10.1016/S0006-8993(97)00521-0 (doi:10.1016/S0006-8993(97)00521-0) [DOI] [PubMed] [Google Scholar]
- Knudsen R., Amundsen P. A., Klemetsen A.2003Inter- and intra-morph patterns in helminth communities of sympatric whitefish morphs. J. Fish Biol. 62, 847–859 10.1046/j.1095-8649.2003.00069.x (doi:10.1046/j.1095-8649.2003.00069.x) [DOI] [Google Scholar]
- Kortet R., Rantala M. J., Hedrick A.2007Boldness in anti-predator behaviour and immune defence in field crickets. Evol. Ecol. Res. 9, 185–197 [Google Scholar]
- Krause J., Godin J. G. J.1994Influence of parasitism on the shoaling behavior of banded killifish, Fundulus diaphanus. Can. J. Zool. 72, 1775–1779 10.1139/z94-240 (doi:10.1139/z94-240) [DOI] [Google Scholar]
- Krause J., James R., Croft D. P.2010Personality in the context of social networks. Phil. Trans. R. Soc. B 365, 4099–4106 10.1098/rstb.2010.0216 (doi:10.1098/rstb.2010.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuris A. M., et al. 2008Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454, 515–518 10.1038/nature06970 (doi:10.1038/nature06970) [DOI] [PubMed] [Google Scholar]
- Lafferty K. D.1992Foraging on prey that are modified by parasites. Am. Nat. 140, 854–867 10.1086/285444 (doi:10.1086/285444) [DOI] [Google Scholar]
- Lafferty K. D.1999The evolution of trophic transmission. Parasitol. Today 15, 111–115 10.1016/S0169-4758(99)01397-6 (doi:10.1016/S0169-4758(99)01397-6) [DOI] [PubMed] [Google Scholar]
- Lafferty K. D., Kuris A. M.1999How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 44, 925–931 10.4319/lo.1999.44.3_part_2.0925 (doi:10.4319/lo.1999.44.3_part_2.0925) [DOI] [Google Scholar]
- Lafferty K. D., Morris A. K.1996Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77, 1390–1397 10.2307/2265536 (doi:10.2307/2265536) [DOI] [Google Scholar]
- Lafferty K. D., Dobson A. P., Kuris A. M.2006Parasites dominate food web links. Proc. Natl Acad. Sci. USA 103, 11 211–11 216 10.1073/pnas.0604755103 (doi:10.1073/pnas.0604755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. D., et al. 2008Parasites in food webs: the ultimate missing links. Ecol. Lett. 11, 533–546 10.1111/j.1461-0248.2008.01174.x (doi:10.1111/j.1461-0248.2008.01174.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochmillar R., Deerenberg C.2000Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98 10.1034/j.1600-0706.2000.880110.x (doi:10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- MacColl A. D. C.2009Parasite burdens differ between sympatric three-spined stickleback species. Ecography 32, 153–160 10.1111/j.1600-0587.2008.05486.x (doi:10.1111/j.1600-0587.2008.05486.x) [DOI] [Google Scholar]
- Madden J. R., Drewe J. A., Pearce G. P., Clutton-Brock T. H.2009The social network structure of a wild meerkat population: 2. Intragroup interactions. Behav. Ecol. Sociobiol. 64, 81–95 10.1007/s00265-009-0820-8 (doi:10.1007/s00265-009-0820-8) [DOI] [Google Scholar]
- Mazzi D., Bakker T. C. M.2003A predator's dilemma: prey choice and parasite susceptibility in three-spined sticklebacks. Parasitology 126, 339–347 10.1017/S0031182003003019 (doi:10.1017/S0031182003003019) [DOI] [PubMed] [Google Scholar]
- McElreath R., Strimling P.2006How noisy information and individual asymmetries can make 'personality' an adaptation: a simple model. Anim. Behav. 72, 1135–1139 10.1016/j.anbehav.2006.04.001 (doi:10.1016/j.anbehav.2006.04.001) [DOI] [Google Scholar]
- McElreath R., Luttbeg B., Fogarty S. P., Brodin T., Sih A.2007Evolution of animal personalities. Nature 450, E5–E5 10.1038/nature06326 (doi:10.1038/nature06326) [DOI] [PubMed] [Google Scholar]
- Milinski M.1985Risk of predation of parasitized sticklebacks (Gasterosteus aculeatus L) under competition for food. Behaviour 93, 203–215 10.1163/156853986X00883 (doi:10.1163/156853986X00883) [DOI] [Google Scholar]
- Moore J.2002Parasites and the behavior of animals. Oxford, UK: Oxford University Press [Google Scholar]
- Mooring M. S., Hart B. L.1995Differential grooming rate and tick load of territorial male and female impala Aepyceros melampus. Behav. Ecol. 6, 94–101 10.1093/beheco/6.1.94 (doi:10.1093/beheco/6.1.94) [DOI] [Google Scholar]
- Morand S., Manning S. D., Woolhouse M. E. J.1996Parasite–host coevolution and geographic patterns of parasite infectivity and host susceptibility. Proc. R. Soc. Lond. B. 263, 119–128 10.1098/rspb.1996.0019 (doi:10.1098/rspb.1996.0019) [DOI] [PubMed] [Google Scholar]
- Moskat C., Szentpeteri J., Barta Z.2002Adaptations by great reed warblers brood parasitism: a comparison of populations in sympatry and allopatry with the common cuckoo. Behaviour 139, 1313–1329 10.1163/156853902321104181 (doi:10.1163/156853902321104181) [DOI] [Google Scholar]
- Pasternak A. F., Huntingford F. A., Crompton D. W. T.1995Changes in metabolism and behavior of the fresh-water copepod Cyclops strenuus abyssorum infected with Diphyllobothrium spp. Parasitology 110, 395–399 10.1017/S0031182000064738 (doi:10.1017/S0031182000064738) [DOI] [PubMed] [Google Scholar]
- Perkins S. E., Cagnacci F., Stradiotto A., Arnoldi D., Hudson P. J.2009Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. J. Anim. Ecol. 78, 1015–1022 10.1111/j.1365-2656.2009.01557.x (doi:10.1111/j.1365-2656.2009.01557.x) [DOI] [PubMed] [Google Scholar]
- Petney T. N., Andrews R. H.1998Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 28, 377–393 10.1016/S0020-7519(97)00189-6 (doi:10.1016/S0020-7519(97)00189-6) [DOI] [PubMed] [Google Scholar]
- Pike T. W., Samanta M., Lindstrom J., Royle N. J.2008Behavioural phenotype affects social interactions in an animal network. Proc. R. Soc. B 275, 2515–2520 10.1098/rspb.2008.0744 (doi:10.1098/rspb.2008.0744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateaux L.1972Sur les modifications produites chez une fourmi par la presence d'un parasite cestode. Ann. Sci. Nat. (Zool.) 14, 203–220 [Google Scholar]
- Pontier D., Fromont E., Courchamp F., Artois M., Yoccoz N. G.1998Retroviruses and sexual size dimorphism in domestic cats (Felis catus L.). Proc. R. Soc. Lond. B 265, 167–173 10.1098/rspb.1998.0278 (doi:10.1098/rspb.1998.0278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R.1994The evolution of parasite manipulation of host behavior—a theoretical analysis. Parasitology 109, S109–S118 [DOI] [PubMed] [Google Scholar]
- Poulin R.2007Evolutionary ecology of parasites. Princeton, NJ: Princeton University Press [Google Scholar]
- Poulin R.2010Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Stud. Behav. 41, 151–186 10.1016/S0065-3454(10)41005-0 (doi:10.1016/S0065-3454(10)41005-0) [DOI] [Google Scholar]
- Quinn J. L., Patrick S. C., Bouwhuis S., Wilkin T. A., Sheldon B. C.2009Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215 10.1111/j.1365-2656.2009.01585.x (doi:10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
- Rau M. E., Caron F. R.1979Parasite-induced susceptibility of moose to hunting. Can. J. Zool. 57, 2466–2468 10.1139/z79-321 (doi:10.1139/z79-321) [DOI] [Google Scholar]
- Read A. F.1990Parasites and the evolution of host sexual behaviour. In Parasitism and host behaviour (eds Barnard C. J., Behnke J. M.), pp. 117–157 London, UK: Taylor and Francis [Google Scholar]
- Réale D., Festa-Bianchet M.2003Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65, 463–470 10.1006/anbe.2003.2100 (doi:10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Reimchen T. E.1994Predators and morphological evolution in threespine stickleback. In Evolutionary biology of the threespine stickleback (eds Bell M. A., Foster S. A.), pp. 240–273 Oxford, UK: Oxford University Press [Google Scholar]
- Sheldon B., Verhulst S.1996Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C.2004aBehavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004bBehavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Adv. Stud. Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Hanser S. F., McHugh K. A.2009Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 63, 975–988 10.1007/s00265-009-0725-6 (doi:10.1007/s00265-009-0725-6) [DOI] [Google Scholar]
- Smith B. R., Blumstein D. T.2008Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stirling D. G., Réale D., Roff D. A.2002Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289 10.1046/j.1420-9101.2002.00389.x (doi:10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- Tain L., Perrot-Minnot M. J., Cézilly F.2006Altered host behaviour and brain serotonergic activity caused by acanthocephalans: evidence for specificity. Proc. R. Soc. B 273, 3039–3045 10.1098/rspb.2006.3618 (doi:10.1098/rspb.2006.3618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain L., Perrot-Minnot M. J., Cézilly F.2007Differential influence of Pomphorhynchus laevis (Acanthocephala) on brain serotonergic activity in two congeneric host species. Biol. Lett. 3, 68–71 10.1098/rsbl.2006.0583 (doi:10.1098/rsbl.2006.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F., Adamo S., Moore J.2005Parasitic manipulation: where are we and where should we go? Behav. Process. 68, 185–199 10.1016/j.beproc.2004.06.010 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Tierney J. F., Huntingford F. A., Crompton D. W. T.1993The relationship between infectivity of Schistocephalus solidus (Cestoda) and antipredator behavior of its intermediate host, the 3-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 46, 603–605 10.1006/anbe.1993.1229 (doi:10.1006/anbe.1993.1229) [DOI] [Google Scholar]
- Urdal K., Tierney J. F., Jakobsen P. J.1995The tapeworm Schistocephalus solidus alters the activity and response, but not the predation susceptibility of infected copepods. J. Parasitol. 81, 330–333 10.2307/3283949 (doi:10.2307/3283949) [DOI] [PubMed] [Google Scholar]
- van de Laar M. J. W., Termorshuizen F., Slomka M. J., van Doornum G. J. J., Ossewaarde J. M., Brown D. W. G., Coutinho R. A., van den Hoek J. A. R.1998Prevalence and correlates of herpes simplex virus type 2 infection: evaluation of behavioural risk factors. Int. J. Epidemiol. 27, 127–134 10.1093/ije/27.1.127 (doi:10.1093/ije/27.1.127) [DOI] [PubMed] [Google Scholar]
- van Overveld T., Mattysen E.2010Personality predicts spatial responses to food manipulations in free-ranging great tits (Parus major). Biol. Lett. 6, 187–190 10.1098/rsbl.2009.0764 (doi:10.1098/rsbl.2009.0764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeperre P., Lepolain B., Carael M., Nzaramba D., Zissis G., Butzler J. P.1987HIV antibodies in a remote rural area in Rwanda, Central Africa—an analysis of potential risk factors for HIV seropositivity. AIDS 1, 213–215 [PubMed] [Google Scholar]
- Vyas A., Kim S. K., Giacomini N., Boothroyd J. C., Sapolsky R. M.2007Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl Acad. Sci. USA 104, 6442–6447 10.1073/pnas.0608310104 (doi:10.1073/pnas.0608310104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J. P.1994The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology 109, 583–589 10.1017/S0031182000076460 (doi:10.1017/S0031182000076460) [DOI] [PubMed] [Google Scholar]
- Webster J. P., Brunton C. F. A., Macdonald D. W.1994Effect of Toxoplasma gondii upon neophobic behavior in wild brown rats Rattus norvegicus. Parasitology 109, 37–43 10.1017/S003118200007774X (doi:10.1017/S003118200007774X) [DOI] [PubMed] [Google Scholar]
- Wedekind C., Milinski M.1996Do three-spined sticklebacks avoid consuming copepods, the first intermediate host of Schistocephalus solidus? An experimental analysis of behavioural resistance. Parasitology 112, 371–383 10.1017/S0031182000066609 (doi:10.1017/S0031182000066609) [DOI] [Google Scholar]
- Wilson D. S., Coleman K., Clark A. B., Biederman L.1993Shy–bold continuum in pumpkinseed sunfish (Lepomis gibbosus)—an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260 10.1037/0735-7036.107.3.250 (doi:10.1037/0735-7036.107.3.250) [DOI] [Google Scholar]
- Wolf M., Weissing F. J.2010An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–585 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J.2008Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]