Abstract
Many animals exhibit behavioural syndromes—consistent individual differences in behaviour across two or more contexts or situations. Here, we present adaptive, state-dependent mathematical models for analysing issues about behavioural syndromes. We find that asset protection (where individuals with more ‘assets’ tend be more cautious) and starvation avoidance, two state-dependent mechanisms, can explain short-term behavioural consistency, but not long-term stable behavioural types (BTs). These negative-feedback mechanisms tend to produce convergence in state and behaviour over time. In contrast, a positive-feedback mechanism, state-dependent safety (where individuals with higher energy reserves, size, condition or vigour are better at coping with predators), can explain stable differences in personality over the long term. The relative importance of negative- and positive-feedback mechanisms in governing behavioural consistency depends on environmental conditions (predation risk and resource availability). Behavioural syndromes emerge more readily in conditions of intermediate ecological favourability (e.g. medium risk and medium resources, or high risk and resources, or low risk and resources). Under these conditions, individuals with higher initial state maintain a tendency to be bolder than individuals that start with low initial state; i.e. later BT is determined by state during an early ‘developmental window’. In contrast, when conditions are highly favourable (low risk, high resources) or highly unfavourable (high risk, low resources), individuals converge to be all relatively bold or all relatively cautious, respectively. In those circumstances, initial differences in BT are not maintained over the long term, and there is no early developmental window where initial state governs later BT. The exact range of ecological conditions favouring behavioural syndromes depends also on the strength of state-dependent safety.
Keywords: behavioural syndromes, animal personality, state-dependent behaviour, state-dependent safety, boldness, predation risk
1. Introduction
In recent years, numerous studies have found that within species, animals exhibit personalities or behavioural syndromes—consistent individual differences in behaviour across two or more situations (Clark & Ehlinger 1987; Gosling 2001; Dall et al. 2004; Sih et al. 2004a,b; Réale et al. 2007; Biro & Stamps 2008; Sih & Bell 2008). For example, although individuals typically alter their aggressiveness or boldness depending on the ecological situation (e.g. resource availability or predation risk), some are consistently more aggressive or bold than others in multiple situations (Huntingford 1976; Riechert & Hedrick 1993; Wilson et al. 1994; Sih et al. 2003; Duckworth 2006; Bell & Sih 2007; Cote & Clobert 2007; Johnson & Sih 2007; Kortet & Hedrick 2007). That is, individuals differ in behavioural type (BT), with some having a BT that is consistently more bold or aggressive than others. The result is a positive correlation between behaviours expressed in different situations. Interestingly, while many studies have found evidence of behavioural syndromes, others have not found behavioural correlations over time or across contexts. The goal of this paper is to provide an adaptive, state-dependent framework for understanding this variation in behavioural syndromes.
To clarify terminology, a behavioural syndrome involves both within- and between-individual consistency in behaviour across either different situations or contexts (Sih et al. 2004a). A context is a functional behavioural category—e.g. feeding, mating, predator avoidance or parental care contexts. A situation is the set of conditions at a particular time. Different situations could involve different levels along an environmental gradient (e.g. different levels of predation risk) or different conditions across time (e.g. breeding versus non-breeding seasons). Within-individual consistency (having a BT) is an individual characteristic—the tendency for a given individual to behave consistently across contexts or situations. Between-individual consistency is a population characteristic—the tendency for a set of individuals to exhibit consistent individual differences in behaviour across contexts or situations (e.g. rank-order consistency). The result is a behavioural correlation across contexts or situations. Our analysis focuses on behavioural consistency across situations that differ in predation risk; however, our basic logic should also apply to other situations and contexts.
Sih et al. (2004a,b) suggested that behavioural correlations across situations might be particularly important for ecology and evolution when: (i) tradeoffs exist such that different behaviours are favoured in different situations and (ii) behavioural consistency across situations results in less than optimal plasticity across situations. Point (i) is a central tenet of behavioural ecology; e.g. predator–prey behavioural ecology typically assumes that being bolder is riskier, but yields more resources (e.g. food, mates), while being less bold is safer, but less rewarding (Sih 1992; Werner & Anholt 1993; Lima 1998; Stamps 2007; Wolf et al. 2007a). Thus, in high-risk situations, cautious behaviour is often favoured, while in low-risk situations, selection favours being bolder. Point (ii) alone does not necessarily pose a problem for animals. Individuals can, in principle, exhibit the optimal behaviour in all situations. However, adding behavioural consistency (point (ii)) potentially generates a costly tradeoff associated with having a BT. For example, while bold individuals might do well in low-risk situations, a tradeoff arises if their bold BT carries over to result in inappropriately bold behaviour (and thus low survival) in high-risk situations (e.g. Riechert & Hedrick 1993; Sih et al. 2003). Conversely, while individuals with a cautious BT might hide well in high-risk situations, a tradeoff across situations exists if the cautious BT results in relatively low feeding rates even when risk is low (Sih et al. 2003; Brodin & Johansson 2004).
Numerous studies have quantified effects of behaviour per se on components of fitness in different situations (e.g. Lima 1998; Brodin & Johansson 2004), and a moderate number of studies have measured effects of BT on fitness in the laboratory. Relatively few studies have quantified how BT affects fitness in the field (Smith & Blumstein 2008). Notably, however, several field studies have shown that different BTs are favoured in different ecological conditions depending on resource levels or predation risk (Réale & Festa-Bianchet 2003; Dingemanse et al. 2004; Boon et al. 2007). To emphasize, in each ecological condition examined, some BTs do well, but others fare poorly.
In this context, an interesting question is: if behavioural correlations can result in apparently suboptimal behaviour in some situations, why should these correlations persist? Should not natural selection decouple correlations that reduce fitness? Why should individuals show stable, consistent BTs if their BT causes apparently suboptimal behaviour? Why should animals, including humans, have personalities?
Behavioural consistency can be viewed at two main time scales. On a long time scale, some studies have looked for whether BTs are stable over an entire lifetime, or at least over a substantial part of the life cycle (Réale et al. 2000; Dingemanse et al. 2002; Bell & Stamps 2004; Caspi et al. 2005; Johnson & Sih 2005; Roberts et al. 2006). In contrast, presumably owing to the difficulty of following individual BTs over long periods, many studies have examined behavioural consistency over only a few hours or days (e.g. Moretz et al. 2007; Salonen & Peuhkuri 2006; Croft et al. 2009). While a behavioural carryover over just a short period seems less striking than stable BTs over a lifetime, even short carryovers can be ecologically important. When individuals with an active BT remain inappropriately active for a few hours after predators appear, the result is often lethal (Sih et al. 2003).
A growing number of both short- and long-term studies have found that significant behavioural correlations are sometimes, but not always, detected (e.g. Bell 2005; Bell & Sih 2007; Dingemanse et al. 2007; Moretz et al. 2007; Nelson et al. 2008; Snekser et al. 2009; Sinn et al. 2010). In some cases, the inability to detect a significant correlation might be due to low statistical power (Dingemanse et al. 2010a). In addition, in most cases, empirical studies have not applied new statistical methods championed by Dingemanse et al. (2010b) to partition behavioural correlations into between- versus within-individual components. Our interest is, in particular, in behavioural correlations owing to between-individual differences in BT (i.e. between-individual correlations). Nonetheless, extant evidence suggests a clear possibility that behavioural correlations vary in strength in different situations. In most cases, studies have not identified factors or mechanisms to explain this variation in the strength of behavioural correlations. One exception involves the correlation between boldness and aggressiveness in stickleback fish. Positive correlations between these behaviours were found primarily in populations with higher predation risk (Bell 2005; Bell & Sih 2007; Dingemanse et al. 2007; but see Dingemanse et al. 2010a). Under low risk, the correlation was not significant. Another example involves the observation with damselfish that an aggression syndrome was observed under natural conditions, but not after an experimental manipulation enhanced overall habitat quality (Snekser et al. 2009). A major issue is thus to explain variation in behavioural syndromes. What mechanisms explain why we sometimes, but not always see behavioural correlations and long-term persistence of stable BTs?
Here, our goals are to: (i) use an adaptive, state-dependent modelling framework to identify mechanisms that can explain both short- and long-term stability of BTs and (ii) examine how variation in feedbacks between state and behaviour and in key ecological factors (resource availability and predation risk) might explain both when behavioural syndromes do and do not occur.
(a). Adaptive behavioural syndromes
Much of the thinking on why animals exhibit behavioural consistency has emphasized proximate constraints: neuroendocrine profiles that can have a genetic basis (Capitanio et al. 1998; Koolhaas et al. 1999, 2007; Bell et al. 2007), or metabolic differences associated with variation in metabolic organ size (Biro & Stamps 2008; Careau et al. 2008). A recent, alternative approach emphasizes that having a consistent BT can be adaptive when either: (i) consistency is the best response to environmental uncertainty (McElreath & Strimling 2006), (ii) there are social benefits associated with being predictable (Dall et al. 2004; McNamara et al. 2009), (iii) selection favours consistent (rather than fluctuating) growth rates (Stamps 2007), or (iv) adaptive behaviour is ‘anchored’ to a more stable state variable such as size, energy reserves or life-history type (Wolf et al. 2007a; Sih & Bell 2008; Dingemanse & Wolf 2010; Wolf & Weissing 2010). The present paper builds on this final idea to not only explain why behavioural consistency can be favoured, but also to explain both when it is and when it is not favoured.
The fundamental logic underlying adaptive, state-dependent explanations for behavioural syndromes is that although behaviour can, in principle, be extremely plastic, if optimal behaviour is connected to a slow-changing state variable (e.g. size, energy reserves, condition, morphology, reproductive value), then behaviour should also be slow-changing (consistent over time or across situations). Wolf et al. (2007a) examined a model that connected BTs to stable, life-history types that differ in assets. They posited that early in life, some individuals explore more, gain more information about high-quality habitats and thus have good potential to enjoy high reproductive success later in life (i.e. they have high assets), while others reproduce more early in life, explore little and thus have lower future assets. In essence, in their model, different BTs represent alternative strategies for coping with a life-history tradeoff between early reproduction and later fitness. Following the asset protection principle (Clark 1994), high explorers with large assets should be cautious (not bold, not aggressive) to protect their large assets, while low explorers with less to lose should be bolder and aggressive (e.g. Biro et al. 2005; Heithaus et al. 2007).
Asset protection, however, is inherently a negative-feedback mechanism that results in convergence in state (assets) over time, rather than maintenance of differences (Clark 1994; McElreath et al. 2007). Individuals that begin with high assets should be cautious and unaggressive, which should cause their assets to erode over time. Conversely, individuals that begin with low assets should be bold and aggressive, which, assuming that they survive, should increase assets over time. Thus, McElreath et al. (2007) suggest that unless other mechanisms come into play that offset the negative feedback, standard state-dependent models do not explain the long-term stability of BTs. Wolf et al. (2007a,b) suggest that despite the negative feedback inherent in asset protection, their life-history-based model can explain behavioural syndromes if: (i) behavioural consistency is only for short periods of time or (ii) behaviour has relatively little effect on state, e.g. if new assets are used (e.g. converted to reproduction) immediately. While these points might apply to some examples of behavioural syndromes, they do not appear to explain long-term behavioural consistency in other systems, including stable personalities in humans.
Another negative-feedback mechanism that is often included in state-dependent models is a starvation threshold (Houston & McNamara 1999; Clark & Mangel 2000). Animals that have very low state must be bold in response to the danger of starving to death. In contrast, animals that are not close to the starvation threshold can be cautious without a chance of imminent starvation. This is, like asset protection, a negative-feedback process because bold foraging by animals near the starvation threshold should raise their energy reserves and thus allow them to be more cautious in the future. Although a starvation threshold has not been featured in models of adaptive behavioural syndromes, the logic outlined here suggests that it alone should not favour long-term behavioural consistency.
In contrast, McElreath et al. (2007) suggested that positive-feedback mechanisms hold great promise for explaining behavioural syndromes (also see Sih & Bell 2008; Wolf et al. 2008; Wolf & Weissing 2010; Dingemanse & Wolf 2010). In a positive-feedback loop between assets and behaviour, individuals that already have high state (assets) would be bold, and thus gain more resources that maintain their high state. Conversely, individuals with low state would be relatively cautious (contra the asset protection or starvation threshold mechanisms) and thus would not gain the resources to substantially increase their state. Via this process, individual differences in state would be maintained, or would even diverge over the long term. The differences and divergence in state can then explain long-term stability of divergent BTs.
If positive-feedback mechanisms drive divergence in state, then small differences in initial state can be important in setting the long-term trajectory for different BTs. What might cause variation in initial state? If the period when individuals can express a BT begins when they become independent from parental care, then differences in initial state could be due to differences in parental investment. Alternatively, if the relevant time period is the onset of a new growing season, or time after metamorphosis, differences in initial state could be due to carryovers from a previous year or from the pre-metamorphic (e.g. larval) period.
An example of a common, positive-feedback mechanism in nature is state-dependent safety. Numerous studies show that individuals that have higher state (e.g. larger size or energy reserves, better condition or vigour) cope better with predators either by fleeing faster or by being better at defending themselves (e.g. Temple 1987; Chase 1999; Downes 2002; Iriarte-Diaz 2002; Caro 2005; Lindstrom et al. 2006; Alzaga et al. 2008; Basolo 2008; Hoefler et al. 2008; Arendt 2009; Stankowich 2009). Accordingly, although foragers feeding on immobile prey often prefer larger prey with more energy (i.e. high-state prey), predators attacking mobile prey often avoid larger, high-state prey (Sih & Christensen 2001). In these systems, animals with higher state thus have lower predation risk while being bold and should thus be bolder than low-state individuals. Many studies indeed show that larger, more vigorous prey hide less from predators than smaller, more susceptible prey (e.g. Sih 1980; Lima 1998).
Reality presumably features a mix of asset protection, starvation avoidance and state-dependent safety. We seek to better understand how these three mechanisms interact to determine conditions where we expect to see short- or long-term behavioural syndromes. We focus on boldness as a BT. Following the norm in behavioural ecology, we assume a risk–reward tradeoff where bolder individuals take more risks, but have the potential to gain more resources. First, we analyse a model that only has negative-feedback mechanisms (both asset protection and starvation thresholds). As expected (Clark 1994), if behaviour is state dependent, then as long as individuals differ in state, they differ in BT. However, because of the negative feedback, over the long term, state and thus behaviour converges. Models with only negative feedback do not yield long-term stable differences in BT. If, however, we add state-dependent safety (a positive-feedback mechanism) to the existing negative-feedback mechanisms, the positive feedback can produce divergence in state and thus long-term stable differences in state-dependent BTs (see §3). The key issue is when does one mechanism dominate over the others? To address this, we examine how environmental variation in resource levels and predation risk and variation in the strength of state-dependent safety influence the likelihood of persistence of consistent, adaptive behavioural syndromes. To emphasize, our adaptive, state-dependent modelling approach (Houston & McNamara 1999; Clark & Mangel 2000) addresses stability of BTs and divergence (as opposed to convergence) of different BTs over ontogeny within a lifetime. Heritability of BTs and maintenance of genetic variation across evolutionary time is a separate question that we do not explicitly address.
2. Methods
We examine state-dependent adaptive behaviour during a growing season without reproduction that could also represent an ontogenetic, developmental period from the onset of independent foraging until reproduction. During this period, animals forage, potentially increase a state variable such as size, energy or condition, and suffer a risk of predation. To account for environmental unpredictability, the duration of this overall period (T) is variable (uniformly distributed between 51 and 100 time units) and unknown to individuals. To address behavioural consistency across situations, we compare behaviour of the same individuals in high- versus low-risk situations, where between each time step there is a probability (γ = 0.1) of the environmental state switching between high (dh) and low predation risk (dl). This represents a situation where the proximity or the number of predators present varies through time with a positive temporal autocorrelation.
For simplicity, we assume that terminal fitness, the fitness an individual achieves if it survives to the final time period T, is linearly related to the individual's state, x, at the time horizon (e.g. at the end of the season or at the onset of reproduction). At the beginning of each time unit, individuals choose a level of foraging effort (e; its boldness), which ranges from 0 (hiding) to 1 (maximum effort). The probability of an individual finding food during one time unit is equal to its foraging effort, and the state units gained if it finds food is r. r is thus a measure of the environment's resource availability. Individuals lose state units to metabolic costs with a baseline rate of 0.05 units per time period and a behaviourally dependent component that increases at a fixed rate of 0.4 with foraging effort; i.e. if animals forage at 100 per cent effort, their energy costs are eight times higher than the baseline rate. Additional unpublished analyses (not shown here) show that qualitative results do not depend on this specific assumption about energy costs.
An individual's state in the next time step is thus,
| 2.1 |
For all models, animals starve to death if x falls to 0 or less. The maximum cap on x was set at 180, high enough that individuals could not reach the cap.
We developed two models that differed in how an individual's predation risk depends on their behaviour and their state. Since the optimal behaviour is state dependent, we solve for optimal behaviours using stochastic dynamic state variable models (Clark & Mangel 2000).
(a). Asset protection model
Following Clark (1994) and Wolf et al. (2007a), we first assume that predation risk depends only on an individual's foraging effort and the predation risk environment; not on the individual's state per se. This examines state-dependent behaviour in the absence of state-dependent safety as a ‘control’ for then illustrating the effect of state-dependent safety.
We assume that as foraging effort increases predation risk, μ, increases at an accelerating rate, μ = de1.5. Maximum foraging effort results in the maximum predation risk (μ = d). Given the above terminal fitness function, the expected fitness of an individual at time t with state x and environmental state d is
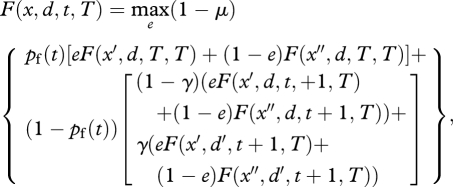 |
2.2 |
with pf(t) being the probability of time period t being the final time period of the season (which is 1 divided by the maximum number of possible time periods left if t > 50 and 0 otherwise), x′ and x″ being the energy state, respectively, if the individual does or does not find food and d′ is the presence of the other environmental state. The equation was solved using backward iteration (Clark & Mangel 2000) for e between 0 and 1 at intervals of 0.01. The e associated with maximum expected fitness was recorded as the optimal foraging effort for the given x, d and t.
(b). State-dependent safety model
Here, we assume that the risk of predation increases in an accelerating manner as foraging increases, but that the risk decreases linearly as the state of an individual, x, increases. The risk of predation decreases until individuals reach state = θ at which point predation risk is 10 per cent of the base level of risk,
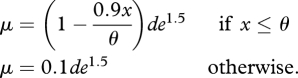 |
2.3 |
Larger values of θ correspond to a weaker effect of state on safety. For simplicity, we assumed a linear relationship between state and safety. Analyses with selected nonlinear functions for state-dependent safety did not alter qualitative results. State-dependent safety introduces a positive feedback into the system. As an individual gains x, its predation risk decreases, which lowers the cost of subsequent foraging. Optimal behaviours for this model were found using equation (2.2) with the state-dependent mortality incorporated.
In baseline runs of the asset protection and the state-dependent safety models, we set r = 1.3, dh = 0.09, dl = 0.045 and θ = 60. These values were not chosen to fit any particular system. Instead, we explored parameter space to elucidate general insights on how variation in ecological conditions (average predation risk (keeping the same ratio of predation risk in high- versus low-risk conditions) and resource levels), as well as the strength of state-dependent safety (altered by varying θ), influences the emergence of long-term consistent BTs. We first show results in some detail for a few sets of parameter values to illustrate major classes of outcomes that differ in whether individuals exhibit long-term behavioural consistency. We then show a plot of outcomes for the overall parameter space; this plot indicates ecological conditions that result in adaptive behavioural syndromes.
(c). Forward simulations
Both models produce a decision matrix that specifies optimal behaviours for every combination of individual and environmental state variables. We then ran forward simulations to view patterns of optimal foraging effort (boldness), as prescribed by the decision matrices (with interpolation between solutions). To visualize adaptive behavioural syndromes, we plot trajectories of optimal state-dependent behaviour over a season for individuals with different initial states, x0. As noted earlier, this could reflect, for example, differences in parental investment prior to the beginning of the model. Prey exhibit a behavioural syndrome if some individuals are consistently bolder (have higher foraging effort), while others are consistently less bold in both high and low predator density conditions. Not surprisingly, differences in initial state influenced initial behaviour early in the season. A stable, long-term behavioural syndrome exists if the trajectories diverge or at least remain consistently different over much of the season. In contrast, if trajectories converge, individuals might exhibit short-term differences in BT; however, they do not show a stable, long-term behavioural syndrome.
In the forward simulations, individuals perceived predation risk and estimated the probability that the season was going to end at the next time step. However, to get a sample of the behaviour across the whole season in the forward simulations, we did not allow predation to occur and all seasons lasted 100 time steps. We stress that this does not affect the behaviour of individuals, because their behavioural rules have been shaped by the probabilities of predation or the end of the season occurring. Across the seasonal time track, we recorded x, the foraging efforts of individuals, and whether the environmental state was currently low or high predation risk.
3. Results
Figures 1, 3 and 4 show time tracks of optimal boldness, and state for individuals that began with low versus high initial states (x0). In all models, individuals experienced unpredictable, alternating periods of high and low predation risk. To visualize what animals should do at each time point under both low and high risk, we averaged values from 1000 individuals. In all cases, we ran 1000 runs for all integer values of x0 from 1 to 25, but here, to illustrate patterns without too much clutter, we only show results for the lowest and highest initial states. Trajectories for behaviour and state for intermediate x0 always fell between the extremes shown here.
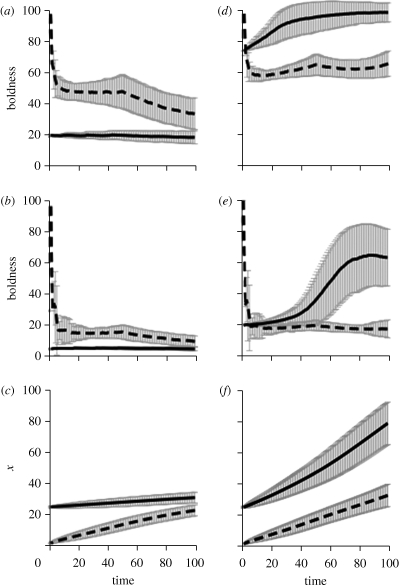
Figure 1.
Time series of the average boldness (±s.d.) of 1000 individuals that started with low (x0 = 1, dashed line) versus high (x0 = 25, solid line) initial state, x with r = 1.3 and θ = 60. (a–c) Asset protection model (which also includes avoidance of starvation) and (d–f) the state-dependent safety model (which also includes asset protection and avoidance of starvation). (a,d) Behaviour during periods of low predation risk (dl = 0.045) and (b,e) behaviour during periods of high risk (dt = 0.09). (c,f) Average levels of state, x, across the season.
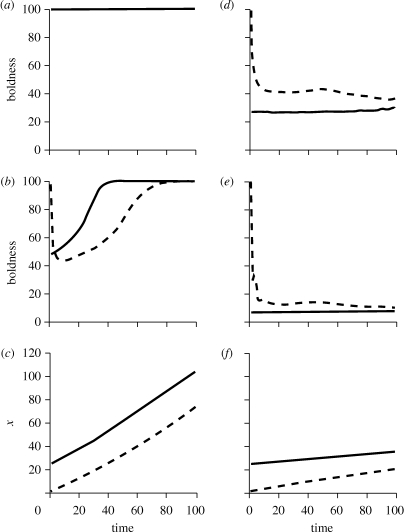
Figure 3.
Effects of altering overall average predation risk on the outcome of the state-dependent safety model holding r = 1.3 and θ = 60. The dashed lines show average results for 1000 individuals that started with low initial state (x0 = 1) while the solid lines show average results for 1000 individuals that started with high initial state (x0 = 25). With overall average risk reduced, (a) shows average boldness during periods of lower risk (dl = 0.02) while (b) shows average boldness during periods of higher risk (dh = 0.04). (c) Resulting levels of x. With overall average risk increased, (d) shows average boldness during periods of lower risk (dl = 0.07) while (e) shows average boldness during periods of higher risk (dh = 0.14). (f) Shows the resulting levels of x.
Figure 4.
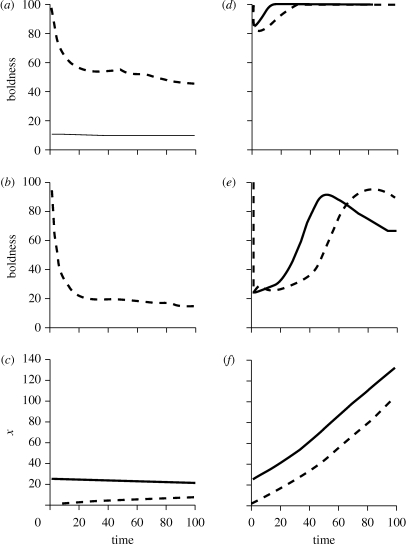
Effects of altering resource levels on the outcome of the state-dependent safety model with θ = 60. The dashed lines show average results for 1000 individuals that started with low initial state (x0 = 1) while the solid lines show average results for 1000 individuals that started with high initial state (x0 = 25). With resource levels reduced to r = 0.8, (a) shows average boldness during periods of low risk (dl = 0.045), while (b) shows average boldness during high risk (dh = 0.09). (c) Resulting levels of x. With resource levels increased to r = 1.8, the comparable graphs are in panels (d–f).
Although stochasticity in foraging success and environmental state generated variation in behavioural trajectories for any given level of initial state (figure 1), clear patterns still emerged. Not surprisingly, in all situations examined, individuals showed higher boldness during periods of low risk as compared with periods of high risk (figures 1, 3 and 4, compare panels (a) to (b) and (d) to (e)); i.e. individuals avoided predation risk. Our primary interest is in conditions that result in behavioural syndromes, consistent differences between individuals in behaviour, as indicated by stable or even increasing differences in behavioural trajectories over time.
(a). Asset protection model
In the standard asset protection model, initial differences in behaviour (driven by large differences in initial state) eroded over time (figure 1a–c). Early in the season, low-state individuals exhibited high foraging effort during both high and low predation risk. This reflected both avoidance of starvation and the fact that they had few assets to protect (i.e. little to lose). In contrast, to protect high assets, high-state individuals were cautious during both high and low predation risk. However, because low-state individuals were initially bold, they accumulated assets (state) at a faster rate than individuals that initially had higher state. By the end of the season, individuals had largely converged in state (figure 1c), and thus converged in behaviour (figure 1a,b); all individuals had moderate-high state (assets) and thus owing to asset protection, all individuals were cautious.
State dependence per se can explain the existence of behavioural syndromes in the short term, but not in the longer term. If individuals began with large differences in the initial state, then in the short term (i.e. over a few time units), particularly early in the season, they maintained consistent differences in BT. Individuals with low state were bolder than those with high state. However, owing to negative-feedback mechanisms (asset protection and avoiding starvation), differences in BT were not maintained in the longer term.
(b). State-dependent safety model
Under baseline ecological conditions, adding state-dependent safety produced a brief, initial period of unstable differences in BT followed by stable BTs over the longer term (figure 1d–f). Initially, individuals that started with low x0 were very bold (to avoid starvation) under high and low predation risk. Within a few time steps, however, after they gained enough state to avoid imminent starvation, low-state individuals settled into a consistent BT that featured moderate foraging effort during periods of lower risk (figure 1d) and low foraging effort during periods of higher risk (figure 1e). Their low-moderate boldness represented a balance between three forces. Their state was high enough that neither starvation risk nor asset protection was an important factor, but their state was not high enough to yield strong state-dependent safety.
Individuals that began with high x0 early in the season were bold during low predation risk, but cautious during high risk. Over the course of the season, however, as they accumulated additional state and thus became safer, they gradually increased their boldness during high predation risk (figure 1e). By the end of the season, they exhibited high foraging effort during high and low risk.
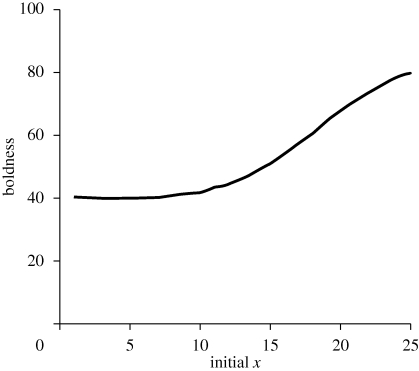
Examining the full range of x0 from 1 to 25 showed that under the default ecological conditions, initial state had strong effects on later BT (defined as average boldness in the last 20 time units; figure 2). Overall, key points are: (i) state-dependent safety produces divergence into a range of consistent long-term BTs, (ii) high-state individuals (e.g. those in better condition, or more vigorous) are bolder than low-state individuals (to emphasize, the asset protection model predicts the opposite), and (iii) individual differences in BT are most apparent during periods of relatively high predation risk.
Figure 2.
From the state-dependent safety model, effects of initial state, x, on the average boldness of 1000 individuals. Shown are averages pooling across periods of low and high predation risk, dl = 0.045 and dh = 0.09, during time periods 71–80. r = 1.3 and θ = 60.
(c). Variation in predation risk
We next examined effects of varying the average level of predation risk, retaining state-dependent safety and the 2 : 1 ratio of predation risk during periods of higher and lower risk.
When average risk was low, BTs emerged only during higher risk periods in the middle of the season (figure 3a,b). During periods of lower risk, all individuals used the maximum foraging effort throughout the season regardless of initial or current state (figure 3a). During periods of higher risk, individuals with high x0 rapidly increased their foraging effort, accumulated higher state, and thus increased their safety (figure 3b). By midway through the season, their state and safety were high enough that they exhibited the maximum foraging effort in both risk environments. In contrast, individuals with low x0 after an initial very brief period of boldness (to avoid starvation) were relatively cautious, only gradually building enough state (figure 3c) to be safe enough to facilitate higher foraging effort. However, by the end of the season, behavioural differences disappeared because individuals that began with low x0 had accumulated enough state that they too used the maximum foraging effort during periods of both lower and higher risk.
Key points are that with low average risk: (i) long-term BTs are not maintained over time, individuals converge in behaviour over time and (ii) when consistent differences in behaviour are observed, they are only expressed during periods of relatively high risk.
In contrast, when average predation risk was high, results were similar to the asset protection model. Although animals that differed in x0 initially differed substantially in behaviour, both behaviour and state converged over time (figure 3d–f). Individuals that began with low x0 were very bold for a brief period to avoid starvation, but after that, exhibited moderate boldness during periods of lower risk and low boldness during periods of higher risk. Individuals generally decreased their boldness over time in response to the increasing need for asset protection. In contrast, individuals that started with high x0 were cautious throughout the season. They had moderate assets to protect and predation risk was too high to favour using high foraging efforts to build up state and thus safety from predation.
Figures 1d–f and 3a–c show that in environments with low to moderate average risk, it is generally beneficial to be bold early in the season to gain state and thus enjoy state-dependent safety that allows high foraging effort later in the season. In contrast, with high average risk, because it is too dangerous to be bold early on, individuals never accumulate enough state and safety to have high foraging effort later in the season (figure 3d–f). As a result, individuals had much lower assets at the end of the season in environments with high average risk (compare figures 1f, 3c and 3f).
With high average risk, similar to the asset protection model, low-state individuals were consistently bolder than high-state individuals; however, behaviour and state converged over time. By the end of the season, individuals that initially differed substantially in behaviour and state exhibited very similar behaviour; behavioural syndromes were not well maintained over the long-term.
(d). Variation in resources
In environments with low resources (and moderate average predation risk), qualitative patterns resembled those under high average predation risk though with slower convergence (compare figures 4a–c with 3d–f). In essence, when r is low, boldness is not beneficial except to avoid imminent starvation. Even for individuals that started with high x0, resource levels were insufficient to justify being bold to increase state for future safety. Instead, individuals with high x0 were very cautious throughout the season, foraging just enough to maintain existing assets. Individuals with low x0 were initially bold to avoid starvation, but then settled into being moderately cautious during periods of lower risk, and very cautious during periods of higher risk. Individuals maintained consistent differences in BT, but over time, these differences decreased.
With high resources (and moderate average risk), patterns qualitatively resembled those in low average risk environments (compare figures 4d–f with 3a–c). During low-risk periods, regardless of initial state, the substantial benefits of boldness in terms of future safety drove animals to exhibit very high foraging effort (figure 4d). During high-risk periods, individuals that began with high x0 were initially cautious, but as their state (and safety) increased (owing largely to high foraging effort during low-risk periods), they rapidly increased their boldness (figure 4e). Individuals with low x0 followed the same trajectory, but with a time lag. By late in the season, animals gained such high levels of state that asset protection drove them to become cautious during periods of high risk. As a result, over the course of the season, individuals that began with different initial state exhibited reversals in relative boldness (figure 4e).
Overall, moving along a gradient of increasing resources, we observed shifts in the relative importance of negative- and positive-feedback mechanisms. With low resources, individuals cannot increase their state rapidly enough to take advantage of state-dependent safety. Since negative-feedback mechanisms (asset protection and starvation avoidance) dominated over state-dependent safety, behaviour converged over time (figure 4a,b). With intermediate resources, a positive-feedback mechanism, state-dependent safety, was most important, resulting in clear-cut BTs (figure 1d,e), and with high resources, all individuals, regardless of x0, attained state-dependent safety. By the end of the season, asset protection was again most important.
(e). Ecological conditions resulting in adaptive behavioural syndromes
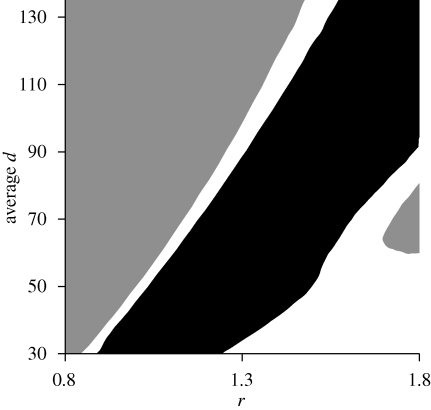
Figures 1, 3 and 4 show three main types of outcomes: (i) behavioural convergence, where all individuals end up being cautious regardless of initial state, but those that started with higher state are particularly cautious (figures 1a–c, 3d–f and 4a–c), (ii) behavioural convergence, where all individuals end up being bold regardless of initial state (figures 3a–c and 4d–f), and (iii) divergence to distinct, consistent BTs, where higher state individuals are bolder (figure 1d–f). Exploration of the full parameter space bracketed by the five ecological scenarios shown in figures 1, 3 and 4 reveals the overall effects of variation in average risk and resource levels on the emergence of consistent BTs (figure 5). The dark region across the diagonal indicates conditions where positive feedback through state-dependent safety produces stable differences in BT with boldness positively correlated to initial and later state (as in figure 1d–f). In essence, behavioural syndromes emerged when resources and risk were ‘matched’ (low resources and low risk, or medium resources and medium risk, or high resources and high risk) such that overall conditions were of intermediate favourability. When conditions were highly favourable (low risk and high resources, the lower right region, in white), all individuals ended up being bold, whereas when conditions were highly unfavourable (high risk and low resources, the upper left region, in grey), all individuals ended up being cautious. Further analyses varying τ show that the exact range of intermediate conditions that result in stable BTs and a behavioural syndrome depends on the strength of state-dependent safety. If state-dependent safety is easier to attain, a broader range of conditions end with everyone being bold.
Figure 5.
Effects of resource levels (r) and predation risk (average d) on main outcomes in terms of differences in average boldness (from time period 71–80) for individuals that started with x0 = 1 versus x0 = 25. Risk is scaled by 0.001; i.e. a value of 50 is d = 0.05. In the black region, individuals diverged in behaviour and state (as in figure 1d–f) and those that started initially with higher state ended up more bold (at least 0.05 higher effort) than individuals that started with low state. Positive feedback dominated under these conditions. In the main grey region (upper left), individuals with different initial states converged in state and behaviour over time, they were generally cautious, and those starting with higher state were even more cautious (at least 0.05 lower effort) than those that started with lower state. This happened when risk was relatively high (as in figure 3d–f) or resources were relatively low (as in figure 4a–c). Negative feedback was the predominant force in this region. White regions are where the difference in average boldness between individuals that started with different initial states was less than 0.05. In highly favourable conditions (the lower right region), when risk was low (as in figure 3a–c) or resources were high, regardless of initial state, all individuals converged on being very bold.
4. Discussion
A fascinating evolutionary mystery involves the existence of BTs (also known as animal personalities) that can result in apparently suboptimal behaviour, particularly when behavioural carryovers go across situations that favour very different behaviours (e.g. Sih et al. 2003; Johnson & Sih 2005). If behavioural syndromes are sometimes associated with reduced fitness, why are they so common? Or, perhaps even more interesting is the fact that they do not always exist. Some studies find behavioural correlations across situations, but others do not. What explains this variation in behavioural syndromes?
One approach to explaining behavioural syndromes focuses on proximate constraints underlying BTs (Koolhaas et al. 1999; Biro & Stamps 2008; Careau et al. 2008). An alternative, adaptive approach explains both within-individual behavioural consistency and maintenance of consistent behavioural differences among individuals by connecting behaviour to a less plastic state variable (McElreath & Strimling 2006; McElreath et al. 2007; Wolf et al. 2007a,b; Dingemanse & Wolf 2010; Wolf & Weissing 2010). Previous theory on this issue considered a state-dependent model based on asset protection (Wolf et al. 2007a). Here, we expanded on previous work by analysing models that include three state-dependent mechanisms: asset protection, starvation thresholds and state-dependent safety. These three mechanisms have conflicting effects on optimal behaviour. With asset protection and starvation risk, higher state individuals are more cautious and lower state individuals are bolder; thus, these mechanisms involve negative-feedback loops that should not maintain differences in states. In contrast, in some ecological conditions, state-dependent safety causes higher state individuals to be bolder than lower state individuals. This is a positive-feedback loop that can maintain differences in state and thus differences in behaviour.
Because state variables generally change relatively slowly, any tight connection of optimal behaviour to a state variable can explain short-term stability of BTs. State variables that might play this role for behavioural syndromes include: size, energy reserves, condition, vigour, informational state (knowledge or skills), physiological capacity and morphology. Individual differences in any of these variables are likely to persist in at least the short–medium term, and thus could explain somewhat stable BTs. Given that behaviour often is state dependent (Houston & McNamara 1999; Clark & Mangel 2000), and state almost always varies within populations, we should often expect to see short-term (but not necessarily, long-term) individual differences in BT. Indeed, many empirical examples of behavioural carryovers occur over relatively short time scales (e.g. hours, days or weeks).
Although short-term carryovers can have important impacts on fitness (Sih et al. 2003), long-term stable BTs have the potential to have even larger ecological and fitness consequences. Thus, one of our primary goals has been to explain long-term consistency of BTs. We found that long-term stability in BTs depends on the interplay between negative-feedback mechanisms (asset protection, avoidance of starvation) that tend to result in convergence in state and behaviour and positive-feedback loops (e.g. state-dependent safety) that maintain differences or even drive divergence in state and behaviour. A previous model based on state-dependence and asset protection produced short-term BTs (Wolf et al. 2007a), but not long-term BTs (McElreath et al. 2007), except in situations where feedbacks between behaviour and changes in state (assets) were decoupled (Wolf et al. 2007b). This decoupling might occur if, for example, animals do not allow their energy reserves to accumulate, but instead use them immediately. Then, even though bold/aggressive animals gather more energy, this does not increase their energy reserves. Most state-dependent models, however, assume that behaviour does influence future state; in particular, that bold, active, aggressive behaviour increases state (if the animal survives). In that case, positive-feedback mechanisms appear necessary to explain long-term, stable BTs.
Comparisons of behavioural trajectories under different risk and resource regimes, and with different strengths of state-dependent safety, illustrate how initial state and conditions early in the season play a critical role. The fact that initial conditions can have major effects on subsequent outcomes is, of course, a common feature of models with positive-feedback loops. Here, with state-dependent safety, early in the season, animals with high initial state should often be bold both because this has immediate benefits in terms of increased foraging returns and because it tends to increase individual state, which enhances future safety and thus future foraging returns. In contrast, animals with low initial state are often cautious, do not increase in state, do not gain state-dependent safety and thus tend to play it safe over the long term. Boldness early on is favoured if the benefits of early boldness are relatively large (e.g. if resources are abundant enough to allow bold animals to rapidly increase in state), and the costs are relatively small (e.g. if risk is low enough to allow animals to be bold without too much risk). The size of the benefit in future safety also depends on both the degree to which higher state increases safety and the time remaining in the season. With state-dependent safety, a longer season favours boldness because a longer season allows animals more time to ‘cash in’ on the foraging benefits of future safety.
We identified ecological situations where animals with different initial states should follow these divergent behavioural trajectories, and thus exhibit behavioural syndromes. Importantly, our analyses showed that this divergence in BT does not always occur. Under some conditions, despite some positive feedback, all individuals should converge in behaviour over time or exhibit reversals in relative boldness over time.
If resource levels are too low, the positive-feedback loop revolving around state-dependent safety does not get started for anyone. In that case, only individuals that are close to starvation should be bold. It does not pay for animals with moderate-high initial state to be bold because bold foraging cannot increase their state quickly enough to gain enough benefits in terms of future safety. Instead, animals with relatively high state protect their assets by playing it safe. In the long term, the negative feedbacks inherent in asset protection and starvation avoidance cause behavioural convergence. A similar dynamic arises in environments with very high predation risk. If it is too dangerous early on for moderate-high state animals to be bold, then state-dependent safety never plays an important role.
In contrast, with very high resources and only low-moderate risk, early on, it can be beneficial for all individuals to be bold. Then, they all eventually accumulate enough state to enjoy state-dependent safety. Because individuals that begin with different levels of initial state have offset trajectories (figure 4d,e), variation in state and behaviour persists in the population, but these differences are not stable over time. A parallel dynamic where all individuals should be bold emerges in environments with very low risk and moderate resources.
Stable, long-term differences in BT emerged most clearly in environments with matched risk and resources (low levels for both, medium levels for both or high levels for both). In these conditions, individuals that start with high-enough state can enter into the positive-feedback loop that results in long-term boldness, while others that start with lower state are cautious and never accumulate state or future safety. Our explicit prediction is thus that behavioural syndromes involving boldness should emerge most readily in conditions with matched risk and resources, strong state-dependent safety and substantial differences in initial state. We are not aware of extant studies that test these ideas; however, with the recent, rapid increase in studies measuring behavioural correlations, we might soon have enough examples to elucidate empirical patterns on how risk, resources and state-dependent safety influence the prevalence of behavioural syndromes. Alternatively, future studies could experimentally manipulate initial state, and risk and resources early on to test their influence on the development of BTs and behavioural syndromes over ontogeny. In particular, empiricists should first test whether their system features state-dependent safety, and if it does, then to span a range of conditions that should produce different outcomes (figure 5); experiments might hold risk constant and contrast low, medium and high food levels, or conversely hold food levels constant and contrast three levels of risk.
Note that in our model with state-dependent safety, individuals that started with higher initial state had higher average fitness than individuals that started with lower initial state. They either survive better or have higher eventual reproductive success, or both. That is, although individuals that start with low state exhibit adaptive behaviour, that behaviour still represents making the ‘best of a bad job’. Other analyses have explained the maintenance of genetic variation in personalities (e.g. van Oers et al. 2005) by invoking mechanisms (e.g. frequency dependence) that result in equal fitness for all BTs (Penke et al. 2007; Stamps 2007; Wolf et al. 2007a,b). Here, we note the possibility that different BTs might not have equal fitness. Maintenance of genetic variation in BT might then be explained by mechanisms that maintain genetic variation in inherent condition (Rowe & Houle 1996) or in parental investment per offspring (Roff 2002).
We focused on one axis of BT—boldness, in particular, in a foraging/antipredator behaviour context. We did not explicitly consider the issue of correlations across behavioural axes such as the commonly observed positive correlation between boldness and aggressiveness (see earlier references). Stamps (2007) hypothesized that the positive correlation between boldness and aggressiveness arises because the two often are complementary behaviours that represent alternative strategies for balancing the growth–mortality tradeoff. Some individuals are both bold and aggressive to gain more resources despite the risks, while others are both cautious and unaggressive to minimize mortality risk. Interestingly, empirical work suggests that the correlation between boldness and aggressiveness occurs in environments with moderate-high predation risk, but not in relatively safe environments (Bell 2005; Bell & Sih 2007; Dingemanse et al. 2007). Our analysis of boldness suggests the hypothesis that individual differences in boldness (and a correlation between boldness in low- versus high-risk conditions) should arise mainly in conditions with matched risk and resources. If aggressiveness is another way to gain resources while taking risks, then the rationale in our models also predicts that while the positive correlation between boldness and aggressiveness might hold in the short term under a broad range of risk-resource conditions, the correlation should only be maintained in the long term under conditions with matched risks and resources. An important complexity that is not in our models is the fact that aggressiveness has a game aspect. The rewards and costs associated with aggressiveness depend on the aggressiveness of other individuals. State-dependent foraging and boldness can also have a game aspect that can favour individual consistency in behaviour (Rands et al. 2003). Thus, future models analysing adaptive connections between ecological factors, state-dependence and behavioural syndromes involving boldness and aggressiveness should account for frequency-dependent games.
Our model emphasized state-dependent safety. Another potential positive-feedback loop involves state-dependent competitive ability. Animals with higher state (e.g. that are larger, have more energy reserves) might be superior foragers either through exploitative or interference competition. Given that higher reward rates per unit of foraging effort should favour higher foraging effort, individuals that already have high state should further increase their state, which would further favour bold/aggressive behaviour. Conversely, if low-state individuals fare poorly in competition and thus gain relatively little reward per unit effort, then they might be cautious and unaggressive, which would tend to keep them in low state. Further analyses of this positive-feedback mechanism are required to see whether it is as effective as state-dependent safety in generating behavioural syndromes.
For either state-dependent safety or state-dependent competitive ability, the positive-feedback mechanism might involve morphological or physiological traits that are not directly associated with energy state (or overall size, or condition). For example, bold individuals that gain high state might divert some of their energy reserves towards building inducible defensive morphologies that increase their future safety. Alternatively, high-state individuals might use some of their energy reserves to build the digestive or metabolic machinery required to assimilate energy faster or more efficiently, or they might build competitive morphologies (e.g. weapons) that allow them to win contests. In both cases, the key is that in order to build morphology that can help enhance future safety or competitive success, animals must use part of the state (e.g. energy reserves) that could, alternatively, be used later to reproduce. This introduces a more complex tradeoff where animals must set not only their behaviour (boldness, aggressiveness), but also their induced physiology or morphology. That is, animals must determine their behaviour, physiology and induced morphology as an integrated package (e.g. DeWitt et al. 1999; Pigliucci & Preston 2004). Although many animals (and plants) are known to exhibit multiple types of plasticity, models and experiments have rarely attempted to examine adaptive integration of these multiple responses. In the context of behavioural syndromes, physiological or morphological traits are often viewed as proximate mechanisms underlying individual differences in BT. Given that the physiological and morphological traits are plastic, and can change in tandem with behaviour (albeit often more slowly than behaviour), our suggestion is that future analyses should treat the suite of traits as a potentially adaptive, integrated package (also see Réale et al. 2010).
Acknowledgements
This work was supported by three grants from the National Science Foundation. The ideas emerged originally from discussions with Sean Fogarty, Richard McElreath and Judy Stamps. They were honed by feedback, mostly positive, following talks on the model at IEC in Rennes, and at Oxford University in 2009, along with ongoing, stimulating interactions with Cait McGaw. Earlier versions of the manuscript were read by numerous people at UC/Davis including Alison Bell, Ann Hedrick and members of the Sih laboratory. The editors of this special issue and two anonymous reviewers also provided influential comments.
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Alzaga V., Vicente J., Villanua D., Acevedo P., Casas F., Gortazar C.2008Body condition and parasite intensity correlates with escape capacity in Iberian hares (Lepus granatensis). Behav. Ecol. Sociobiol. 62, 769–775 10.1007/s00265-007-0502-3 (doi:10.1007/s00265-007-0502-3) [DOI] [Google Scholar]
- Arendt J. D.2009Influence of sprint speed and body size on predator avoidance in New Mexican spadefoot toads (Spea multiplicata). Oecologia 159, 455–461 10.1007/s00442-008-1210-z (doi:10.1007/s00442-008-1210-z) [DOI] [PubMed] [Google Scholar]
- Basolo A. L.2008Evolution of pleiotropic alleles for maturation and size as a consequence of predation. Biol. Lett. 4, 200–203 10.1098/rsbl.2007.0638 (doi:10.1098/rsbl.2007.0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M.2005Differences between individuals and populations of threespined stickleback. J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Sih A.2007Exposure to predation generates personality in threespined sticklebacks. Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Stamps J. A.2004The development of behavioural differences between individuals and populations of stickleback. Anim. Behav. 68, 1339–1348 10.1016/j.anbehav.2004.05.007 (doi:10.1016/j.anbehav.2004.05.007) [DOI] [Google Scholar]
- Bell A. M., Backstrom T., Huntingford F. A., Pottinger T. G., Winberg S.2007Variable behavioural and neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol. Behav. 91, 15–25 10.1016/j.physbeh.2007.01.012 (doi:10.1016/j.physbeh.2007.01.012) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Stamps J. A.2008Life-history productivity is linked to animal personality traits. Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Post J. R., Abrahams M. V.2005Ontogeny of energy allocation reveals selection pressure promoting risk taking behaviour in young fish cohorts. Proc. R. Soc. B 272, 1443–1448 10.1098/rspb.2005.3096 (doi:10.1098/rspb.2005.3096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2007The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecol. Lett. 10, 1094–1104 10.1111/j.1461-0248.2007.01106.x (doi:10.1111/j.1461-0248.2007.01106.x) [DOI] [PubMed] [Google Scholar]
- Brodin T., Johansson F.2004Conflicting selection pressures on the growth/predation risk trade-off in a damselfly. Ecology 85, 2927–2932 10.1890/03-3120 (doi:10.1890/03-3120) [DOI] [Google Scholar]
- Capitanio J. P., Mendoza S. P., Lerche N. W.1998Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): evidence for temporal and situational consistency. Am. J. Primatol. 44, 29–41 (doi:10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- Careau J., Thomas M., Humphries M., Réale D.2008Metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Caro T.2005Antipredator defenses in birds and mammals. Chicago, IL: University of Chicago Press [Google Scholar]
- Caspi A., Roberts B. W., Shiner R. L.2005Personality development, stability and change. Annu. Rev. Psychol. 56, 453–484 10.1146/annurev.psych.55.090902.141913 (doi:10.1146/annurev.psych.55.090902.141913) [DOI] [PubMed] [Google Scholar]
- Chase J. M.1999To grow or reproduce? The role of life-history plasticity in food web dynamics. Am. Nat. 154, 571–586 10.1086/303261 (doi:10.1086/303261) [DOI] [PubMed] [Google Scholar]
- Clark C. W.1994Antipredator behaviour and the asset-protection principle. Behav. Ecol. 5, 159–170 10.1093/beheco/5.2.159 (doi:10.1093/beheco/5.2.159) [DOI] [Google Scholar]
- Clark A. B., Ehlinger T. J.1987Pattern and adaptation in individual behavioural differences. In Perspectives in ethology (eds G Bateson P. P., Klopfer P. H.), pp. 1–47 New York, NY: Plenum Press [Google Scholar]
- Clark C. W., Mangel M.2000Dynamic state variable models in ecology. Oxford, UK: Oxford University Press [Google Scholar]
- Cote J., Clobert J.2007Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390 10.1098/rspb.2006.3734 (doi:10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D. P., Krause J., Darden S. K., Ramnarine I. W., Faria J. J., James R.2009Behavioural trait assortment in a social network: patterns and implications. Behav. Ecol. Sociobiol. 63, 1495–1503 10.1007/s00265-009-0802-x (doi:10.1007/s00265-009-0802-x) [DOI] [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- DeWitt T. J., Sih S., Hucko J. A.1999Trait compensation and cospecialization in a freshwater snail: size, shape and antipredator behaviour. Anim. Behav. 58, 397–407 10.1006/anbe.1999.1158 (doi:10.1006/anbe.1999.1158) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Wolf M.2010Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Van Oers K., Van Noordwijk A. J.2002Repeatability and heritability of exploratory behaviour in great tits in the wild. Anim. Behav. 64, 929–938 10.1006/anbe.2002.2006 (doi:10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M.2004Fitness consequences of avian personality in a fluctuating environment. Proc. R. Soc. Lond. B 278, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Thomas D. K., Wright J., Kazem A. J. N., Koese B., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between twelve populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Dochterman N., Wright J.2010aA method for exploring the structure of behavioural syndromes to allow formal comparison within and between data sets. Anim. Behav. 79, 439–450 10.1016/j.anbehav.2009.11.024 (doi:10.1016/j.anbehav.2009.11.024) [DOI] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010bBehavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Downes S. J.2002Size-dependent predation by snakes: selective foraging or different prey vulnerability? Behav. Ecol. 13, 551–560 10.1093/beheco/13.4.551 (doi:10.1093/beheco/13.4.551) [DOI] [Google Scholar]
- Duckworth R. A.2006Behavioural correlations across breeding contexts provide a mechanism for a cost of aggression. Behav. Ecol. 17, 1011–1019 10.1093/beheco/arl035 (doi:10.1093/beheco/arl035) [DOI] [Google Scholar]
- Gosling S. D.2001From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- Heithaus M. R., Frid A., Wirsing A. J., Dill L. M., Fourqurean J. W., Burkholder D., Thomas J., Bejder O.2007State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 76, 837–844 10.1111/j.1365-2656.2007.01260.x (doi:10.1111/j.1365-2656.2007.01260.x) [DOI] [PubMed] [Google Scholar]
- Hoefler C. D., Persons M. H., Rypstra A. L.2008Evolutionarily costly courtship displays in a wolf spider a test of viability indicator theory. Behav. Ecol. 19, 974–979 10.1093/beheco/arn055 (doi:10.1093/beheco/arn055) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1999Models of adaptive behaviour: an approach based on state. New York, NY: Cambridge University Press [Google Scholar]
- Huntingford F. A.1976The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback. Anim. Behav. 24, 245–260 10.1016/S0003-3472(76)80034-6 (doi:10.1016/S0003-3472(76)80034-6) [DOI] [Google Scholar]
- Iriarte-Diaz J.2002Differential scaling of locomotor performance in small and large terrestrial mammals. J. Exp. Biol. 205, 2897–2908 [DOI] [PubMed] [Google Scholar]
- Johnson J., Sih A.2005Pre-copulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioural syndromes. Behav. Ecol. Sociobiol. 58, 390–396 10.1007/s00265-005-0943-5 (doi:10.1007/s00265-005-0943-5) [DOI] [Google Scholar]
- Johnson J. C., Sih A.2007Fear, food, sex and parental care: a syndrome of boldness in the fishing spider, Dolomedes triton. Anim. Behav. 74, 1131–1138 10.1016/j.anbehav.2007.02.006 (doi:10.1016/j.anbehav.2007.02.006) [DOI] [Google Scholar]
- Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping styles in animals: current status in behaviour and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., De Boer S. F., Buwalda M., Van Reenen K.2007Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 70, 218–226 10.1159/000105485 (doi:10.1159/000105485) [DOI] [PubMed] [Google Scholar]
- Kortet R., Hedrick A.2007A behavioural syndrome in the field cricket Gryllus integer: intrasexual aggression is correlated with activity in a novel environment. Biol. J. Linn. Soc. 91, 475–482 10.1111/j.1095-8312.2007.00812.x (doi:10.1111/j.1095-8312.2007.00812.x) [DOI] [Google Scholar]
- Lima S. L.1998Stress and decision making under the risk of predation: recent developments from behavioural, reproductive, and ecological perspectives. Adv. Study Behav. 27, 215–290 10.1016/S0065-3454(08)60366-6 (doi:10.1016/S0065-3454(08)60366-6) [DOI] [Google Scholar]
- Lindstrom L., Ahtianinen J. J., Mappes J., Kotiaho J. S., Lyytinen A., Alatalo R. V.2006Negatively condition dependent predation cost of a positively condition dependent sexual signalling. J. Evol. Biol. 19, 649–656 10.1111/j.1420-9101.2005.01043.x (doi:10.1111/j.1420-9101.2005.01043.x) [DOI] [PubMed] [Google Scholar]
- McElreath R., Strimling P.2006How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim. Behav. 72, 1135–1139 10.1016/j.anbehav.2006.04.001 (doi:10.1016/j.anbehav.2006.04.001) [DOI] [Google Scholar]
- McElreath R., Luttbeg B., Fogarty S. P., Brodin T., Sih A.2007Evolution of animal personalities. Nature 450, E5. 10.1038/nature06326 (doi:10.1038/nature06326) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Stephens P. A., Dall S. R. X., Houston A. I.2009Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B 276, 605–613 10.1098/rspb.2008.1182 (doi:10.1098/rspb.2008.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretz J., Martins E., Robison B.2007Behavioural syndromes and the evolution of correlated behaviour in zebrafish. Behav. Ecol. 18, 556–562 10.1093/beheco/arm011 (doi:10.1093/beheco/arm011) [DOI] [Google Scholar]
- Nelson X. J., Wilson D. R., Evans C. S.2008Behavioural syndromes in stable social groups: an artifact of external constraints? Ethology 114, 1154–1165 10.1111/j.1439-0310.2008.01568.x (doi:10.1111/j.1439-0310.2008.01568.x) [DOI] [Google Scholar]
- Penke L., Denissen J. J. A., Miller G. F.2007The evolutionary genetics of personality. Eur. J. Personality 21, 549–587 10.1002/per.629 (doi:10.1002/per.629) [DOI] [Google Scholar]
- Pigliucci M., Preston K.2004Phenotypic integration studying the ecology and evolution of complex phenotypes. Oxford, UK: Oxford University Press [Google Scholar]
- Rands S. A., Cowlishaw G., Pettifor S. A., Rowcliffe J. M., Johnstone R. A.2003Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434 10.1038/nature01630 (doi:10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- Réale D., Festa-Bianchet M.2003Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65, 463–470 10.1006/anbe.2003.2100 (doi:10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- Réale D., Gallant B. Y., Leblanc M., Festa-Bianchet M.2000Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597 10.1006/anbe.2000.1530 (doi:10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Réale D., Garant D., Humphries M. M., Bergeron P., Careau V., Montiglio P.-O.2010Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063 10.1098/rstb.2010.0208 (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechert S. E., Hedrick A. V.1993A test for correlations among fitness-linked behavioural traits in the spider Agelenopsis aperta. Anim. Behav. 46, 669–675 10.1006/anbe.1993.1243 (doi:10.1006/anbe.1993.1243) [DOI] [Google Scholar]
- Roberts B. W., Walton K. E., Viechtbauer W.2006Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol. Bull. 132, 1–25 10.1037/0033-2909.132.1.1 (doi:10.1037/0033-2909.132.1.1) [DOI] [PubMed] [Google Scholar]
- Roff D. A.2002Life history evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- Rowe L., Houle D.1996The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421 10.1098/rspb.1996.0207 (doi:10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- Salonen A., Peuhkuri N.2006The effect of captive breeding on aggressive behaviour of European grayling, Thymallus thymallus, in different contexts. Anim. Behav. 72, 819–825 10.1016/j.anbehav.2005.12.012 (doi:10.1016/j.anbehav.2005.12.012) [DOI] [Google Scholar]
- Sih A.1980Optimal behaviour: can foragers balance two conflicting demands ? Science 210, 1041–1043 10.1126/science.210.4473.1041 (doi:10.1126/science.210.4473.1041) [DOI] [PubMed] [Google Scholar]
- Sih A.1992Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 139, 1052–1069 10.1086/285372 (doi:10.1086/285372) [DOI] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioural syndromes. Adv. Stud. Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Christensen B.2001Optimal diet theory: when does it work and when and why does it fail? Anim. Behav. 61, 379–390 10.1006/anbe.2000.1592 (doi:10.1006/anbe.2000.1592) [DOI] [Google Scholar]
- Sih A., Kats L. B., Maurer E. F.2003Behavioural correlations across situations and the evolution of antipredator behaviour in a sunfish-salamander system. Anim. Behav. 65, 29–44 10.1006/anbe.2002.2025 (doi:10.1006/anbe.2002.2025) [DOI] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C.2004aBehavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R.2004bBehavioural syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Moltschaniwskyj N. A., Wapstra E., Dall S. R. X.2010Are behavioural syndromes invariant? Spatiotemporal variation in shy/bold behaviour in squid. Behav. Ecol. Sociobiol. 64, 693–702 10.1007/s00265-009-0887-2 (doi:10.1007/s00265-009-0887-2) [DOI] [Google Scholar]
- Smith B. R., Blumstein D. T.2008Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- Snekser J. L., Leese J., Ganim A., Itzkowitz M.2009Caribbean damselfish with varying territory quality: correlated behaviours but not a syndrome. Behav. Ecol. 20, 124–130 10.1093/beheco/arn123 (doi:10.1093/beheco/arn123) [DOI] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stankowich T.2009When predators become prey: flight decisions in jumping spiders. Behav. Ecol. 20, 318–327 10.1093/beheco/arp004 (doi:10.1093/beheco/arp004) [DOI] [Google Scholar]
- Temple S. A.1987Do predators always capture substandard individuals disproportionately from prey populations? Ecology 68, 669–674 10.2307/1938472 (doi:10.2307/1938472) [DOI] [Google Scholar]
- van Oers K., de Jong G., van Noordwijk A. J., Kempenaers B., Drent P. J.2005Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206 10.1163/156853905774539364 (doi:10.1163/156853905774539364) [DOI] [Google Scholar]
- Werner E. E., Anholt B. R.1993Ecological consequences of the trade-off between growth and mortality-rates mediated by foraging activity. Am. Nat. 142, 242–272 10.1086/285537 (doi:10.1086/285537) [DOI] [PubMed] [Google Scholar]
- Wilson D. S., Clark A. B., Coleman K., Dearstyne T.1994Shyness and boldness in humans and other animals. Trends Ecol. Evol. 11, 442–446 10.1016/0169-5347(94)90134-1 (doi:10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- Wolf M., Weissing F. J.2010An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007aLife history tradeoffs favour the evolution of personality. Nature 447, 581–585 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007bWolf et al. reply. Nature 450, E5–E6 10.1038/nature06327 (doi:10.1038/nature06327) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J.2008Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. 105, 15 825–15 830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]