Abstract
The pace-of-life syndrome (POLS) hypothesis specifies that closely related species or populations experiencing different ecological conditions should differ in a suite of metabolic, hormonal and immunity traits that have coevolved with the life-history particularities related to these conditions. Surprisingly, two important dimensions of the POLS concept have been neglected: (i) despite increasing evidence for numerous connections between behavioural, physiological and life-history traits, behaviours have rarely been considered in the POLS yet; (ii) the POLS could easily be applied to the study of covariation among traits between individuals within a population. In this paper, we propose that consistent behavioural differences among individuals, or personality, covary with life history and physiological differences at the within-population, interpopulation and interspecific levels. We discuss how the POLS provides a heuristic framework in which personality studies can be integrated to address how variation in personality traits is maintained within populations.
Keywords: pace-of-life, personality, life-history strategies, metabolism, immune system, trait interactions
1. Introduction
Ecological conditions affect the evolution of life-history strategies in a population (Roff 1992; Stearns 1992). The pace-of-life syndrome (hereafter POLS) hypothesis suggests that closely related species should differ in a suite of physiological (e.g. metabolic, hormonal, immunity) traits that have coevolved with the life-history particularities of each species (Ricklefs & Wikelski 2002; Wikelski et al. 2003; Martin et al. 2006). Accordingly, a given set of ecological conditions that favours a particular life-history strategy could affect this whole series of traits. The idea of the POLS thus finds its roots in the classic concept of r- and K-selection (MacArthur & Wilson 1967; Pianka 1970; Reznick et al. 2002). It also extends the more recent concept of a fast–slow life-history continuum (e.g. Gaillard et al. 1989; Bielby et al. 2007; Jones et al. 2008) by expanding the examination of life-history differences among species to include physiological traits (see also Hennemann 1983). POLS has been successfully tested at the interspecies level (Tieleman et al. 2005; Wiersma et al. 2007) and interpopulation levels (Wikelski et al. 2003). Notably, it has been shown that tropical bird species or populations are long-lived and produce few offspring, develop slowly and mature relatively late in life, and also have a low metabolic rate (Wikelski et al. 2003; Wiersma et al. 2007). Hence, relative to their temperate zone counterparts, tropical birds have a slow pace of life along both physiological and life-history axes of variation.
In this paper, we note that two potentially important facets of the POLS concept have been neglected: (i) despite potentially strong links between metabolism, hormones and behaviour, no clear framework has been developed to incorporate behavioural traits within the POLS (Careau et al. 2008); (ii) at the within-population level, one aspect of behaviour that would be very relevant to the POLS hypothesis is the fact that individuals show consistent behavioural differences over time or across situations, in other words personality differences (Wilson et al. 1994; Gosling 2001; Sih et al. 2004; Réale et al. 2007). Recent theoretical studies have emphasized that individual behavioural differences should be linked to life-history differences (Stamps 2007; Wolf et al. 2007). Yet only a few empirical studies have shown a link between life history and behaviour within a species (Boon et al. 2007; Biro & Stamps 2008; Réale et al. 2009), and even fewer have established a clear link between personality and metabolic rate (Careau et al. 2008) or immunity (Koolhaas 2008).
Here, we review recent evidence that consistent individual behavioural differences covary with physiological and life-history traits at the within-population, interpopulation and interspecific levels, and describe opportunities and challenges associated with including personality within the POLS. We conclude by considering some ecological and evolutionary consequences of a POLS integration of behavioural, physiological and life-history traits. Recent work on personality has highlighted integrative aspects of research on the topic (Sih et al. 2004; Réale et al. 2007), in particular, how the concepts of personality and behavioural plasticity are tightly linked (Dingemanse et al. 2010). This paper is in the same vein, defending the use of a more integrative approach to the study of behaviour. Whereas theoretical studies have primarily focused on the evolution of personality owing to feedback loops with variation in state (Dingemanse & Wolf 2010; Luttbeg & Sih 2010; Wolf & Weissing 2010), here we focus, instead, upon how long-term selection pressures could have led to the coevolution of suites of behavioural, physiological and life-history traits. Because we are primarily interested in the presence of among-individual variance in the average level of behaviour, we will use the terms personality and behaviour interchangeably throughout the paper and will not be considering behavioural plasticity within individuals here.
2. Trait interactions contributing to the pace-of-life syndrome
Individuals in a population vary widely in life-history strategies and other ecologically important traits (Roff & Fairbairn 2007). Individual differences in life-history strategies are mainly attributed to the existence of evolutionary (i.e. genetic and physiological) trade-offs between life-history traits (Williams 1966; Roff & Fairbairn 2007) and age-structured mortality resulting from predation, parasitism or the heterogeneous quality of resources in space and time (Stearns 1976; Reznick et al. 2002). We thus expect individuals that differ in their behavioural, physiological and life-history characteristics to be affected differently by changes in density and resource abundance (Chitty 1967). Correlations between physiological, behavioural and life-history traits have been assumed to illustrate potential evolutionary constraints related to both pleiotropic genetic effects and common physiological pathways underlying multiple traits (Sih et al. 2004), probably resulting from past selection pressures on the developmental stability and homeostasis of an organism. Correlational selection pressures, associated with differences in fitness outcomes among the different possible combinations of phenotypes, could also generate and maintain such correlations among traits (Cheverud 1982; Sinervo & Svensson 2002; Sih et al. 2004; McGlothlin & Ketterson 2008). Correlational selection is probably a central force acting on the integration of traits and leading to their coadaptation (Sinervo & Svensson 2002). In a POLS perspective, correlational selection should thus play a particularly important role in the coadaptation of personality, hormonal, metabolic and life-history traits. For example, if being aggressive facilitates acquiring and monopolizing resources it would potentially have coevolved with a high growth rate. On the other hand, aggression may also increase mortality risk, which should lead to selection for early sexual maturity and more intense reproductive effort early in life (Wolf et al. 2007; Biro & Stamps 2008). These correlations may also reflect possible constraints on their future independent evolution (Sih et al. 2004). Other types of selection can help explain the amount of variance observed in personality and other traits, but are less effective in accounting for the links between traits (see Dingemanse & Réale 2010; Réale & Dingemanse 2010).
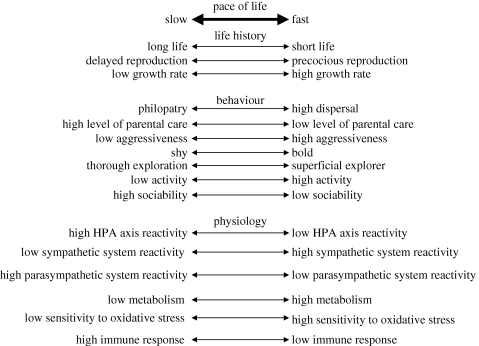
Correlations and mechanistic linkages between hormones and personality, personality and metabolism, and metabolism and life history provide an intriguingly plausible path of causality that would manifest as a POLS spanning physiology, behaviour and life histories (figure 1). Below we describe one such potential path, before critically re-examining the assumptions and interpretations involved and identifying alternative causal pathways and critical uncertainties.
Figure 1.
Schematic of the potential integration of different traits along a pace-of-life continuum. Double arrows illustrate presumed continuous variation in life-history strategies among individuals in a population, and its assumed relationship with personality and physiological traits.
(a). Within-population studies
The first general finding on personality is that behavioural traits often form a suite of correlated traits. More specifically, aggressiveness has been shown to be positively associated with activity, boldness or superficial exploration in a number of species (Koolhaas et al. 1999; Sih et al. 2004; Groothuis & Carere 2005; Boon et al. 2008; Sih & Bell 2008; Réale et al. 2009). These correlations have been speculated to originate from common underlying neuroendocrine pathways, such as the hypothalamic–pituitary–adrenal (HPA) axis (but see, Coppens et al. 2010), or the sympathetic and parasympathetic nervous systems. For instance, Koolhaas et al. (1999) showed that mice artificially selected for high aggressiveness are active, superficial explorers, and produce little corticosterone in response to a stressful situation (i.e. express low reactivity of the HPA axis). Highly aggressive mice are also characterized by elevated adrenaline production and heart rate under stress (i.e. high reactivity of the sympathetic system). High values of these traits generally characterize ‘proactive’ individuals whereas low aggressiveness, low activity, thorough exploration and shyness characterize ‘reactive’ individuals (Koolhaas et al. 1999). The existence of correlations between neuroendocrine and behavioural traits has been found in diverse taxa including mammals, birds, fish and reptiles (Koolhaas et al. 1999; Groothuis & Carere 2005; Øverli et al. 2007), and suggests that these suites of traits have been maintained throughout evolution (Øverli et al. 2007).
There is increasing evidence that personality phenotypes are also linked to specific life-history strategies. Biro & Stamps (2008), for example, found numerous links between proactivity (i.e. high boldness, aggressiveness and activity) and growth rate and food intake. By contrast, the links between proactivity and age and size at maturity, fecundity and longevity were more equivocal (Biro & Stamps 2008). A recent review by Smith & Blumstein (2008) emphasized that boldness is generally related to high reproductive success but also to lower survival. Docility in bighorn rams (Ovis canadensis) is associated with both delayed reproduction and longevity (Réale et al. 2009), whereas in females boldness and docility are associated with an earlier age at first reproduction and weaning success increases with boldness (Réale et al. 2000). In red squirrels (Tamiasciurus hudsonicus), females differing in level of aggressiveness and activity exhibit contrasting reproductive success depending on the abundance of food resources (Boon et al. 2007), and aggressiveness and activity were correlated with higher mortality, apparently associated with specific spatial activity patterns (Boon et al. 2008). Numerous other examples from long-term studies have confirmed that humans with different personalities also differ in their life histories (Figueredo et al. 2004; reviewed in Réale & Dingemanse 2010).
In turn, the metabolic machinery required to support a proactive, fast lifestyle may generate a positive relationship between metabolism and proactive personality traits (Careau et al. 2008). Resting metabolism should increase with the relative size of metabolically active organs (Daan et al. 1990; Brzek et al. 2007; Russell & Chappell 2007). Because many of these organs are highly recruited during aggressive interactions, risk-taking, and aerobic activity in general, proactive individuals should be characterized by higher rates of metabolism than reactives, even when at rest (Careau et al. 2008). Indeed higher resting metabolic rates in proactive than in reactive individuals have been reported (Cyprinus carpio, Huntingford et al. 2010; Peromyscus maniculatus, V. Careau, D. Thomas, F. Pelletier, L. Turki, F. Landry, D. Garant & D. Réale unpublished). Viewed from a top-down perspective, correlational selection could also occur because a large metabolic machinery is presumably necessary for successful (adaptive) proactive behaviour (Careau et al. 2010). High rate of metabolism is also one potential component of a fast life history that may lead to correlated selection for other fast traits such as high fecundity and early reproduction. Caloric restriction has been shown to extend lifespan in a wide array of organisms. One hypothesis for links between metabolism and lifespan is the production of reactive oxygen species (ROS) as by-products of metabolism (adenosine triphosphate production), which can lead to oxidative damage in the absence of compensatory repair mechanisms and antioxidants (Beckman & Ames 1998; Finkel & Holbrook 2000). High levels of aggressiveness and boldness may facilitate access to and monopolization of the resources necessary for high growth and early reproduction (Biro & Stamps 2008), but are also associated with a higher risk of mortality (Wolf et al. 2007).
Other theoretical and empirical progress offers new opportunities to uncover potential links between personality, immunity and disease sensitivity, which could themselves affect survival and reproductive patterns (Koolhaas et al. 1999; Koolhaas 2008). These links can be of two kinds. First, depending on their personality, individuals may behave in ways that increase their likelihood of encountering parasites or contracting diseases (Barber & Dingemanse 2010). This has been shown recently in Siberian chipmunks (Tamias sibiricus), where superficial explorers use larger home ranges and host a larger number of ticks (Boyer et al. 2010). Second, different coping styles can result in different immune capacities (Koolhaas 2008; Barber & Dingemanse 2010). Immunological defence mechanisms carry different costs (Sheldon & Verhulst 1996), traded off along an axis of fast, cheap, non-specific defence versus slow, costly, specific defence. For example, immunity, personality, reproduction and survival are all affected by hormones such as testosterone (Koolhaas et al. 1999; Kempenaers et al. 2008).
Finally, other trait associations are well integrated by the POLS concept. Bold, aggressive individuals show a higher tendency to disperse (Fairbairn 1978; Fraser et al. 2001; Dingemanse et al. 2003; Duckworth & Badyaev 2007; Cote et al. 2010). There is also direct evidence that more aggressive males invest very little in parental care compared with reactive males (Duckworth & Badyaev 2007), although in other species aggressiveness can be related to higher maternal care (Benus & Röndig 1996) and maternal performance varies with maternal aggressiveness depending on the availability of resources (Boon et al. 2007). Sociability has been found to affect dispersal (Cote & Clobert 2007; Blumstein et al. 2009; Cote et al. 2010), and is suspected to increase the chance of acquiring and transmitting parasites and diseases (Barber & Dingemanse 2010).
In this section, we can thus picture the POLS as a complex of interwoven behaviour, physiological and life-history traits that are affecting each other. It is also necessary to understand how ecological factors such as predation, parasites, and spatio-temporal heterogeneity in the resources that affect a population can reinforce or on the contrary curtail the associations between these traits.
(b). Potential pace-of-life syndromes at the interpopulation and interspecies levels
The purpose of the interspecific approach to the study of POLS should be to identify both the possible evolutionary trade-offs and the key ecological and evolutionary forces driving the coevolution between component traits. Here, we provide some examples of studies at the interpopulation and interspecies levels that show possible associations among traits according to the model shown in figure 1.
In the Trinidadian guppy (Poecilia reticulata), populations living upstream under low predation regimes show a slower pace of life than populations living downstream and subject to high predation pressures. In downstream populations guppies exhibit an earlier age and smaller size at maturity, shorter inter-brood intervals and high reproductive investment (Reznick et al. 1996). Interestingly, in a separate study, guppies from downstream populations were also shown to be bolder and more tenacious than upstream populations when being fed in the presence of predator signs (Fraser & Gilliam 1987). Similar effects of predation on both life-history characteristics (Walsh & Reznick 2009) and boldness (Fraser & Gilliam 1987) have been found in another species of this ecosystem, Hart's rivulus (Rivulus hartii). Although there are no data on metabolic rate in these two species, results on opercular beat rate in another small tropical poeciliid, Brachyrhaphis episcopi, living under similarly contrasting predation regimes suggest a higher metabolic rate in fish found in high predation sites (Brown et al. 2005). Interestingly, Jennions & Telford (2002) also found that Brachyrhaphis spp. living in a high predation environment exhibited faster pace of life than low predation populations. Although this provides only indirect evidence, we can predict that fish from a high predation regime should exhibit higher standard metabolic rates than fish from lower predation regimes.
Another good example is the Atlantic silverside (Menidia menidia). In this species, fish from a high-latitude population (Nova Scotia, Canada) showed a higher growth rate and food intake, reduced locomotory performance, reduced survival and higher vulnerability to predators, compared with fish from a low-latitude population (SC, USA; Billerbeck et al. 2001; Lankford et al. 2001). Furthermore, high-latitude fast-growing M. menidia genotypes had a higher standard metabolic rate and were bolder (i.e. more willing to feed in presence of a predator) than low latitude ones (Arnott et al. 2006; Chiba et al. 2007). This pattern is suggested to be shaped by shorter growth seasons favouring an aggressive growth strategy at northern latitudes, while higher predation pressures favour evasiveness in southern populations (Billerbeck et al. 2001; Lankford et al. 2001). Other documented effects of predation upon life history (Abrams & Rowe 1996) or behaviour (Grand 1999; Sih et al. 2003; Urban 2007) suggest that such a syndrome might exist in more populations.
Interspecies comparative studies, using data from tests classically performed in personality studies (e.g. novel object, novel environment and flight initiation distance (FID) tests) have also looked at the links between personality and ecological factors (Mettke-Hofmann et al. 2002, 2005; Tebbich et al. 2009), or life history, behaviour and/or metabolic rate (Blumstein 2006; Møller 2009). For example, Lovegrove (2001) has shown that body-armoured mammal species have lower basal metabolic rate (BMR) and lower activity than non-armoured species. Careau et al. (2009) combined published data on open-field behaviour in muroid rodents (Wilson et al. 1976; Webster et al. 1979) with data on life history (Duncan et al. 2007) and metabolism (Lovegrove 2000; White & Seymour 2003) and showed that interspecific variation in exploratory behaviour was positively correlated with age at first reproduction, and that both of these traits were negatively correlated with BMR. This comparative study suggests that environmental productivity and predictability might play an important role in coordinating behavioural, physiological and life-history traits with each other to form the POLS.
In another study, Careau et al. (2010) combined standardized personality data on dog breeds (Draper 1995) with published estimates of mortality rate (Bonnett et al. 1997) and energy requirements (cf. multiple sources). They showed that more trainable breeds live longer than disobedient breeds and that aggressive breeds have higher levels of energy expenditure than unaggressive breeds. Because behavioural differences among dog breeds have a genetic basis (Saetre et al. 2006) and can be regarded as remnants from past selection targeted at personality (Svartberg 2006), the genetic component underlying these correlations is presumably high.
As a whole, the comparative study of the POLS will help to understand how physiological, life-history and behavioural traits coevolve to create suites of interlinked traits at multiple levels of biological variation.
3. Can the pace-of-life syndrome be generalized to all populations or species?
(a). Evidence contradicting the general model
Although the causal pathway outlined above (and in figure 1) is plausible and intuitively appealing, there are many potential exceptions and alternatives to the correlations and causal relationships we have suggested, and no general pattern regarding the direction of the association between many of the traits seems to emerge yet (see also Biro & Stamps 2008; Adriaenssens & Johnsson 2009).
First, personality traits are not systematically associated in a behavioural syndrome (Réale et al. 2007), and the link between personality and life history may not always be as predicted in figure 1. For example, Bell (2005) and Dingemanse et al. (2007) have found that correlations among behavioural traits in three-spined sticklebacks (Gasterosteus aculeatus) differ in their sign and strength depending on the population. In both studies, predation was suggested to be an important factor shaping the correlation among traits, and partly supported by experimental work (Bell & Sih 2007). Réale et al. (2009) have found that contrary to predictions (and to previous results published on docility) bold bighorn rams survived longer than shy ones, and suggest that ecological conditions may affect the degree of association between all these traits. Furthermore, variable patterns of correlation among physiological, behavioural and life-history traits have been documented in different species of insects showing a flight-reproduction or migratory syndrome (Zera et al. 1997; Dingle 2006; Roff & Fairbairn 2007), which could represent a case of POLS. In many insect species, two wing-morphs coexist in the same population: a long-winged, flight capable morph (LW) and a short-winged, flight incapable morph (SW; Dingle 2006; Roff & Fairbairn 2007; Guerra in press). The evolutionary coexistence of two morphs and a flight–reproduction syndrome constituted of a series of life history, morphological and physiological traits has been explained by an energetic trade-off between the ability to fly and reproductive effort, and the costs/benefits associated with each morph in a heterogeneous environment where resources can vary in both space and time. The LW morph is able to disperse, and thus has the advantage of colonizing new habitats and founding new populations. This morph is characterized by large body size, fully developed wings and flight apparatus (e.g. flight muscles). The SW morph on the other hand is philopatric, short lived, and devotes a large part of its energy to competition and reproduction. Indeed, in several species, SW morphs show very fast histolysis of their flight muscle, females reproduce faster and show a high fecundity, and males are highly aggressive and devote a lot of their time to courting females (Guerra in press). Most of the traits involved within a migratory syndrome thus seem to represent coadaptations related to the importance of delaying reproduction after settling down in a new, uncertain and low density habitat for dispersers (i.e. LW), or of competing for rare resources in a highly populated habitat in philopatric (i.e. SW) individuals. However, for many species, expected trait associations are not observed (Dingle 2006; Guerra in press). For example, high aggressiveness in this case is related to low metabolic rate, probably owing to the energetic constraints associated with flight, and in contrast to vertebrate studies (see above) dispersers are not good competitors (Guerra in press).
Second, patterns contradicting the model in figure 1 can also be observed for the links between behaviour, metabolism and physiology. Contradictory results regarding the endocrine–personality relationships can be caused by the multiple intrinsic and extrinsic sources of variation and covariation in hormones and behaviour between individuals, that are not always considered in studies (Kempenaers et al. 2008). Although we suggest that proactive animals should have higher metabolic rates than reactive ones, according to an alternative allocation model described by Careau et al. (2008), proactive animals may instead have lower BMR, because only then can they afford the high energetic overheads of a proactive lifestyle (Careau et al. 2008). Demonstrating that large and active organ systems contribute to high metabolic rates has proved difficult, particularly at intraspecific scales of comparison. Laboratory experiments with knockout mice are not supportive of an important role for ROS and oxidative stress in dictating lifespan, and even the more fundamental association between elevated metabolism, ROS, and short lifespan is far from ubiquitous (Speakman et al. 2004). In particular, the ‘uncoupling to survive’ hypothesis predicts positive correlation between metabolic rate and longevity (Brand 1990). Correlations between personality and antioxidant capacity may further complicate the picture. For example, mice artificially selected for short attack latency (i.e. the most aggressive, proactive line) had similar ROS levels but lower serum antioxidant capacity compared with mice selected for long attack latency (i.e. the reactive line; Costantini 2008).
Some studies at the interspecific level have also produced low support for a POLS widely applicable to every species. For example, analysing FID data in 150 species of birds, Blumstein (2006) could not provide unequivocal evidence for an association between risk-taking and life-history traits, with age at first reproduction being the only trait positively related to FID. At the interspecific level, Møller (2009) showed that FID in birds was positively (albeit weakly) correlated with BMR, whereas we would predict these traits to be negatively linked (i.e. risk-taking should be associated with high metabolism; Careau et al. 2008). That high BMR is associated with long FIDs is possibly a consequence of reduced predation pressures on both traits (Møller 2009).
(b). Some cautions about the pace-of-life syndrome model
As we saw in §3a there are many potential alternative routes of correlation and causation between physiology, personality and life history beyond those outlined in figure 1. We thus suggest that future research avoids the temptation to generalize and simplify a complex reality, in a way that has been detrimental to the r- and K-selection model in ecology in the 1980s (Reznick et al. 2002; Roff 2002). Importantly, most of the alternatives reviewed above do not cast doubt on our broader premise that there are substantial links between physiology, personality and life history. But they do introduce fundamental uncertainties about the direction and mechanistic basis of such linkages. A much more detailed examination of those relationships, in a wide diversity of organisms experiencing a wide array of natural ecological circumstances, is therefore warranted. Identifying and testing these alternatives will be as challenging as it is important. Below we suggest a few ideas to examine when studying the POLS.
First, we need to consider how the combination of multiple ecological factors affecting mortality and reproduction patterns can shape the evolution of life-history strategies in a population. Different combinations of traits within a population are expected, in association with specific survival and reproduction rates that are themselves affected by environmental stability, predation, resource fluctuation and predictability, diseases, population density, resource distribution and monopolization (Stearns 1992; Reznick et al. 2002). The evolution of the direction of a correlation between traits may be affected by the fitness outcome of the combination of two trait values, which may depend on the particular ecological conditions of the population. For example, dispersal may be positively associated with high aggressiveness and boldness when the survival and reproduction of dispersers depend strongly on their ability to acquire a territory and survive in an unknown environment (Duckworth & Badyaev 2007). By contrast, in systems where resources can be monopolized and overt competition among individuals is probable, the most philopatric individuals may also be the most aggressive and boldest individuals, as shown in some cricket species (Roff & Fairbairn 2007; Guerra in press). In this case, slow, long-lived genotypes may have higher probabilities of colonizing a new, uncertain habitat and establishing new populations in which competition will be lower. We thus expect that the type of competition (i.e. contest versus scramble competition) among individuals, and the life-stage at which the competition occurs, will affect the association between specific traits in a POLS.
Second, we need more information on how changes in selection pressures in space and time related to fluctuating environmental conditions (i) shape the pattern of covariation among traits; (ii) favour the maintenance of different life-history strategies within a population; and (iii) promote the emergence of a POLS. The maintenance of alternative life-history strategies, as well as different behavioural and physiological traits, may be related to spatial or temporal fluctuation in resources driven by factors such as environmental conditions or density variation (see Sinervo et al. 2000; Grant & Grant 2002). Such instability could induce fluctuating selective pressures on traits and thus favour different life-history phenotypes or genotypes depending on environmental conditions. From a pace-of-life perspective, according to recent studies (Boon et al. 2007; Stamps 2007; Biro & Stamps 2008) poorer environmental conditions should potentially favour individuals with suites of traits which are representative of the slower phenotypes. Alternatively, increasingly high resource availability should favour faster phenotypes. However, the link between resource abundance and fast–slow phenotypes may not be as simple as indicated here, and other factors such as the type of competition, the possibility of monopolizing resources and the costs associated with resource defence may interact to alter the association between food availability and POL type.
Third, although in the present paper we have simplified the situation by focusing on stable inherent difference in behaviour, physiology and life history, there is some evidence that developmental effects on these traits can change the patterns of correlation among them at the local level (i.e. Stamps & Groothuis 2010a,b). For example, individuals may experience a range of contrasting environmental conditions throughout life (Magnhagen & Borcherding 2008; Sinn et al. 2010), and these changes may weaken or even reverse the association between personality and other traits. Detailed investigation of the developmental aspects of personality traits, the effects of genotype by environment interaction, and of genotype/environment correlation on these traits have recently been advocated (e.g. Dingemanse et al. 2010; Stamps & Groothuis 2010a,b), which will help us provide more rigorous tests of the POLS.
Finally, in a metapopulation context, if environmental conditions differ among sub-populations, migration and gene flow between them will potentially disrupt local coadaptations and the POLS (Guillaume & Whitlock 2007). Furthermore, genetic correlations can change depending on the environment (Sgrò & Hoffmann 2004), and thus studies of the POLS may obtain different estimates of correlations under variable environmental conditions. Studies will thus have to consider gene flow and the effect of the environment as potential explanations for the correlations observed among the traits studied (see below).
4. Consequences for the evolutionary and ecological study of populations
A POLS perspective can have important consequences for behavioural ecology studies; it stimulates the integration of behaviour within a metapopulation context and the inclusion of other important ecological, demographic and genetic factors that could affect the links observed among behavioural traits and between these and other traits (see above). Such processes have important consequences for the evolutionary and ecological study of populations. Here, we describe some of these consequences and suggest a few perspectives that could potentially be integrated in future studies.
(a). Assessing selection on multiple traits and at multiple levels
Variation in pace of life at the scale of the population should lead to the assessment of the direction and strength of selection acting on traits representative of each strategy and under different environments. Few studies have yet considered the consequences of changing environmental conditions for a large suite of integrated traits, probably because of the methodological challenges associated with the multivariate approach. It is generally assumed that life-history traits (e.g. age at first reproduction or longevity) depend on numerous underlying traits (e.g. hormones or body size; Price & Schluter 1991; Réale et al. 2007). Given such a hierarchical organization of traits, should one consider that selection is acting mainly on the variation in life-history strategies with top-down effects on a whole series of traits, or alternatively that selection is acting mainly on lower level traits with bottom-up effects on life-history strategies? As such categorization of the organization of traits might be rather arbitrary in most cases, all levels should be considered simultaneously to get a sense of what ‘type’ of pace-of-life applies to a group of organisms. The way forward is thus to assess the strength of selective pressures acting at each level using either a multivariate selection perspective (Blows & Hoffmann 2005; Blows 2007) or path-analysis approach to selection (Scheiner et al. 2000; see Kotiainen et al. 2009 for an example of this approach on personality). While the great majority of previous studies of selection have considered only linear and univariate selection (Kingsolver et al. 2001; Siepielski et al. 2009), the study of personality and POLS in particular—and of behaviour in general—should consider the multivariate (multi-traits) selection approach, as is now generally recognized in evolutionary biology (see Blows 2007). Multivariate selection models provide a useful framework to address the relative strength of selection depending on the level of organization (e.g. Blows & Brooks 2003). Such approaches thus represent a promising way to dissect the main underlying drivers of the organization of traits (given the probable importance of correlational selection in this context—see §2), despite their primary challenges and logistical difficulties (Kruuk & Garant 2007). Describing selection on traits related to pace of life from this multivariate perspective should also help explain one of the main contradictions in natural populations: namely that there is maintenance of variation at the genetic level for traits under relatively strong selection.
(b). Fluctuation of resources in space and time and the mosaic of life-history strategies
Spatial and temporal fluctuations in ecological conditions should promote variation in pace of life at the interpopulation level (Dingemanse & Réale 2010). The change observed within each population in terms of life-history characteristics and other related traits as a result of environmental variability may generate a mosaic of life-history traits at the metapopulation level. More specifically, one could argue that (i) the spatial variation in resource abundance among populations and (ii) the differences in resource fluctuation regime within each population could be coupled with (iii) the variation among years in resource availability at a larger landscape scale, to explain the variation in strength and type of selective pressures observed at a local scale. Furthermore, the possibility for resource monopolization, the costs associated with it and the type of competition over resources (scramble or contest competition) may all favour different associations between traits such as activity, risk-taking, aggression and metabolic rate. Combinations of ecological factors at different spatial scales could also account for the mixture of life histories observed at the larger scale of the metapopulation (see Goldwasser et al. 1994). Theoretical expectations are that any divergence in life-history characteristics should be magnified by local selection forces acting within each population and in contrast be reduced by the homogenizing effects of gene flow between populations (or even between species when hybridization occurs; Slatkin 1987; Hendry et al. 2001; reviewed in Garant et al. 2007). Another possibility is that dispersal itself could influence the population at certain sites (typically those with low carrying capacity), elevating local densities above carrying capacity, which will reduce average fitness of individuals and potentially disrupt local adaptation (Holt & Gomulkiewicz 1997; Gomulkiewicz et al. 1999; reviewed in Garant et al. 2007 and in Edelaar et al. 2008). The very idea of the POLS and the integration of personality should thus stimulate development of the study of the behavioural ecology of metapopulations. Such studies would benefit from recognizing that dispersal bias associated with individual characteristics, such as personality, will impact the dynamics of the metapopulation (Cote et al. 2010). A modelling approach could be useful in generating testable predictions about the multiple interactions of behavioural traits, other traits and ecological conditions, and their effects on the associations among traits in a metapopulation context.
(c). Predicting the distribution of traits along ecological gradients or different ecological conditions
Another advantage of the POLS perspective is to provide a framework in which we can predict the distribution of behavioural traits and their associations along different ecological gradients or between populations subjected to differing ecological conditions. For example, one could test possible effects of biogeography on the POLS. Not only is it interesting to examine potential differences between populations along latitudinal (Wikelski et al. 2003; Tieleman et al. 2005; Wiersma et al. 2007; see also the case of M. menidia above) or altitudinal gradients (Bears et al. 2009; Tieleman 2009), but it is also possible to analyse how such gradients affect the combinations of traits within each population. More importantly, studying these links will provide valuable information on how changes in ecological conditions along a gradient can lead to the coupling or decoupling of these traits in specific areas.
Furthermore, we may expect that pace of life will influence species invasion and variation along the colonization gradient (see also Clobert et al. 2009). For example, one could predict that populations at the forefront of a species distribution should be characterized by individuals which are on average more active, more mobile and more aggressive—in other words more proactive and with a fast pace of life, whereas populations located closer to the core area of a species distribution should be characterized by sedentary and more reactive individuals, with a slow pace of life. Evidence that frontal populations of an invasive species are composed of more active individuals than the core population has been found in cane toads (Bufo marinus) in Australia (Phillips et al. 2007).
POLS could also be related to variation between habitats in the quality of their resources, and to the trade-off between acquisition and allocation of resources (van Noordwijk & de Jong 1986; Reznick et al. 2001). Under the allocation principle, one could predict that in poor habitats with low food availability—where the capacity for high acquisition rate is somewhat less advantageous—‘slow’ individuals will do better than ‘fast’ ones. However, this association may be affected by potential interference with local competition, frequency-dependent selection associated with local social conditions (potentially linked to resource abundance itself) and predation, all of which should also be considered.
Other cases include islands, where there may be evolution of specific life histories that should affect the personality and metabolism of insular species (see, the Glanville fritillary butterfly, Melitaea cinxia, study system in the Ålands Islands in Finland, details in Hanski 1999). Therefore, we should expect combinations of traits to differ between islands as a result of personality-biased migration, competitive abilities and resource acquisition/allocation. In this way, the study of POLS, with more emphasis on incorporating personality variation, may have interesting implications at the biogeographical level.
(d). Pace of life and anthropogenic activities
Anthropogenic activities such as hunting or fishing, which affect survival and/or reproduction, have been shown to greatly shape life-history strategies of natural populations (Coltman et al. 2003; Hutchings & Fraser 2008). Similarly, domestication can rapidly change a whole set of traits involved in the POLS (i.e. growth, boldness, aggressiveness and fast life; Johnsson 1993). Such actions are therefore likely to result in changes at the behavioural or physiological levels as well, with potential feedback effects on the sensitivity of animals to anthropogenic pressures (Biro & Post 2008). As mentioned above, risk-taking behaviour is typically associated with shorter lifespan and earlier maturity and reproduction. If hunting or fishing pressures select for genotypes that in ‘pristine’ conditions survive longer (because hunters have a higher chance of killing large, old individuals), we might expect hunting to increase risk-taking in the population and witness evolution towards a more proactive type of individual. On the other hand, under harsher conditions and at lower prey densities hunters may manage to select only the bolder individuals, which should eventually lead to reduced risk-taking in the population and evolution towards a more reactive type of individual. In the same way release of domestic animals into the wild and introgression with wild populations may affect the whole POLS of a wild population with important demographic and ecological consequences. For example, fast-growing, bold domesticated strains of trout were more subjects of predation than were their wild counterparts (Biro et al. 2004). Introgression of domestic characteristics into wild populations may thus change the way individuals cope with the challenges of predation, resource fluctuation and habitat selection. Therefore, the concept of POLS can have important implications for the conservation of species in human influenced contexts.
5. Conclusion
In this paper, we review the evidence that evolutionary ecologists can benefit from merging the study of behavioural, hormonal, metabolic and immunological traits and their associations within a general life-history framework. The POLS perspective at the population level presented here extends ideas previously developed in life-history theory (i.e. r- and K-selection, fast–slow continuum) by proposing personality traits as a central element in the study of life-history strategies (see also Wolf et al. 2007; Biro & Stamps 2008). It clearly reflects the general trend towards an increasing interest in individual variation and the use of a more integrative approach towards the study of traits of ecological importance (Bolnick et al. 2003; Sih et al. 2004; Réale et al. 2007; Careau et al. 2008; Kempenaers et al. 2008; McGlothlin & Ketterson 2008; Williams 2008). The novelty of the thesis defended in this paper has two main aspects: (i) although other authors have previously suggested a potential association between personality, and either life history, metabolism or ecology, this paper is the first attempt to provide a global, holistic framework (i.e. multi-factorial and multi-level) for the study of personality; (ii) it demonstrates that there is an obvious lack of predictions concerning the strength and sign of correlations between traits potentially involved in a POLS, the interactive effects of different ecological factors on these correlations, and the variation in these traits among subpopulations. Our understanding of how ecological and evolutionary processes shape trait associations among and within populations is still limited, and no clear overall prediction can be made yet regarding the possible associations between some of the traits considered here. Furthermore, it is still not clear to what extent patterns found at one level of analysis (e.g. within-population correlation) can be used to infer or predict patterns at other levels (i.e. among-population or among-species correlations) and vice versa. Nevertheless, we would argue that by permitting establishment of strong links between ecological (e.g. predators, parasites, resource fluctuations) and demographic factors (e.g. migration, competition, metapopulation structure, density- and frequency-dependence) and the variation in numerous physiological, behavioural and life-history traits, the POLS provides a heuristic framework in which personality studies can build models, elaborate hypotheses and generate empirical tests to account for the maintenance of variation in personality traits in natural animal populations.
Acknowledgements
We dedicate this paper to Don Thomas, who sadly died too soon to contribute to the writing of this paper, but whose ideas, influence and encouragement underlie much of what we present here. We would like to thank Niels Dingemanse, Anahita Kazem, Jon Wright as well as two reviewers for their thoughtful and constructive comments on the paper. This work is part of our reflections related to a research project on the Eastern chipmunk (Tamias striatus) in Eastern Townships, Québec, funded by the FQRNT. During the writing of this paper D.R., D.G. and M.M.H. were individually funded by NSERC discovery grants. V.C. and P.B. were funded by NSERC fellowships, and P.M. by a FQRNT fellowship, for their Ph.D. dissertations.
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Abrams P. A., Rowe L.1996The effects of predation on the age and size of maturity of prey. Evolution 50, 1052–1061 10.2307/2410646 (doi:10.2307/2410646) [DOI] [PubMed] [Google Scholar]
- Adriaenssens B., Johnsson J. I.2009Personality and life-history productivity: consistent or variable association? Trends Ecol. Evol. 24, 179–180 10.1016/j.tree.2008.12.003 (doi:10.1016/j.tree.2008.12.003) [DOI] [PubMed] [Google Scholar]
- Arnott S. A., Chiba S., Conover D. O.2006Evolution of intrinsic growth rate: metabolic costs drive trade-offs between growth and swimming performance in Menidia menidia. Evolution 60, 1269–1278 [PubMed] [Google Scholar]
- Barber I., Dingemanse N. J.2010Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc B 365, 4077–4088 10.1098/rstb.2010.0182 (doi:10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bears H., Martin K., White G. C.2009Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. J. Anim. Ecol. 78, 365–375 10.1111/j.1365-2656.2008.01491.x (doi:10.1111/j.1365-2656.2008.01491.x) [DOI] [PubMed] [Google Scholar]
- Beckman K. B., Ames B. N.1998The free radical theory of aging matures. Physiol. Rev. 78, 547–581 [DOI] [PubMed] [Google Scholar]
- Bell A. M.2005Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Sih A.2007Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- Benus R. F., Röndigs M.1996Patterns of maternal effort in mouse lines bidirectionally selected for aggression. Anim. Behav. 51, 67–75 10.1006/anbe.1996.0006 (doi:10.1006/anbe.1996.0006) [DOI] [Google Scholar]
- Bielby J., Mace G. M., Bininda-Emonds O. R. P., Cardillo M., Gittleman J. L., Jones K. E., Orme C. D. L., Purvis A.2007The fast-slow continuum in mammalian life history: an empirical reevaluation. Am. Nat. 169, 748–757 10.1086/516847 (doi:10.1086/516847) [DOI] [PubMed] [Google Scholar]
- Billerbeck J. M., Lankford T. E., Jr, Conover D. O.2001Evolution of intrinsic growth and energy acquisition rates. I. Trade-offs with swimming performance in Menidia menidia. Evolution 55, 1863–1872 10.1111/j.0014-3820.2001.tb00835.x (doi:10.1111/j.0014-3820.2001.tb00835.x) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Post J. R.2008Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922 10.1073/pnas.0708159105 (doi:10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P. A., Stamps J. A.2008Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Abrahams M. V., Post J. R., Parkinson E. A.2004Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc. R. Soc. Lond. B 271, 2233–2237 10.1098/rspb.2004.2861 (doi:10.1098/rspb.2004.2861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blows M. W.2007A tale of two matrices: multivariate approaches in evolutionary biology. J. Evol. Biol. 20, 1–8 10.1111/j.1420-9101.2006.01164.x (doi:10.1111/j.1420-9101.2006.01164.x) [DOI] [PubMed] [Google Scholar]
- Blows M. W., Brooks R.2003Measuring nonlinear selection. Am. Nat. 162, 815–820 10.1086/378905 (doi:10.1086/378905) [DOI] [PubMed] [Google Scholar]
- Blows M. W., Hoffmann A. A.2005A reassessment of genetic limits to evolutionary change. Ecology 86, 1371–1384 10.1890/04-1209 (doi:10.1890/04-1209) [DOI] [Google Scholar]
- Blumstein D. T.2006Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim. Behav. 71, 389–399 10.1016/j.anbehav.2005.05.010 (doi:10.1016/j.anbehav.2005.05.010) [DOI] [Google Scholar]
- Blumstein D. T., Wey T. W., Tang K.2009A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc. R. Soc. B 276, 3007–3012 10.1098/rspb.2009.0703 (doi:10.1098/rspb.2009.0703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick D. I., Svanbäck R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L.2003The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 10.1086/343878 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- Bonnett B. N., Egenvall A., Olson P., Hedhammar A.1997Mortality in insured Swedish dogs: rates and causes of death in various breeds. Vet. Rec. 141, 40–44 [DOI] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2007The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecol. Lett. 10, 1094–1104 10.1111/j.1461-0248.2007.01106.x (doi:10.1111/j.1461-0248.2007.01106.x) [DOI] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2008Personality, habitat use, and their consequences for survival in North American red squirrels (Tamiasciurus hudsonicus). Oikos 117, 1321–1328 10.1111/j.0030-1299.2008.16567.x (doi:10.1111/j.0030-1299.2008.16567.x) [DOI] [Google Scholar]
- Boyer N., Réale D., Marmet J., Pisanu B., Chapuis J. L.2010Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J. Anim. Ecol. 79, 538–547 10.1111/j.1365-2656.2010.01659.x (doi:10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- Brand M. D.1990The proton leak across the mitochondrial inner membrane. Biochim. Biophys. Acta 1018, 128–133 10.1016/0005-2728(90)90232-S (doi:10.1016/0005-2728(90)90232-S) [DOI] [PubMed] [Google Scholar]
- Brown C., Gardner C., Braithwaite V. A.2005Differential stress responses in fish from areas of high- and low-predation pressure. J. Comp. Physiol. B 175, 305–312 10.1007/s00360-005-0486-0 (doi:10.1007/s00360-005-0486-0) [DOI] [PubMed] [Google Scholar]
- Brzek P., Bielawska K., Ksiazek A., Konarzewski M.2007Anatomic and molecular correlates of divergent selection for basal metabolic rate in laboratory mice. Physiol. Biochem. Zool. 80, 491–499 10.1086/520617 (doi:10.1086/520617) [DOI] [PubMed] [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Réale D.2008Energy metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Careau V., Bininda-Emonds O. R. P., Thomas D., Humphries M. M., Réale D.2009Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct. Ecol. 23, 150–156 10.1111/j.1365-2435.2008.01468.x (doi:10.1111/j.1365-2435.2008.01468.x) [DOI] [Google Scholar]
- Careau V., Réale D., Humphries M. M., Thomas D.2010The pace of life under artificial selection: personality, energy expenditure and longevity are correlated in domestic dogs. Am. Nat. 175, 753–758 10.1086/652435 (doi:10.1086/652435) [DOI] [PubMed] [Google Scholar]
- Cheverud J. M.1982Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution 36, 499–516 10.2307/2408096 (doi:10.2307/2408096) [DOI] [PubMed] [Google Scholar]
- Chiba S., Arnott S. A., Conover D. O.2007Coevolution of foraging behavior with intrinsic growth rate: risk-taking in naturally and artificially selected growth genotypes of Menidia menidia. Oecologia 154, 237–246 10.1007/s00442-007-0825-9 (doi:10.1007/s00442-007-0825-9) [DOI] [PubMed] [Google Scholar]
- Chitty D.1967The natural selection of self-regulatory behaviour in animal populations. Proc. Ecol. Soc. Austral. 2, 51–78 [Google Scholar]
- Clobert J., Le Galliard J. F., Cote J., Meylan S., Massot M.2009Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209 10.1111/j.1461-0248.2008.01267.x (doi:10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- Coltman D. W., O'Donoghue P., Jorgenson J. T., Hogg J. T., Strobeck C., Festa-Bianchet M.2003Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 10.1038/nature02177 (doi:10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- Coppens C. M., de Boer S. F., Koolhaas J. M.2010Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc B 365, 4021–4028 10.1098/rstb.2010.0217 (doi:10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D.2008Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251 10.1111/j.1461-0248.2008.01246.x (doi:10.1111/j.1461-0248.2008.01246.x) [DOI] [PubMed] [Google Scholar]
- Cote J., Clobert J.2007Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390 10.1098/rspb.2006.3734 (doi:10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J., Clobert J., Brodin T., Fogarty S., Sih A.2010Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc B 365, 4065–4076 10.1098/rstb.2010.0176 (doi:10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S., Masman D., Groenewold A.1990Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am. J. Physiol. 259, R333–R340 [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Réale D.2010What is the evidence for natural selection maintaining animal personality variation? In Animal personalities: behaviour, physiology and evolution (eds Carere C., Maestripieri D.). Chicago, IL: The University of Chicago Press [Google Scholar]
- Dingemanse N. J., Wolf M.2010Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., van Noordwijk A. J., Rutten A. L., Drent P. J.2003Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 10.1098/rspb.2002.2300 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Dingle H.2006Animal migration: is there a common migratory syndrome? J. Ornithol. 147, 212–220 10.1007/s10336-005-0052-2 (doi:10.1007/s10336-005-0052-2) [DOI] [Google Scholar]
- Draper T. W.1995Canine analogs of human personality factors. J. Gen. Psychol. 122, 241–252 10.1080/00221309.1995.9921236 (doi:10.1080/00221309.1995.9921236) [DOI] [PubMed] [Google Scholar]
- Duckworth R. A., Badyaev A. V.2007Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. P., Forsyth D. M., Hone J.2007Testing the metabolic theory of ecology: allometric scaling exponents in mammals. Ecology 88, 324–333 10.1890/0012-9658(2007)88[324:TTMTOE]2.0.CO;2 (doi:10.1890/0012-9658(2007)88[324:TTMTOE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Edelaar P., Siepielksi A. M., Clobert J.2008Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472 10.1111/j.1558-5646.2008.00459.x (doi:10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- Fairbairn D. J.1978Behaviour of dispersing deer mice Peromyscus maniculatus. Behav. Ecol. Sociobiol. 3, 265–282 10.1007/BF00296313 (doi:10.1007/BF00296313) [DOI] [Google Scholar]
- Figueredo A. J., Vasquez G., Brumbach B. H., Schneider S. M.2004The heritability of life history strategy: the K-factor, covitality, and personality. Soc. Biol. 51, 121–143 10.1080/19485565.2004.9989090 (doi:10.1080/19485565.2004.9989090) [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N. J.2000Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 10.1038/35041687 (doi:10.1038/35041687) [DOI] [PubMed] [Google Scholar]
- Fraser D. F., Gilliam J. F.1987Feeding under predation hazard: response of the guppy and Hart's rivulus from sites with contrasting predation hazard. Behav. Ecol. Sociobiol 21, 203–209 10.1007/BF00292500 (doi:10.1007/BF00292500) [DOI] [Google Scholar]
- Fraser D. F., Gilliam J. F., Daley M. J., Le A. N., Skalski G. T.2001Explaining leptokurtik movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135 10.1086/321307 (doi:10.1086/321307) [DOI] [PubMed] [Google Scholar]
- Gaillard J. M., Pontier D., Allainé D., Lebreton J. D., Trouvilliez J., Clobert J.1989An analysis of demographic tactics in birds and mammals. Oikos 56, 59–76 10.2307/3566088 (doi:10.2307/3566088) [DOI] [Google Scholar]
- Garant D., Forde S. E., Hendry A. P.2007The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 21, 434–443 10.1111/j.1365-2435.2006.01228.x (doi:10.1111/j.1365-2435.2006.01228.x) [DOI] [Google Scholar]
- Goldwasser L., Cook J., Silverman E. D.1994The effects of variability on metapopulation dynamics and rates of invasion. Ecology 75, 40–47 10.2307/1939380 (doi:10.2307/1939380) [DOI] [Google Scholar]
- Gomulkiewicz R., Holt R. D., Barfield M.1999The effects of density dependence and immigration on local adaptation and niche evolution in a black-hole sink environment. Theor. Popul. Biol. 55, 283–296 10.1006/tpbi.1998.1405 (doi:10.1006/tpbi.1998.1405) [DOI] [PubMed] [Google Scholar]
- Gosling S. D.2001From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- Grand T.1999Risk-taking behaviour and the timing of life history events: consequences of body size and season. Oikos 85, 467–480 10.2307/3546696 (doi:10.2307/3546696) [DOI] [Google Scholar]
- Grant P. R., Grant B. R.2002Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711 10.1126/science.1070315 (doi:10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- Groothuis T. G. G., Carere C.2005Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150 10.1016/j.neubiorev.2004.06.010 (doi:10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Guerra P. A.In press Evaluating the life history trade-off between dispersal capability and reproduction in wing dimorphic insects: a meta-analysis. Biol. Rev. [DOI] [PubMed] [Google Scholar]
- Guillaume F., Whitlock M. C.2007Effects of migration on the genetic covariance matrix. Evolution 61, 2398–2409 10.1111/j.1558-5646.2007.00193.x (doi:10.1111/j.1558-5646.2007.00193.x) [DOI] [PubMed] [Google Scholar]
- Hanski I.1999Metapopulation ecology. Oxford, UK: Oxford University Press [Google Scholar]
- Hendry A. P., Day T., Taylor E. B.2001Population mixing and the adaptive divergence of quantitative traits in discrete populations: a theoretical framework for empirical tests. Evolution 55, 459–466 10.1554/0014-3820(2001)055[0459:PMATAD]2.0.CO;2 (doi:10.1554/0014-3820(2001)055[0459:PMATAD]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Hennemann W. W., III1983Relationship among body mass, metabolic rate, and the intrinsic rate of natural increase in mammals. Oecologia 56, 104–108 10.1007/BF00378224 (doi:10.1007/BF00378224) [DOI] [PubMed] [Google Scholar]
- Holt R. D., Gomulkiewich R.1997How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am. Nat. 149, 563–572 10.1086/286005 (doi:10.1086/286005) [DOI] [Google Scholar]
- Huntingford F. A., Andrew G., Mackenzie S., Morera D., Coyle S. M., Pilarczyk M., Kadri S.2010Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J. Fish Biol. 76, 1576–1591 10.1111/j.1095-8649.2010.02582.x (doi:10.1111/j.1095-8649.2010.02582.x) [DOI] [PubMed] [Google Scholar]
- Hutchings J. A., Fraser D. J.2008The nature of fisheries- and farming-induced evolution. Mol. Ecol. 17, 294–313 10.1111/j.1365-294X.2007.03485.x (doi:10.1111/j.1365-294X.2007.03485.x) [DOI] [PubMed] [Google Scholar]
- Jennions M. D., Telford S. R.2002Life-history phenotypes in populations of Brachyrhaphis episcopi (Poeciliidae) with different predator communities. Oecologia 132, 44–50 10.1007/s00442-002-0942-4 (doi:10.1007/s00442-002-0942-4) [DOI] [PubMed] [Google Scholar]
- Johnsson J. I.1993Big and brave: selection affects foraging under risk of predation in juvenile rainbow trout, Oncorhynchus mykiss. Anim. Behav. 45, 1219–1225 10.1006/anbe.1993.1143 (doi:10.1006/anbe.1993.1143) [DOI] [Google Scholar]
- Jones O. R., et al. 2008Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol. Lett. 11, 664–673 10.1111/j.1461-0248.2008.01187.x (doi:10.1111/j.1461-0248.2008.01187.x) [DOI] [PubMed] [Google Scholar]
- Kempenaers B., Peters A., Foerster K.2008Sources of individual variation in plasma testosterone levels. Phil. Trans. R. Soc. B 363, 1711–1723 10.1098/rstb.2007.0001 (doi:10.1098/rstb.2007.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver J. G., Hoekstra H. E., Berrigan J. M., Vignieri S. N., Hill C. E., Hoang A., Gibert P., Beerli P.2001The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 10.1086/319193 (doi:10.1086/319193) [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M.2008Coping style and immunity in animals: making sense of individual variation. Brain Behav, Immun. 22, 662–667 10.1016/j.bbi.2007.11.006 (doi:10.1016/j.bbi.2007.11.006) [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping style in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Kruuk L. E. B., Garant D.2007A wake-up call for studies of natural selection? J. Evol. Biol. 20, 30–33 10.1111/j.1420-9101.2006.01223.x (doi:10.1111/j.1420-9101.2006.01223.x) [DOI] [PubMed] [Google Scholar]
- Lankford T. E., Jr, Billerbeck J. M., Conover D. O.2001Evolution of intrinsic growth and energy acquisition rates. II. Trade-offs with vulnerability to predation in Menidia menidia. Evolution 55, 1873–1881 10.1111/j.0014-3820.2001.tb00836.x (doi:10.1111/j.0014-3820.2001.tb00836.x) [DOI] [PubMed] [Google Scholar]
- Lovegrove B. G.2000The zoogeography of mammalian basal metabolic rate. Am. Nat. 156, 201–219 10.1086/303383 (doi:10.1086/303383) [DOI] [PubMed] [Google Scholar]
- Lovegrove B.2001The evolution of body armor in mammals: plantigrade constraints of large body size. Evolution 55, 1464–1473 [DOI] [PubMed] [Google Scholar]
- Luttbeg B., Sih A.2010Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc B 365, 3977–3990 10.1098/rstb.2010.0207 (doi:10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R. H., Wilson E. O.1967The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- Magnhagen C., Borcherding J.2008Risk-taking behaviour in foraging perch: does predation pressure influence age-specific boldness? Anim. Behav 75, 509–517 10.1016/j.anbehav.2007.06.007 (doi:10.1016/j.anbehav.2007.06.007) [DOI] [Google Scholar]
- Martin L. B., Hasselquist D., Wikelski M.2006Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147, 565–575 10.1007/s00442-005-0314-y (doi:10.1007/s00442-005-0314-y) [DOI] [PubMed] [Google Scholar]
- McGlothlin J. W., Ketterson E. D.2008Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 10.1098/rstb.2007.0002 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettke-Hofmann C., Winkler H., Leisler B.2002The significance of ecological factors for exploration and neophobia in parrots. Ethology 108, 249–272 10.1046/j.1439-0310.2002.00773.x (doi:10.1046/j.1439-0310.2002.00773.x) [DOI] [Google Scholar]
- Mettke-Hofmann C., Wink M., Winkler H., Leisler B.2005Exploration of environmental changes relates to lifestyle. Behav. Ecol. 16, 247–254 10.1093/beheco/arh159 (doi:10.1093/beheco/arh159) [DOI] [Google Scholar]
- Møller A. P.2009Basal metabolic rate and risk-taking behaviour in birds. J. Evol. Biol. 22, 2420–2429 10.1111/j.1420-9101.2009.01850.x (doi:10.1111/j.1420-9101.2009.01850.x) [DOI] [PubMed] [Google Scholar]
- Noordwijk A. J., van Jong G. de.1986Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat 128, 137–142 [Google Scholar]
- Øverli Ø., Sørensen C., Pulman K. G. T., Pottinger T. G., Korzan W., Summers C. H., Nilsson G. E.2007Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 31, 396–412 10.1016/j.neubiorev.2006.10.006 (doi:10.1016/j.neubiorev.2006.10.006) [DOI] [PubMed] [Google Scholar]
- Phillips B. L., Brown G. P., Greenlees M., Webb J. K., Shine R.2007Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral. Ecol. 32, 169–176 10.1111/j.1442-9993.2007.01664.x (doi:10.1111/j.1442-9993.2007.01664.x) [DOI] [Google Scholar]
- Pianka E. R.1970On r- and K-selection. Am. Nat. 104, 592–597 [Google Scholar]
- Price T., Schluter D.1991On the low heritability of life-history traits. Evolution 45, 853–861 10.2307/2409693 (doi:10.2307/2409693) [DOI] [PubMed] [Google Scholar]
- Réale D., Dingemanse N. J.2010Selection and evolutionary explanation for the maintenance of personality differences. In The Evolution of personality and individual differences (eds Buss D., Hawley P.). Oxford, UK: Oxford University Press [Google Scholar]
- Réale D., Gallant B. Y., Leblanc M., Festa-Bianchet M.2000Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597 10.1006/anbe.2000.1530 (doi:10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Réale D., Martin J., Coltman D. W., Poissant J., Festa-Bianchet M.2009Male personality, life-history strategies and reproductive success in a promiscuous mammal. J. Evol. Biol. 22, 1599–1607 10.1111/j.1420-9101.2009.01781.x (doi:10.1111/j.1420-9101.2009.01781.x) [DOI] [PubMed] [Google Scholar]
- Reznick D. N., Rodd F. H., Cardenas M.1996Life-history evolution in guppies (Poecilia reticulata: Poeciliidae). IV. Parallelism in life-history phenotypes. Am. Nat 147, 319–338 10.1086/285854 (doi:10.1086/285854) [DOI] [Google Scholar]
- Reznick D., Nunney L., Tessier A.2001Big house, big cars, superfleas and the cost of reproduction. Trends Ecol. Evol. 15, 421–425 10.1016/S0169-5347(00)01941-8 (doi:10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- Reznick D., Bryant M. J., Bashey F.2002r- and K-selection revisited: the role of population regulation in life history evolution. Ecology 83, 1509–1520 10.1890/0012-9658(2002)083[1509:RAKSRT]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[1509:RAKSRT]2.0.CO;2) [DOI] [Google Scholar]
- Ricklefs R. E., Wikelski M.2002The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.1016/S0169-5347(02)02578-8 (doi:10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- Roff D. A.1992The evolution of life histories. Theory and analysis. New York, NY: Chapman & Hall [Google Scholar]
- Roff D. A.2002Life history evolution. Sunderland, MA: Sinauer [Google Scholar]
- Roff D. A., Fairbairn D. J.2007The evolution and genetics of migration in insects. BioScience 57, 155–164 10.1641/B570210 (doi:10.1641/B570210) [DOI] [Google Scholar]
- Russell G. A., Chappell M. A.2007Is BMR repeatable in deer mice? Organ mass correlates and the effects of cold acclimation and natal altitude. J. Comp. Physiol. B 177, 75–87 10.1007/s00360-006-0110-y (doi:10.1007/s00360-006-0110-y) [DOI] [PubMed] [Google Scholar]
- Saetre P., Strandberg E., Sundgren P. E., Pettersson U., Jazin E., Bergstrom T. F.2006The genetic contribution to canine personality. Genes Brain Behav. 5, 240–248 10.1111/j.1601-183X.2005.00155.x (doi:10.1111/j.1601-183X.2005.00155.x) [DOI] [PubMed] [Google Scholar]
- Scheiner S. M., Mitchell R. J., Callahan H. S.2000Using path analysis to measure natural selection. J. Evol. Biol. 13, 423–433 10.1046/j.1420-9101.2000.00191.x (doi:10.1046/j.1420-9101.2000.00191.x) [DOI] [Google Scholar]
- Sgrò C. M., Hoffmann A. A.2004Genetic correlations, tradeoffs and environmental variation. Heredity 93, 241–248 10.1038/sj.hdy.6800532 (doi:10.1038/sj.hdy.6800532) [DOI] [PubMed] [Google Scholar]
- Sheldon B. C., Verhulst S.1996Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- Siepielski A. M., DiBattista J., Carlson S.2009It's about time: the temporal dynamics of phenotypic selection in the wild. Ecol. Lett. 11, 1261–1276 10.1111/j.1461-0248.2009.01381.x (doi:10.1111/j.1461-0248.2009.01381.x) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Adv. Stud. Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Kats L. B., Maurer E. F.2003Behavioural correlation across situations and the evolution of antipredator behaviour in a sunfish-salamander system. Anim. Behav 65, 29–44 10.1006/anbe.2002.2025 (doi:10.1006/anbe.2002.2025) [DOI] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- Sinervo B., Svensson E.2002Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338 10.1038/sj.hdy.6800148 (doi:10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- Sinervo B., Svensson E., Comendant T.2000Density cycles and an offspring quantity and quality game driven by natural selection. Nature 406, 985–988 10.1038/35023149 (doi:10.1038/35023149) [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Moltschaniwskyj N. A., Wapstra E., Dall S. R. X.2010Are behavioral syndromes invariant? Spatiotemporal variation in shy/bold behavior in squid. Behav. Ecol. Sociobiol. 64, 693–702 10.1007/s00265-009-0887-2 (doi:10.1007/s00265-009-0887-2) [DOI] [Google Scholar]
- Slatkin M.1987Gene flow and the geographic structure of natural populations. Science 236, 787–792 10.1126/science.3576198 (doi:10.1126/science.3576198) [DOI] [PubMed] [Google Scholar]
- Smith B. R., Blumstein D. L.2008Fitness consequences of personality: a metanalysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- Speakman J. R., et al. 2004Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3, 87–95 10.1111/j.1474-9728.2004.00097.x (doi:10.1111/j.1474-9728.2004.00097.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Groothuis T. G. G.2010aThe development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325 10.1111/j.1469-185X.2009.00103.x (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Groothuis T. G. G.2010bDevelopmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc B 365, 4029–4041 10.1098/rstb.2010.0218 (doi:10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S. C.1976Life-history tactics: a review of the ideas. Q. Rev. Biol. 51, 3–47 10.1086/409052 (doi:10.1086/409052) [DOI] [PubMed] [Google Scholar]
- Stearns S. C.1992The evolution of life histories. New York, NY: Oxford University Press [Google Scholar]
- Svartberg K.2006Breed-typical behaviour in dogs—historical remnants or recent constructs? Appl. Anim. Behav. Sci. 96, 293–313 10.1016/j.applanim.2005.06.014 (doi:10.1016/j.applanim.2005.06.014) [DOI] [Google Scholar]
- Tebbich S., Fessl B., Blomqvist D.2009Exploration and ecology in Darwin's finches. Evol. Ecol. 23, 591–605 10.1007/s10682-008-9257-1 (doi:10.1007/s10682-008-9257-1) [DOI] [Google Scholar]
- Tieleman B. I.2009High and low, fast or slow: the complementary contributions of altitude and latitude to understand life-history variation. J. Anim. Ecol. 78, 293–295 10.1111/j.1365-2656.2008.01522.x (doi:10.1111/j.1365-2656.2008.01522.x) [DOI] [PubMed] [Google Scholar]
- Tieleman B. I., Williams J. B., Ricklefs R. E., Klasing K. C.2005Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc. R. Soc. B 272, 1715–1720 10.1098/rspb.2005.3155 (doi:10.1098/rspb.2005.3155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. C.2007Risky prey behavior evolves in risky habitats. Proc. Natl Acad. Sci. USA 104, 14 377–14 382 10.1073/pnas.0704645104 (doi:10.1073/pnas.0704645104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. R., Reznick D. N.2009Phenotypic diversification across an environmental gradient: a role for predators and resource availability on the evolution of life histories. Evolution 63, 3201–3213 10.1111/j.1558-5646.2009.00785.x (doi:10.1111/j.1558-5646.2009.00785.x) [DOI] [PubMed] [Google Scholar]
- Webster D. G., Baumgardner D. J., Dewsbury D. A.1979Open-field behavior in eight taxa of muroid rodents. Bull. Psychol. Soc. 13, 90–92 [Google Scholar]
- White C. R., Seymour R. S.2003Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl Acad. Sci. USA 100, 4046–4049 10.1073/pnas.0436428100 (doi:10.1073/pnas.0436428100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma P., Muñoz-Garcia A., Walker A., Williams J. B.2007Tropical birds have a slow pace of life. Proc. Natl Acad. Sci. USA 104, 9340–9345 10.1073/pnas.0702212104 (doi:10.1073/pnas.0702212104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M., Spinney L., Schelsky W., Scheuerlein A., Gwinner E.2003Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc. R. Soc. Lond. B 270, 2383–2388 10.1098/rspb.2003.2500 (doi:10.1098/rspb.2003.2500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. C.1966Natural selection, the cost of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690 10.1086/282461 (doi:10.1086/282461) [DOI] [Google Scholar]
- Williams T. D.2008Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Phil. Trans. R. Soc. B 363, 1687–1698 10.1098/rstb.2007.0003 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. C., Thomas V., Lanier D. L., Dewsbury D. A.1976Open-field behavior in Muroid rodents. Behav. Biol. 17, 495–506 10.1016/S0091-6773(76)90901-9 (doi:10.1016/S0091-6773(76)90901-9) [DOI] [PubMed] [Google Scholar]
- Wilson D. S., Clark A. B., Coleman K., Dearstyne T.1994Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446 [DOI] [PubMed] [Google Scholar]
- Wolf M., Weissing F. J.2010An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Zera A. J., Sall J., Grudzinsky K.1997Flight-muscle polymorphism in the cricket Gryllus firmus: muscle characteristics and their influence on the evolution of flightlessness. Physiol. Zool. 70, 519–529 10.1086/515865 (doi:10.1086/515865) [DOI] [PubMed] [Google Scholar]