Abstract
We develop a conceptual framework for the understanding of animal personalities in terms of adaptive evolution. We focus on two basic questions. First, why do behavioural types exhibit limited behavioural plasticity, that is, behavioural correlations both across contexts and over time? Second, how can multiple behavioural types coexist within a single population? We emphasize differences in ‘state’ among individuals in combination with state-dependent behaviour. Some states are inherently stable and individual differences in such states can explain stable differences in suites of behaviour if it is adaptive to make behaviour in various contexts dependent on such states. Behavioural stability and cross-context correlations in behaviour are more difficult to explain if individual states are potentially more variable. In such cases stable personalities can result from state-dependent behaviour if state and behaviour mutually reinforce each other by feedback mechanisms. We discuss various evolutionary mechanisms for the maintenance of variation (in states and/or behaviour), including frequency-dependent selection, spatial variation with incomplete matching between habitat and phenotype, bet-hedging in a temporally fluctuating environment, and non-equilibrium dynamics. Although state differences are important, we also discuss how social conventions and social signalling can give rise to adaptive personality differences in the absence of state differences.
Keywords: behavioural syndromes, animal personality, state-dependent behaviour, frequency-dependent selection, bet hedging, non-equilibrium dynamics
1. Introduction
In many animal species, individuals of the same sex, age and size differ consistently in whole suites of correlated behavioural tendencies, comparable to human personalities (e.g. Clark & Ehlinger 1987; Digman 1990; Gosling 2001; Sih et al. 2004a). Birds often differ consistently in the way they explore their environment and these differences are associated with, for example, differences in boldness and aggressiveness (Groothuis & Carere 2005). Rodents, such as mice and rats, differ consistently in the way they deal with environmental challenges and such differences encompass, for example, differences in attack, avoidance and nest-building behaviour (Koolhaas et al. 1999). Interestingly, consistent behavioural differences are often associated with consistent differences in physiology, for example, in metabolism (Careau et al. 2008) and stress physiology (Koolhaas et al. 1999). Consistent individual differences in behaviour have been termed animal personalities (also coping styles, Koolhaas et al. 1999; temperament, Réale et al. 2007; behavioural syndromes, Sih et al. 2004a). It should be stressed that the concept of personalities does not require that individuals are completely consistent in their behaviour but rather that individual differences are consistently maintained over time and across contexts (Dingemanse et al. 2010). The emerging notion that individual differences may be expressions of different behavioural types—rather than the result of stochastic noise—has provoked a large body of empirical research in recent years (reviewed in Sih et al. 2004b), most of which aims at understanding the structure and the proximate causes of animal personalities.
Here we develop a conceptual framework for the understanding of animal personalities in terms of adaptive evolution. To this end, we focus on two basic questions associated with animal personalities. Why do behavioural types exhibit behavioural correlations both across contexts and over time (§3)? And how can multiple behavioural types coexist within a single population (§4)? Our goal is to review the evolutionary principles that are relevant for these questions, and to provide a systematic categorization of these explanatory principles. A companion paper in this issue (Dingemanse & Wolf 2010) discusses how the more specific explanations employed by recent models for adaptive personality differences fit into this explanatory framework.
2. State-dependent behaviour
The majority of models for adaptive personality differences explain the differences in behaviour on the basis of differences in state. Since the term state is used in very different connotations in personality research, we first want to clarify how the term should be interpreted in the rest of this paper. When talking about states, animal physiologists typically refer to the psychological state of an individual, like its state of arousal or its motivational state. Here, we use the different (and also well-established) concept of state as it is used in life-history theory (Stearns 1992) and evolutionary game theory (Maynard Smith 1982). In this context, the state of an animal refers to all those features that are strategically relevant, i.e. features that should be taken into consideration in the behavioural decisions in order to increase fitness (McNamara & Houston 1996; Houston & McNamara 1999; Clark & Mangel 2000). These features include:
— the age, size and morphology of an individual;
— the physical and physiological condition of an individual (e.g. level of energy reserves, parasite load, body temperature);
— the information available to an individual (e.g. experienced individuals may be better able to judge a situation);
— the type of environment an individual finds itself in (e.g. type of habitat), including the social environment (e.g. the quality of an individual's mate).
State differences can thus be externally induced or the result of natural selection. Note that environmental features (like good or bad weather conditions) are subsumed under the definition of state if they are of strategic importance. This is in clear contrast to the psychological interpretation of state mentioned above. A state can be strategically relevant in a variety of ways. For example, the state of an individual can constrain an individual's action (e.g. a moulting bird is not able to fly) or it can affect the performance of an individual (e.g. level of energy reserves of a predator may affect its ability to catch a prey).
States are important since they should give rise to state-dependent behaviour (condition-dependent behaviour, phenotypic plasticity). Hence, individual differences in state should be reflected in individual differences in behaviour. Importantly, single states are often relevant for different types of behaviour in different contexts. As a consequence, the behaviours in these contexts will tend to be correlated if they reflect the same underlying state. Hence, stable differences in states in combination with state-dependent behaviour provide a powerful framework for explaining adaptive differences in suites of correlated behavioural traits that are stable over time.
3. Adaptive behavioural correlations
We now turn to the observation that behavioural types often exhibit (i) time-consistency of behaviour (i.e. stability through part of the ontogeny), and (ii) suites of correlated behavioural traits (e.g. types that are more aggressive towards conspecifics are also bolder when confronted with a predator). Both types of behavioural correlations indicate limited behavioural plasticity to a degree that is often surprising (Wilson 1998; Dall et al. 2004). Consider, for example, voracity in fishing spiders. Young voracious spiders tend to be highly successful in catching prey. Voracity, however, is correlated with intraspecific aggression which in the case of adult females results in a low mating success, since voracious females tend to attack and cannibalize males before copulation (Johnson & Sih 2005). In these and other examples, one would expect a more flexible structure of behaviour that is fine-tuned to the local circumstances (e.g. being voracious when confronted with prey, being only mildly aggressive when confronted with potential mates).
On a proximate level, behavioural correlations can often be understood in terms of the architecture of behaviour, that is, the genetic, physiological, neurobiological and cognitive systems underlying behaviour. This architecture gives rise to behavioural correlations whenever multiple traits are affected by a common underlying mechanism within this architecture. Such mechanisms are ubiquitous; examples include pleiotropic genes (Mackay 2004), hormones (Ketterson & Nolan 1999; Lessells 2008), neurotransmitters (Bond 2001) and emotions (Rolls 2000) affecting multiple traits at the same time. In the case of fishing spiders, for example, both voracity and aggressiveness against males might be regulated by the same hormone. This would explain the correlation between voracity and aggressiveness, but it would not explain why the dependence of the two traits on a single hormone has not been uncoupled in the course of evolution. In other words, why has natural selection not led to a more flexible architecture of behaviour?
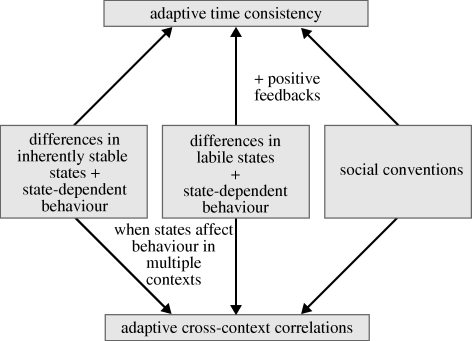
In this section we discuss the causes of adaptive behavioural correlations. Whether or not behavioural types are associated with state differences and whether or not the state differences involved have some inherent stability will be crucial for this discussion. Figure 1 provides a summary of the mechanisms discussed in this section.
Figure 1.
Explaining adaptive behavioural correlations. We consider three possible explanations for the evolution of behaviour that is consistent in time and correlated across contexts. The first is straightforward: when individual behaviour is dependent on states that are inherently stable (like sex or caste), the time consistency of state differences will be reflected in time-consistent behaviour; moreover cross-context correlations will result if the same state is of selective importance in different contexts. This explanation can be extended to potentially variable, labile states (like energy reserves, blood pressure or hormone levels) if positive feedbacks between state and behaviour induce time consistency in states. In the case of social interactions, consistent and correlated behaviour can evolve in the absence of state differences. Examples are social conventions (like winner–loser effects) or the coevolution of social responsiveness and behavioural consistency.
(a). Differences in inherently stable states
If the state of an individual is stable over time, adaptive state-dependent behaviour will also be time consistent, that is, not easily changed on a short-term perspective. Moreover, if the same stable state affects the behaviour of an individual in multiple contexts, differences in inherently stable states can also explain adaptive behavioural correlations across contexts. Accordingly, differences in stable individual states provide an obvious and straightforward explanation for animal personalities. It is thus not surprising if large and irreversible state differences (e.g. male/female, different castes in social insects) result in stable and consistent differences in behaviour.
This explanation applies to states that are ‘inherently stable’. By this we mean features of an organism that are very costly, time-consuming or even impossible to change. Examples for inherently stable states abound. For example, morphological, physiological and neurobiological features of an animal often emerge through a complex and time-consuming developmental process. Once such features are developed, substantial changes are often difficult to achieve. Males and females in sexually reproducing organisms, castes in social insects and alternative developmental trajectories are obvious examples. Evidence is accumulating, however, that less apparent features of animals may act as inherently stable states underlying personalities. Examples include differences in organ size (Biro & Stamps 2008), basal metabolic rates (Careau et al. 2008; Millidine et al. 2009) and stress response systems (Koolhaas et al. 1999; Schjolden & Winberg 2007) and their associated physiological morphology, structural differences in the organization of the brain (e.g. strength of cerebral lateralization, Reddon & Hurd 2009), and differences in cognitive mechanisms (e.g. learning ability, Kotrschal & Taborsky 2010). We presume that detecting such ‘non-apparent’ inherently stable states associated with personalities will be a key area for future research on animal personalities.
Note that since inherently stable states may reflect either a genetic polymorphism or phenotypic plasticity, the same holds true for behavioural correlations caused by inherently stable states.
It should be emphasized that the environment of an individual can also be a state that may in some cases be difficult to change and, hence, be inherently stable. This is obvious in the case of organisms with limited mobility. The same often holds true for the social environment of animals. Human societies, for example, encompass a large diversity of social positions (e.g. teachers, managers, bureaucrats). While it is in principle possible for an individual to change its position, such changes are typically very costly to the individual (e.g. in terms of required training or education).
(b). Differences in labile states
We will from now on focus on ‘labile states’, that is on states that can change easily and are thus potentially highly variable in time. Examples of such features include gene expression patterns, levels and composition of hormones and neurochemicals, receptor sensitivity and density, blood pressure, energy reserves, the experience that an animal has with a particular behaviour or environment and its skill levels. These states can easily be changed by many different factors, including the individual's own behaviour.
Behaviour that is made dependent on labile states provides a challenge to personality research, since such behaviour will only be stable and consistent in time if, for whatever reason, the underlying state does not change too much. Why should this be the case for labile states, which, by definition, are potentially highly variable in time?
In some situations the state and the behaviour of individuals are coupled by a positive feedback mechanism (Sih & Bell 2008; Wolf et al. 2008a; Luttbeg & Sih 2010). Initial state differences give rise to differences in behaviour, which act to stabilize or even increase the initial differences in state. Similarly, initial differences in behaviour can give rise to differences in state, which then may stabilize the differences in behaviour. Such a positive feedback mechanism can give rise to consistency in labile states and associated state-dependent behaviours. Several recent models for adaptive personality differences are based on this idea of labile state differences that are stabilized by positive feedback mechanisms (reviewed in Dingemanse & Wolf 2010): Rands et al. (2003; see also Dall et al. 2004), for example, investigate how feedbacks between energy reserves and foraging behaviour can give rise to ‘leaders’ and ‘followers’; Van Doorn et al. (in Wolf 2009) study how feedbacks between risk-taking behaviour and residual reproductive value can promote differences in risk-taking behaviour; and Luttbeg & Sih (2010) investigate how feedbacks between states that affect predation risk (e.g. size, energy reserves) and boldness can give rise to consistent individual differences in boldness.
A potentially important positive feedback is mediated by the positive effect that experience often has on the performance of an individual. Individuals perform better with increased experience (Rosenzweig & Bennett 1996; Kleim et al. 1998; Brown & Laland 2003) since processes such as learning, training and skill formation give rise to lower costs or higher benefits for the same action when repeated, which in turn favours adaptive consistency in this behaviour (Wolf et al. 2008a). Animals often learn how to recognize predators (Griffin 2004), which in turn reduces the cost of exploring and foraging in a risky habitat. Whenever the experience gained in one context affects the costs and benefits of behavioural actions in other contexts (e.g. learning to assess the strength of conspecific competitors might improve the ability to assess predators), such feedbacks can explain stable differences in whole suites of correlated behaviours.
Positive feedback need not act via behaviour directly. The cost and benefits of behavioural traits that are related to resource acquisition (e.g. aggression, boldness), for example, often depend on characteristics of the individual that are affected by the resources available to an individual (e.g. size, strength, resource holding potential) and this interaction can give rise to a positive feedback loop (Sih & Bell 2008; Luttbeg & Sih 2010). The strength of an individual positively affects its fighting ability, for example, which in turn gives rise to more resources that positively affect its strength. This feedback can explain adaptive consistency in suites of traits related to the characteristic.
Positive feedbacks can also act via physiological characteristics of the individual. It has been suggested, for example, that deviations from an initially chosen growth rate are costly to the individual (Stamps 2007; Biro & Stamps 2008). Compensatory growth, for example, often comes at the cost of increased risk of disease, higher mortality rates or decreased physiological capacity later in life (Metcalfe & Monaghan 2001; Mangel & Munch 2005). Similarly, deviations from any other physiological characteristic (e.g. blood pressure, gene expression pattern) may be costly to the individual—thus potentially explaining adaptive consistency in suites of traits related to these characteristics.
Initial variation in states (or behaviour) in combination with positive feedback mechanisms can thus explain adaptive behavioural consistency. In some cases positive feedback mechanisms will lead to the divergence of small initial differences in state; differences in experience with a certain environment or task, for example, might be small initially but substantial and difficult to change after longer periods of time.
(c). No relevant state differences
As indicated above, most recent models for adaptive personality differences are based on state-dependent behaviour and differences in state (Dingemanse & Wolf 2010). Yet, adaptive associations of different behaviours can also arise in the absence of relevant state differences. Here, we discuss some examples that, in our view, do not yet receive sufficient attention in the literature. The common feature of these examples is that behavioural consistency is selectively favoured in particular types of social interactions.
In the first example (Dall et al. 2004; Wolf et al. in press), the coevolution of social responsiveness and behavioural consistency leads to stable differences in behaviour. Consider a population of individuals that interact in Hawk–Dove like encounters (Maynard Smith 1982). Some individuals are responsive: they observe the behaviour of their future opponents in encounters with others and they adapt their own behaviour accordingly. In the Hawk–Dove game, Hawk is the best response to Dove, while Dove is the best response to Hawk (Maynard Smith 1982). Provided that there is some consistency in the behaviour of their opponents, responsive individuals can exploit this consistency by following the strategy ‘if the current opponent played Hawk in a previously witnessed interaction with a third individual, choose Dove, otherwise choose Hawk’ (Johnstone 2001; Dall et al. 2004; Wolf et al. in press). Indeed there is evidence for eavesdropping on aggressive interactions in several taxa (Peake 2005). Consistency can thus favour responsiveness since it allows the responsive individual to choose the best response to the behaviour of its opponent. Conversely, the presence of responsive individuals can favour consistency. This is, for example, the case in the Hawk–Dove game. If an individual played Hawk when being eavesdropped, the responsive eavesdropper should respond with Dove. This in turn should induce the first individual to stick to Hawk, since Hawk is the best response to the eavesdropper's behaviour Dove. Similarly, an individual that played Dove before should stick to Dove in a confrontation with an eavesdropper, since Dove is the best response to the eavesdropper's behaviour Hawk. In situations like the Hawk–Dove game, the feedback between social responsiveness and consistency can result in an evolutionarily stable state where the individuals show consistent behaviour even in the absence of state differences (like differences in fighting ability). The same phenomenon occurs in other social interactions. For example, McNamara et al. (2009) showed that the interplay between trust and trustworthiness can lead to consistent behaviour. As in the eavesdropping game, consistency results from the fact that socially responsive individuals can ‘exploit’ variation in trustworthiness, and that the existence of responsive individuals selects for consistency.
We would like to stress that there are also situations where the presence of socially responsive individuals results in inconsistent behaviour. An example is the rock–scissors–paper game (Maynard Smith 1982; Weissing 1991), where individuals that specialize on either of the three pure strategies (and, hence, exhibit consistent behaviour) can stably coexist in the absence of responsive individuals. As soon as responsive individuals are present, the consistent use of a single behaviour, say Rock, can be heavily exploited. It turns out that the only way to escape such exploitation is to be as unpredictable (and, hence, inconsistent) as possible and to employ a randomized strategy.
Behavioural consistency can also result from behavioural conventions. Social dominance associated with winner–loser effects is a good example. In many species of animals, individuals become more aggressive once they have won a fight, while they become less aggressive once they have lost a fight. These behavioural tendencies stabilize the dominance hierarchies found in many animals. With a variety of evolutionary models, Van Doorn et al. (2003a,b) showed that winner–loser effects can evolve as ‘social conventions’ even in the absence of differences in state (like differences in fighting ability).
Finally, consistent behavioural differences may arise in the context of animal communication. Adaptive correlations between behaviours may arise if individuals use their behaviour in one context (or point in time) as a signal in a different context (point in time). Individuals might, for example, use their boldness towards predators to signal their willingness to behave aggressively in intra-specific competition. In fact it has been suggested in a different context that male guppies use their boldness towards predators as a behavioural signal towards females (Godin & Dugatkin 1996). Animal signals have been studied quite extensively (Maynard Smith & Harper 2003), yet the possibility that individuals may use their behaviour in one context as a signal in a different context has been largely overlooked up to now. Interestingly, evolved signalling and communication systems can themselves be a source of individual variation in behaviour (Botero et al. in press).
4. Adaptive coexistence of behavioural types
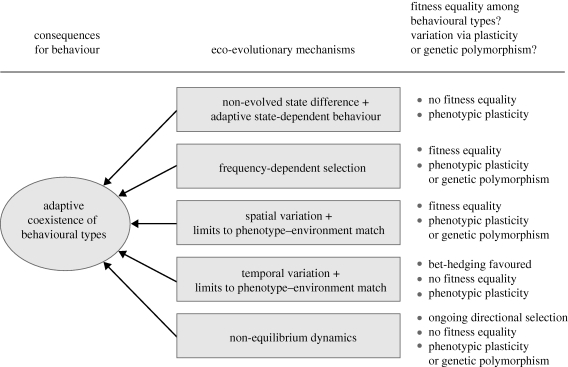
We now turn to the question of how different behavioural types can coexist within a single population. We first discuss the case where behavioural differences are caused by externally induced state differences. In such situations, behavioural types can coexist within a population without achieving the same fitness. We then focus on situations in which individuals either do not differ in any relevant state, or where state differences are evolved, that is, the result of a ‘strategic decision’ of an individual. In these situations, behavioural types can typically only coexist if all types achieve the same fitness (but see our discussion of bet-hedging below). Fitness equality can only be achieved if the fitness of various types is not constant, but dependent on the state of the population and/or the local environment. We argue that context-dependent fitness is the rule rather than the exception, and we discuss various eco-evolutionary processes leading to such context-dependence. We conclude this section by discussing how non-equilibrium dynamics can give rise to the coexistence of behavioural types despite sustained variation in fitness. Figure 2 provides a summary of the mechanisms discussed and their consequences for behaviour and fitness.
Figure 2.
Explaining the adaptive coexistence of behavioural types. Several eco-evolutionary mechanisms can explain the adaptive coexistence of multiple behavioural types within a population. Dependent on the mechanism, behavioural types may or may not obtain equal fitness and phenotypic variation may or may not be associated with genetic variation.
(a). Externally induced differences in states
Different behavioural types can adaptively coexist whenever individuals differ in state and behavioural variation among types reflects a state-dependent response of individuals (McNamara & Houston 1986; Houston & McNamara 1988). In some cases, state differences reflect an evolved feature, such as an evolved system of sex determination. In these cases, an adaptive theory has to consider the coevolution of state on the one hand and state-dependent behaviour on the other. Here, we first consider the simpler case where state differences are externally induced.
Many aspects of the state of an individual are affected by factors that are not under the control of the individual. Early life experiences often differ between individuals (e.g. environmental conditions in a critical period of development, accidents, windfall); the success of strategies often differs owing to stochastic events (e.g. one individual coincidentally finds a food source and thus increases its nutritional condition relative to another individual); and many other important events in the life of animals vary randomly among individuals (e.g. one individual gets infected by a parasite while another one does not). In view of the huge number of external factors that can potentially contribute to state differences among individuals, such difference may be viewed as the rule rather than the exception. This immediately explains the coexistence of behavioural types, if individuals differing in state tend to behave differently in an adaptive way. It does not, however, resolve the consistency problem (§3), since externally induced differences in state are not necessarily stable in time.
Two points are worth mentioning here. Whenever variation in behavioural types is owing to externally induced variation in states in combination with state-dependent behaviour, (i) different phenotypes need not obtain identical fitness in order to coexist (since individuals in a low-fitness state make ‘the best of a bad job’, Charnov 1993; Lucas & Howard 1995) and (ii) behavioural variation does not reflect a genetic polymorphism but phenotypic plasticity.
(b). Frequency-dependent selection
Fitness equality between behavioural types can be the outcome of selection if the fitness associated with different behavioural types is frequency-dependent, that is, if the fitness of behavioural types depends on the distribution of types present in the population.
Producer–scrounger situations (Barnard & Sibly 1981) are a typical example. In groups of foraging animals, individuals often have the choice between two behavioural roles: actively search for hidden food sources (‘producer’) or exploit food sources discovered by others (‘scrounger’). In these scenarios, the benefits associated with one behavioural type depend negatively on the frequency of that type in the population: the higher the frequency of scroungers in a group, the less beneficial this role becomes, since more scroungers compete for fewer resources. Such situations give rise to the negative frequency-dependent selection (Maynard Smith 1982), that is, selection where the rare phenotype has a selective advantage. This rareness advantage can explain the adaptive coexistence of two behavioural types in stable frequencies.
In scenarios with more than two behavioural types, other forms of frequency dependence can give rise to the adaptive coexistence of multiple behavioural types in stable frequencies. A prototype example is the rock–scissors–paper game (Maynard Smith 1982), where the interaction of negative and positive frequency dependence may lead to an equilibrium of multiple behavioural types or to the ‘dynamic’ coexistence of multiple behavioural types at continuously changing frequencies (Maynard Smith 1982; Weissing 1991).
Negative frequency-dependent selection can, as in the producer–scrounger game above, explain the coexistence of behavioural types that do not differ in state. Similarly, negative frequency-dependent selection can explain adaptive state differences among individuals (Wolf et al. 2007a,b, 2008b). One obvious example is the coexistence of the two sexes in stable frequencies caused by frequency-dependent sex ratio evolution. However, differences in much less apparent features of animals might also be explained by frequency-dependent selection. The benefits to a particular physiological or cognitive architecture (e.g. level of stress responsiveness, learning rule employed), for example, might depend negatively on how common this architecture is in the population, thus promoting the coexistence of different architectures (Wolf et al. 2008a).
Negative frequency dependence is common in natural populations (Sinervo & Calsbeek 2006). Below we list three general mechanisms that give rare strategies a systematic advantage over more common ones:
— Competition avoidance. Competition for limited resources is typically most intense among phenotypes that resemble each other closely and consequently compete for the same resources. Rare strategies can have a fitness advantage if they make use of a less competed part of the resource spectrum.
— Enemy avoidance. Predators and pathogens often focus on the most abundant prey, either because of selection in the past or by phenotypic adaptation (e.g. the development of a ‘search image’). Rarer strategies are therefore often less subject to predation, giving them a fitness advantage.
— Complementation. Pairs of individuals may benefit when specializing in different behavioural roles, thereby avoiding intra-pair competition and allowing complementation (e.g. by division of labour or exploitation of different parts of the resource spectrum). Rare strategies profit more often from such benefits than common strategies, since they can more easily team up with a different type of strategy (the common one).
It should be stressed that variation in behaviour caused by frequency-dependent selection may or may not be associated with genetic variation (Wilson 1994; Leimar 2005, 2009) since a phenotypic polymorphism (such as 25% scroungers and 75% producers) can be realized by either a genetic polymorphism (such as the coexistence of the pure strategies ‘always behave as a scrounger’ and ‘always behave as a producer’) or a genetic monomorphism (i.e. a population where the single genotype corresponds to the mixed strategy ‘play scrounger in 25% of the cases and producer otherwise’).
(c). Spatio-temporal variation in the environment
The fitness associated with a certain behavioural type typically depends on the local environment. Environmental conditions vary in space and time, leading to spatio-temporal variation in selection pressures. It is often thought that such variation in selection pressures can explain the coexistence of behavioural types. Whether this is indeed the case depends on the details of the situation (Hedrick et al. 1976; Hedrick 1986; Seger & Brockmann 1987; Moran 1992; Leimar 2005, 2009), in particular, on whether the population faces spatial or temporal variation in the environment (for an alternative classification, see Frank & Slatkin 1990; Leimar 2005, 2009; Donaldson-Matasci et al. 2008) and on how well individuals can match their phenotype to the local environment.
Consider first spatial variation in the environment, for example, an array of low-risk and high-risk habitats. Assume that the optimal behavioural type in low-risk habitats is different from that in high-risk habitats. The optimal strategy of an individual would be to make its behaviour dependent on the habitat it finds itself in, that is, to show a state-dependent strategy. Hence, whenever individuals can, in an error- and cost-free manner, adapt their behaviour to the environment (adaptive phenotypic plasticity) or adjust their environment to their behavioural type (e.g. via habitat choice), only a single state-dependent strategy will be maintained at the population level. Moreover, the population will also be monomorphic at the local level (all individuals show the same behaviour in a low-risk or high-risk habitat, respectively). The situation changes if individuals are constrained in their ability to match their behaviour to their local environment (e.g. because of incomplete information or costs of plasticity). In such a case, variation in behavioural types can be maintained both at a population level and within each habitat (Seger & Brockmann 1987). This is because the coexisting behavioural types experience different environments in such a way that each type will, on average, experience more often the environment to which it is better adapted.
Spatial variation in combination with limits to phenotype–environment matching can thus explain the coexistence of behavioural types. It should be clear from the above that whenever different environments favour different states (e.g. different physiological or cognitive set-up), spatial variation can also explain the coexistence of behavioural types associated with adaptive state differences. As in the case of frequency-dependent selection, this variation can in principle be realized by behavioural plasticity or a genetic polymorphism.
Next to spatial variation, temporal variation in environmental conditions has often been used to explain the coexistence of behavioural types. For example, Dingemanse et al. (2004) explain the coexistence of bold and shy individuals in great tits by temporal fluctuations in environmental conditions which favour boldness at some times and shyness at others. However, temporal variation may not be as general an explanation of the coexistence of behavioural types as many biologists seem to assume. Consider the scenario where the environment varies across generations, but where all individuals within one generation face the same environment. If the individuals are able to adjust their behaviour in an error- and cost-free manner to their current environment, they should all choose the same optimal behaviour in this environment; no behavioural variation is to be expected. The same conclusion holds in the opposite situation where individuals are constrained in their ability to adjust their behaviour to the current conditions (e.g. because of incomplete information or costs of plasticity). Even if some behavioural type (e.g. boldness) is favourable in some environments and another type (e.g. shyness) is favourable in another environment, these types will typically not be able to coexist in a long-term perspective. Long-term fitness reflects the performance over many generations (e.g. geometric mean fitness over the years), and there is generally a single strategy that maximizes this long-term measure of evolutionary success (but see Reinhold 2000). Therefore, a global monomorphism is to be expected. However, the resulting genotype will often be a diversifying ‘bet-hedging’ strategy (Seger & Brockmann 1987; Philippi & Seger 1989), that is, a strategy that does not produce one type of behaviour but a stochastic distribution over two or more phenotypes (e.g. low-risk and high-risk behavioural types). This can be seen as a ‘risk-spreading’ strategy, since no matter how the environment turns out, some of the individuals harbouring this strategy are well adapted to the local conditions. More technically, a diversifying bet-hedger can reduce its variance in fitness in an optimal way, thereby increasing its geometric mean fitness.
Bet-hedging can thus explain the coexistence of behavioural types (but see Hopper et al. 2003). Whenever different times favour different states (e.g. different physiological or cognitive set-ups), bet-hedging can also explain the coexistence of behavioural types associated with adaptive state differences (e.g. offspring size: Marshall et al. 2008). It should be noted that the variation caused by bet-hedging is only phenotypic (i.e. all phenotypes have the same genotype).
(d). Non-equilibrium dynamics
Until now, we considered environments that are either constant in space and time or randomly fluctuating owing to external factors. Our analysis was largely based on the premise that natural selection gives rise to an equilibrium where strategies coexist in stable frequencies. In many cases, however, the dynamics of selection will not lead to equilibrium but to ongoing oscillations or even to chaotic fluctuations. Such non-equilibrium dynamics can be caused by various factors, including resource competition, frequency-dependent selection (e.g. Weissing 1991), and sexual selection (Van Doorn & Weissing 2006). In several examples, it has been demonstrated that non-equilibrium conditions have a high potential for maintaining variation even in cases where equilibrium theory would predict the dominance of a single behavioural type (e.g. Huisman & Weissing 1999; Van Doorn & Weissing 2006).
A good example for non-equilibrium coexistence is the co-variation of dispersal and colonizing ability observed in many species (Chitty 1967; Hanski et al. 2006; Duckworth & Badyaev 2007). In these species, some individuals disperse while others stay at home. Dispersers typically have a phenotype allowing them to colonize unoccupied space, but this same phenotype is selectively disadvantageous under crowded conditions (e.g. Duckworth & Kruuk 2009). Such a ‘colonist’ behavioural type could probably not persist under constant and stable equilibrium conditions. In a perturbed environment, however, where empty spaces are created once in a while, the colonists can flourish because they can exploit these opportunities. Once the empty spaces are filled, however, the settlers succumb to their own success, since they create an environment that can be more efficiently exploited by alternative phenotypes that do better under crowded conditions.
Non-equilibrium dynamics can thus explain the coexistence of behavioural types that may be associated with adaptive differences in underlying states. The phenotypic variation may or may not be associated with genetic variation. At each point in time there is ongoing directional selection; different behavioural types will thus typically achieve different fitness.
5. Concluding remarks
In this paper, we have developed an explanatory conceptual framework for adaptive animal personalities. We identified two major types of adaptive explanations for the coexistence of animal personalities: (i) differences in state in combination with state-dependent behaviour; and (ii) responsive strategies and conventions in social interactions. The first type of explanation is reasonably well understood. There is already a rich theory for adaptive variation in morphology, growth patterns, physiology, etc., that means for adaptive variation in (evolved) states. The main questions in this area revolve around the time consistency of states, since otherwise state differences cannot explain behavioural consistency, a characteristic aspect of animal personalities. Positive feedback between states and the induced state-dependent behaviour can explain that seemingly minor and labile differences in state are enhanced into major and stable differences (Wolf et al. 2008a; Luttbeg & Sih 2010). While the majority of models on the evolution of animal personalities are focusing on state differences (Dingemanse & Wolf 2010), it is important to note that consistent behavioural differences can also result from the evolution of conditional strategies in social interactions. Due to the lack of models in this area, we could only discuss a few examples, but we anticipate that this kind of ‘strategic’ explanation for adaptive differences in behaviour will play a prominent role in the near future. It is conceivable that a major function of an individual's personality is to signal the individual's future intentions. Although signalling intentions can be disadvantageous under certain conditions (Maynard Smith & Harper 2003), it may provide both the sender and the receiver a crucial advantage in strategically complex situations. Such situations are characterized by a huge number of equilibria (Van Doorn et al. 2003a,b), and the coordination of players may be required to avoid low-fitness equilibria and to achieve a high-fitness outcome.
Acknowledgements
The authors thank Alasdair Houston, Marc Mangel and the guest editors of this special issue for critical feedback and D. Visser for preparing the figures.
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Barnard C. J., Sibly R. M.1981Producers and scroungers—a general model and its application to captive flocks of house sparrows. Anim. Behav. 29, 543–550 10.1016/S0003-3472(81)80117-0 (doi:10.1016/S0003-3472(81)80117-0) [DOI] [Google Scholar]
- Biro P. A., Stamps J. A.2008Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Bond A. J.2001Neurotransmitters, temperament and social functioning. Eur. Neuropsychopharmacol. 11, 261–274 10.1016/S0924-977X(01)00094-3 (doi:10.1016/S0924-977X(01)00094-3) [DOI] [PubMed] [Google Scholar]
- Botero C. A., Pen I., Komdeur J., Weissing F. J.In press The evolution of individual variation in communication strategies. Evolution. [DOI] [PubMed] [Google Scholar]
- Brown C., Laland K. N.2003Social learning in fishes: a review. Fish Fish. 4, 280–288 10.1046/j.1467-2979.2003.00122.x (doi:10.1046/j.1467-2979.2003.00122.x) [DOI] [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Réale D.2008Energy metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Charnov E. L.1993Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford, UK: Oxford University Press [Google Scholar]
- Chitty D.1967The natural selection of self-regulatory behaviour in animal populations. Proc. Ecol. Soc. Austral. 2, 51–78 [Google Scholar]
- Clark A., Ehlinger T.1987Pattern and adaptation in individual behavioral differences. In Perspectives in ethology (eds Bateson P., Klopfer P.), pp. 403–420 New York, NY: Plenum [Google Scholar]
- Clark C. W., Mangel M.2000Dynamic state variable models in ecology. New York, NY: Oxford University Press [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- Digman J. M.1990Personality structure—emergence of the 5-Factor Model. Annu. Rev. Psychol. 41, 417–440 [Google Scholar]
- Dingemanse N. J., Wolf M.2010Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M.2004Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Donaldson-Matasci M. C., Lachmann M., Bergstrom C. T.2008Phenotypic diversity as an adaptation to environmental uncertainty. Evol. Ecol. Res. 10, 493–515 [Google Scholar]
- Duckworth R. A., Badyaev A. V.2007Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R. A., Kruuk L. E. B.2009Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977 10.1111/j.1558-5646.2009.00625.x (doi:10.1111/j.1558-5646.2009.00625.x) [DOI] [PubMed] [Google Scholar]
- Frank S. A., Slatkin M.1990Evolution in a variable environment. Am. Nat. 136, 244–260 10.1086/285094 (doi:10.1086/285094) [DOI] [Google Scholar]
- Godin J. G., Dugatkin L. A.1996Female mating preference for bold males in the guppy, Poecilia reticulata. Proc. Natl Acad. Sci. USA 93, 10 262. 10.1073/pnas.93.19.10262 (doi:10.1073/pnas.93.19.10262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling S. D.2001From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- Griffin A. S.2004Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140 [DOI] [PubMed] [Google Scholar]
- Groothuis T. G. G., Carere C.2005Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150 10.1016/j.neubiorev.2004.06.010 (doi:10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Hanski I., Saastamoinen M., Ovaskainen O.2006Dispersal-related life-history trade-offs in a butterfly metapopulation. J. Anim. Ecol. 75, 91–100 10.1111/j.1365-2656.2005.01024.x (doi:10.1111/j.1365-2656.2005.01024.x) [DOI] [PubMed] [Google Scholar]
- Hedrick P. W.1986Genetic polymorphism in heterogeneous environments—a decade later. Annu. Rev. Ecol. Syst. 17, 535–566 10.1146/annurev.es.17.110186.002535 (doi:10.1146/annurev.es.17.110186.002535) [DOI] [Google Scholar]
- Hedrick P. W., Ginevan M. E., Ewing E. P.1976Genetic polymorphism in heterogeneous environments. Annu. Rev. Ecol. Syst. 7, 1–32 10.1146/annurev.es.07.110176.000245 (doi:10.1146/annurev.es.07.110176.000245) [DOI] [Google Scholar]
- Hopper K. R., Rosenheim J. A., Prout T., Oppenheim S. J.2003Within-generation bet hedging: a seductive explanation? Oikos 101, 219–222 10.1034/j.1600-0706.2003.12051.x (doi:10.1034/j.1600-0706.2003.12051.x) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1988Fighting for food: a dynamic version of the Hawk–Dove game. Evol. Ecol. 2, 51–64 10.1007/BF02071588 (doi:10.1007/BF02071588) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1999Models of adaptive behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- Huisman J., Weissing F. J.1999Biodiversity of plankton by species oscillations and chaos. Nature 402, 407–410 10.1038/46540 (doi:10.1038/46540) [DOI] [Google Scholar]
- Johnson J. C., Sih A.2005Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav. Ecol. Sociobiol. 58, 390–396 10.1007/s00265-005-0943-5 (doi:10.1007/s00265-005-0943-5) [DOI] [Google Scholar]
- Johnstone R. A.2001Eavesdropping and animal conflict. Proc. Natl Acad. Sci. USA 98, 9177–9180 10.1073/pnas.161058798 (doi:10.1073/pnas.161058798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson E. D., Nolan V.1999Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25 10.1086/303280 (doi:10.1086/303280) [DOI] [PubMed] [Google Scholar]
- Kleim J. A., Barbay S., Nudo R. J.1998Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol. 80, 3321. [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Kotrschal A., Taborsky B.2010Environmental change enhances cognitive abilities in fish. PLoS Biol. 8, e1000351. 10.1371/journal.pbio.1000351 (doi:10.1371/journal.pbio.1000351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimar O.2005The evolution of phenotypic polymorphism: randomized strategies versus evolutionary branching. Am. Nat. 165, 669–681 10.1086/429566 (doi:10.1086/429566) [DOI] [PubMed] [Google Scholar]
- Leimar O.2009Environmental and genetic cues in the evolution of phenotypic polymorphism. Evol. Ecol. 23, 125–135 10.1007/s10682-007-9194-4 (doi:10.1007/s10682-007-9194-4) [DOI] [Google Scholar]
- Lessells C. M.2008Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity? Phil. Trans. R. Soc. B 363, 1589–1598 10.1098/rstb.2007.0008 (doi:10.1098/rstb.2007.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. R., Howard R. D.1995On alternative reproductive tactics in anurans: dynamic games with density and frequency-dependence. Am. Nat. 146, 365–397 10.1086/285805 (doi:10.1086/285805) [DOI] [Google Scholar]
- Luttbeg B., Sih A.2010Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990 10.1098/rstb.2010.0207 (doi:10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F. C.2004The genetic architecture of quantitative traits: lessons from Drosophila. Curr. Opin. Genet. Dev. 14, 253–257 10.1016/j.gde.2004.04.003 (doi:10.1016/j.gde.2004.04.003) [DOI] [PubMed] [Google Scholar]
- Mangel M., Munch S. B.2005A life-history perspective on short- and long-term consequences of compensatory growth. Am. Nat. 166, E155–E176 10.1086/444439 (doi:10.1086/444439) [DOI] [PubMed] [Google Scholar]
- Marshall D. J., Bonduriansky R., Bussière L. F.2008Offspring size variation within broods as a bet-hedging strategy in unpredictable environments. Ecology 89, 2506–2517 10.1890/07-0267.1 (doi:10.1890/07-0267.1) [DOI] [PubMed] [Google Scholar]
- Maynard Smith J.1982Evolution and the theory of games. Cambridge, UK: Cambridge University Press [Google Scholar]
- Maynard Smith J., Harper D.2003Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- McNamara J. M., Houston A. I.1986The common currency for behavioral decisions. Am. Nat. 127, 358–378 10.1086/284489 (doi:10.1086/284489) [DOI] [Google Scholar]
- McNamara J. M., Houston A. I.1996State-dependent life histories. Nature 380, 215–221 10.1038/380215a0 (doi:10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Stephens P. A., Dall S. R. X., Houston A. I.2009Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B 276, 605–613 10.1098/rspb.2008.1182 (doi:10.1098/rspb.2008.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe N. B., Monaghan P.2001Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- Millidine K. J., Armstrong J. D., Metcalfe N. B.2009Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proc. R. Soc. B 276, 2103–2108 10.1098/rspb.2009.0080 (doi:10.1098/rspb.2009.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A.1992The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 10.1086/285369 (doi:10.1086/285369) [DOI] [Google Scholar]
- Peake T. M.2005Eavesdropping in communication networks. In Animal communication networks (ed. McGregor P. K.), pp. 13–37 Chicago, IL: Chicago University Press [Google Scholar]
- Philippi T., Seger J.1989Hedging one's evolutionary bets, revisited. Trends Ecol. Evol. 4, 41–44 10.1016/0169-5347(89)90138-9 (doi:10.1016/0169-5347(89)90138-9) [DOI] [PubMed] [Google Scholar]
- Rands S. A., Cowlishaw G., Pettifor R. A., Rowcliffe J. M., Johnstone R. A.2003Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434 10.1038/nature01630 (doi:10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Reddon A. R., Hurd P. L.2009Individual differences in cerebral lateralization are associated with shy–bold variation in the convict cichlid. Anim. Behav. 77, 189–193 10.1016/j.anbehav.2008.09.026 (doi:10.1016/j.anbehav.2008.09.026) [DOI] [Google Scholar]
- Reinhold K.2000Maintenance of a genetic polymorphism by fluctuating selection on sex-limited traits. J. Evol. Biol. 13, 1009–1014 10.1046/j.1420-9101.2000.00229.x (doi:10.1046/j.1420-9101.2000.00229.x) [DOI] [Google Scholar]
- Rolls E. T.2000Precis of the brain and emotion. Behav. Brain Sci. 23, 177–233 10.1017/S0140525X00002429 (doi:10.1017/S0140525X00002429) [DOI] [PubMed] [Google Scholar]
- Rosenzweig M. R., Bennett E. L.1996Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 78, 57–65 10.1016/0166-4328(95)00216-2 (doi:10.1016/0166-4328(95)00216-2) [DOI] [PubMed] [Google Scholar]
- Schjolden J., Winberg S.2007Genetically determined variation in stress responsiveness in rainbow trout: behavior and neurobiology. Brain Behav. Evol. 70, 227–238 10.1159/000105486 (doi:10.1159/000105486) [DOI] [PubMed] [Google Scholar]
- Seger J., Brockmann H. J.1987What is bet-hedging? Oxford surveys in evolutionary biology, vol. 4, pp. 182–211 Oxford, UK: Oxford University Press [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Adv. Study Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C.2004aBehavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004bBehavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- Sinervo B., Calsbeek R.2006The developmental, physiological, neural, and genetical causes and consequences of frequency-dependent selection in the wild. Annu. Rev. Ecol. Evol. Syst. 37, 581–610 10.1146/annurev.ecolsys.37.091305.110128 (doi:10.1146/annurev.ecolsys.37.091305.110128) [DOI] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits' in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stearns S. C.1992The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- Van Doorn G. S., Weissing F. J.2006Sexual conflict and the evolution of female preferences for indicators of male quality. Am. Nat. 168, 742–757 10.1086/508634 (doi:10.1086/508634) [DOI] [PubMed] [Google Scholar]
- Van Doorn G. S., Hengeveld G. M., Weissing F. J.2003aThe evolution of social dominance—II: multi-player models. Behaviour 140, 1333–1358 10.1163/156853903771980611 (doi:10.1163/156853903771980611) [DOI] [Google Scholar]
- Van Doorn G. S., Hengeveld G. M., Weissing F. J.2003bThe evolution of social dominance—I: two-player models. Behaviour 140, 1305–1332 10.1163/156853903771980602 (doi:10.1163/156853903771980602) [DOI] [Google Scholar]
- Weissing F. J.1991Evolutionary stability and dynamic stability in a class of evolutionary normal form games. Game equilibrium models I. Evolution and game dynamics (ed. Selten R.), pp. 29–97 Berlin, Germany: Springer [Google Scholar]
- Wilson D. S.1994Adaptive genetic variation and human evolutionary psychology. Ethol. Sociobiol. 15, 219–235 [Google Scholar]
- Wilson D. S.1998Adaptive individual differences within single populations. Phil. Trans. R. Soc. Lond. B 353, 199–205 10.1098/rstb.1998.0202 (doi:10.1098/rstb.1998.0202) [DOI] [Google Scholar]
- Wolf M.2009Adaptive individual differences—the evolution of animal personalities. PhD thesis, University of Groningen, Groningen, The Netherlands: (http://dissertations.ub.rug.nl/faculties/science/2009/m.wolf/) [Google Scholar]
- Wolf M., Van Doorn G. S., Leimar O., Weissing F. J.2007aEvolution of animal personalities. Reply. Nature 450, E5–E6 10.1038/nature06327 (doi:10.1038/nature06327) [DOI] [PubMed] [Google Scholar]
- Wolf M., Van Doorn G. S., Leimar O., Weissing F. J.2007bLife-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Wolf M., Van Doorn G. S., Weissing F. J.2008aEvolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Van Doorn G. S., Leimar O., Weissing F. J.2008bDo animal personalities emerge? Reply. Nature 451, E9–E10 10.1038/nature06744 (doi:10.1038/nature06744) [DOI] [PubMed] [Google Scholar]
- Wolf M., Van Doorn G. S., Weissing F. J.In press On the coevolution of social responsiveness and behavioural consistency. Proc. R. Soc. B. 10.1098/rspb.2010.1051 (doi:10.1098/rspb.2010.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]