Abstract
The combination of a long-acting delivery system for the agonist [D-Trp6]luteinizing hormone-releasing hormone ([D-Trp6]LH-RH) with modern somatostatin analogs was studied in the Dunning R-3327H rat prostate cancer model. Microcapsules of [D-Trp6]LH-RH releasing 25 micrograms/day were injected once a month. In the first experiment the adjunct was the somatostatin analog D-Phe-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2 (RC-121), administered at a dose of 2.5 micrograms twice a day, and the therapy was continued for 70 days. Tumor volume was significantly decreased by [D-Trp6]LH-RH microcapsules or RC-121 given alone. The combination of microcapsules and analog RC-121 caused a greater inhibition of tumor growth than the single agents. Similar effects were seen when the percent increase in the tumor volume was examined. The inhibition of tumor growth caused by the [D-Trp6]LH-RH microcapsules was greater than that caused by RC-121. The combination of the two agents was again the most effective, resulting in the smallest increase in tumor volume. Tumor weights were much lower in the groups treated with microcapsules or RC-121 alone than in controls. The lowest tumor weights were obtained in the group that received the combination of [D-Trp6]LH-RH microcapsules and RC-121. Similar results were obtained in the second experiment, in which the animals were treated for a period of 83 days with microcapsules containing the somatostatin analog D-Phe-Cys-Tyr-D-Trp-Lys-Val-Cys-Trp-NH2 (RC-160) that released 5 micrograms/day and were injected twice a month alone or in combination with microcapsules of [D-Trp6]LH-RH. Microcapsules of analog RC-160 given alone significantly decreased tumor growth as measured by the final tumor volume, the percentage change from the initial tumor volume, and the reduction in tumor weight. The inhibition of tumor growth induced by [D-Trp6]LH-RH microcapsules was greater than that caused by RC-160. The most striking decrease in tumor weight and volume was obtained in animals treated with microcapsules of [D-Trp6]LH-RH combined with the delayed delivery system for RC-160. The overall response to the combination therapy could reflect the inhibition by somatostatin analogs of the proliferation of prostate cancer cells through a decrease in growth hormone and prolactin release and interference with endogenous growth factors, in addition to the main effect, which is the suppression by [D-Trp6]LH-RH of the growth of androgen-dependent tumor cells. Our results indicate that somatostatin analogs enhance the inhibitory effects of [D-Trp6]LH-RH on the growth of prostate tumors. The administration of somatostatin analogs in combination with microcapsules of [D-Trp6]LH-RH might improve clinical response in patients with advanced prostate carcinoma.
Full text
PDF
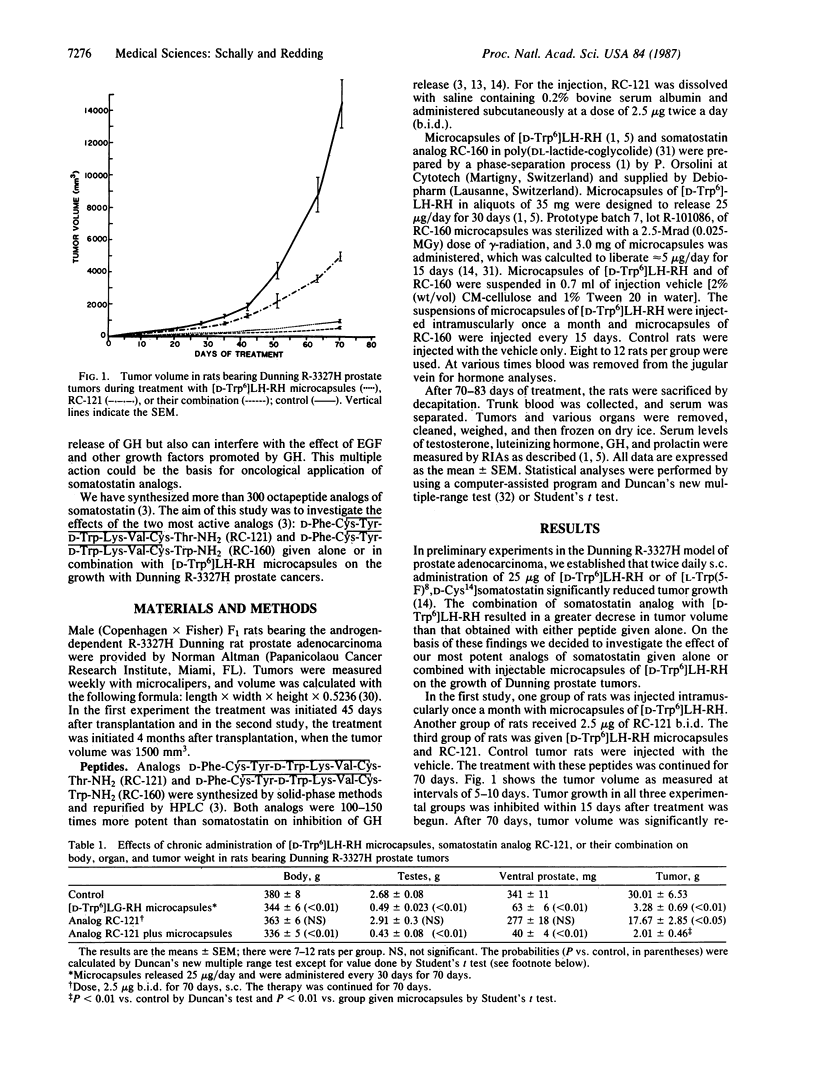
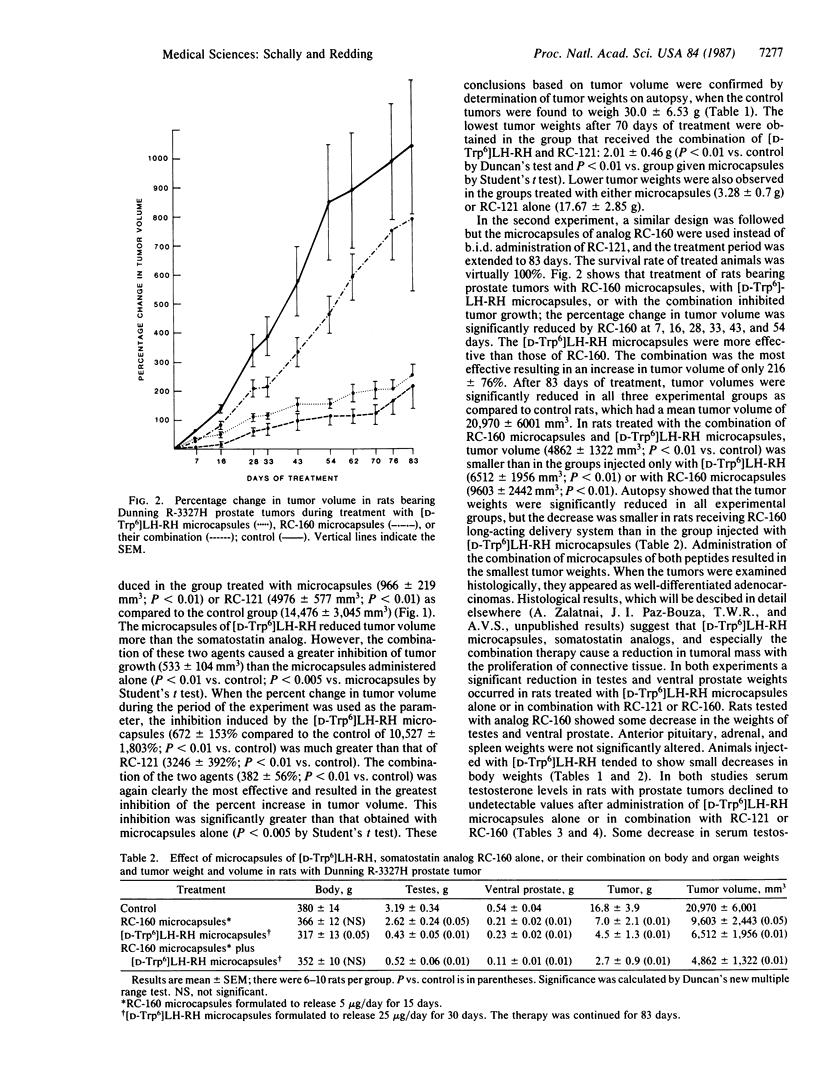


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimos D., Smith C., Lee C., Grayhack J. T. Action of prolactin in regressing prostate: independent of action mediated by androgen receptors. Prostate. 1984;5(6):589–595. doi: 10.1002/pros.2990050604. [DOI] [PubMed] [Google Scholar]
- Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Synthesis and biological activity of highly potent octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coert A., Nievelstein H., Kloosterboer H. J., Loonen P., van der Vies J. Effects of hyperprolactinemia on the accessory sexual organs of the male rat. Prostate. 1985;6(3):269–276. doi: 10.1002/pros.2990060306. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoren J. B., Hsueh A. J. Growth hormone increases ovarian levels of immunoreactive somatomedin C/insulin-like growth factor I in vivo. Endocrinology. 1986 Feb;118(2):888–890. doi: 10.1210/endo-118-2-888. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- GRAYHACK J. T. PITUITARY FACTORS INFLUENCING GROWTH OF THE PROSTATE. Natl Cancer Inst Monogr. 1963 Oct;12:189–199. [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hierowski M. T., Liebow C., du Sapin K., Schally A. V. Stimulation by somatostatin of dephosphorylation of membrane proteins in pancreatic cancer MIA PaCa-2 cell line. FEBS Lett. 1985 Jan 7;179(2):252–256. doi: 10.1016/0014-5793(85)80529-9. [DOI] [PubMed] [Google Scholar]
- Holland J. M., Lee C. Effects of pituitary grafts on testosterone stimulated growth of rat prostate. Biol Reprod. 1980 Mar;22(2):351–355. doi: 10.1093/biolreprod/22.2.351. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Böhlen P., Fava R., Baldwin J. H., Kleeman G., Armour R. Purification of kidney epithelial cell growth inhibitors. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5989–5992. doi: 10.1073/pnas.77.10.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J. T., Coffey D. S. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981 Dec;41(12 Pt 1):5070–5075. [PubMed] [Google Scholar]
- Iversen O. H. What is new in endogenous growth stimulators and inhibitors (chalones). Pathol Res Pract. 1985 Jul;180(1):77–80. doi: 10.1016/S0344-0338(85)80079-0. [DOI] [PubMed] [Google Scholar]
- Iwata K. K., Fryling C. M., Knott W. B., Todaro G. J. Isolation of tumor cell growth-inhibiting factors from a human rhabdomyosarcoma cell line. Cancer Res. 1985 Jun;45(6):2689–2694. [PubMed] [Google Scholar]
- Janik P., Briand P., Hartmann N. R. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res. 1975 Dec;35(12):3698–3704. [PubMed] [Google Scholar]
- Johnson M. P., Thompson S. A., Lubaroff D. M. Differential effects of prolactin on rat dorsolateral prostate and R3327 prostatic tumor sublines. J Urol. 1985 Jun;133(6):1112–1120. doi: 10.1016/s0022-5347(17)49392-x. [DOI] [PubMed] [Google Scholar]
- Karashima T., Schally A. V. Inhibitory effects of somatostatin analogs on prolactin secretion in rats pretreated with estrogen or haloperidol. Proc Soc Exp Biol Med. 1987 May;185(1):69–75. doi: 10.3181/00379727-185-42518. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Kasid A., Gelmann E., Davidson N., McManaway M., Huff K., Bronzert D., Bates S., Swain S. Autocrine and paracrine growth regulation of human breast cancer. J Steroid Biochem. 1986 Jan;24(1):147–154. doi: 10.1016/0022-4731(86)90044-0. [DOI] [PubMed] [Google Scholar]
- Mascardo R. N., Sherline P. Somatostatin inhibits rapid centrosomal separation and cell proliferation induced by epidermal growth factor. Endocrinology. 1982 Oct;111(4):1394–1396. doi: 10.1210/endo-111-4-1394. [DOI] [PubMed] [Google Scholar]
- Mathé G., Schally A. V., Comaru-Schally A. M., Mauvernay R. Y., Vovan M. L., Machover D., Misset J. L., Court B., Bouchard P., Duchier J. Phase II trial with D-Trp-6-LH-RH in prostatic carcinoma: comparison with other hormonal agents. Prostate. 1986;9(4):327–342. doi: 10.1002/pros.2990090404. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Russell E. K., O'Donohue T. L., Linden C. D., Gazdar A. F. Bombesin-like peptides in small cell lung cancer: biochemical characterization and secretion from a cell line. Life Sci. 1983 Jan 31;32(5):487–493. doi: 10.1016/0024-3205(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Mukamel E., Nissenkorn I., Servadio C. Early combined hormonal and chemotherapy for metastatic carcinoma of prostate. Urology. 1980 Sep;16(3):257–260. doi: 10.1016/0090-4295(80)90037-0. [DOI] [PubMed] [Google Scholar]
- Müntzing J., Kirdani R., Murphy G. P., Sandberg A. A. Hormonal control of zinc uptake and binding in the rat dorsolateral prostate. Invest Urol. 1977 May;14(6):492–495. [PubMed] [Google Scholar]
- Nilsson A., Isgaard J., Lindahl A., Dahlström A., Skottner A., Isaksson O. G. Regulation by growth hormone of number of chondrocytes containing IGF-I in rat growth plate. Science. 1986 Aug 1;233(4763):571–574. doi: 10.1126/science.3523759. [DOI] [PubMed] [Google Scholar]
- Parmar H., Phillips R. H., Lightman S. L., Edwards L., Allen L., Schally A. V. Randomised controlled study of orchidectomy vs long-acting D-Trp-6-LHRH microcapsules in advanced prostatic carcinoma. Lancet. 1985 Nov 30;2(8466):1201–1205. doi: 10.1016/s0140-6736(85)90739-1. [DOI] [PubMed] [Google Scholar]
- Paz-Bouza J. I., Redding T. W., Schally A. V. Treatment of nitrosamine-induced pancreatic tumors in hamsters with analogs of somatostatin and luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1112–1116. doi: 10.1073/pnas.84.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotka E. D., Seal U. S., Letellier M. A., Verme L. J., Ozoga J. J. Early effects of pinealectomy on LH and testosterone secretion in white-tailed deer. J Endocrinol. 1984 Oct;103(1):1–7. doi: 10.1677/joe.0.1030001. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V. Inhibition of cell growth by a hypothalamic peptide. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7014–7018. doi: 10.1073/pnas.79.22.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V., Tice T. R., Meyers W. E. Long-acting delivery systems for peptides: inhibition of rat prostate tumors by controlled release of [D-Trp6]luteinizing hormone-releasing hormone from injectable microcapsules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5845–5848. doi: 10.1073/pnas.81.18.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V., Coy D. H., Meyers C. A. Hypothalamic regulatory hormones. Annu Rev Biochem. 1978;47:89–128. doi: 10.1146/annurev.bi.47.070178.000513. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Kook A. I., Monje E., Redding T. W., Paz-Bouza J. I. Combination of a long-acting delivery system for luteinizing hormone-releasing hormone agonist with Novantrone chemotherapy: increased efficacy in the rat prostate cancer model. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8764–8768. doi: 10.1073/pnas.83.22.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V., Redding T. W. Combination of long-acting microcapsules of the D-tryptophan-6 analog of luteinizing hormone-releasing hormone with chemotherapy: investigation in the rat prostate cancer model. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2498–2502. doi: 10.1073/pnas.82.8.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck C. M., King L. E., Jr Role of epidermal growth factor in carcinogenesis. Cancer Res. 1986 Mar;46(3):1030–1037. [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Weber S., Zuckerman J. E., Bostwick D. G., Bensch K. G., Sikic B. I., Raffin T. A. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985 Jan;75(1):306–309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Green H. The generation of insulin-like growth factor-1--sensitive cells by growth hormone action. Science. 1986 Aug 1;233(4763):551–553. doi: 10.1126/science.3726546. [DOI] [PubMed] [Google Scholar]



