Abstract
A coping style (also termed behavioural syndrome or personality) is defined as a correlated set of individual behavioural and physiological characteristics that is consistent over time and across situations. This relatively stable trait is a fundamental and adaptively significant phenomenon in the biology of a broad range of species, i.e. it confers differential fitness consequences under divergent environmental conditions. Behavioural flexibility appears to be an important underlying attribute or feature of the coping style that might explain consistency across situations. Proactive coping is characterized by low flexibility expressed as rather rigid, routine-like behavioural tendencies and reduced impulse control (behavioural inhibition) in operant conditioning paradigms. This article summarizes some of the evidence that individual differentiation in behavioural flexibility emerges as a function of underlying variability in the activation of a brain circuitry that includes the prefrontal cortex and its key neurochemical signalling pathways (e.g. dopaminergic and serotonergic input). We argue that the multidimensional nature of animal personality and the terminology used for the various dimensions should reflect the differential pattern of activation of the underlying neuronal network and the behavioural control function of its components. Accordingly, unravelling the molecular mechanisms that give rise to individual differences in the coping style will be an important topic in biobehavioural neurosciences, ecology and evolutionary biology.
Keywords: coping, prefrontal cortex, serotonin, dopamine, behavioural flexibility, proximate mechanism
1. Introduction
During the last decades, a wide variety of scientific disciplines have shifted their interest towards the causes and consequences of individual variation. Ecologists and evolutionary biologists aim at understanding the ecological function of individual variation in behaviour and its consequences for evolutionary fitness (Sih et al. 2004; Réale et al. 2007; Wolf et al. 2008). Understanding individual disease vulnerability and personalized medicine has become a major area of research in the biomedical sciences (Ginsburg & Willard 2009), and in the behavioural neurosciences much research effort is devoted to gene–environment interaction in the development of adult phenotypes and the underlying molecular and physiological mechanisms (Barr et al. 2003). Although the boundaries between these disciplines gradually disappear, we feel that much can be gained by a further integration of both levels of analysis, in terms of concepts, terminology and design of experiments.
Naturalistic studies in a variety of animal species show that individuals can be categorized in distinct behavioural phenotypes. These studies are all based on two observations: (i) within an individual, behaviours are often correlated independent of the environmental situation and (ii) correlated behaviours result in only a limited number of phenotypes across individuals. Several terms are used for this phenomenon. Sih et al. (2004) used the term behavioural syndrome, whereas Groothuis & Carere (2005) preferred the term behavioural profile. More specifically, research has focused on two distinct patterns of reaction to stressful conditions or coping style. Rodent research distinguishes between proactive and reactive coping (Koolhaas et al. 1999) and researchers of fish and birds often use the terms shyness and boldness (Wilson et al. 1994). Whatever term is used exactly, they all refer to alternative response patterns in reaction to challenges that are stable over time and across various situations (Koolhaas et al. 1999). For example, animals characterized by a proactive coping style are offensive towards male conspecific rivals, are impulsive in decision-making, score high in frustration tests, take risks in the face of potential dangers and are novelty seekers (David et al. 2004; Groothuis & Carere 2005; Steimer & Driscoll 2005). Although a unidimensional approach of individual variation is useful in these early stages of animal personality research, several studies emphasize the need to consider individual variation being composed of several independent trait characteristics (Steimer & Driscoll 2005; Van Reenen et al. 2005; Koolhaas et al. 2007). These authors suggest a two-tier model in which a coping style axis reflects how an animal responds to a challenge (qualitative dimension) and an emotional reactivity axis reflects how strongly it responds (quantitative dimension). These allow the characterization of individuals on two independent scales in a two-dimensional space. In view of the tests used to characterize individual fish or birds as shy or bold, it is conceivable that this phenotypic characterization includes both qualitative and quantitative aspects. The dimensions are generally determined using principle component analyses of the variation in behaviour between individuals tested in various conditions. In human personality research, this has resulted in five independent dimensions (the big five) or axes at which individuals may vary (Goldberg 1990). The fact that individual variation in behaviour can be reduced to variation in a limited number of independent dimensions is important.
From an evolutionary perspective, variable trait characteristics are the subject of selection pressure. Hence, the various dimensions may reflect independent components of individual fitness. From the point of view of behavioural neuroscience, it is reasonable to suggest that these dimensions somehow reflect underlying causal mechanisms. The idea is that certain behaviours are correlated because they share the same neurobiological, neuroendocrine and/or genetic mechanisms (Bell 2007; Bell et al. 2007). The present paper aims at one of these causal mechanisms. Since we focus on individual behavioural characteristics that are stable across situations, one has to look for variation in causal mechanisms or behavioural control functions that are activated in different contexts in one and the same animal. Inter-individual variations in behaviour in these contexts should consequently be reflected in a differential activation of the underlying causal mechanisms. This line of reasoning also implies that the dimensions used to describe individual variation in behaviour should reflect variation in the main proximate mechanisms controlling the behaviour.
It is beyond the scope of this paper to review all causal mechanisms underlying individual trait characteristics. We will rather explore the neurobiology of behavioural flexibility as an important underlying attribute or feature of general coping style that might explain consistency of individual behaviour across a wide variety of environmental conditions. We will mainly use data derived from rodent studies in laboratory settings. An elaborate overview of the evolutionary basis of coping styles and the underlying physiology is given by Overli et al. (2007). However, they do not specifically address the mechanisms of behavioural flexibility as defined below.
2. Behavioural flexibility
Behavioural flexibility is an ill-defined concept. Evolutionary ecology uses the term behavioural plasticity to indicate that the expression of behavioural traits is not fixed within genotypes or individuals (Dingemanse et al. 2007). Applied to individuals, behavioural plasticity is defined as the slope of the relationship between behaviour (response variable) over an environmental gradient: a behavioural reaction norm. This slope can thus be viewed as an index for the number of phenotypes a single genotype can produce in a given set of environments (Dingemanse et al. 2009). Behavioural neuroscience does not use the concept of behavioural reaction norm. In this field of science, behavioural flexibility includes a range of behavioural control functions of an animal aimed to directly respond and adjust its behaviour to environmental stimuli. This includes impulsivity (impulsive action/impulsive choice), reversal learning/response perseveration, etc. (Dalley et al. 2004). Behavioural flexibility is defined as the ability of an individual to directly respond and adjust its behaviour to environmental stimuli. Here, we will consider the individual variation in the underlying behavioural control functions. Behavioural flexibility reflects the degree to which behaviour is guided by stimuli from the environment, which can be considered an important fundamental and rather stable differential characteristic of coping styles.
So far, flexibility of behaviour in relation to coping style has mainly been tested in laboratory settings using rodents. A wide range of studies suggest that actions of the proactive coping style are principally based on rather rigid internally organized (i.e. ‘brain-engrained’) predictions of the actual environment. This is in contrast to the reactive coping style in which there is a more direct actual stimulus–response relationship. For example, rats or mice can easily be trained to run a maze for a food reward. After reaching a stable task performance, the reaction to a small change in the maze is often studied. In one experiment on mice, a small piece of tape was put on the floor in one of the alleys of the maze, while in another experiment the maze was turned 90° with respect to the extra-maze cues. In both experiments, the proactive coping males paid little or no attention to the change; i.e. there was no increase in time to complete the task and no increase in the number of errors made in the maze. Reactive coping males on the other hand started exploring the maze again and hence took much more time to get to the goal box and made more errors in the task (Benus et al. 1990). This suggests that the reactive coping style may be much more guided by environmental stimuli, while the proactive coping style seems to rely on routines. Similar results were obtained in a study of coping styles in pigs. Piglets that struggle a lot in the back-test (proactive, high resisters) are less successful in reversal learning of a T-maze task compared with animals that hardly show any resistance (reactive, low resisters) in the back-test. High resisting, proactive coping pigs had more difficulties in inhibiting their previously reinforced response, which is consistent with the idea that these animals rely on previous experience and develop routines (Bolhuis et al. 2004).
Differences in behavioural flexibility can be demonstrated in several other situations where the animal has to switch suddenly from a familiar situation to a new one. For example, the two coping styles differ strongly in response to a 12 h shift in light/dark cycle. Proactive coping male mice stay in their original day–night rhythm for a few days, after which their rhythm gradually shifts to the new cycle. Reactive coping males on the other hand start to shift their rhythm immediately; they are twice as fast in adapting to the new light–dark cycle as the proactive coping males (Benus et al. 1988). This suggests that the rhythm of the reactive animals is more determined by the extrinsic light/dark cycle. Similar studies in non-mammalian species are hardly available. However, in their work on great tits as an avian model of coping styles, Verbeek et al. (1994) also concluded that the fast-exploring (i.e. proactive) birds seem to rely on routines.
Besides maze tests, operant conditioning tasks are often employed as well to test for behavioural flexibility. In these tasks, animals are trained to perform an operant (usually to press a lever or turn a wheel) to trigger a reinforcement (usually a food reward). An operant conditioning paradigm allows precise experimental control of the stimuli that the animals can respond to, and of the responses they make. Hence, a more refined analysis of the various behavioural control mechanisms that determine behavioural flexibility is thus possible. These include impulsive responding or behavioural inhibition, response perseveration and attention. One of the studies aimed at documenting the relationship between coping styles and behavioural flexibility using an operant conditioning paradigm has been performed in hamsters (Cervantes & Delville 2007). High-aggressive hamsters perform impulsively compared with low-aggressive hamsters in a two-lever delay-discounting paradigm. High-aggressive hamsters were more likely to press a lever for an immediate but small reward, whereas low-aggressive animals showed a preference for a delayed but larger reward (Cervantes & Delville 2007). In a similar study by David and co-workers, hamsters were trained in operant conditioning chambers for immediate reinforcement and were later tested for their response to a delayed reward. They showed that all animals increase their frequency of lever pressing initially. However, low-aggressive animals were able to adapt to the delay and showed a decreased rate of lever pressing per reward within 5 days, reaching a significantly higher feeding efficiency than the high-aggressive males (David et al. 2004). Similar results were obtained in a genetic model of coping styles; the Roman high (RHA)- and Roman low (RLA)-avoidance rats. These animals were genetically selected for their avoidance behaviour in an active shock avoidance paradigm. Extensive studies show that the RLA animals are also less aggressive in a social interaction test and are more efficient in a delayed reinforcement task than RHA rats (Zeier et al. 1978). Apparently, non-aggressive males are better in inhibiting their actions when required.
These experiments all demonstrate different aspects of behavioural flexibility. It is important to note that these aspects of behavioural flexibility all seem to be correlated with the individual level of aggression, suggesting that behavioural flexibility can be considered as a rather fundamental and presumably stable component of the coping style dimension. It might also explain the consistency of individual behaviour across different contexts. A reduced capacity of behavioural inhibition will not only affect the way an animal deals with its social environment but also how it deals with food shortage. The proactive animal acts primarily on the basis of previous experience (feed-forward control), which is fast but may be inaccurate. The reactive coping animal tends to rely more on the detailed information available in the environment, which may take time to acquire but is probably more accurate information on current environmental conditions. This fundamental difference in behavioural control may also relate to the adaptive nature of the two coping styles. A proactive coping animal may be adapted to stable environmental conditions, whereas the reactive coping style may do better under variable and unpredictable environmental conditions. Although it is intriguing to notice that the above-mentioned experimental paradigms developed in behavioural neuroscience are reminiscent of the paradigms used to measure optimal foraging in behavioural ecology (Shapiro et al. 2008), these predictions have hardly been tested under more natural conditions. In the great tit model, food availability in the field was found to be a major determinant in the differential survival of fast- and slow-exploring animals (Dingemanse et al. 2004). In a recent field experiment, van Overveld & Matthysen (2010) showed that fast-exploring juvenile tits more rapidly invaded new food resources than slow-exploring birds after a sudden drop in food availability, consistent with our thesis. Clearly, this topic needs a much more elaborate experimental approach using carefully characterized animals that preferably vary along only one dimension of personality.
3. Causal mechanisms
As argued above, the dimensions of personality are likely to reflect individual variation in the pattern of activity of underlying causal physiological mechanisms. We feel that the terminology used to describe the dimensions of animal personalities should somehow be consistent with the behavioural control function of the underlying brain structures. Moreover, a careful analysis of the key components of these proximate mechanisms is not only essential for an evidence-based candidate gene approach of animal personalities, but also important in unravelling variable trait characteristics that might be subjected to selection pressure.
Many studies have considered neuroendocrine parameters as part of such a common causal mechanism for behavioural syndromes. For example, the shy individual is usually considered to be characterized by a high reactivity of the hypothalamus–pituitary–adrenocortical (HPA) axis. However, in view of the multidimensional nature of behavioural syndromes as discussed above, one has to ask the question how neuroendocrine mechanisms relate to these dimensions. This has been discussed in a recent review for the HPA axis, the sympathetic adrenomedullary (SAM) system and the hypothalamus–pituitary–gonadal (HPG) system (Koolhaas et al. 2010). There, it is argued that, with the exception of the HPG axis, it is unlikely that there is a direct causal relationship between these neuroendocrine systems and the coping style dimension. In other words, neither corticosteroids nor plasma catecholamines determine the qualitative type of behavioural response to a challenge. It is more likely that the activity of these neuroendocrine systems reflects individual variation at the emotionality axis. However, it is important to notice that both the HPA axis and the SAM system have an important function in the metabolic support of behaviour as well. Therefore, it cannot be excluded that the magnitude of these physiological responses may be a direct consequence of differences in the physical activity. Consequently, one has to consider the possibility that the correlations between behavioural syndromes and neuroendocrine stress reactivity are secondary to the individual differences in the behavioural activity (Koolhaas et al. 2010).
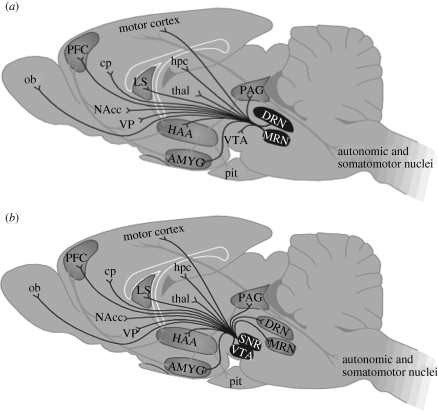
The brain circuitry that has been associated with various personality dimensions is depicted in figure 1. The amygdala, hypothalamus and periaqueductal grey are mainly involved in the emotional reactivity of the organism. The neuronal network involved in behavioural flexibility involves the prefrontal cortex (PFC), the nucleus accumbens (NAcc) and their dopaminergic and serotonergic input. It is beyond the scope of this paper to review the available literature on the function of this circuitry in behaviour in detail. A more extensive review of the role of this circuitry in cue dependency of behaviour, habit formation and behavioural flexibility is given by Everitt & Robbins (2005). Here, we will focus on the question to what extent individual variation in behaviour on the coping style axis is related to variation in (components of) this latter neuronal circuitry.
Figure 1.
Overview of the main brain structures and their connections involved in (a) aggressive behaviour and their serotonergic input from the dorsal raphe and (b) dopaminergic input from the ventral tegmental area. AMYG, amygdala; AVP, arginine vasopressin; cp, caudate putamen; DRN, dorsal raphe nucleus; HAA, hypothalamic attack area; hpc, hippocampus; LS, lateral septum; MRN, medial raphe nucleus; NAcc, nucleus accumbens; ob, olfactory bulb; PAG, periaqueductal grey; PFC, prefrontal cortex; pit, pituitary; SNR, substantia nigra; thal, thalamus; VP, ventral pallidum; VTA, ventral tegmental area.
(a). Prefrontal cortex and behavioural flexibility
Several of the tasks used to measure behavioural flexibility in rodents are derived from tests of PFC functioning. In general, the PFC has been associated with both aggressive behaviour (Blair 2004; Siever 2008) and various aspects of behavioural flexibility such as impulsive action and impulsive choice (Dalley et al. 2008). Similarly in birds, the nidopallium, which is considered the avian homologue of the mammalian PFC, has an important function in choice behaviour and optimal foraging (Matsushima et al. 2008). In mammals, the PFC can be divided into several sub-regions, each with a somewhat different function in the control of behaviour. Its involvement in aggressive behaviour seems to be secondary to its primary role in behavioural inhibition, decision-making, working memory and planning of behaviour (Dalley et al. 2004). Lesions of the orbital PFC in rats induced an increase in impulsive behaviour as measured by a reduced performance in a delayed reinforcement task and a preference for smaller and more immediate reward (Mobini et al. 2002). In view of the current discussion on proximate mechanisms of coping styles, we will now consider the question to what extent individual variation in behaviour is reflected in variation at the level of the PFC.
The PFC receives important input from the evolutionary ancient neurotransmitter system serotonin originating in the dorsal raphe nucleus (figure 1a). Throughout the animal kingdom, serotonin is involved in the regulation of aggression (Kravitz 2000; Kravitz & Huber 2003; Miczek et al. 2007) and seems to have an evolutionarily well-conserved function in behavioural flexibility as well (Kravitz 2000; Evers et al. 2007). The serotonergic input of the PFC plays a causal role in the individual variation in both aggression and behavioural flexibility. Low levels of serotonin in the PFC have been associated with both aggression and impulsive behaviour at the level of the PFC (van Erp & Miczek 2000; De Boer et al. 2003; Winstanley et al. 2006; Caramaschi et al. 2007; Miczek et al. 2007). Several studies show that proactive and reactive coping rats and mice differ in the serotonergic input of the PFC. Rats with extensive experience of aggressive behaviour have lower levels of release of serotonin (5-hydroxytryptamine; 5-HT) in the PFC (van Erp & Miczek 2000; De Boer et al. 2003; Ferrari et al. 2003; Caramaschi et al. 2007; Miczek et al. 2007). Similarly, aggressive mice strains have significantly lower levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the PFC (Caramaschi et al. 2007).
A decrease in serotonergic function has also been implicated in impulsive action in various paradigms of impulsivity in both humans and rodents (Roberts et al. 1994; Fletcher 1995; Harrison et al. 1997; Crean et al. 2002; Homberg et al. 2007). In the five-choice serial reaction time task (5-CSRTT), a task that has been developed to test for the inhibitory control of behaviour, 5-HT depletion has been found to increase premature responding (Harrison et al. 1997). In addition, administration of the 5-HT releasing agent d-fenfluramine has been shown to decrease premature responding in the 5-CSRTT (Carli & Samanin 1992). The role of serotonin in behavioural inhibition is confirmed by the behavioural disinhibition induced by 5-HT lesions of the raphe nuclei in rats using a selective neurotoxin (Fletcher 1995). In serotonin transporter (SERT) knockout rats, a continuously enhanced level of PFC serotonin is associated with reduced aggression as measured in a resident–intruder paradigm. SERT knockout rats also show improved inhibitory control in a 5-CSRTT, but unchanged behavioural flexibility investigated in a reversal learning task (Homberg et al. 2007). Control of impulsive choice and action (behavioural inhibition) seems to be mediated by the medial PFC, because a delay-discounting paradigm enhances 5-HT efflux in the medial PFC but not in the orbital PFC (Winstanley et al. 2006).
Taken together, brain serotonin is causally involved in both aggression and behavioural flexibility. Individual variation in the serotonergic input to the medial PFC may explain the correlated individual variation in the coping style dimension. This is consistent with the hamster studies in an operant conditioning paradigm by Cervantes & Delville (2007, 2009) mentioned before in which aggressive hamsters had less 5-HT innervation of the PFC and were more impulsive than their non-aggressive counterparts.
(b). Mesolimbic dopamine system and reward processing
The fact that aggressive hamsters prefer an immediate small reward over a delayed large reward indicates that individuals may differ in the processing of reward-related cues (Cervantes & Delville 2009). The mesolimbic dopamine system has an important role in the processing of natural rewards. This system has its cell bodies in the ventral tegmental area and innervates not only the NAcc, but also the PFC (figure 1b). This circuit is extensively studied for its involvement in natural reward processing and the development of drug addiction (Kelley & Berridge 2002). Several studies show that individual variation in coping with environmental challenges is related to differences at the level of this mesolimbic dopamine system. For example, in the Roman rat lines, the density of dopamine D1 receptors and D3 receptor binding in the NAcc is consistently higher in RHA than in RLA rats (Guitart-Masip et al. 2006; Giorgi et al. 2007). Furthermore, RHA rats show remarkable behavioural and neurochemical responses to the acute administration of morphine and psychostimulants (Corda et al. 2005; Giorgi et al. 2007) and are more susceptible, compared with RLA rats, to the reinforcing properties of cocaine (Fattore et al. 2009). An extensive clinical and preclinical literature shows that impulsivity appears to be a major vulnerability factor in the development of substance abuse (de Wit 2009). With regard to the argument of the present paper, these data support the view that individual differences in reward processing and the underlying neurobiology are important components of animal personality and behavioural flexibility that might explain the consistency of individual trait characteristics across contexts.
4. Concluding remarks
The present paper argues that the behavioural expression of different coping styles, animal personalities or behavioural syndromes should be related to individual variation in the underlying causal neurobiological mechanisms. Behavioural flexibility seems to be an important underlying component of a coping style that might explain consistency of individual differentiation across a wide variety of behaviours. Indeed, the lower flexibility observed in proactive coping animals as a reduced behavioural inhibition does explain not only short attack latencies in an aggressive interaction or an escape situation, but also the choice for immediate small rewards in a food-related situation. Behavioural flexibility seems to relate to the degree to which behaviour is guided by environmental input. The proactive individual behaves mainly on the basis of internally organized predictions, which is fast but can be inaccurate. At the same time, behavioural flexibility includes aspects of behavioural inhibition.
The medial PFC has a key role in the neuronal network involved in behavioural flexibility and planning of behaviour in time. An increasing number of studies show individual differentiation in the pattern of activation of the various components of this neuronal network in relation to phenotypic differences in behavioural flexibility. The functional differentiation in dopaminergic and serotonergic input of the PFC as discussed above is a prerequisite for a candidate gene approach of these two neurotransmitter systems. Indeed, several studies show that this might be a promising avenue. For example, polymorphisms in the promoter region of the SERT gene have been associated both with a functional change in the transporter capacity and with individual variation in aggression and personality in humans and in rhesus monkeys (Lesch & Merschdorf 2000; Suomi 2006). Similarly, a single nucleotide polymorphism in the gene coding for the dopamine-4 receptor has been associated with individual variation in novelty seeking and behavioural inhibition in humans and animals (Savitz & Ramesar 2004; Munafo et al. 2008; Korsten et al. 2010). However, the nature of such a differentiation in neurobiology and underlying genetics in terms of independent dimensions of individual variation as discussed above has hardly been addressed. This would require an experimental approach of the question whether a manipulation in a certain component of the network affects behavioural characteristics of one dimension without affecting the characteristics of other dimensions. Such information is important to understand in more detail the individual behavioural characteristics that might be subjected to selection pressures.
Finally, it is tempting to consider the possibility that behavioural flexibility is a prerequisite for phenotypic plasticity at the within-individual level. Studies aimed at understanding individual stress vulnerability show that the behavioural flexible, reactive coping mouse shows the strongest stress-induced changes at the level of behaviour, neuroendocrinology and neurobiology (Veenema et al. 2004). These changes have often been interpreted as signs of stress-induced pathology. However, these changes might just as well reflect the behavioural and physiological underpinning of individual adaptation. This line of reasoning suggests indeed that high behavioural flexibility is associated with a high capacity to adapt to a changing environment.
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Barr C. S., Newman T. K., Becker M. L., Parker C. C., Champoux M., Lesch K. P., Goldman D., Suomi S. J., Higley J. D.2003The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2, 336–340 10.1046/j.1601-1848.2003.00051.x (doi:10.1046/j.1601-1848.2003.00051.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M.2007Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761 10.1098/rspb.2006.0199 (doi:10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M., Backstrom T., Huntingford F. A., Pottinger T. G., Winberg S.2007Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol. Behav. 91, 15–25 10.1016/j.physbeh.2007.01.012 (doi:10.1016/j.physbeh.2007.01.012) [DOI] [PubMed] [Google Scholar]
- Benus R. F., Koolhaas J. M., van Oortmerssen G. A.1988Aggression and adaptation to the light-dark cycle: role of intrinsic and extrinsic control. Physiol. Behav. 43, 131–137 10.1016/0031-9384(88)90228-4 (doi:10.1016/0031-9384(88)90228-4) [DOI] [PubMed] [Google Scholar]
- Benus R. F., Den Daas S., Koolhaas J. M., van Oortmerssen G. A.1990Routine formation and flexibility in social and non-social behaviour of aggressive and non-aggressive male mice. Behaviour 112, 176–193 10.1163/156853990X00185 (doi:10.1163/156853990X00185) [DOI] [Google Scholar]
- Blair R. J.2004The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 55, 198–208 10.1016/S0278-2626(03)00276-8 (doi:10.1016/S0278-2626(03)00276-8) [DOI] [PubMed] [Google Scholar]
- Bolhuis J. E., Schouten W. G. P., Leeuw J. A. D., Schrama J. W., Wiegant V. M.2004Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav. Brain Res. 152, 351–360 10.1016/j.bbr.2003.10.024 (doi:10.1016/j.bbr.2003.10.024) [DOI] [PubMed] [Google Scholar]
- Caramaschi C., De Boer S. F., Koolhaas J. M.2007Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol. Behav. 90, 590–601 10.1016/j.physbeh.2006.11.010 (doi:10.1016/j.physbeh.2006.11.010) [DOI] [PubMed] [Google Scholar]
- Carli M., Samanin R.1992Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats' performance differently in a five-choice serial reaction time task. Psychopharmacology (Berl) 106, 228–234 10.1007/BF02801977 (doi:10.1007/BF02801977) [DOI] [PubMed] [Google Scholar]
- Cervantes M. C., Delville Y.2007Individual differences in offensive aggression in golden hamsters: a model of reactive and impulsive aggression? Neuroscience 150, 511–521 10.1016/j.neuroscience.2007.09.034 (doi:10.1016/j.neuroscience.2007.09.034) [DOI] [PubMed] [Google Scholar]
- Cervantes M. C., Delville Y.2009Serotonin 5-HT1A and 5-HT3 receptors in an impulsive-aggressive phenotype. Behav. Neurosci. 123, 589–598 10.1037/a0015333 (doi:10.1037/a0015333) [DOI] [PubMed] [Google Scholar]
- Corda M. G., Piras G., Lecca D., Fernández-Teruel A., Driscoll P., Giorgi O.2005The psychogenetically selected Roman rat lines differ in the susceptibility to develop amphetamine sensitization. Behav. Brain Res. 157, 147–156 10.1016/j.bbr.2004.06.016 (doi:10.1016/j.bbr.2004.06.016) [DOI] [PubMed] [Google Scholar]
- Crean J., Richards J. B., de Wit H.2002Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav. Brain Res. 136, 349–357 10.1016/S0166-4328(02)00132-8 (doi:10.1016/S0166-4328(02)00132-8) [DOI] [PubMed] [Google Scholar]
- Dalley J. W., Cardinal R. N., Robbins T. W.2004Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 28, 771–784 10.1016/j.neubiorev.2004.09.006 (doi:10.1016/j.neubiorev.2004.09.006) [DOI] [PubMed] [Google Scholar]
- Dalley J. W., Mar A. C., Economidou D., Robbins T. W.2008Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 90, 250–260 10.1016/j.pbb.2007.12.021 (doi:10.1016/j.pbb.2007.12.021) [DOI] [PubMed] [Google Scholar]
- David J. T., Cervantes M. C., Trosky K. A., Salinas J. A., Delville Y.2004A neural network underlying individual differences in emotion and aggression in male golden hamsters. Neuroscience 126, 567–578 10.1016/j.neuroscience.2004.04.031 (doi:10.1016/j.neuroscience.2004.04.031) [DOI] [PubMed] [Google Scholar]
- De Boer S. F., Van Der Vegt B. J., Koolhaas J. M.2003Individual variation in aggression of feral rodent strains: a standard for the genetics of aggression and violence? Behav. Genet. 33, 485–501 10.1023/A:1025766415159 (doi:10.1023/A:1025766415159) [DOI] [PubMed] [Google Scholar]
- de Wit H.2009Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 14, 22–31 10.1111/j.1369-1600.2008.00129.x (doi:10.1111/j.1369-1600.2008.00129.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M.2004Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Kazem A. J., Reale D., Wright J.2009Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Robbins T. W.2005Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489 10.1038/nn1579 (doi:10.1038/nn1579) [DOI] [PubMed] [Google Scholar]
- Evers E. A., van d. V., Fekkes D., Jolles J.2007Serotonin and cognitive flexibility: neuroimaging studies into the effect of acute tryptophan depletion in healthy volunteers. Curr. Med. Chem. 14, 2989–2995 10.2174/092986707782794032 (doi:10.2174/092986707782794032) [DOI] [PubMed] [Google Scholar]
- Fattore L., Piras G., Corda M. G., Giorgi O.2009The Roman high- and low-avoidance rat lines differ in the acquisition, maintenance, extinction, and reinstatement of intravenous cocaine self-administration. Neuropsychopharmacology 34, 1091–1101 10.1038/npp.2008.43 (doi:10.1038/npp.2008.43) [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., van Erp A. M., Tornatzky W., Miczek K. A.2003Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur. J. Neurosci. 17, 371–378 10.1046/j.1460-9568.2003.02447.x (doi:10.1046/j.1460-9568.2003.02447.x) [DOI] [PubMed] [Google Scholar]
- Fletcher P. J.1995Effects of combined or separate 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei on responding maintained by a DRL 20s schedule of food reinforcement. Brain Res. 675, 45–54 10.1016/0006-8993(95)00037-Q (doi:10.1016/0006-8993(95)00037-Q) [DOI] [PubMed] [Google Scholar]
- Ginsburg G. S., Willard H. F.2009Genomic and personalized medicine: foundations and applications. Transl. Res. 154, 277–287 10.1016/j.trsl.2009.09.005 (doi:10.1016/j.trsl.2009.09.005) [DOI] [PubMed] [Google Scholar]
- Giorgi O., Piras G., Corda M. G.2007The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci. Biobehav. Rev. 31, 148–163 10.1016/j.neubiorev.2006.07.008 (doi:10.1016/j.neubiorev.2006.07.008) [DOI] [PubMed] [Google Scholar]
- Goldberg L. R.1990An alternative ‘description of personality’: the big-five factor structure. J. Pers. Soc. Psychol. 59, 1216–1229 [DOI] [PubMed] [Google Scholar]
- Groothuis T. G., Carere C.2005Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150 10.1016/j.neubiorev.2004.06.010 (doi:10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M., Johansson B., Fernández-Teruel A., Cañete T., Tobeña A., Terenius L., Giménez-Llort L.2006Divergent anatomical pattern of D1 and D3 binding and dopamine- and cyclic AMP-regulated phosphoprotein of 32 kDa mRNA expression in the Roman rat strains: implications for drug addiction. Neuroscience 142, 458–466 [DOI] [PubMed] [Google Scholar]
- Harrison A. A., Everitt B. J., Robbins T. W.1997Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 133, 329–342 10.1007/s002130050410 (doi:10.1007/s002130050410) [DOI] [PubMed] [Google Scholar]
- Homberg J. R., Pattij T., Janssen M. C., Ronken E., De Boer S. F., Schoffelmeer A. N., Cuppen E.2007Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur. J. Neurosci. 26, 2066–2073 10.1111/j.1460-9568.2007.05839.x (doi:10.1111/j.1460-9568.2007.05839.x) [DOI] [PubMed] [Google Scholar]
- Kelley A. E., Berridge K. C.2002The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 22, 3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A., Blokhuis H. J.1999Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., De Boer S. F., Buwalda B., Van Reenen C. G.2007Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 70, 218–226 10.1159/000105485 (doi:10.1159/000105485) [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., De Boer S. F., Coppens C. M., Buwalda B.2010Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 31, 307–321 10.1016/j.yfrne.2010.04.001 (doi:10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- Korsten P., et al. 2010Association between DRD4 gene polymorphism and personality variation in great tits: a test across four wild populations. Mol. Ecol. 19, 832–843 10.1111/j.1365-294X.2009.04518.x (doi:10.1111/j.1365-294X.2009.04518.x) [DOI] [PubMed] [Google Scholar]
- Kravitz E. A.2000Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A 186, 221–238 10.1007/s003590050423 (doi:10.1007/s003590050423) [DOI] [PubMed] [Google Scholar]
- Kravitz E. A., Huber R.2003Aggression in invertebrates. Curr. Opin. Neurobiol. 13, 736–743 10.1016/j.conb.2003.10.003 (doi:10.1016/j.conb.2003.10.003) [DOI] [PubMed] [Google Scholar]
- Lesch K. P., Merschdorf U.2000Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav. Sci. Law 18, 581–604 (doi:10.1002/1099-0798(200010)18:5<581::AID-BSL411>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- Matsushima T., Kawamori A., Bem-Sojka T.2008Neuro-economics in chicks: foraging choices based on amount, delay and cost. Brain Res. Bull. 76, 245–252 10.1016/j.brainresbull.2008.02.007 (doi:10.1016/j.brainresbull.2008.02.007) [DOI] [PubMed] [Google Scholar]
- Miczek K. A., de Almeida R. M., Kravitz E. A., Rissman E. F., De Boer S. F., Raine A.2007Neurobiology of escalated aggression and violence. J. Neurosci. 27, 11 803–11 806 10.1523/JNEUROSCI.3500-07.2007 (doi:10.1523/JNEUROSCI.3500-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S., Body S., Ho M. Y., Bradshaw C. M., Szabadi E., Deakin J. F., Anderson I. M.2002Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 160, 290–298 10.1007/s00213-001-0983-0 (doi:10.1007/s00213-001-0983-0) [DOI] [PubMed] [Google Scholar]
- Munafo M. R., Yalcin B., Willis-Owen S. A., Flint J.2008Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol. Psychiatry 63, 197–206 10.1016/j.biopsych.2007.04.006 (doi:10.1016/j.biopsych.2007.04.006) [DOI] [PubMed] [Google Scholar]
- Overli O., Sorensen C., Pulman K. G., Pottinger T. G., Korzan W., Summers C. H., Nilsson G. E.2007Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 31, 396–412 10.1016/j.neubiorev.2006.10.006 (doi:10.1016/j.neubiorev.2006.10.006) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 82, 291–318 (PM:17437562) [DOI] [PubMed] [Google Scholar]
- Roberts D. C. S., Loh E. A., Baker G. B., Vickers G.1994Lesions of central serotonin systems affect responding on a progressive ratio schedule reinforced either by intravenous cocaine or by food. Pharmacol. Biochem. Behav. 49, 177–182 10.1016/0091-3057(94)90473-1 (doi:10.1016/0091-3057(94)90473-1) [DOI] [PubMed] [Google Scholar]
- Savitz J. B., Ramesar R. S.2004Genetic variants implicated in personality: a review of the more promising candidates. Am. J. Med. Genet. B Neuropsychiatr. Genet. 131B, 20–32 10.1002/ajmg.b.20155 (doi:10.1002/ajmg.b.20155) [DOI] [PubMed] [Google Scholar]
- Shapiro M. S., Siller S., Kacelnik A.2008Simultaneous and sequential choice as a function of reward delay and magnitude: normative, descriptive and process-based models tested in the European starling (Sturnus vulgaris). J. Exp. Psychol. Anim. Behav. Process. 34, 75–93 10.1037/0097-7403.34.1.75 (doi:10.1037/0097-7403.34.1.75) [DOI] [PubMed] [Google Scholar]
- Siever L. J.2008Neurobiology of aggression and violence. Am. J. Psychiatry 165, 429–442 10.1176/appi.ajp.2008.07111774 (doi:10.1176/appi.ajp.2008.07111774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A., Johnson J. C.2004Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Steimer T., Driscoll P.2005Inter-individual versus line/strain differences in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: neuroendocrine and behavioural aspects. Neurosci. Biobehav. Rev. 29, 99–112 10.1016/j.neubiorev.2004.07.002 (doi:10.1016/j.neubiorev.2004.07.002) [DOI] [PubMed] [Google Scholar]
- Suomi S. J.2006Risk, resilience, and gene × environment interactions in rhesus monkeys. Ann. N. Y. Acad. Sci. 1094, 52–62 10.1196/annals.1376.006 (doi:10.1196/annals.1376.006) [DOI] [PubMed] [Google Scholar]
- van Erp A. M., Miczek K. A.2000Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci. 20, 9320–9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overveld T., Matthysen E.2010Personality predicts spatial responses to food manipulations in free-ranging great tits (Parus major). Biol. Lett. 6, 187–190 10.1098/rsbl.2009.0764 (doi:10.1098/rsbl.2009.0764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reenen C. G., O'Connell N. E., Van der Werf J. T., Korte S. M., Hopster H., Jones R. B., Blokhuis H. J.2005Responses of calves to acute stress: individual consistency and relations between behavioral and physiological measures. Physiol. Behav. 85, 557–570 10.1016/j.physbeh.2005.06.015 (doi:10.1016/j.physbeh.2005.06.015) [DOI] [PubMed] [Google Scholar]
- Veenema A. H., Koolhaas J. M., de Kloet E. R.2004Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann. N. Y. Acad. Sci. 1018, 255–265 10.1196/annals.1296.030 (doi:10.1196/annals.1296.030) [DOI] [PubMed] [Google Scholar]
- Verbeek M. E. M., Drent P. J., Wiepkema P. R.1994Consistent individual differences in early exploratory behavior of male great tits. Anim. Behav. 48, 1113–1121 10.1006/anbe.1994.1344 (doi:10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- Wilson D. S., Clark A. B., Coleman K., Dearstyne T.1994Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446 10.1016/0169-5347(94)90134-1 (doi:10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- Winstanley C. A., Theobald D. E., Dalley J. W., Cardinal R. N., Robbins T. W.2006Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb. Cortex 16, 106–114 10.1093/cercor/bhi088 (doi:10.1093/cercor/bhi088) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J.2008Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier H., Baettig K., Driscoll P.1978Acquisition of DRL-20 behavior in male and female, Roman high- and low-avoidance rats. Physiol. Behav. 20, 791–793 10.1016/0031-9384(78)90307-4 (doi:10.1016/0031-9384(78)90307-4) [DOI] [PubMed] [Google Scholar]