Abstract
In this paper we review recent models that provide adaptive explanations for animal personalities: individual differences in behaviour (or suites of correlated behaviours) that are consistent over time or contexts. We start by briefly discussing patterns of variation in behaviour that have been documented in natural populations. In the main part of the paper we discuss models for personality differences that (i) explain animal personalities as adaptive behavioural responses to differences in state, (ii) investigate how feedbacks between state and behaviour can stabilize initial differences among individuals and (iii) provide adaptive explanations for animal personalities that are not based on state differences. Throughout, we focus on two basic questions. First, what is the basic conceptual idea underlying the model? Second, what are the key assumptions and predictions of the model? We conclude by discussing empirical features of personalities that have not yet been addressed by formal modelling. While this paper is primarily intended to guide empiricists through current adaptive theory, thereby stimulating empirical tests of these models, we hope it also inspires theoreticians to address aspects of personalities that have received little attention up to now.
Keywords: adaptive individual variation, behavioural syndromes, personality, evolution, model, theory
1. Introduction
Individuals within single populations often differ consistently in their behavioural tendencies across time and contexts (Wilson 1998; Sih et al. 2004a,b; Réale et al. 2007). Male great tits (Parus major), for example, differ consistently in whole suites of correlated traits, with more aggressive individuals also tending to be more explorative towards novel objects and unfamiliar environments than less aggressive ones (Verbeek et al. 1996). Over the last few years, the notion that such personality types, or behavioural syndromes, exist in a wide range of animal species has stimulated empirical research on the proximate and ultimate factors shaping such variation (Dingemanse & Réale 2005; Sih & Bell 2008; Stamps & Groothuis 2010a,b). At the same time, researchers have started to develop conceptual frameworks for understanding the basic phenomena associated with animal personalities (Wilson 1998; Dall et al. 2004; Sih et al. 2004a,b; Réale et al. 2007; Sih & Bell 2008; Wolf & Weissing 2010; Dingemanse et al. 2010b). In parallel, various theoretical models have been developed to explain and predict particular aspects of animal personalities—these recent models are the focus of this paper.
We start by briefly outlining patterns of individual differences in behaviour that require explanation (§2). We then review formal (and some verbal) models for adaptive personality differences (§3), where we focus on two main questions. First, what is the basic conceptual idea underlying the model? Second, what are the key assumptions and predictions of the model? We conclude by discussing features of consistent individual differences that have not yet been addressed by models (§4). The aim of this review is to guide empiricists through recent models for adaptive animal personalities and stimulate tests of the assumptions and predictions of these models.
2. Patterns of individual variation requiring explanation
The empirical literature on animal personality has reported three types of behavioural patterns that require adaptive explanation in the context of animal personality variation (Dall et al. 2004; Sih et al. 2004b; Dingemanse et al. 2010b). First, consistent individual differences exist in single behaviours. Second, consistent individual differences exist in suites of functionally distinct behaviours. Third, consistent individual differences exist in behavioural plasticity (also called responsiveness). In all cases, consistency refers to both stability over time (in terms of date or age) and/or contexts (environmental gradients, e.g. predation risk).
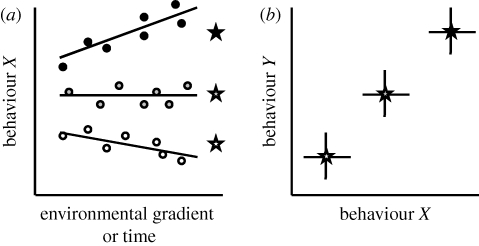
Patterns consistent with consistent individual variation in a single behaviour are illustrated in figure 1a, which depicts behavioural phenotypes over time (or alternatively an environmental gradient) for each of three individuals (black, grey and white), where lines depict their reaction norms (sensu Sarkar 1999). The key feature here is that the rank order differences between individuals are maintained over time or contexts (Sih et al. 2004a,b; Bell 2007).
Figure 1.
Three types of consistent individual variation in behaviour. Panel (a) illustrates the presence of consistent individual variation in a single behaviour using a reaction norm plot. Dots represent phenotypic values measured for each of three individuals (black, grey, white) along a contextual gradient (which could be time); lines depict the reaction norm of each individual, and stars give their average phenotype. Individuals differ consistently in average level of behaviour because rank order differences are maintained over an environmental gradient (which could be time in terms of age or date). Individual variation in plasticity is also depicted (as individuals differ in reaction norm slope). Panel (b) illustrates the presence of consistent individual variation in suites of behavioural traits, because individual means (stars) are correlated across behaviours (X and Y), where vertical and horizontal lines acknowledge the presence of within-individual variation (due to plasticity or measurement error).
In many species, individuals differ consistently not only in single behaviours but also these differences involve whole suites of behaviours (van Oers et al. 2005; Bell 2007; Réale et al. 2007), resulting in correlations across functionally distinct behaviours at the population level (figure 1b). In many populations of three-spined stickleback (Gasterosteus aculeatus), for example, activity, aggressiveness, exploratory behaviour and boldness are positively correlated across individuals (Huntingford 1976; Bell 2005; Dingemanse et al. 2007; Brydges et al. 2008). Note that we refer here to correlations between an individual's behavioural mean (illustrated with a star in figure 1a) across two or more behavioural traits (figure 1b), i.e. the between-individual (as opposed to within-individual) correlations (Dingemanse et al. 2010b).
Individuals differ not only in their average behaviour but also in their level of behavioural plasticity (responsiveness) (Boyce & Ellis 2005; Nussey et al. 2007; Smiseth et al. 2008; but see Martin & Réale 2008; Dingemanse et al. 2010b). This phenomenon is also illustrated in figure 1a, which depicts a scenario where individuals differ both in their behavioural mean and in their behavioural plasticity (e.g. black circle individuals are more responsive than grey circle individuals). In laboratory rodents, for example, certain individuals adjust their aggressiveness with social context, whereas others do not (Koolhaas et al. 1999; e.g. Fuller et al. 2005). Moreover, it has recently been suggested that individual variation in plasticity (also referred to as ‘behavioural flexibility’; Coppens et al. 2010) might be correlated across traits, i.e. certain individuals might be consistently more plastic in a variety of functionally distinct behaviours when compared with others (Boyce & Ellis 2005; Sih & Bell 2008), resulting in plasticity syndromes. There is further evidence suggesting that individual variation in plasticity may also covary with mean levels of behaviour (Dingemanse et al. 2010b) (as in figure 1a). In Ural Owls (Strix uralensis), for example, mothers that are on average aggressive in nest defence against humans show greater plasticity in aggressiveness when compared with mothers that are less aggressive (Kontiainen et al. 2009).
3. Models for adaptive personality differences
Here we review formal modelling studies that have explicitly addressed adaptive personality differences, though we have also included two studies (Stamps 2007; Biro & Stamps 2008) that were not based on formal models. Following the classification developed by Wolf & Weissing (2010), these studies were categorized into three non-exclusive types: (i) models that investigate how differences in state give rise to consistent individual differences in (suites of correlated) behaviours, (ii) models that investigate how feedbacks between state and behaviour can stabilize initial differences among individuals over time and (iii) models based on alternative patterns of explanation (i.e. those not based on variation in state).
(a). Models based on differences in state
State-dependent personality models are centred around the idea that individuals differ in state, where state can be defined broadly as those features of an organism (e.g. morphological, physiological, neurobiological or environmental) that affect the balance between the costs and benefits of its behavioural actions (Houston & McNamara 1999). For a full discussion of state variables in the context of animal personalities see Wolf & Weissing (2010). Consistent differences in state in combination with state-dependent behaviour potentially provide a powerful explanation for adaptive behavioural differences in suites of correlated behaviours, because (i) variation in state can give rise to state-dependent behaviour (condition-dependent behaviour or individual plasticity) and (ii) single states often simultaneously affect behaviour in multiple contexts. This idea underlies several models that investigated how adaptive personality differences can result from individual differences in states (table 1), such as energy reserves (Rands et al. 2003; Luttbeg & Sih 2010), body size (McElreath & Strimling 2006), residual reproductive value (RRV, Wolf et al. 2007a), productivity (Stamps 2007; Biro & Stamps 2008), metabolic rate (Houston 2010) or fighting ability (Botero et al. in press). For example, Wolf et al. (2007a) demonstrated that natural selection should favour individuals possessing low RRV (also termed assets) to act consistently more boldly and aggressively compared to individuals with high RRV, and Stamps (2007) argued that individuals with relatively high growth rates should differ in suites of ‘risky’ behaviours (those behaviours contributing to the trade-off between growth and mortality) compared to individuals with comparatively low growth rates. Table 1 summarizes the basic assumptions and predictions for each of these models.
Table 1.
Models that investigate how individual differences in state can give rise to adaptive individual differences in behaviour.
| state difference | behavioural context and predicted behavioural differences | basic assumptions of model | origin and stability of state differences | reference |
|---|---|---|---|---|
| energy reserves | context: pair of foragers with repeated choice between remaining in a safe refuge and emerging to forage under the risk of predation. | A. foraging in a pair is advantageous (resulting in decreased predation risk or increased energetic gain). |
origin: stochastic initial differences. stability: short-term stability due to positive feedback between foraging behaviour and energy reserves. |
Rands et al. (2003) |
| predictions: the individual with lower energy reserves is more willing to take risks (i.e. emerges from refuge first and returns last). | ||||
| body sizea | context: foraging context. Individuals can assess environment for imperfect cue that a predator is present. After observing cue, individuals can either forage or run away. predictions: three behavioural types that exhibit cross-context correlations: large individuals always forage, intermediate individuals are responsiveb, small individuals never forage. | A1. individuals have imperfect information about whether or not predators are present |
origin: not addressedc. stability: not addressed. |
McElreath et al. (2006) |
| A2. body size affects the chance of being eaten by predator: larger individuals are less likely to be eaten. | ||||
| RRVd | context: risky choices, i.e. behaviours that put the animal's life in danger. | A. fecundity benefits and mortality risk associated with risky choices are identical for individuals with different RRV. | origin: frequency-dependent selectionc. | Wolf et al. (2007a) |
| predictions: individuals with higher RRV are less willing to take risk than individuals with lower RRV. These differences correlate across risky contexts (in the model: ‘aggression’ and ‘boldness’ context). | stability: stable over timee. | |||
| productivityf,g | context: behaviours related to the acquisition of food resources that increase both productivity and mortality. | A. a trade-off exists between productivity and survival: higher productivity is associated with increased mortality. |
origin: life-history trade-offsh. stability: stable since deviations from initial productivity path are costly to the individual. |
Stamps (2007); Biro & Stamps (2008) |
| predictions: productive individuals are more willing to take actions that increase productivity at the cost of increased mortality than individuals with low productivity. These differences are correlated across behaviours that increase both productivity and mortality (e.g. foraging under predation risk, aggressive defence of feeding territories). | ||||
| metabolic rate | context: foraging behaviour that influences intake rate and mortality. | A. a trade-off exists between energy intake and predation risk: high foraging intensity results in a high intake rate but also a high rate of mortality. | origin: coevolution of metabolic rate and foraging intensity. | Houston (2010) |
| predictions: in certain situations, different combinations of foraging intensity and metabolic rate can have equal fitness. | stability: stable over time. | |||
| fighting ability | context: agonistic interactions where individuals can, before choosing whether to attack or not, signal their fighting ability to rivals. | A1. individuals have imperfect information about their own fighting ability. A2. costs of signals increase with signal intensity but decrease with fighting ability. A3. genetic mechanism that allows for correlation of sender and receiver behaviour. |
origin: stochastic differencesc. stability: stable over timei. |
Botero et al. (in press) |
| predictions: coexistence of behavioural types that differ both in their communication strategies and aggressiveness (i.e. for the same fighting ability, behavioural types show different signalling behaviour and different levels of aggression). |
aAuthors note that identical results may be obtained for differences in skill, energy reserves, experience and immune condition, and discuss an application of their model to differences in awareness or ability to process cues.
bResponsive individuals forage when cue is absent and run away when cue is present.
cThe origin of the state differences is not important for the predictions of this model, i.e. state differences could arise either stochastically or owing to natural selection (see Wolf & Weissing 2010).
dResidual reproductive value. Terms in the literature that are used synonymously: future fitness expectation, assets.
eFollow-up work showed that stability depends on whether or not feedbacks between state and behaviour are present and whether these feedbacks act to increase (van Doorn et al. 2009) or decrease (Luttbeg & Sih 2010) initial differences in RRV; for a brief discussion of this issue see McElreath et al. (2007) and Wolf et al. (2007b).
fProductivity refers to either growth rate or rate of offspring production (Biro & Stamps 2008).
gThe predictions of this work are not based on formal models but on verbal arguments.
hMangel & Stamps (2001) show how trade-offs between growth and mortality can lead to the maintenance of individual variation in growth rates through nearly equal fitness for individuals growing at different rates.
iEach individual has a behavioural reaction norm that is stable over time by assumption.
Explaining personality variation via differences in state leaves us with two basic problems (Wolf & Weissing 2010): first, why should there be variation in state in the first place, i.e. what is the origin of state differences? Second, why should state differences among individuals be stable over time? Some of the models in table 1 do not address the origin of state differences explicitly (McElreath & Strimling 2006; Botero et al. in press), while others use stochastic effects acting on states (Rands et al. 2003), frequency-dependent selection (Wolf et al. 2007a) or spatio-temporal forms of selection (Stamps 2007; Biro & Stamps 2008) to explain state differences among individuals (table 1); we believe that future models should explicitly discuss the mechanism maintaining the variation in states that are investigated. We discuss the question of consistency of state differences in §3b.
To work out whether a state-dependent model explains behavioural variation associated with personalities in real animals, it would be useful to design experimental studies to test model predictions and assumptions (tables 1 and 2). To the extent that a model deals with behavioural time, a straightforward experimental test would be to manipulate the state variable of interest and investigate whether this manipulation results in the predicted behavioural change (table 1). For instance, based on the verbal model of Stamps (2007), we would predict that food restrictions resulting in decreased growth rates should affect the expression of any behaviour that positively affects growth at the cost of survival—but not other types of behaviour (table 1). Similarly, manipulation of RRV should affect the willingness of individuals to take risky actions (i.e. actions that increase fecundity at the cost of mortality), as predicted by the model of Wolf et al. (2007a) (table 1). Importantly, we note that none of the models listed in table 1 explicitly predicts whether the link between state and behaviour should be underpinned by phenotypic plasticity (i.e. within genotypes or individuals, environmentally induced changes in state produce changes in behaviour) or by a genetic correlation between state and behaviour (at the population level). Plasticity can involve either an early environmental influence producing individual differences in state with long-lasting effects on behaviour (cf. permanent environment effects), or ongoing and often reversible fluctuations in state over an animal's lifetime. Given that most behavioural studies operate at the latter level, it should be emphasized that if an experimental manipulation of state fails to result in the predicted behavioural change(s), the possibility that a state-behaviour link involves either developmental plasticity or a genetic correlation should be investigated before concluding that the assumptions or predictions of the model were not supported.
Table 2.
Models that investigate the mutual feedback between state and behaviour.
| state | behaviour | feedback and its basic assumptions | references |
|---|---|---|---|
| energy reserves | willingness to emerge from refuge and forage under predation risk | feedback: positive feedback stabilizes initial differences for pair of foragers: the individual with lower energy reserves is consistently more willing to take risks (i.e. emerge from refuge first and return last). | Rands et al. (2003) |
| basic assumptions: foraging in a pair is advantageous (resulting in decreased predation risk or increased energetic gain). | |||
| experience with responsive behaviour | responsiveness to environmental stimuli | feedback: positive feedback stabilizes initial behavioural differences. Individuals differ consistently in their level of responsiveness. | Wolf et al. (2008) |
| basic assumptions: individuals who were responsive in the past face lower cost (or higher benefits) of being responsive again. | |||
| RRVa | willingness to take risksb | feedback: positive feedback stabilizes initial differences in state and gives rise to consistency in risk-taking behaviour. | van Doorn et al. (2009); Luttbeg & Sih (2010) |
| basic assumptions: trade-off between the immediacy of benefits associated with risky actions and their risk (e.g. risky actions increase current fecundity while less risky actions increase future fecundity) (van Doorn et al. 2009). | |||
| feedback: negative feedback erodes initial differences. | |||
| basic assumptions: fecundity benefits associated with risky choices accumulate over time such that risk-taking individuals accumulate assets (Luttbeg & Sih 2010). | |||
| size, energy reserves, condition, vigour | boldness in a foraging context | feedback: positive feedback stabilizes initial differences. | Luttbeg & Sih (2010) |
| basic assumptions: individuals with higher state face lower risk of predation while being bold and bold individuals increase their state relative to less bold individuals. |
aResidual reproductive value. Terms in the literature that are used synonymously: future fitness expectation, assets.
bActions that put the animal's life in danger.
In many study systems, experimental approaches might not be feasible. In such cases, initial support for state-dependent models would have to come from studies that investigate the direction, sign and strength of covariation between state variables and behavioural traits within a population. For example, Wilson et al. (2010) found—as predicted by Wolf et al. (2007a)—that (a proxy for) the RRV of individuals was positively correlated with their boldness. Where covariation is found, such studies may provide insight into how the link between state and behaviour was encoded (see above), if repeated measures of state and behaviour can be obtained for individuals within a given population. In this case, intra-individual correlations would imply links encoded at the phenotypic level alone, whereas an inter-individual correlation in the absence of intra-individual correlations might represent a genetically encoded link between state and behaviour. Quantitative genetic approaches can then be used to explore the extent to which the observed phenotypic correlation at the population level is due to underlying genetic versus permanent environmental correlations.
State-dependent personality variation can also be studied by assessing whether the amount of interindividual variation in behaviour can be predicted based on the amount of interindividual variation in state. For example, the extent of individual differentiation in those behaviours that contribute to growth-survival trade-offs in the model of Stamps (2007) should disappear in life-history phases where there is no individual variation in growth rates.
We conclude this section by stressing that, to date, few empirical studies exist that have explicitly tested predictions derived from state-dependent personality models (but see Biro & Stamps 2008; Smith & Blumstein 2008; Harcourt et al. 2009; Kobler et al. 2009; Réale et al. 2009; Wilson et al. 2010). Such studies are now needed since a dynamic interaction between theoretical and empirical results provides the key to furthering our understanding of animal personalities.
(b). Models investigating the feedback between state and behaviour
State variables differ in their stability: some states are inherently stable, either because they are very costly (e.g. time-consuming) or even impossible to change, other state variables are much more labile (Wolf & Weissing 2010). Interestingly, several of the reviewed models (table 1) use apparently labile states to explain consistent differences in behaviour (e.g. energy reserves, fighting ability). In order for this type of state difference to provide a good explanation for animal personalities, we thus need an explanation for why the differences between individuals in labile states should be stable over time.
Feedback mechanisms between labile states and behaviour might provide such an explanation (Sih & Bell 2008; Luttbeg & Sih 2010; Wolf & Weissing 2010): the state of an individual affects its optimal behaviour, which in turn might feedback on its state. The more experience (state) an individual has with a certain behavioural pattern, for example, the more advantageous it might be to exhibit this pattern, which in turn increases the experience with this behaviour. Such positive feedbacks between state and behaviour act to stabilize any initial differences in labile states (e.g. individuals with more experience get even more experienced over time). The idea of positive feedbacks is attractive because even minor initial differences in either state or behaviour can be amplified and stabilized through such feedback. Positive feedbacks thus provide a potentially powerful explanatory framework for animal personalities associated with labile state differences.
It should be stressed that feedbacks between state and behaviour need not always reinforce initial differences: state differences might give rise to behavioural differences, which in turn act to decrease initial differences in state. Individuals with low reserves (state), for example, might show a high foraging intensity to avoid starvation and thus increase their reserves. Such negative feedbacks tend to erode initial differences in state.
Several models have addressed the dynamic feedback between labile state and behaviour (table 2), and we here detail the four feedback mechanisms that have been investigated upto now.
(i). Feedback between energy reserves and foraging behaviour under predation risk
Individuals with low energy reserves are expected to be bolder in a foraging context than those with higher reserves because they have to avoid the risk of starvation. This relation between energy reserves and boldness involves a negative feedback that erodes initial differences in state (and thus behaviour): individuals with low reserves are bolder than individuals with high reserves; they consequently acquire more reserves, thus leading to the convergence of states and therefore behaviour among individuals over time (Luttbeg & Sih 2010).
However, this need not always be the case. Rands et al. 2003 (see Rands et al. 2008 for an extended analysis) investigated a scenario where a pair of foragers is repeatedly confronted with the choice between remaining in a safe refuge and emerging to forage under the risk of predation. The authors considered the situation where the two individuals differed initially in energy reserves and showed that—provided that foraging in a pair is advantagous (table 2)—such differences can be stabilized by the feedback between energy reserve and foraging behaviour. The individual with lower energy reserves is consistently willing to take on greater risk (i.e. emerges from refuge first and returns last), yet it will remain in poorer energetic condition. In such a situation, differences in energy reserves and risk-taking behaviour will thus be stable for at least some period of time.
(ii). Feedback between performance and experience
Individuals often perform better with increased experience (Rosenzweig & Bennett 1996; Brown & Laland 2003), and processes like learning, training and skill formation often increase the abilities and success of behavioural patterns when repeated. It is relatively easy to envisage that such positive feedbacks between behaviour and experience with that behaviour gives rise to stable behavioural differences among individuals: small initial differences in behaviour give rise to differences in experience with the behaviour, which act to reinforce initial behavioural differences.
While these verbal ideas are well known, they have rarely been investigated in formal models. One recent example is provided by Wolf et al. (2008), who focussed on a scenario where individuals could repeatedly choose between a responsive and an unresponsive behavioural tactic (frequency-dependent selection maintained both tactics in the population). In the absence of feedbacks (i.e. where individuals do not perform better with increasing experience), individuals are identical at the evolutionary equilibrium and play the same mixed strategy that randomizes between the two behavioural alternatives. However, whenever experience with one of the tactics decreases the costs (or increases the benefits) of employing this tactic again (i.e. individuals perform better with increasing experience), stable behavioural differences among individuals evolve. While this analysis was performed in the context of responsiveness, it should apply to any choice situation where (i) individuals repeatedly have a choice between two behavioural actions (e.g. hawk versus dove, cooperate versus defect, produce versus scrounge), (ii) the two actions are maintained by frequency-dependent selection in the population and (iii) positive feedback is present.
(iii). Feedback between RRV and risk-taking behaviour
The asset-protection principle (Houston & McNamara 1989; Clark 1994) provides a link between the RRV (future fitness expectations, assets) of an individual and its risk-taking behaviour: individuals with low assets have little to lose and should therefore be more willing to take risky actions (i.e. actions that increase their fecundity at the cost of increased mortality) than individuals possessing high assets. Wolf et al. (2007a) showed that this principle can explain consistent individual differences in suites of risky traits. We should stress that risk-taking here refers to behaviours that put the life of an individual in danger, and not to behaviours that are associated with high variance in outcomes.
At first sight it may seem that asset protection involves a negative feedback that erodes differences in assets over time (McElreath et al. 2007; Sih & Bell 2008; Luttbeg & Sih 2010). Individuals with low assets are bolder than individuals with high assets. Consequently, the former acquire more resources than the latter, giving rise to the convergence of assets and thus behaviour over time. Asset protection, however, is not always associated with negative feedbacks (Wolf et al. 2007b; van Doorn et al. 2009). We provide three examples. First, the benefits of everyday risky behaviour may often be small relative to the underlying individual differences in fitness expectations. In such cases, initial differences in assets will not be eroded over time and asset protection predicts stable behavioural differences (Wolf et al. 2007a,b). Second, the benefits associated with risky actions may not directly increase the assets of the acting individual but that of kin members (e.g. risky parental care, risky foraging in cooperative breeding animals; Cant & Field 2001). In such a situation, individuals do not experience a direct increase in RRV when taking a risky action. Consequently, differences in risk-taking behaviour do not affect the underlying differences in assets, and behavioural differences are predicted to be stable over time. Third, whenever there is a trade-off between the immediacy of benefits associated with risky actions and their risk (e.g. risky actions increase current fecundity, while less risky actions increase future fecundity) assets and risk-taking behaviour are coupled by a positive feedback (van Doorn et al. 2009): behavioural differences among individuals are predicted to be stable over time and even emerge in cases where differences in fitness expectations are initially absent.
(iv). Feedback associated with state-dependent safety
Luttbeg & Sih (2010) considered state variables (e.g. size, energy reserves, condition and vigour) that are characterized by the following two features: (i) individuals with a higher state face a lower predation risk (e.g. are better at fleeing or defending themselves) and (ii) bold individuals increase their state relative to shy individuals. Because high-state individuals face a lower mortality risk when compared with low-state individuals, the former should be bolder under predation risk than the latter. This gives rise to a positive feedback since, by being bold, high-state individuals increase their state even more, relative to low-state conspecifics. Initial individual differences in state are thus predicted to increase over time thereby giving rise to stable behavioural differences in boldness.
(c). Models that are not based on state differences
Up to now our discussion focussed on models that are based on state differences. However, adaptive personality differences need not always reflect state differences among individuals (Wolf & Weissing 2010). We here detail three types of model that have investigated the emergence of stable individual differences in the absence of state differences.
(i). Adaptive variation in responsiveness by frequency-dependent selection
Wolf et al. (2008) developed a model to investigate how spatial and temporal variation in the environment can give rise to adaptive individual differences in the responsiveness to environmental stimuli. In this model individuals have a choice between two behavioural options (e.g. a risky versus safe patch). The payoffs associated with these options depend on the current state of the environment, which is changing over time or space. Individuals can be either responsive or unresponsive. Responsive individuals sample their environment for cues about its current state and can therefore show behavioural plasticity, i.e. their behaviour is fine-tuned to the current environmental conditions. In contrast, unresponsive individuals (which do not pay the cost of sampling) do not take such cues into account and exhibit a non-plastic behaviour that is good on average. The authors showed that this basic set-up gives rise to frequency-dependent selection on responsiveness. In short, responsive individuals can exploit environmental opportunities (e.g. switch to the more profitable patch). The benefits that are associated with these opportunities, however, will often decrease with the frequency of individuals that exploit these opportunities (e.g. via density-dependent competition for resources) and thus with the frequency of responsive individuals in the population. The benefits of responsiveness are thus negatively frequency-dependent, which promotes the coexistence of responsive and unresponsive individuals. Interestingly, this modelling framework has recently been applied to explain individual variation in responsiveness in a natural population of pike (Esox lucius) (Kobler et al. 2009).
(ii). Variation, responsiveness and adaptive personality differences
Natural populations typically harbour substantial amounts of variation (e.g. due to mutations). A series of recent models have shown that the amount of variation present in a population may have substantial effects on the expected outcome of evolution (Johnstone 2001; McNamara et al. 2004, 2008, 2009; Wolf et al. in press). The basic idea is that whenever variation in social contexts is present, responsive (socially aware, eavesdropping) strategies that make their behaviour dependent on certain features of their social partners (e.g. physical features, reputation or behavioural history) may be favoured. The presence of responsive individuals, in turn, often drastically changes the selection pressures for the monitored traits.
This coevolutionary process between responsive strategies and the strategies that are monitored, triggered through some initial variation in the monitored trait, can also give rise to animal personalities. McNamara et al. (2009) provide an example in which individuals interact with each other in a trust game. In the absence of variation in trustworthiness, costly sampling (i.e. information acquisition about other individuals) is not beneficial. Whenever there is sufficient variation in trustworthiness, however, samplers are favoured. The presence of samplers, in turn, induces disruptive selection on trustworthiness which gives rise to the coexistence of trustworthy and untrustworthy individuals.
Dall et al. (2004) verbally discuss a related scenario. They consider aggressive interactions in a hawk–dove game. In the absence of variation in aggressiveness (i.e. the probability of playing hawk), all individuals should evolve to the same mixed strategy that randomizes between the two behavioural actions (hawk and dove). Whenever sufficient variation among individuals is present, responsive (here: eavesdropping) strategies should be favoured. The presence of responsive strategies, in turn, should favour behaviourally consistent individuals (i.e. individuals that either always play hawk or always play dove). Wolf et al. (in press) extended these arguments formally and showed that (i) these processes indeed give rise to polymorphic populations in which individuals are either always responsive, hawks or doves, and (ii) more generally, these results apply to all scenarios that can be represented as matrix games with two pure strategies (e.g. hawk–dove or snowdrift games).
(iii). Signalling, communication and adaptive personality differences
Botero et al. (in press) developed a model to investigate the joint evolution of signal emission and signal interpretation in the context of aggressive interactions. Individuals differing in quality (fighting ability) can express an ornament potentially reflecting this quality. The relationship between ornament size and quality is a heritable reaction norm (a sender code) that can evolve under the influence of mutation and selection. An individual's fighting strategy is determined by an evolvable receiver code, which specifies the probability of attack in a hawk–dove game as a function of the individual's own quality and the opponent's ornament size. Interestingly, the authors find that in the presence of errors in signal production, a single signalling type (i.e. a combination of a sender strategy and interpretation strategy) did not evolve but, depending upon the magnitude of error in signal production, evolution would favour the stable coexistence of two, three or four signalling types. In other words, the evolved populations were polymorphic and individuals differed systematically in the way they sent signals and interpreted the signals sent by others. These differences in signalling strategy, in turn, gave rise to consistent individual differences in behaviour. For moderate levels of error in signal production, for example, the model predicts the emergence and coexistence of two distinct types: aggressive individuals that have a high probability of producing a large ornament and a tendency to attack, and conservative individuals that produce smaller ornaments and have a lower tendency to attack during fights.
4. Discussion
How successful have theoreticians been in explaining and predicting patterns of individual differences in behaviour observed in natural populations? And what is still to be done? In our view, the current set of adaptive models should be regarded as a first step—much remains to be done.
(a). More states
Our review illustrates that the majority of current explanations for animal personalities are based on state differences among individuals. Several state variables have been investigated (table 1). Many others, however, which might be equally or even more important for understanding animal personalities have received little or no attention from theoreticians up to now. There is, for example, accumulating evidence that behavioural differences are often associated with physiological differences, such as metabolic rate (Careau et al. 2008; Millidine et al. 2009) and stress responsiveness (Koolhaas et al. 1999; Schjolden & Winberg 2007), differences in brain structure (Reddon & Hurd 2009) and cognitive mechanisms such as learning ability (Kotrschal & Taborsky 2010). At present, little is known about how natural selection shapes variation in such states and how such variation might be associated with personalities (but see Houston 2010).
(b). More traits
The majority of models published to date have focussed on a small number of behavioural traits like aggressiveness and boldness. In fact, all models except those by McNamara et al. (2008, 2009) and Wolf et al. (2008) address aspects of the aggressiveness–boldness syndrome. While this syndrome appears to be widespread, several other axes of behavioural variation are present in both humans and animals (Pervin & John 1999; Gosling 2001). Important examples include variation in cooperativeness (Schürch et al. 2010), responsiveness (Dingemanse et al. 2010b), diet specialization (Bolnick 2004), parental care (Smiseth et al. 2008) and sexual promiscuity (Schuett et al. 2010). Those axes have received very little attention by theoreticians up to now.
(c). More ecology
Empiricists are now starting to understand how key ecological variables (such as habitat stability, predation regime) are related to the presence (or absence) and structure of animal personalities in natural populations (Bell & Sih 2007; Dingemanse et al. 2007; Brydges et al. 2008; Sinn et al. 2010). In contrast, very few models discussed above have provided explanations for spatial or temporal variation in personality structure. One interesting exception is the recent model by Luttbeg & Sih (2010), who aimed to reveal the ecological conditions (dis)favouring animal personality variation, providing a set of predictions regarding when personalities should versus should not be expected to evolve. A pattern in need of explanation, for example, is that environments with higher predation risk appear to favour tighter associations between boldness and aggressiveness when compared to those with lower predation risk (Bell & Sih 2007; Dingemanse et al. 2007). One might also investigate systematically which features of environments favour adaptive diversification in states—Barber and Dingemanse (2010) use spatial variation in the presence and diversity of parasites as a worked example. It should be noted, however, that empiricists have only recently started to employ sophisticated statistical techniques that enable detailed insight into how animal personality might differ between populations, years or habitats (Dochtermann & Jenkins 2007; Dingemanse et al. 2010a), and details of how populations might differ in personality structure therefore largely remain to be unravelled. Nevertheless, we believe that a more systematic investigation of how key ecological conditions affect the presence and the structure of personalities would constitute a necessary next step in understanding animal personalities.
(d). When heritable?
Animal personality traits can be underpinned by heritable variation (Penke et al. 2007; Réale et al. 2007; van Oers & Mueller 2010), result from environmental factors (Quinn et al. 2009; Oosten et al. 2010) or be shaped by interactions between genes and environments (Carere et al. 2005; van Oers et al. 2005; Dingemanse et al. 2009). Most of the models we discussed above do not make specific predictions about the extent to which personalities are shaped by either of these factors, including whether links between state and behaviour are caused by phenotypic plasticity or genetic correlations (§3a), and we believe that future research should address this issue more explicitly. Furthermore, data from real animals show that behavioural variation is repeatable only over short time spans in certain species (e.g. Sih et al. 2003; Bell & Stamps 2004) but over long time spans in others (e.g. Réale et al. 2000; Dingemanse et al. 2002). Therefore, it would appear useful for theoreticians to develop adaptive models to address the conditions favouring short- versus long-term consistency, and specify the timescale of their analysis.
(e). Why variation in plasticity?
Behavioural ecologists have recently discovered that certain classes of individuals are behaviourally more consistent than others, and that such individual variation in consistency is caused by individual differences in their behavioural plasticity (Smiseth et al. 2008; Coppens et al. 2010; Dingemanse et al. 2010b; Réale & Dingemanse 2010; Stamps & Groothuis 2010a; figure 1a), e.g. aggressive types are less plastic than non-aggressive ones (Koolhaas et al. 1999). Similar patterns have been observed in humans (Boyce & Ellis 2005; Ellis et al. 2006). However, few models have yet addressed individual differences in behavioural plasticity (but see Wolf et al. 2008; Botero et al. in press). It has been argued recently that because individuals can differ in both their average level of behaviour and behavioural plasticity, it would be useful to apply reaction norm approaches to the study of behaviour (Smiseth et al. 2008; Dingemanse et al. 2010b; Nettle & Penke 2010; Stamps & Groothuis 2010a,b). This approach considers that behavioural variation comes about because individuals can differ both in their average level of behaviour (the elevation of a reaction norm) and level of behavioural plasticity (the slope of a reaction norm). Such an approach essentially treats differences between individuals in their behavioural consistency as potentially meaningful (Dingemanse et al. 2010b), and might ultimately enable us to better understand links between personality and plasticity within a single adaptive framework (see Botero et al. (in press) for a model on adaptive personality variation using a reaction norm approach).
(f). More testing
We think that the time has come for empiricists to start testing the assumptions and predictions derived from adaptive models presented in the literature more explicitly (tables 1 and 2). Such feedbacks between empirical and theoretical approaches are now well on its way and will undoubtedly deepen our understanding of personality variation, exemplified by empirical tests of Rands et al. (2003, 2008) models concerning leaders and followers (Harcourt et al. 2009), Wolf et al.'s (2007a,b) model on risk-taking behaviour (Réale et al. 2009; Wilson et al. 2010), Stamps' (2007) model concerning relationships between growth and risky behaviours (Biro & Stamps 2008) and Wolf et al.'s (2008) model on individual differences in responsiveness (Kobler et al. 2009).
5. Conclusions
In this paper, we have reviewed recent models for adaptive personality differences in order to guide empiricists through current adaptive theory. Throughout we have focused on the basic conceptual ideas underlying these models, and their key assumptions and predictions. We argue that empiricists should now start designing studies targeted at testing the assumptions and predictions of existing models. At the same time, there is a need for new theoretical models explaining (i) variation in personality axes other than the aggressiveness–boldness syndrome, (ii) the links between ecological factors (like predation risk) and the presence and structure of personality variation, (iii) individual differences in behavioural plasticity, (iv) heritable versus environmentally determined personality variation and (v) conditions favouring personality variation not associated with variation in states.
Acknowledgements
We would like to thank Peter Bednekoff, Anahita Kazem, Denis Réale and Jonathan Wright for constructive comments on the ideas presented in this paper. This work was supported by the Max Planck Society (M.P.G.).
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Barber I., Dingemanse N. J.2010Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088 10.1098/rstb.2010.0182 (doi:10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M.2005Behavioral differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M.2007Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761 10.1098/rspb.2006.0199 (doi:10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M., Sih A.2007Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Stamps J. A.2004Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Anim. Behav. 68, 1339–1348 10.1016/j.anbehav.2004.05.007 (doi:10.1016/j.anbehav.2004.05.007) [DOI] [Google Scholar]
- Biro P. A., Stamps J. A.2008Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Bolnick D. I.2004Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution 58, 608–618 10.1111/j.0014-3820.2004.tb01683.x (doi:10.1111/j.0014-3820.2004.tb01683.x) [DOI] [PubMed] [Google Scholar]
- Botero C. A., Pen I., Komdeur J., Weissing F. J.In press The evolution of individual variation in signalling strategies. Evolution. [DOI] [PubMed] [Google Scholar]
- Boyce W. T., Ellis B. J.2005Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 17, 271–301 10.1017/S0954579405050145 (doi:10.1017/S0954579405050145) [DOI] [PubMed] [Google Scholar]
- Brown C., Laland K. N.2003Social learning in fishes: a review. Fish. Fish. 4, 280–288 10.1046/j.1467-2979.2003.00122.x (doi:10.1046/j.1467-2979.2003.00122.x) [DOI] [Google Scholar]
- Brydges N. M., Colegrave N., Heathcote R. J. P., Braithwaite V. A.2008Habitat stability and predation pressure affect temperament behaviours in populations of three-spined sticklebacks. J. Anim. Ecol. 77, 229–235 10.1111/j.1365-2656.2007.01343.x (doi:10.1111/j.1365-2656.2007.01343.x) [DOI] [PubMed] [Google Scholar]
- Cant M. A., Field J.2001Helping effort and future fitness in cooperative animal societies. Proc. R. Soc. Lond. B 268, 1959–1964 10.1098/rspb.2001.1754 (doi:10.1098/rspb.2001.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Réale D.2008Energy metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Carere C., Drent P. J., Koolhaas J. M., Groothuis T. G. G.2005Epigenetic effects on personality traits: early food provisioning and sibling competition. Behaviour 142, 1335–1361 10.1163/156853905774539328 (doi:10.1163/156853905774539328) [DOI] [Google Scholar]
- Clark C. W.1994Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159–170 10.1093/beheco/5.2.159 (doi:10.1093/beheco/5.2.159) [DOI] [Google Scholar]
- Coppens C. M., de Boer S. F., Koolhaas J. M.2010Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028 10.1098/rstb.2010.0217 (doi:10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- Dingemanse N. J., Réale D.2005Natural selection and animal personality. Behaviour 142, 1165–1190 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., van Oers K., van Noordwijk A. J.2002Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–937 10.1006/anbe.2002.2006 (doi:10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., van der Plas F., Wright J., Réale D., Schrama M., Roff D. A., van der Zee E., Barber I.2009Individual experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc. R. Soc. B 276, 1285–1293 10.1098/rspb.2008.1555 (doi:10.1098/rspb.2008.1555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Dochtermann N. A., Wright J.2010aA method for exploring the structure of behavioural syndromes to allow formal comparison within and between datasets. Anim. Behav. 79, 439–450 10.1016/j.anbehav.2009.11.024 (doi:10.1016/j.anbehav.2009.11.024) [DOI] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010bBehavioural reaction norms: where animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Dochtermann N. A., Jenkins S. H.2007Behavioural syndromes in Merriam's kangaroo rats (Dipodomys merriami): a test of competing hypotheses. Proc. R. Soc. B 274, 2343–2349 10.1098/rspb.2007.0622 (doi:10.1098/rspb.2007.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B. J., Jackson J. J., Boyce W. T.2006The stress response systems: universality and adaptive individual differences. Dev. Rev. 26, 175–212 10.1016/j.dr.2006.02.004 (doi:10.1016/j.dr.2006.02.004) [DOI] [Google Scholar]
- Fuller T., Sarkar S., Crews D.2005The use of norms of reaction to analyze genotypic and environmental influences on behavior in mice and rats. Neurosci. Biobehav. Rev. 29, 445–456 10.1016/j.neubiorev.2004.12.005 (doi:10.1016/j.neubiorev.2004.12.005) [DOI] [PubMed] [Google Scholar]
- Gosling S. D.2001From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- Harcourt J. L., Ang T. Z., Sweetman G., Johnstone R. A., Manica A.2009Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252 10.1016/j.cub.2008.12.051 (doi:10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- Houston A. I.2010Evolutionary models of metabolism, behaviour and personality. Phil. Trans. R. Soc. B 365, 3969–3975 10.1098/rstb.2010.0161 (doi:10.1098/rstb.2010.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston A. I., McNamara J. M.1989The value of food: effects of open and closed economies. Anim. Behav. 37, 546–562 10.1016/0003-3472(89)90034-1 (doi:10.1016/0003-3472(89)90034-1) [DOI] [Google Scholar]
- Houston A. I., McNamara J. M.1999Models of adaptive behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- Huntingford F. A.1976The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 24, 245–260 10.1016/S0003-3472(76)80034-6 (doi:10.1016/S0003-3472(76)80034-6) [DOI] [Google Scholar]
- Johnstone R. A.2001Eavesdropping and animal conflict. Proc. Natl Acad. Sci. USA 98, 9177–9180 10.1073/pnas.161058798 (doi:10.1073/pnas.161058798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobler A., Klefoth T., Mehner T., Arlinghaus R.2009Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia 161, 837–847 10.1007/s00442-009-1415-9 (doi:10.1007/s00442-009-1415-9) [DOI] [PubMed] [Google Scholar]
- Kontiainen P., Pietiäinen H., Huttunen K., Karell P., Kolunen H., Brommer J. E.2009Aggressive Ural Owl mothers recruit more offspring. Behav. Ecol. 20, 789–796 10.1093/beheco/arp062 (doi:10.1093/beheco/arp062) [DOI] [Google Scholar]
- Koolhaas J. M., Korte S. M., de Boer S. F., van der Vegt B. J., van Reenen C. G., Hopster H., de Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Kotrschal A., Taborsky B.2010Environmental change enhances cognitive abilities in fish. PLoS Biol. 8, e1000351. 10.1371/journal.pbio.1000351 (doi:10.1371/journal.pbio.1000351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttbeg B., Sih A.2010Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990 10.1098/rstb.2010.0207 (doi:10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel M., Stamps J. A.2001Trade-offs between growth and mortality and the maintenance of individual varation in growth. Evol. Ecol. Res. 3, 538–593 [Google Scholar]
- Martin J. G. A., Réale D.2008Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim. Behav. 75, 309–318 10.1016/j.anbehav.2007.05.026 (doi:10.1016/j.anbehav.2007.05.026) [DOI] [Google Scholar]
- McElreath R., Strimling P.2006How noisy information and individual asymmetries can make 'personality' an adaptation: a simple model. Anim. Behav. 72, 1135–1139 10.1016/j.anbehav.2006.04.001 (doi:10.1016/j.anbehav.2006.04.001) [DOI] [Google Scholar]
- McElreath R., Luttbeg B., Fogarty S. P., Brodin T., Sih A.2007Evolution of animal personalities. Nature 450, E5. 10.1038/nature06326 (doi:10.1038/nature06326) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Barta Z., Houston A. I.2004Variation in behaviour promotes cooperation in the Prisoner's Dilemma game. Nature 428, 745–748 10.1038/nature02432 (doi:10.1038/nature02432) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Barta Z., Fromhage L., Houston A. I.2008The coevolution of choosiness and cooperation. Nature 451, 189–192 10.1038/nature06455 (doi:10.1038/nature06455) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Stephens P. A., Dall S. R. X., Houston A. I.2009Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B 276, 605–613 10.1098/rspb.2008.1182 (doi:10.1098/rspb.2008.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millidine K. J., Armstrong J. D., Metcalfe N. B.2009Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proc. R. Soc. B 276, 2103–2108 10.1098/rspb.2009.0080 (doi:10.1098/rspb.2009.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D., Penke L.2010Personality: bridging the literatures from human psychology and behavioural ecology. Phil. Trans. R. Soc. B 365, 4043–4050 10.1098/rstb.2010.0061 (doi:10.1098/rstb.2010.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey D. H., Wilson A. J., Brommer J. E.2007The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 10.1111/j.1420-9101.2007.01300.x (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- Oosten J. E., Magnhagen C., Hemelrijk C. K.2010Boldness by habituation and social interactions: a model. Behav. Ecol. Sociobiol. 64, 793–802 10.1007/s00265-009-0896-1 (doi:10.1007/s00265-009-0896-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L., Denissen J. J. A., Miller G. F.2007The evolutionary genetics of personality. Eur. J. Personality 21, 549–587 10.1002/per.629 (doi:10.1002/per.629) [DOI] [Google Scholar]
- Pervin L., John O. P.1999Handbook of personality: theory and research. New York, NY: Guilford Press [Google Scholar]
- Quinn J. L., Patrick S. C., Bouwhuis S., Wilkin T. A., Sheldon B. C.2009Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215 10.1111/j.1365-2656.2009.01585.x (doi:10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
- Rands S. A., Cowlishaw G., Pettifor R. A., Rowcliffe J. M., Johnstone R. A.2003Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434 10.1038/nature01630 (doi:10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- Rands S. A., Cowlishaw G., Pettifor R. A., Rowcliffe J. M., Johnstone R. A.2008The emergence of leaders and followers in foraging pairs when the qualities of individuals differ. BMC Evol. Biol. 8, 51. 10.1186/1471-2148-8-51 (doi:10.1186/1471-2148-8-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D., Dingemanse N. J.2010Personality and individual social specialisation. In Social behaviour: genes, ecology and evolution (eds Szekely T., Moore A., Komdeur J.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Réale D., Gallant B. Y., Leblanc M., Festa-Bianchet M.2000Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597 10.1006/anbe.2000.1530 (doi:10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P., Dingemanse N. J.2007Integrating temperament in ecology and evolutionary biology. Biol. Rev. Camb. Phil. Soc. 82, 291–318 [DOI] [PubMed] [Google Scholar]
- Réale D., Martin J., Coltman D. W., Poissant J., Festa-Bianchet M.2009Male personality, life-history strategies and reproductive success in a promiscuous mammal. J. Evol. Biol. 22, 1599–1607 10.1111/j.1420-9101.2009.01781.x (doi:10.1111/j.1420-9101.2009.01781.x) [DOI] [PubMed] [Google Scholar]
- Reddon A. R., Hurd P. L.2009Individual differences in cerebral lateralization are associated with shy–bold variation in the convict cichlid. Anim. Behav. 77, 189–193 10.1016/j.anbehav.2008.09.026 (doi:10.1016/j.anbehav.2008.09.026) [DOI] [Google Scholar]
- Rosenzweig M. R., Bennett E. L.1996Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 78, 57–65 10.1016/0166-4328(95)00216-2 (doi:10.1016/0166-4328 (95)00216-2) [DOI] [PubMed] [Google Scholar]
- Sarkar S.1999From the Reaktionsnorm to the adaptive norm: the norm of reaction, 1909–1960. Biol. Phil. 14, 235–252 10.1023/A:1006690502648 (doi:10.1023/A:1006690502648) [DOI] [Google Scholar]
- Schjolden J., Winberg S.2007Genetically determined variation in stress responsiveness in rainbow trout: behavior and neurobiology. Brain Behav. Evol. 70, 227–238 10.1159/000105486 (doi:10.1159/000105486) [DOI] [PubMed] [Google Scholar]
- Schuett W., Tregenza T., Dall S. R. X.2010Sexual selection and animal personality. Biol. Rev. Camb. Phil. Soc. 85, 217–246 [DOI] [PubMed] [Google Scholar]
- Schürch R., Rothenberger S., Heg D.2010The building-up of social relationships: behavioural types, social networks and cooperative breeding in a cichlid. Phil. Trans. R. Soc. B 365, 4089–4098 10.1098/rstb.2010.0177 (doi:10.1098/rstb.2010.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Adv. Study Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Kats L. B., Maurer E. F.2003Behavioural correlations across situations and the evolution of ineffective antipredator behaviour in a sunfish–salamander system. Anim. Behav. 65, 29–44 10.1006/anbe.2002.2025 (doi:10.1006/anbe.2002.2025) [DOI] [Google Scholar]
- Sih A., Bell A., Johnson J. C.2004aBehavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004bBehavioural syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Moltschaniwskyj N. A., Wapstra E., Dall S. R. X.2010Are behavioral syndromes invariant? Spatiotemporal variation in shy/bold behavior in squid. Behav. Ecol. Sociobiol. 64, 693–702 10.1007/s00265-009-0887-2 (doi:10.1007/s00265-009-0887-2) [DOI] [Google Scholar]
- Smiseth P. T., Wright J., Kölliker M.2008Parent–offspring conflict and co-adaptation: behavioural ecology meets quantitative genetics. Proc. R. Soc. B 275, 1823–1830 10.1098/rspb.2008.0199 (doi:10.1098/rspb.2008.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. R., Blumstein D. T.2008Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits' in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stamps J., Groothuis T. G. G.2010aThe development of animal personality: relevance, concepts and perspectives. Biol. Rev. Camb. Phil. Soc. 85, 301–325 [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Groothuis T. G. G.2010bDevelopmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc. B 365, 4029–4041 10.1098/rstb.2010.0218 (doi:10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn G. S., Wolf M., Leimar O., Weissing F. J.2009Animal personalities and the divergence of life histories. In Adaptive individual differences (ed. Wolf M.). PhD thesis, University of Groningen, The Netherlands. (see http://dissertations.ub.rug.nl/science/2009/m.wolf/) [Google Scholar]
- van Oers K., Mueller J. C.2010Evolutionary genomics of animal personality. Phil. Trans. R. Soc. B 365, 3991–4000 10.1098/rstb.2010.0178 (doi:10.1098/rstb.2010.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K., de Jong G., van Noordwijk A. J., Kempenaers B., Drent P. J.2005Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1191–1212 10.1163/156853905774539364 (doi:10.1163/156853905774539364) [DOI] [Google Scholar]
- Verbeek M. E. M., Boon A., Drent P. J.1996Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour 133, 945–963 10.1163/156853996X00314 (doi:10.1163/156853996X00314) [DOI] [Google Scholar]
- Wilson D. S.1998Adaptive individual differences within single populations. Phil. Trans. R. Soc. B 353, 199–205 10.1098/rstb.1998.0202 (doi:10.1098/rstb.1998.0202) [DOI] [Google Scholar]
- Wilson A. D. M., Godin J. G. J., Ward A. J. W.2010Boldness and reproductive fitness correlates in the eastern mosquitofish, Gambusia holbrooki. Ethology 116, 96–104 10.1111/j.1439-0310.2009.01719.x (doi:10.1111/j.1439-0310.2009.01719.x) [DOI] [Google Scholar]
- Wolf M., Weissing F. J.2010An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007aLife-history trade-offs favour the evolution of animal personalities. Nature 447, 581–585 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007bEvolution of animal personalities: reply. Nature 450, E5–E6 10.1038/nature06327 (doi:10.1038/nature06327) [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J.2008Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15825–15830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J.In press On the coevolution of social responsiveness and behavioural consistency. Proc. R. Soc. B 10.1098/rspb.2010.1051 (doi:10.1098/rspb.2010.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]