Abstract
This introduction to the themed issue on Evolutionary and ecological approaches to the study of personality provides an overview of conceptual, theoretical and methodological progress in research on animal personalities over the last decade, and places the contributions to this volume in context. The issue has three main goals. First, we aimed to bring together theoreticians to contribute to the development of models providing adaptive explanations for animal personality that could guide empiricists, and stimulate exchange of ideas between the two groups of researchers. Second, we aimed to stimulate cross-fertilization between different scientific fields that study personality, namely behavioural ecology, psychology, genomics, quantitative genetics, neuroendocrinology and developmental biology. Third, we aimed to foster the application of an evolutionary framework to the study of personality.
Keywords: personality, adaptive theory, evolutionary ecology, life-history variation, behavioural development, genetics
1. Introduction
In almost any species of animal studied, including humans, individuals differ consistently in numerous aspects of their behaviour. Behavioural differences between individuals that are consistent over time and across situations are referred to as personality by an increasing number of psychologists and biologists (Gosling 2001; Sih et al. 2004; Réale et al. 2007). How can we explain the existence of such a diversity of behavioural phenotypes within single populations (Wilson 1998)? This question represents the main challenge that students of personality currently face (Sih et al. 2004; Réale et al. 2007), and exemplifies a genuine increase in research interest in a major unsolved issue in biology: why do individuals from the same population often differ consistently in aspects of their phenotype, and is this variation adaptive (Bolnick et al. 2003; Careau et al. 2008; Kempenaers et al. 2008; McGlothlin & Ketterson 2008; Williams 2008)?
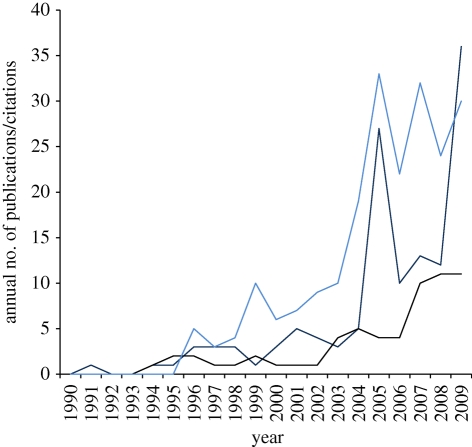
The study of personality traces its roots to the early twentieth century, and has experienced a notable research effort and development in psychology over the last century (Gosling 2001, 2008; Penke et al. 2007; Nettle & Penke 2010). In contrast, despite pioneering work on the subject in the 1970–1980s (e.g. Huntingford 1976; Clark & Ehlinger 1987), personality has been literally ignored by behavioural ecologists, who have only started to work on it in the last decade (Réale et al. 2007; Gosling 2008; Nettle & Penke 2010). The tenfold increase in the number of annual publications since Wilson et al.'s (1994) seminal paper on shyness and boldness in humans and other animals illustrates the success of personality as a major concept within behavioural ecology. Furthermore, the recent appearance of papers on personality in general ecological and evolutionary journals indicates that the concept of personality is now spreading rapidly beyond the realm of behavioural ecology (figure 1). Nevertheless, despite the recent burgeoning of publications on the topic, our understanding of the evolutionary ecology of personality remains scanty.
Figure 1.
Temporal trends in the number of publications and citations on animal personality from 1990 to 2009. We performed a query in Scopus using the terms ‘personality', ‘temperament', ‘coping style', ‘behavioural syndrome' or ‘boldness' in the title, abstract or keywords. Behavioural journals considered were, in alphabetical order: Animal Behaviour, Behavioral Ecology, Behavioral Ecology and Sociobiology, Behaviour, Behavioural Processes and Ethology. Ecological and evolutionary journals considered were: American Naturalist, Biology Letters, Ecology, Ecology Letters, Evolution, Functional Ecology, Journal of Animal Ecology, Journal of Evolutionary Biology, Proceedings of the National Academy of Sciences USA, Proceedings of the Royal Society of London B, and Trends in Ecology and Evolution. Blue line, number of citations of Wilson et al. (1994); navy blue line, publications in journals specializing in the study of behaviour; black line, publications in journals in ecology and evolution.
In this introduction to the special issue of Philosophical Transactions on Evolutionary and ecological approaches to the study of personality, we provide a brief overview of conceptual, theoretical and methodological progress over the last decade, and place the contributions to this volume in context. The issue has three main goals. First, we aimed to bring together theoreticians to contribute to the development of models providing adaptive explanations for animal personality that could guide empiricists, and stimulate exchange of ideas between the two groups of researchers. Second, we aimed to stimulate cross-fertilization between the multiple scientific fields that study personality. In our view, personality is best studied by bridging gaps between major fields in biology, such that useful tools and concepts can be applied to new problems. We therefore asked contributors to explore links between behavioural ecology and other disciplines, such as psychology, genomics, quantitative genetics, neuroendocrinology and developmental biology. Finally, we aimed to foster the application of an evolutionary framework to the study of personality. Various papers included in this special issue therefore specifically explore the interface between personality studies and evolutionary biology.
2. A brief semantic appraisal
The debate concerning the definition of personality has been a vigorous one in behavioural ecology over the past decade (Réale et al. 2007), and is similar to the types of discussion that have preoccupied differential psychologists for many years (Nettle & Penke 2010). After this first phase of discussion and reflection, two main definitions of personality now appear to coexist among behavioural ecologists. For some, personality simply corresponds to the presence of behavioural differences between individuals that are consistent and can involve any type of behaviour; we view this as the broad-sense definition of personality. Others have been interested in consistent individual differences in specific suites of behaviours, typically expressed in a novel or challenging context; we view this as the narrow-sense definition of personality.
The former definition of personality is close to the notion of a behavioural syndrome (Clark & Ehlinger 1987; Sih et al. 2004), which does not make any assumption about the type of behaviour concerned, and addresses the study of correlations at the population level either between the same behavioural trait in two different environmental contexts or between two distinct behavioural traits. The broad-sense definition of personality has the advantage that any behaviour can be placed under the magnifying glass and scrutinized within a general evolutionary theoretical framework (see below). It is not surprising that this definition is adopted in many theoretical papers that are part of this volume (Dingemanse & Wolf 2010; Houston 2010; Luttbeg & Sih 2010; Wolf & Weissing 2010), since their interest primarily lies in understanding general patterns of behaviour within an adaptive framework.
The second definition is closer to the one used in psychology (Gosling 2008; Nettle & Penke 2010) and behavioural physiology (where it is called coping style; Koolhaas et al. 1999; Coppens et al. 2010), both of which emphasize the multi-faceted nature of the phenomenon, and explicitly infer links between behavioural expression and aspects of emotionality. This axis of variation is probably associated with a limited number of neuroendocrinological characteristics (Coppens et al. 2010; Koolhaas et al. 2010). Again, it is not surprising that contributions to the volume detailing the proximate underpinning of personality structure (Coppens et al. 2010; van Oers & Mueller 2010) by and large adopt the narrow-sense definition.
As guest editors of this volume, we were primarily interested in contributions providing general insights into patterns of consistent individual variation in behaviour within single populations, and we therefore welcomed papers focusing on either narrow- or broad-sense personality.
3. The theoretical foundations of the presence (or absence) of personality differences
(a). Recent advances on the theoretical front
The dynamics and health of any discipline should be characterized by intense feedback and integration between conceptual, theoretical and empirical studies, each feeding one another as the field progresses. The recent success of personality research in behavioural ecology has been associated with an explosion of ideas, and new research directions are developing rapidly as increasing numbers of scientists are attracted to this area. However, recent debates at academic conferences indicate a pressing need for a strong theoretical and conceptual foundation to clarify the reasons why personality variation is important and the context within which it should be studied (Réale 2006; Katsnelson 2010). How far have we come in this respect?
Despite the annual publication of 30–50 papers on personality in behavioural and ecological journals over the last decade, there is a paucity of theoretical papers on the topic. Dingemanse & Wolf (2010) provide the first comprehensive review of the formal models developed so far to explain the adaptive significance of personality differences, while Wolf & Weissing (2010) provide a general framework for analyses of personality variation. These papers show that although state-dependent models have been the most used to explain the maintenance of personality variation, other very fruitful options such as frequency-dependent selection, spatial variation under specific conditions and bet-hedging or non-equilibrium dynamics can be explored. The lack of a general theoretical framework for personality variation until now might explain the disproportionate number of descriptive papers published recently that simply present the syndrome structure of a specific model species. A quick glance at table 1 supports the notion of such a deficit in theoretical models despite a very dynamic production of new ideas (typified by the large number of review, perspective and opinion papers that have appeared in the recent behavioural ecology literature). It thus seems that personality research is mainly conceptual and empirical—not the traditional approach in behavioural ecology, where key theoretical models seem to be produced at a much faster rate than empirical studies can test them (Owens 2006).
Table 1.
Selected studies demonstrating the potential implication of personality for different facets of ecology or evolutionary biology in wild animals.a
aHere, we focus on within-species differences.
bC = conceptual (no data analysed); T = formal theoretical model or simulation; E = empirical.
cStudy also showing evidence for selection on personality traits.
One way of providing a more robust theoretical framework for personality variation is to develop adaptive models to reveal the conditions favouring consistent individual differences. Two of the contributions in this special issue do just that. Houston's (2010) model shows that when foraging intensity and metabolic rate coevolve, different combinations of these two traits can have equal—and not merely similar—fitness, a result that has important implications for the evolution of individual differences in behaviour. However, a rather counterintuitive result emerging from this modelling exercise is that high resting metabolic rate need not always be associated with high daily energy expenditure, high risk-taking or high food availability. The link between metabolic rate and personality thus appears important, but may not prove to be a straightforward one. Luttbeg & Sih (2010) explore the effects of risk and resources in a state-dependent adaptive behavioural syndrome. In contrast to the asset protection or avoidance of starvation previously considered as the main factors generating individual differences in risk-taking (e.g. Wolf et al. 2007), these authors have focused on state-dependent safety to explain stable differences in personality over the long term. They show that the emergence of behavioural syndromes is favoured by conditions of intermediate ecological favourability (medium rewards and medium risk, high rewards offset by high risk or low rewards compensated for by low risk). However, highly favourable conditions favour population convergence towards bold individuals, whereas highly unfavourable conditions lead to a convergence towards cautious individuals (i.e. scenarios where personalities do not evolve).
(b). State-dependence and other adaptive explanations for personality
Most adaptive models of personality thus far have employed a state-dependent modelling framework (Dingemanse & Wolf 2010; Wolf & Weissing 2010). The classical approach with state-dependent models is to assume that behaviour (a type of trait very labile in principle) is stable because it is linked to a relatively consistent trait qualified as a state variable. Models have therefore successfully shown that state-dependence can be responsible for the maintenance of individual behavioural differences. However, these models do not generally explain how the correlation between state and behaviour initially evolved, nor how state variation is maintained. Wolf & Weissing (2010) provide explanations for the maintenance of variation in state variables underpinning variation in personality.
One area that has yet to receive attention is the notion that ‘risk-taking’ in the true sense of risk- (variance-) sensitivity and variation in fitness rewards within and between individuals may have implications for long-term personality evolution (i.e. in the form of bet-hedging), a point that Wolf & Weissing (2010) briefly mention. Although no formal personality model using bet-hedging has yet been published, one might speculate that conservative bet-hedging should favour an absence of personality in order to reduce fitness variances over many generations (even if it is favoured in the short-term), while diversification bet-hedging (e.g. in the form of maternal effects to vary offspring state) should favour personalities because they might buffer fitness variances against environmental unpredictability. More research is clearly required in order to resolve such issues in this promising area for personality research.
(c). Theoretical foundations: classic evolutionary explanations
Ecologists working on animal personality have been inspired by other fields of evolutionary ecology, such as quantitative genetics, evolutionary and developmental biology (e.g. Réale & Festa-Bianchet 2003; Sih et al. 2003; Dingemanse et al. 2004, 2009, 2010; Bell 2005; Sinn et al. 2006; Duckworth & Kruuk 2009; Quinn et al. 2009; Réale et al. 2009). Classical evolutionary explanations for the maintenance of genetic variance in quantitative traits (e.g. mutation-selection balance, pleiotropy, trade-offs between traits, spatio-temporal heterogeneity and fluctuating selection) can be applied to any type of trait (Roff 1997; Mousseau et al. 2000; Penke et al. 2007), and so may provide initial insight into why personality variation might persist (but see Dall 2004; Dingemanse & Réale 2005, 2010; Stamps 2007). For example, two papers in this volume outline why it might be important to consider the evolution of personality in a metapopulation context where selection, gene flow, and dispersal favour the maintenance of personality types (Cote et al. 2010b; Réale et al. 2010). Furthermore, correlational selection (Brodie 1993; Sinervo & Svensson 2002) on the link between different traits might provide a powerful adaptive mechanism for the existence of suites of correlated traits (Bell & Sih 2007). Similarly, indirect genetic effects (Kölliker et al. 2005; Harris et al. 2007; Roulin et al. 2010) offer interesting options to explain co-adaptation between personality and other traits. Finally, from a mechanistic point of view, progress in the study of coping styles has generated predictions concerning how hormonal, physiological and behavioural reactions should be correlated (Koolhaas et al. 1999; Groothuis & Carere 2005; see §4b).
However, while classic evolutionary explanations can adequately explain variation between individuals if they are already taken to be consistent (stable) in their behaviour by assumption (e.g. variation between individuals might be genetically determined), they do not offer satisfactory explanations for all aspects of personality, owing to their failure to account for why individuals might show consistency in their behaviour, a key aspect of personality variation (Dall 2004; Dingemanse & Réale 2005, 2010; Stamps 2007). Hence, adaptive studies of personality require additional explanations that one might not consider when asking general questions concerning the maintenance of variation in continuous traits within populations, and this is what makes personality research different from studies of many other traits.
4. Mechanisms and ontogeny of personality differences
(a). Alternative viewpoints on behaviour
There are two contrasting conceptions of the architecture of behavioural traits and their functional role. The first, prevalent in behavioural ecology, considers behaviours as highly plastic traits with individuals being capable of rapidly changing the expression of behaviour in response to changes in the surrounding environmental conditions (i.e. unlimited plasticity: Sih et al. 2004). In other words, most of the variation observed in a behavioural trait may be explained by environmental factors (e.g. the presence or absence of predators; manipulation by a parasite; the abundance of food; the density of conspecifics) or intrinsic state differences (e.g. age, body condition). The idea is that every individual could potentially provide an adaptive behavioural response (strategy) to any change in conditions it experiences; such an adaptive strategy would result from the long-term effect of selection (Krebs & Davies 1997). Alternatively, each individual might be limited in its expression of a behavioural trait relative to the overall expression of that trait in the population (Réale & Dingemanse 2010). The concept of personality thus changes substantially the perception of behavioural adaptation, with a shift of interest from a highly plastic conception (i.e. depending mostly on past experience or the immediate environmental conditions) to a conception of behaviour as an intrinsic (i.e. non-flexible) and constrained characteristic of an individual. Selection often also acts on the correlation between seemingly unrelated traits, such that focusing on a single trait might result in a mismatch with the predictions of adaptive models (Sih et al. 2004). Reality probably lies somewhere between these highly plastic versus highly constrained conceptions (Dingemanse et al. 2010), and the challenge for students of the evolutionary ecology of personality is to integrate both within- and between-individual variation within the same adaptive framework (Dingemanse et al. 2010; Nettle & Penke 2010; Réale & Dingemanse 2010).
(b). Timescale of behavioural consistency
Another important and neglected aspect of personality is the timescale over which consistency of behavioural variation is considered. Indeed, individuals might be consistent over only a few hours or days (e.g. because of short-term variation in state across individuals), or individual consistency might be maintained across the entire lifetime (e.g. when encoded genetically or owing to early permanent environmental effects). Although all short- and long-term forms of consistency can be ecologically important (Sih et al. 2003), their consequences at the ecological or evolutionary level differ substantially. Short-term consistency is the modus operandi of some models (see Luttbeg & Sih 2010; Wolf & Weissing 2010). For instance, in the case of anti-predator behaviour, Luttbeg & Sih (2010) indicate that if individuals with an active behavioural type remain inappropriately active for even a few hours after predators appear, the result is often lethal. However, a polymorphism in anti-predator behaviour can also be caused by underlying heritable genetic differences (i.e. Brodie 1993). If selection can act on both types of behavioural consistency, in the first case it will affect the genes involved in producing a phenotypically plastic response to predators, whereas in the second case it will instead affect the anti-predator genetic polymorphism itself. This distinction has two main consequences for theoretical and empirical studies of personality. First, it is important to indicate clearly at which temporal scale within-individual consistency is being considered. Second, studying the mechanisms underlying personality differences will be an important step towards a better understanding of the ecology of personality (Groothuis & Carere 2005), while theoretical modellers could usefully provide adaptive scenarios favouring short- versus long-term consistency, and heritable versus non-heritable encoding of personality (Dingemanse & Wolf 2010). Studies of proximate mechanisms will help us by highlighting the relative importance of short-term plasticity, developmental plasticity and genetic differences for personality differences between individuals.
(c). New tools to study proximate mechanisms
Recent progress in genetics and genomics are a good example of how tools can change our ability to examine personality variation in the wild and quantify its link with fitness. Van Oers & Mueller (2010) review recent methodological advances in the evolutionary genomics of animal personality. While the phenotypic approach can measure current selection on personality traits, they argue that to understand their evolutionary origins one needs to identify polymorphisms at the genomic level. New molecular techniques now allow us to study natural selection at the molecular level, gene interactions and pleiotropic effects, and how gene expression shapes personality phenotypes and the micro-evolutionary processes that maintain them. In contrast, Bell & Aubin-Horth (2010) argue that it would be useful to ask what whole genome expression can tell us about the ecology and evolution of personality. Starting from the principle that personality differences, as with many other ecologically relevant traits, result from a complex set of interacting genes, they argue that the whole genome approach might offer a very fruitful alternative to the candidate gene approach. They also propose ways to use whole genome expression to study behavioural plasticity or the lack thereof. As the concept of personality has traditionally hinged on the idea of heritable individual variance, quantitative genetic tools might also be useful for the study of personality differences (Réale et al. 2007). Dochtermann & Roff (2010) outline exactly why a quantitative genetic approach would be useful; their paper draws attention to the fact that behavioural ecologists have now started to address evolutionary questions regarding personality, but that they are doing this primarily using a phenotypic approach: the authors point out that the assumptions of applying this phenotypic gambit have not been tested (see also Owens 2006; Hadfield et al. 2007). Extensive pedigree analysis of personality traits can, finally, help determine the level at which behavioural consistency occurs: a significant additive genetic variance or maternal genetic effects will, for example, indicate long-term effects (i.e. across generations) on individual consistency, whereas environmental maternal/paternal effects and permanent environmental effects can signal consistency that may be restricted to the individual level.
(d). Developmental sources of behavioural consistency
The idea of within-individual consistency also automatically brings up questions about the ontogeny of personality. However, only a few studies have examined within-individual consistency over the long-term developmental phases (Sinn et al. 2008a; Stamps & Groothuis 2010a,b). Some tools are now available to study the ontogeny of personality. One field that may provide behavioural ecologists with both strong methodological and conceptual frameworks is human personality psychology. Nettle & Penke's (2010) paper explains how behavioural ecologists and personality psychologists might benefit from reading each other's work. For example, psychologists have long explored the proximate mechanisms responsible for personality differences from which we could learn, while they have been inspired by behavioural ecologists to start explaining the adaptive reasons for such differences (see also Buss 2009). Nettle & Penke (2010) finally suggest considering a reaction norm approach to study personality in humans, as has recently been proposed in the field of behavioural ecology (Dingemanse et al. 2010; Stamps & Groothuis 2010a,b).
Other fields of research have also stimulated progress in the study of proximate factors underpinning personality differences. For example, Coppens et al. (2010) discuss the neurophysiological underpinning of differences among individuals in behavioural consistency (or conversely ‘behavioural flexibility’). They also review recent discoveries that challenge the classical view of a unique causal pathway between the hypothalamus–pituitary–adrenal (HPA) axis and coping styles, and even the direction of the causal pathway between the HPA and coping style (see also Koolhaas et al. 2010). Moreover, Stamps & Groothuis (2010b) use insights from developmental biology to show that, at any given age or lifestage, an individual's personality is contingent upon a wide range of experiential factors that occurred earlier in life, from prior to conception through to adulthood. They propose a framework based on the concept of reaction norms from evolutionary biology to aid in studying the development of personality traits, and the consequences for the stability of behavioural correlations across time and contexts.
5. The ecological relevance of the concept of personality
Over the last decade, an increasing number of studies have demonstrated individual differences in a specific behavioural trait over time, between the same behaviour across different environmental conditions, or associations between different behavioural traits. While such studies are necessary to provide the material that will help us generalize the existence of personality or behavioural syndromes across taxa, they are restricted by their descriptive nature, and it is important for the sake of the field that future research integrates personality studies as an important component of more general questions about ecology or evolutionary biology (Bell 2007). It is therefore necessary to move from this descriptive phase of personality studies to the experimental study of the ecological relevance and fitness consequences of personality differences (Sih et al. 2004; Bell & Sih 2007; Réale et al. 2007; Cote et al. 2008).
(a). The importance of ecological factors
A lot can be learned about the evolution of personality by examining in detail how multiple ecological factors can shape—over the short- or long-term—consistent behavioural differences among individuals. Ecological studies of personality have shown that natural selection acts on inter-individual behavioural variation (Dingemanse & Réale 2005, 2010; Smith & Blumstein 2008; table 1). Meanwhile, there is increasing evidence that the concept of personality can be helpful for the study of several seemingly unrelated questions in ecology and evolution (table 1). Réale et al. (2010) argue, for example, that the covariation between behavioural (personality), physiological and life-history traits should be considered in the context of the pace-of-life hypothesis, which can provide a heuristic framework to explain behavioural, physiological or bio-demographic differences within- and between-populations and also species. Réale et al. (2010) propose that empiricists might focus on how spatial and temporal heterogeneity in selection pressures relate to predation regimes, food availability or other ecological factors that could generate the evolution of this pace-of-life syndrome. One such factor could be the occurrence of non-random dispersal of behavioural types. Cote et al. (2010a,b) show that non-random dispersal characterizes many species and that this can be related to personality. They also outline how personality-dependent dispersal can influence the dynamics of metapopulations. Finally, Barber & Dingemanse (2010) discuss how the presence of parasites might generate behavioural syndromes, both from a proximate and ultimate perspective. Between-population variation in personality structure has so far largely been attributed to the predation history of populations (i.e. Bell 2005; Dingemanse et al. 2007) or resource competition. However, Barber & Dingemanse (2010) argue that because the risk of acquiring parasites can be influenced by an individual's behavioural type, variation in local parasite regimes might also generate variation in syndromes in time or space. Here again, there is a need for more formal theoretical models that could provide testable predictions regarding links with ecological factors.
(b). Personality expression and the social context
Many personality traits are expressed within a social context (Réale & Dingemanse 2010) and it is therefore crucial to develop further the social aspects of personality studies (Bergmüller & Taborsky 2010; Bergmüller et al. 2010). Schürch et al. (2010) present empirical data on how behavioural type influences social relationships in a cooperatively breeding cichlid fish. Using a habitat saturation aquarium experiment they showed that personality influences group sizes and the types of dominants/subordinates that individuals accept as group members. They also demonstrate how behavioural type significantly affects the number and quality of connections in both aggressiveness and affiliation social networks. In the context of strong interactions with body size, this represents one of the first demonstrations of the importance of personality in the formation and functioning of complex cooperative social groups. In a similar vein, Krause et al. (2010) outline how social network analysis provides many new metrics to characterize the social fine-scale structure of populations and therefore an opportunity to understand the roles that different personalities may play within groups, and whether individuals assort by personality type (e.g. cooperative tendency) in natural populations. An individual's behavioural tendencies create its network position, which in turn influences the social micro-environment experienced and may continue to shape the individual's personality (see also Stamps & Groothuis 2010a,b). Such analyses can advance our understanding of the powerful selection pressures that may be imposed on behavioural variation by the social environment.
6. Conclusions
Personality is an intriguing phenomenon that fascinates people, perhaps because they intuitively sense that their own personality affects their everyday life and relationships. Personality research is now infiltrating a whole series of domains in the fields of ecology and evolution, for several reasons: (i) consistent behavioural differences between individuals have been reported for a wide variety of animal species, including humans; (ii) the optimality models routinely used in behavioural ecology have neglected the existence of consistent individual variation in natural populations and this shortcoming is now increasingly recognized; (iii) many aspects of an organism's life are affected by personality differences, which can thus provide new perspectives for the study of ecological phenomena; and (iv) research on personality bridges across disciplines and stimulates the development of truly multi-disciplinary research.
Research on personality within an ecological and evolutionary context improves our understanding of the processes that maintain such behavioural differences within populations. For example, with the development of adaptive theoretical models, we are beginning to reveal how a variety of personality types might offer the best strategy to cope with a variety of situations. This view contradicts the current idea commonly accepted in the public domain (e.g. business management, and the workplace) that being bold or proactive is always the best attitude. Personality psychologists are starting to apply these insights from evolutionary biology to explain variability of personality in human populations, discovering new explanations for the existence of personality types that at first glance may not appear to function well in modern society. The development of a specific evolutionary ecology framework for the study of personality will improve the efficacy of research and thus enhance development of applied ideas in this field. Specific fields such as biological conservation, aquaculture and animal welfare, will also benefit from the development of theory and concepts in personality research, which can improve the success of initiatives as diverse as reintroduction programmes and the yields of farmed or managed populations. We therefore hope that this special issue will contribute to this process and provide the beginnings of such an evolutionary and ecological framework for the study and understanding of personalities.
Acknowledgements
We would like to thank Bart Kempenaers, Dany Garant and Fanie Pelletier for their comments on this paper, and Ido Pen for his help as an editorial consultant. Thanks to all the reviewers who have devoted time and effort to improve the papers published in this special issue. D.R. was supported by a NSERC discovery grant and the Canada Research Chair Programme. N.J.D. was supported by the Max Planck Society (MPG). The idea for this special issue originated from a symposium organized at the European Conference for Behavioural Biology in Dijon in 2008.
Footnotes
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Barber I., Dingemanse N. J.2010Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088 10.1098/rstb.2010.0182 (doi:10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M.2005Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M.2007Evolutionary biology: animal personalities. Nature 447, 539–540 10.1038/447539a (doi:10.1038/447539a) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Aubin-Horth N.2010What can whole genome expression data tell us about the ecology and evolution of personality? Phil. Trans. R. Soc. B 365, 4001–4012 10.1098/rstb.2010.0185 (doi:10.1098/rstb.2010.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M., Sih A.2007Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- Bergmüller R., Taborsky M.2007Adaptive behavioural syndromes due to strategic niche specialization. BMC Ecol. 7, 12. 10.1186/1472-6785-7-12 (doi:10.1186/1472-6785-7-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmüller R., Taborsky M.2010Animal personality due to social niche specialisation. Trends Ecol. Evol. 25, 504–511 10.1016/j.tree.2010.06.012 (doi:10.1016/j.tree.2010.06.012) [DOI] [PubMed] [Google Scholar]
- Bergmüller R., Schürch R., Hamilton I. M.2010Evolutionary causes and consequences of consistent individual variation in cooperative behaviour. Phil. Trans. R. Soc. B 365, 2751–2764 10.1098/rstb.2010.0124 (doi:10.1098/rstb.2010.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P. A., Post J. R.2008Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922 10.1073/pnas.0708159105 (doi:10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P. A., Stamps J. A.2008aAre animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Stamps J. A.2008bAre animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Abrahams M. V., Post J. R., Parkinson E. A.2004Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc. R. Soc. B 271, 2233–2237 10.1098/rspb.2004.2861 (doi:10.1098/rspb.2004.2861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick D. I., Svanbäck R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L.2003The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 10.1086/343878 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2007The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecol. Lett. 10, 1094–1104 10.1111/j.1461-0248.2007.01106.x (doi:10.1111/j.1461-0248.2007.01106.x) [DOI] [PubMed] [Google Scholar]
- Boon A. K., Réale D., Boutin S.2008Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117, 1321–1328 10.1111/j.0030-1299.2008.16567.x (doi:10.1111/j.0030-1299.2008.16567.x) [DOI] [Google Scholar]
- Both C., Dingemanse N. J., Drent P. J., Tinbergen J. M.2005Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 74, 667–674 10.1111/j.1365-2656.2005.00962.x (doi:10.1111/j.1365-2656.2005.00962.x) [DOI] [Google Scholar]
- Boyer N., Réale D., Marmet J., Pisanu B., Chapuis J. L.2010Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J. Anim. Ecol. 79, 538–547 10.1111/j.1365-2656.2010.01659.x (doi:10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- Brodie E. D., III1993Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution 46, 1284–1298 [DOI] [PubMed] [Google Scholar]
- Budaev S. V.1997‘Personality’ in the guppy (Poecilia reticulata): a correlational study of exploratory behavior and social tendency. J. Comp. Psychol. 111, 399–411 10.1037/0735-7036.111.4.399 (doi:10.1037/0735-7036.111.4.399) [DOI] [Google Scholar]
- Buss D. M.2009How can evolutionary psychology successfully explain personality and individual differences? Perspect. Psychol. Sci. 4, 359–366 10.1111/j.1745-6924.2009.01138.x (doi:10.1111/j.1745-6924.2009.01138.x) [DOI] [PubMed] [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Réale D.2008Energy metabolism and animal personality. Oikos 117, 641–653 10.1111/j.0030-1299.2008.16513.x (doi:10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- Careau V., Réale D., Humphries M. M., Thomas D.2010The pace of life under artificial selection: personality, energy expenditure and longevity are correlated in domestic dogs. Am. Nat. 175, 753–758 10.1086/652435 (doi:10.1086/652435) [DOI] [PubMed] [Google Scholar]
- Carere C., Welink D., Drent P. J., Koolhaas J. M., Groothuis T. G. G.2001Effect of social defeat in a territorial bird (Parus major) selected for different coping styles. Physiol. Behav. 73, 427–433 10.1016/S0031-9384(01)00492-9 (doi:10.1016/S0031-9384(01)00492-9) [DOI] [PubMed] [Google Scholar]
- Clark A. B., Ehlinger T. J.1987Pattern and adaptation in individual behavioral differences. In Perspectives in ethology (eds Bateson P. P. G., Klopfer P. H.), pp. 1–47 New York, NY: Plenum [Google Scholar]
- Cote J., Dreiss A., Clobert J.2008Social personality traits and fitness. Proc. R. Soc. B 275, 2851–2858 10.1098/rspb.2008.0783 (doi:10.1098/rspb.2008.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J., Fogarty S., Weinersmith K., Brodin T., Sih A.2010aPersonality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579 10.1098/rspb.2009.2128 (doi:10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J., Clobert J., Brodin T., Fogarty S., Sih A.2010bPersonality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076 10.1098/rstb.2010.0176 (doi:10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens C. M., de Boer S. F., Koolhaas J. M.2010Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028 10.1098/rstb.2010.0217 (doi:10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall S. R. X.2004Behavioural biology: fortune favours bold and shy personalities. Curr. Biol. 14, R470–R472 10.1016/j.cub.2004.06.011 (doi:10.1016/j.cub.2004.06.011) [DOI] [PubMed] [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- Dingemanse N. J., de Goede P.2004The relation between dominance and exploratory behavior is context-dependent in wild great tits. Behav. Ecol. 15, 1023–1030 10.1093/beheco/arh115 (doi:10.1093/beheco/arh115) [DOI] [Google Scholar]
- Dingemanse N. J., Réale D.2005Natural selection and animal personality. Behaviour 142, 1159–1184 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- Dingemanse N. J., Réale D.2010What is the evidence for natural selection maintaining ‘animal’ personality variation? In Animal personalities: behaviour, physiology and evolution (eds Carere C., Maestripieri D.). Chicago, IL: University of Chicago Press [Google Scholar]
- Dingemanse N. J., Wolf M.2010Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., van Noordwijk A. J., Rutten A. L., Drent P. J.2003Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 10.1098/rspb.2002.2300 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M.2004Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Van der Plas F., Wright J., Réale D., Schrama M., Roff D. A., Van der Zee E., Barber I.2009Individual experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc. R. Soc. B 276, 1285–1293 10.1098/rspb.2008.1555 (doi: 10.1098/rspb.2008.1555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Dochtermann N. A., Roff D. A.2010Applying a quantitative genetics framework to behavioural syndrome research. Phil. Trans. R. Soc. B 365, 4013–4020 10.1098/rstb.2010.0129 (doi:10.1098/rstb.2010.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R. A., Badyaev A. V.2007Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R. A., Kruuk L. E. B.2009Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977 10.1111/j.1558-5646.2009.00625.x (doi:10.1111/j.1558-5646.2009.00625.x) [DOI] [PubMed] [Google Scholar]
- English S., Nakagawa S., Clutton-Brock T. H.2010Consistent individual differences in cooperative behaviour in meerkats (Suricata suricatta). J. Evol. Biol. 10.1111/j.1420-9101.2010.02025.x (doi:10.1111/j.1420-9101.2010.02025.x) [DOI] [PubMed] [Google Scholar]
- Fraser D. F., Gilliam J. F., Daley M. J., Le A. N., Skalski G. T.2001Explaining leptokurtik movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135 10.1086/321307 (doi:10.1086/321307) [DOI] [PubMed] [Google Scholar]
- Godin J.-G. J., Dugatkin L. A.1996Female mating preference for bold males in the guppy, Poecilia reticulata. Proc. Natl Acad. Sci. USA 93, 10 262–10 267 10.1073/pnas.93.19.10262 (doi:10.1073/pnas.93.19.10262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling S. D.2001From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- Gosling S. D.2008Personality in non-human animals. Soc. Pers. Psychol. Compass 2, 985–1001 10.1111/j.1751-9004.2008.00087.x (doi:10.1111/j.1751-9004.2008.00087.x) [DOI] [Google Scholar]
- Groothuis T. G. G., Carere C.2005Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150 10.1016/j.neubiorev.2004.06.010 (doi:10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Hadfield J. D., Nutall A., Osorio D., Owens I. P. F.2007Testing the phenotypic gambit: phenotypic, genetic and environmental correlations of colour. J. Evol. Biol. 20, 549–557 10.1111/j.1420-9101.2006.01262.x (doi:10.1111/j.1420-9101.2006.01262.x) [DOI] [PubMed] [Google Scholar]
- Harcourt J. L., Ang T. Z., Sweetman G., Johnstone R. A., Manica A.2009Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252 10.1016/j.cub.2008.12.051 (doi:10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- Harris W. E., McKane A. J., Wolf J. B.2007The maintenance of heritable variation through social competition. Evolution 62, 337–347 10.1111/j.1558-5646.2007.00302.x (doi:10.1111/j.1558-5646.2007.00302.x) [DOI] [PubMed] [Google Scholar]
- Houston A. I.2010Evolutionary models of metabolism, behaviour and personality. Phil. Trans. R. Soc. B 365, 3969–3975 10.1098/rstb.2010.0161 (doi:10.1098/rstb.2010.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntingford F. A.1976The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 24, 245–260 10.1016/S0003-3472(76)80034-6 (doi:10.1016/S0003-3472(76)80034-6) [DOI] [Google Scholar]
- Jones K. A., Godin J. G. J.2010Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc. R. Soc. B 277, 625–632 10.1098/rspb.2009.1607 (doi:10.1098/rspb.2009.1607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson A.2010Odd man out. Scientist 24, 35–39 [Google Scholar]
- Kempenaers B., Peters A., Foerster K.2008Sources of individual variation in plasma testosterone levels. Phil. Trans. R. Soc. B 363, 1711–1723 10.1098/rstb.2007.0001 (doi:10.1098/rstb.2007.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker M., Brodie E. D., III, Moore A. J.2005The coadaptation of parental supply and offspring demand. Am. Nat. 166, 506–516 10.1086/491687 (doi:10.1086/491687) [DOI] [PubMed] [Google Scholar]
- Kontiainen P., Pietiainen H., Huttunen K., Karell P., Kolunen H., Brommer J. E.2009Aggressive Ural owl mothers recruit more offspring. Behav. Ecol. 20, 789–796 10.1093/beheco/arp062 (doi:10.1093/beheco/arp062) [DOI] [Google Scholar]
- Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping style in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., De Boer S. F., Coppens C. M.2010Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 31, 307–321 10.1016/j.yfrne.2010.04.001 (doi:10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- Krause J., James R., Croft D. P.2010Personality in the context of social networks. Phil. Trans. R. Soc. B 365, 4099–4106 10.1098/rstb.2010.0216 (doi:10.1098/rstb.2010.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J. R., Davies N. B.1997Behavioural ecology: an evolutionary approach. Sunderland, MA: Sinauer Associates [Google Scholar]
- Kurvers R. H. J. M., Prins H. H. T., Van Wieren S. E., Van Oers K., Nolet B. A., Ydenberg R. C.2010The effect of personality on social foraging: shy barnacle geese scrounge more. Proc. R. Soc. B 277, 601–608 10.1098/rspb.2009.1474 (doi:10.1098/rspb.2009.1474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttbeg B., Sih A.2010Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990 10.1098/rstb.2010.0207 (doi:10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. G. A., Réale D.2008aTemperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim. Behav. 75, 309–318 10.1016/j.anbehav.2007.05.026 (doi:10.1016/j.anbehav.2007.05.026) [DOI] [Google Scholar]
- Martin J. G. A., Réale D.2008bAnimal temperament and human disturbance: implications for the response of wildlife to tourism. Behav. Proc. 77, 66–72 10.1016/j.beproc.2007.06.004 (doi:10.1016/j.beproc.2007.06.004) [DOI] [PubMed] [Google Scholar]
- McElreath R., Strimling P.2006How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim. Behav. 72, 1135–1139 10.1016/j.anbehav.2006.04.001 (doi:10.1016/j.anbehav.2006.04.001) [DOI] [Google Scholar]
- McGlothlin J. W., Ketterson E. D.2008Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 10.1098/rstb.2007.0002 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. M., Stephens P. A., Dall S. R. X., Houston A. I.2009Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B 276, 605–613 10.1098/rspb.2008.1182 (doi:10.1098/rspb.2008.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena P., Jeanson R., Deneubourg J. L., Sibbald A. M.2010Personality and collective decision-making in foraging herbivores. Proc. R. Soc. B 277, 1093–1099 10.1098/rspb.2009.1926 (doi:10.1098/rspb.2009.1926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau T. A., Sinervo B., Endler J. A.2000Adaptive genetic variation in the wild. Oxford, UK: Oxford University Press [Google Scholar]
- Natoli E., Say L., Cafazzo S., Bonanni R., Schmid M., Pontier D.2005Bold attitude makes male urban feral domestic cats more vulnerable to Feline Immunodeficiency Virus. Neurosci. Biobehav. Rev. 29, 151–157 10.1016/j.neubiorev.2004.06.011 (doi:10.1016/j.neubiorev.2004.06.011) [DOI] [PubMed] [Google Scholar]
- Nettle D., Penke L.2010Personality: bridging the literatures from human psychology and behavioural ecology. Phil. Trans. R. Soc. B 365, 4043–4050 10.1098/rstb.2010.0061 (doi:10.1098/rstb.2010.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øverli O., et al. 2004Stress coping style predicts aggression and social dominance in rainbow trout. Horm. Behav. 45, 235–241 10.1016/j.yhbeh.2003.12.002 (doi:10.1016/j.yhbeh.2003.12.002) [DOI] [PubMed] [Google Scholar]
- Owens I. P. F.2006Where is behavioural ecology going? Trends Ecol. Evol. 21, 356–361 10.1016/j.tree.2006.03.014 (doi:10.1016/j.tree.2006.03.014) [DOI] [PubMed] [Google Scholar]
- Penke L., Dennisen J., Miller G.2007The evolutionary genetics of personality. Eur. J. Pers. 21, 549–587 10.1002/per.629 (doi:10.1002/per.629) [DOI] [Google Scholar]
- Pike T. W., Samanta M., Lindström J., Royle N. J.2008Behavioural phenotype affects social interactions in an animal network. Proc. R. Soc. B 275, 2515–2520 10.1098/rspb.2008.0744 (doi:10.1098/rspb.2008.0744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. L., Patrick S. C., Bouwhuis S., Wilkin T. A., Sheldon B. C.2009Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215 10.1111/j.1365-2656.2009.01585.x (doi:10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
- Réale D.2006Do behavioral syndromes represent a paradigm shift? ISBE 2006 Symposium report. ISBE Newsl. 18, 42–43 [Google Scholar]
- Réale D., Dingemanse N. J.2010Personality and individual social specialisation. In Social behaviour: genes, ecology and evolution (eds Székely T., Moore A. J., Komdeur J.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Réale D., Festa-Bianchet M.2003Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65, 463–470 10.1006/anbe.2003.2100 (doi:10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- Réale D., Gallant B. Y., Leblanc M., Festa-Bianchet M.2000Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597 10.1006/anbe.2000.1530 (doi:10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Réale D., Martin J., Coltman D. W., Poissant J., Festa-Bianchet M.2009Male personality, life-history strategies, and reproductive success in a promiscuous mammal. J. Evol. Biol. 22, 1599–1607 10.1111/j.1420-9101.2009.01781.x (doi:10.1111/j.1420-9101.2009.01781.x) [DOI] [PubMed] [Google Scholar]
- Réale D., Garant D., Humphries M. M., Bergeron P., Careau V., Montiglio P.-O.2010Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063 10.1098/rstb.2010.0208 (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehage J. S., Sih A.2004Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol. Invasions 6, 379–391 10.1023/B:BINV.0000034618.93140.a5 (doi:10.1023/B:BINV.0000034618.93140.a5) [DOI] [Google Scholar]
- Roff D. A.1997Evolutionary quantitative genetics. New York, NY: Chapman & Hall [Google Scholar]
- Roulin A., Dreiss A. N., Kölliker M.2010Evolutionary perspective on the interplay between family life, and parent and offspring personality. Ethology 116, 1–10 10.1111/j.1439-0310.2010.01793.x (doi:10.1111/j.1439-0310.2010.01793.x) [DOI] [Google Scholar]
- Schuett W., Tregenza T., Dall S. R. X.2010Sexual selection and animal personality. Biol. Rev. 82, 217–246 10.1111/j.1469-185X.2009.00101.x (doi:10.1111/j.1469-185X.2009.00101.x) [DOI] [PubMed] [Google Scholar]
- Schürch R., Rothenberger S., Heg D.2010The building-up of social relationships: behavioural types, social networks and cooperative breeding in a cichlid. Phil. Trans. R. Soc. B 365, 4089–4098 10.1098/rstb.2010.0177 (doi:10.1098/rstb.2010.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Kats L. B., Maurer E. F.2003Behavioural correlation across situations and the evolution of antipredator behaviour in a sunfish-salamander system. Anim. Behav. 65, 29–44 10.1006/anbe.2002.2025 (doi:10.1006/anbe.2002.2025) [DOI] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Apiolaza L. A., Moltschaniwskyj N. A.2006Heritability and fitness-related consequences of squid personality traits. J. Evol. Biol. 19, 1437–1447 10.1111/j.1420-9101.2006.01136.x (doi:10.1111/j.1420-9101.2006.01136.x) [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Gosling S. D., Moltschaniwskyj N. A.2008aDevelopment of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim. Behav. 75, 442–443 10.1016/j.anbehav.2007.05.008 (doi:10.1016/j.anbehav.2007.05.008) [DOI] [Google Scholar]
- Sinn D. L., While G. M., Wapstra E.2008bMaternal care in a social lizard: links between female aggression and offspring fitness. Anim. Behav. 76, 1249–1257 10.1016/j.anbehav.2008.06.009 (doi:10.1016/j.anbehav.2008.06.009) [DOI] [Google Scholar]
- Sinervo B., Svensson E.2002Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338 10.1038/sj.hdy.6800148 (doi:10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- Smith B. R., Blumstein D. T.2008Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Groothuis T. G. G.2010aThe development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325. 10.1111/j.1469-185X.2009.00103.x (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Groothuis T. G. G.2010bDevelopmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc. B 365, 4029–4041 10.1098/rstb.2010.0218 (doi:10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K., Mueller J. C.2010Evolutionary genomics of animal personality. Phil. Trans. R. Soc. B 365, 3991–4000 10.1098/rstb.2010.0178 (doi:10.1098/rstb.2010.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K., Drent P. J., Dingemanse N. J., Kempenaers B.2008Personality is associated with extrapair paternity in great tits, Parus major. Anim. Behav. 76, 555–563 [Google Scholar]
- Williams T. D.2008Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean'. Phil. Trans. R. Soc. B 363, 1687–1698 10.1098/rstb.2007.0003 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. S.1998Adaptive individual differences within single populations. Phil. Trans. R. Soc. Lond. B 353, 199–205 10.1098/rstb.1998.0202 (doi:10.1098/rstb.1998.0202) [DOI] [Google Scholar]
- Wilson D. S., Clark A. B., Coleman K., Dearstyne T.1994Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446 10.1016/0169-5347(94)90134-1 (doi:10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- Wolf M., Weissing F. J.2010An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J.2007Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- Wright D., Rimmer L. B., Pritchard V. L., Krause J., Butlin R. K.2003Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio). Naturwissenschaften 90, 374–377 10.1007/s00114-003-0443-2 (doi:10.1007/s00114-003-0443-2) [DOI] [PubMed] [Google Scholar]