Abstract
OBJECTIVE
The liver plays a critical role in integrating and controlling glucose metabolism. Thus, it is important that the liver receive and react to signals from other tissues regarding the nutrient status of the body. Leptin, which is produced and secreted from adipose tissue, is a hormone that relays information regarding the status of adipose depots to other parts of the body. Leptin has a profound influence on glucose metabolism, so we sought to determine if leptin may exert this effect in part through the liver.
RESEARCH DESIGN AND METHODS
To explore this possibility, we created mice that have disrupted hepatic leptin signaling using a Cre-lox approach and then investigated aspects of glucose metabolism in these animals.
RESULTS
The loss of hepatic leptin signaling did not alter body weight, body composition, or blood glucose levels in the mild fasting or random-fed state. However, mice with ablated hepatic leptin signaling had increased lipid accumulation in the liver. Further, as male mice aged or were fed a high-fat diet, the loss of hepatic leptin signaling protected the mice from glucose intolerance. Moreover, the mice displayed increased liver insulin sensitivity and a trend toward enhanced glucose-stimulated plasma insulin levels. Consistent with increased insulin sensitivity, mice with ablated hepatic leptin signaling had increased insulin-stimulated phosphorylation of Akt in the liver.
CONCLUSIONS
These data reveal that unlike a complete deficiency of leptin action, which results in impaired glucose homeostasis, disruption of leptin action in the liver alone increases hepatic insulin sensitivity and protects against age- and diet-related glucose intolerance. Thus, leptin appears to act as a negative regulator of insulin action in the liver.
It is well-established that there is a link between diabetes and obesity; however, the molecular mechanisms behind this association are not fully understood. A potential factor linking diabetes and obesity is the hormone leptin. Leptin is a hormone produced predominantly in adipocytes (1) and is typically present in the circulation at levels proportional to fat mass (2). Mice that lack functional leptin (ob/ob) or leptin receptors (db/db) are obese and have a phenotype similar to type 2 diabetes (3).
Although it is often assumed that the diabetic phenotype of these mice is a result of their obesity, the two phenotypes are in fact at least partially independent and several key observations clearly highlight this. In ob/ob and db/db mice, hyperinsulinemia, and thus the disruption of normal glucose homeostasis, precedes changes in body mass (4,5). Doses of recombinant leptin that do not influence body weight are able to normalize glucose levels and reduce the hyperinsulinemia of ob/ob mice with minimal changes in body weight (6,7). Further evidence that leptin has direct effects on glucose metabolism comes from the rare condition of severe lipodystrophy, the near complete loss of adipose mass. Paradoxically, similar to too much fat, too little fat caused by lipodystrophy is associated with a type 2 diabetic phenotype (8) and leptin therapy dramatically ameliorates diabetes in this situation (9). Collectively, these observations indicate that leptin has powerful actions on glucose homeostasis, independent of its well-described central actions on food intake and energy expenditure (10).
Several studies have shown that the weight-independent actions of leptin on glucose metabolism result from activation of central leptin signaling pathways (11–13) that regulate hepatic glucose flux (12,13). Interestingly, leptin receptor transcripts are also expressed in the liver itself (14), including the long signaling isoform, leptin receptor b (Lepr-b) (15–17), and many studies reveal that leptin also has direct action on hepatocytes (17–19). To directly evaluate the contribution of hepatic leptin signaling on aspects of metabolism, Cohen et al. (20) used a Cre-lox approach to allow hepatocyte-specific deletion of the entire leptin receptor. The investigators found that hepatocyte-specific ablation of leptin receptors did not alter body weight, body composition, or free-feeding levels of glucose or insulin (20). We sought to further these studies by investigating metabolism at several different ages, on a high-fat diet, and under the setting of a glucose challenge. For these studies, we too employed a Cre-lox approach. However, distinct from that of the Cohen study, we generated mice with hepatocyte-specific ablation of leptin signaling domains as opposed to ablation of the complete receptor. Similar to Cohen et al., we found that a loss of hepatic leptin signaling does not impact many parameters of glucose metabolism and body composition. Interestingly, however, we discovered that a loss of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. This protection from glucose intolerance correlates with elevated glucose-stimulated plasma insulin levels, increased liver insulin sensitivity, and increased hepatic lipid accumulation. Collectively, our data reveal a previously unappreciated role for hepatic leptin signaling in regulating glucose homeostasis.
RESEARCH DESIGN AND METHODS
Mice.
C57BL/6, db/db and C57BL/6-Tg(Alb-cre)21Mgn (referred to as Albcre tg+) were obtained from The Jackson Laboratory (Bar Harbor, ME). Leprflox/flox Albcre tg+ mice, which lack hepatic leptin signaling, and their Leprflox/flox Albcre tg− littermate controls were generated as described in the supplementary information, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0074/DC1. All procedures with animals were approved by the University of British Columbia Animal Care Committee and carried out in accordance with the Canadian Council on Animal Care guidelines. For additional details, see the supplementary information.
PCR and RT-PCR analysis.
Genomic DNA was prepared with DNeasy kits (Qiagen, Mississauga, Canada). Brain tissue was prepared as a homogenate of the brain minus the hypothalamus. For RNA extractions, the liver was excised and immediately placed in RNAlater (Qiagen). Tissue was homogenized with an Ultra-Turrax and purified using an RNeasy kit (Qiagen). Reverse transcript reactions were performed with a poly T primer using a Superscript First-Strand Synthesis kit (Invitrogen, Burlington, Canada). The generated cDNA was then used for PCR. Primer sequences are available in the supplementary information.
Measurement of lean to lipid mass.
Measurements were performed with a Bruker BioSpec 70/30 7 Tesla magnetic resonance imaging (MRI) scanner (Bruker Biospin, Ettlingen, Germany). The ratio of lean/fat tissue is expressed as weight/weight and was calculated from the nuclear magnetic resonance (NMR) data as described (21). For additional details, see the supplementary information.
Measurement of hepatic lipid content.
Hepatic triglycerides and cholesterol were measured by a modified protocol of Briaud et al. (22). For additional details, see the supplementary information.
Plasma analyte analysis.
Body weight, blood glucose, and insulin were typically measured following a 4-h fast unless specified otherwise. Blood glucose concentration was measured with a OneTouch Ultra Glucometer (LifeScan, Burnaby, Canada) from the saphenous vein. Plasma insulin levels were measured by an Insulin Mouse Ultrasensitive enzyme-linked immunosorbent assay (ELISA) (ALPCO Diagnostics, Salem, NH), and plasma leptin levels were determined using a mouse leptin ELISA (Crystal Chem, Downers Grove, IL).
In vivo oral glucose tolerance, glucose-stimulated insulin levels, and insulin tolerance tests.
Mice were fasted for 4 h and then given either an oral glucose gavage (1.5 mg of glucose per g body weight unless otherwise specified) of a 30% glucose solution or an intraperitoneal injection of 0.75 units/kg (females) or 1.0 units/kg (males) human synthetic insulin (Novolin ge Toronto, Novo Nordisk, Mississauga, Canada). Blood was sampled from the saphenous vein and measured for glucose or insulin as described above.
Measurements of β-cell mass.
Pancreata were removed and fixed in 4% paraformaldehyde overnight at 4°C and then stored in 70% ethanol prior to embedding in paraffin and sectioning. For each mouse, insulin-positive area was assessed on three pancreas sections separated by 35 μm. Details regarding processing, staining, and quantification of β-cell mass are available in the supplementary information.
Hyperinsulinemic-euglycemic clamps.
Hyperinsulinemic-euglycemic clamps were performed as previously described (23). For a brief description, see the supplementary information.
Western blot analysis.
Liver samples were collected immediately after the hyperinsulinemic-euglycemic clamp. Western blots were performed using antibodies against phosphorylated Akt (#4060; Cell Signaling Technologies, Danvers, MA) and total Akt (#9272; Cell Signaling Technologies). See the supplementary information for additional details.
Statistical analysis.
Data are represented as means ± SEM, and significance was set at P ≤ 0.05. Analyses were done by Student's t tests.
RESULTS
Generation of mice with hepatocyte-specific disruption of leptin receptor signaling.
To generate mice with hepatocyte-specific disruption of leptin signaling, we utilized Leprflox/flox mice, which have loxP sites flanking exon 17 of the leptin receptor gene (Lepr) (24,25). Exon 17 of Lepr is present in the long signaling isoform of Lepr-b and encodes the Janus kinase (JAK) binding site. Upon Cre-mediated recombination at the loxP sites, exon 17 is excised (herein referred to as LeprΔ17), and a resulting frame shift mutation generates an altered 3′ terminus (25) that no longer encodes tyrosine 985 and tyrosine 1138, which are sites of JAK-mediated tyrosine phosphorylation (26). Mice homozygous for the LeprΔ17 allele are deficient in leptin-stimulated STAT phosphorylation (27,28). The Leprflox/flox mice were crossed with Albcre tg+ mice, which have the cre transgene under the control of the albumin promoter, conferring hepatocyte-specific expression of Cre (29). The resulting Leprflox/wt mice with and without the Albcre transgene were bred to generate Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− mice.
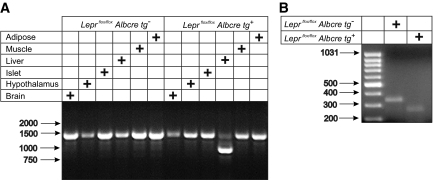
Following generation of Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− littermate controls, we investigated the extent and specificity of Cre-mediated excision of exon 17 of the leptin receptor. In all tissues tested, there was a PCR product of ∼1,370 bp, which corresponds to the expected product size of the Leprflox allele (Fig. 1A). Although present, there was very little of this amplicon generated from liver DNA of Leprflox/flox Albcre tg+ mice, likely contributed by non-hepatocytes. Instead, the major product amplified from liver DNA of Leprflox/flox Albcre tg+ mice was ∼950 bp in size, which corresponds to the expected size of the Leprflox allele following Cre-mediated excision (resulting in LeprΔ17). Thus, it appears that in the Leprflox/flox Albcre tg+ mice, excision at the Leprflox allele among the tissues tested was restricted to the liver and that the majority of hepatocytes carry the LeprΔ17 rather than the Leprflox allele. Consistent with this, an analysis of Lepr-b mRNA transcripts by RT-PCR revealed that while the only amplified product detected from the liver of Leprflox/flox Albcre tg− mice corresponded to the anticipated 343 bp product from the wild-type Lepr-b transcript, in the liver of the Leprflox/flox Albcre tg+ mice, only an amplicon of the predicted size of the LeprΔ17 transcript was detected (Fig. 1B). The LeprΔ17 transcript has been previously shown to result in impaired leptin-stimulated JAK/STAT signaling in mouse lines derived from the same Leprflox/flox mice used in our study (24,28). Thus, our mice, which we have shown to predominantly express the LeprΔ17 allele specifically in the liver, must have impaired leptin-stimulated hepatic JAK/STAT activation.
FIG. 1.
Leprflox/flox Albcre tg+ mice have a liver-specific loss of the leptin receptor signaling domain. A: Genomic DNA from tissues of Leprflox/flox mice with and without the Albcre transgene was used as template for PCR of the Leprflox allele. The predicted product sizes are 1,369 bp for Leprflox and 952 bp for LeprΔ17. B: RNA was extracted from the livers of Leprflox/flox mice and used as template for RT-PCR with primers flanking exon 17. The predicted product sizes are 343 bp for Leprflox and 267 bp for LeprΔ17 transcripts. Arrows to the left mark the migration of molecular weight markers in bp.
Loss of hepatic leptin signaling does not substantially alter body weight or body composition.
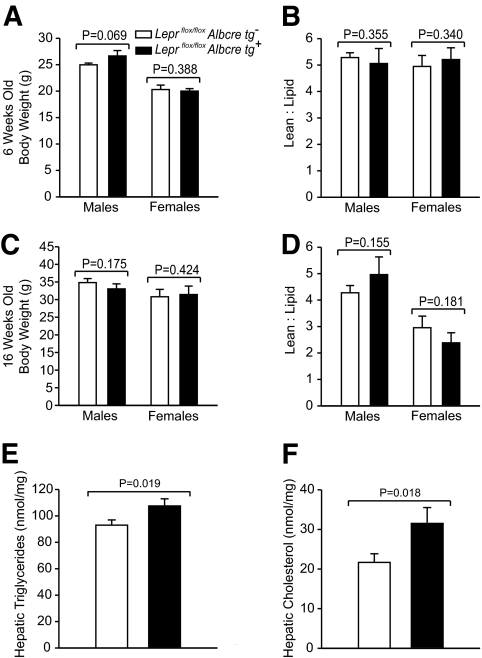
To characterize the physiological effect of ablated hepatic leptin signaling, we first assessed body weight and composition. Unlike mice with a complete loss of leptin signaling (db/db mice), which are much heavier than control mice (C57BL/6), the loss of hepatic leptin signaling did not substantially alter body weight relative to controls in either sex at the various ages tested (Table 1). To assess body composition, the total lean-to-lipid mass ratio of Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− littermate controls was assessed at 6 and 16 weeks of age by NMR. At both ages and in each sex, there was a similar ratio of lean-to-lipid mass in mice with and without hepatic leptin signaling (Figs. 2B and D). However, we found that mice lacking leptin signaling in the liver had 16% more triglycerides and 48% more cholesterol in their livers when compared with littermate controls (Fig. 2E and F). Thus, while a loss of hepatic leptin signaling does not alter body weight or adiposity, it does result in increased lipid accumulation in the liver.
TABLE 1.
Metabolic parameters of mice with and without hepatic leptin signaling
| Metabolic parameter |
||||||
|---|---|---|---|---|---|---|
| Body weight (g) | Fastinga glucose (mM) | Random-fedb glucose (mM) | Fastinga insulin (ng/ml) | |||
| 6 weeks | ♂ | Leprflox/flox Albcre tg− | 25.7 ± 0.5 (22) | 9.7 ± 0.3 (22) | 8.3 ± 0.4 (7) | 1.17 ± 0.13 (5) |
| Leprflox/flox Albcre tg+ | 25.6 ± 0.5 (24) | 9.3 ± 0.3 (24) | 7.6 ± 0.3 (8) | 1.09 ± 0.11 (5) | ||
| C57BL/6 | 20.5 ± 0.5 (8) | 11.1 ± 0.3 (16) | 9.0 ± 0.4 (8) | 0.86 ± 0.06 (8) | ||
| db/db | 30.0 ± 0.7 (8)* | 15.1 ± 1.8 (8)* | 28.1 ± 1.6 (8)* | 7.89 ± 0.91 (8)* | ||
| ♀ | Leprflox/flox Albcre tg− | 20.5 ± 0.3 (18) | 8.3 ± 0.3 (18) | 7.3 ± 0.3 (8) | 0.53 ± 0.15 (6) | |
| Leprflox/flox Albcre tg+ | 19.7 ± 0.3 (17) | 8.5 ± 0.3 (16) | 7.2 ± 0.2 (5) | 0.34 ± 0.05 (3) | ||
| C57BL/6 | 16.0 ± 0.4 (8) | 8.0 ± 0.3 (8) | 8.1 ± 0.4 (8) | 0.53 ± 0.09 (4) | ||
| db/db | 31.6 ± 0.5 (8)* | 22.1 ± 1.6 (8)* | 28.9 ± 0.5 (8)* | 10.18 ± 1.38 (7)* | ||
| 12 weeks | ♂ | Leprflox/flox Albcre tg− | 34.0 ± 0.9 (29) | 10.1 ± 0.3 (29) | 8.3 ± 0.4 (6) | 1.38 ± 0.30 (6) |
| Leprflox/flox Albcre tg+ | 32.6 ± 0.9 (35) | 9.9 ± 0.3 (35) | 8.0 ± 0.3 (7) | 1.32 ± 0.29 (7) | ||
| C57BL/6 | 28.8 ± 1.0 (14) | 11.1 ± 0.4 (14) | 9.0 ± 0.2 (8) | 2.61 ± 0.76 (8) | ||
| db/db | 50.5 ± 0.9 (8)* | 28.0 ± 1.0 (8)* | 28.1 ± 1.1 (8)* | 11.2 ± 2.2 (8)* | ||
| ♀ | Leprflox/flox Albcre tg− | 22.2 ± 0.9 (27) | 7.9 ± 0.3 (27) | 8.0 ± 0.3 (5) | 0.54 ± 0.10 (5) | |
| Leprflox/flox Albcre tg+ | 23.1 ± 0.5 (22) | 7.7 ± 0.2 (22) | 8.0 ± 0.2 (5) | 0.48 ± 0.17 (5) | ||
| C57BL/6 | 18.7 ± 0.6 (8) | 7.2 ± 0.4 (8) | 7.0 ± 0.1 (8) | 0.49 ± 0.06 (8) | ||
| db/db | 51.7 ± 0.6 (8)* | 28.1 ± 2.2 (8)* | 24.8 ± 2.4 (8)* | 7.46 ± 0.76 (8)* | ||
| 16 weeks | ♂ | Leprflox/flox Albcre tg− | 41.6 ± 1.1 (24) | 10.9 ± 0.5 (24) | 7.8 ± 0.4 (5) | 2.00 ± 0.41 (18) |
| Leprflox/flox Albcre tg+ | 39.1 ± 1.1 (25) | 10.6 ± 0.3 (26) | 8.6 ± 0.5 (7) | 1.70 ± 0.25 (23) | ||
| C57BL/6 | 31.7 ± 1.0 (14) | 9.7 ± 0.3 (14) | 8.2 ± 0.5 (8) | 2.32 ± 0.77 (8) | ||
| db/db | 56.7 ± 0.9 (8)* | 25.6 ± 2.8 (8)* | 25.1 ± 1.8 (8)* | 12.0 ± 2.10 (8)* | ||
| ♀ | Leprflox/flox Albcre tg− | 26.9 ± 1.2 (12) | 7.5 ± 0.4 (12) | 7.5 ± 0.3 (5) | 0.82 ± 0.20 (4) | |
| Leprflox/flox Albcre tg+ | 25.4 ± 1.0 (8) | 7.2 ± 0.2 (8) | 7.3 ± 0.3 (5) | 0.41 ± 0.01 (4)* | ||
| C57BL/6 | 20.4 ± 0.7 (8) | 8.4 ± 0.5 (8) | 7.5 ± 0.2 (8) | 0.37 ± 0.06 (8) | ||
| db/db | 57.9 ± 0.6 (8)* | 21.7 ± 2.5 (8)* | 21.0 ± 2.8 (8)* | 9.67 ± 1.42 (6)* | ||
aMice were fasted for 4 h during the light cycle.
bSamples were collected between 11:00 p.m.–1:00 a.m.
*P ≤ 0.05 vs. control (Leprflox/flox Albcre tg− or C57BL/6).
FIG. 2.
Attenuation of hepatic leptin signaling does not alter body composition. A and C: Leprflox/flox Albcre tg+ mice and littermate controls were assessed for body weight as well as (B and D) body composition as measured by NMR at 6 and 16 weeks old. At 6 weeks old, n ≥ 7, and at 16 weeks old, n ≥ 5, for Leprflox/flox Albcre tg+ and littermate controls. E and F: Twenty-one-week-old male mice were fasted for 4 h and then the liver was harvested. Lipids were isolated by a chloroform:methanol extraction and reconstituted into Thesit micelles. Samples were then assayed for triglycerides and cholesterol, n ≥ 8 in each group. Data are mean ± SEM.
Loss of hepatic leptin signaling does not have a major influence on basal metabolic parameters.
We next sought to determine how a loss of hepatic leptin signaling might affect basal metabolic parameters. Plasma leptin levels were not significantly different between groups in either sex at 18 weeks of age (5.66 ± 1.11 ng/ml vs. 4.36 ± 2.63 ng/ml for male Leprflox/flox Albcre tg− vs. Leprflox/flox Albcre tg+, P = 0.308, n ≥ 4 and 4.11 ± 0.78 ng/ml vs. 6.80 ± 1.64 ng/ml for female Leprflox/flox Albcre tg− vs. Leprflox/flox Albcre tg+, P = 0.123, n ≥ 3). Similarly, plasma leptin binding protein levels also appeared to be unchanged between Leprflox/flox Albcre tg+ and littermate controls (supplementary Fig. 1). Further, plasma lipid levels after a 4-h fast were unchanged between Leprflox/flox Albcre tg+ and their littermate controls at 6, 12, and 16 weeks of age (supplementary Table 1). We also measured insulin and blood glucose levels in Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− littermate controls in both the 4-h fasted state as well as the fed state during the light and dark phases, respectively. For comparison, we also measured these parameters in mice lacking functional leptin receptors in all tissues (db/db) and the relevant wild-type controls (C57BL/6). The complete loss of leptin action in the db/db mice resulted in increased body mass, hyperglycemia (in both the fasted and fed state) and fasting hyperinsulinemia in both genders at 6, 12, and 16 weeks of age (Table 1). In contrast, a liver-specific loss of leptin signaling did not significantly alter blood glucose levels (in either fasted or random-fed state) of either sex at any of the ages investigated. Consistent with this, we found no alterations in hepatic PEPCK and glucose-6-phosphatase (G6Pase) transcript levels between male Leprflox/flox Albcre tg+ and tg− littermate controls after a 4-h fast (supplementary Fig. 2A). Further, pyruvate-induced gluconeogenesis after an overnight fast was similar in male Leprflox/flox Albcre tg+ mice and their littermate controls (supplementary Fig. 2B). Fasting plasma insulin levels in males with hepatocyte-specific ablation of leptin signaling were also similar to levels in littermate controls. However, the Leprflox/flox Albcre tg+ females had lower fasting plasma insulin levels compared with their littermate controls at all ages measured, and this difference was statistically significant at 16 weeks of age (P = 0.042).
Loss of hepatic leptin signaling protects against age- and diet-related glucose intolerance.
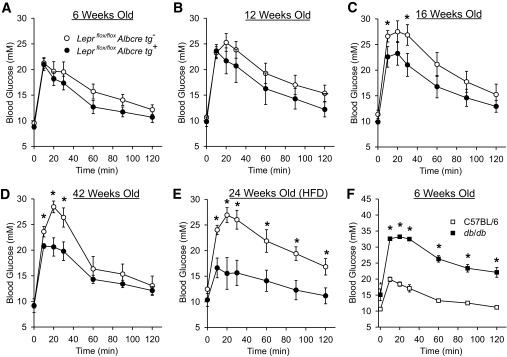
To assess if the response to a nutritional challenge may be altered by a loss of hepatic leptin signaling, oral glucose tolerance tests (OGTTs) were performed on Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− littermate controls at several different ages, along with db/db and C57BL/6 wild-type controls. As expected, the complete absence of leptin signaling in the male db/db mice resulted in striking glucose intolerance in young 6-week-old mice (Fig. 3F). Surprisingly, in contrast to the db/db mice, the 6-week-old male mice lacking hepatic leptin signaling had a trend toward slightly improved glucose tolerance (Fig. 3A). This trend became more prominent as the mice aged and the differences in the peak blood glucose values were statistically significant in 16-week-old mice and beyond (Figs. 3B–D). At 16 and 42 weeks old, the Leprflox/flox Albcre tg+ mice had an 18% improvement in glucose excursion relative to the littermate controls as measured by area under the curve (AUC) analysis. Interestingly, we did not see such a difference in females when comparing the Leprflox/flox Albcre tg+ mice and their littermate controls at the same ages as the males (supplementary Fig. 3). Since it appeared that male mice lacking hepatic leptin signaling were protected from an age-related deterioration of glucose tolerance, we tested if this protection would persist when fed a high-fat diet. Leprflox/flox Albcre tg+ and their littermate controls were fed a high-fat diet for 20 weeks starting at 4 weeks of age, and OGTTs were performed. Similar to the chow-fed mice, the high-fat–fed male mice lacking hepatic leptin signaling had improved glucose tolerance relative to littermate controls (Fig. 3E) despite no differences in body weight (55.9 ± 4.0 g for Leprflox/flox Albcre tg+ and 54.3 ± 2.6 g for Leprflox/flox Albcre tg−, P = 0.365, n ≥ 6). The magnitude of this improvement in glucose tolerance was greater in high-fat–fed mice than the chow-fed mice, as there was a 40% improved glucose excursion (by AUC analysis) in mice lacking hepatic leptin signaling. Thus, the loss of hepatic leptin signaling allows mice to retain normal glucose tolerance as they age even when on a high-fat diet.
FIG. 3.
Loss of hepatic leptin signaling prevents age- and diet-related glucose intolerance. Oral glucose tolerance tests were performed on male (A–E) Leprflox/flox Albcre tg+ mice and littermate controls or (F) db/db mice and C57BL/6 controls at the indicated ages. E: Mice were fed a high-fat diet (HFD) for 20 weeks, fasted for 4 h, and gavaged with 1.22 mg/g glucose. Data are mean ± SEM and n ≥ 6. *P ≤ 0.05 vs. wild-type control.
Since it has been reported that a cre transgene, the RIPcre transgene, itself can cause glucose intolerance (30), we investigated if the differences in glucose tolerance in our studies were a direct result of excision of lepr exon 17 or possibly the presence of the Albcre transgene. To test this, we performed OGTTs in male Leprflox/wt Albcre tg+ and their littermate controls at 16 weeks of age. Glucose excursion was very similar between the two groups of mice when analyzed by AUC analysis (Leprflox/wt Albcre tg− 1,570 ± 140 vs. Leprflox/wt Albcre tg+ 1,556 ± 220 and P = 0.480, n ≥ 7). This reveals that the presence of the cre transgene itself is not the cause of the altered glucose tolerance (Fig. 3) but rather the loss of leptin signaling in hepatocytes, and that the phenotype is only present when both copies of the leptin receptor allele are disrupted.
Increased glucose-stimulated plasma insulin levels and increased insulin sensitivity contribute to improved glucose tolerance in mice lacking hepatic leptin signaling.
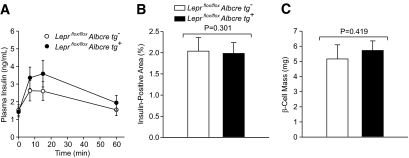
To explore the potential mechanism of improved glucose tolerance in the Leprflox/flox Albcre tg+ mice, we examined aspects of insulin secretion in mice with and without hepatic leptin signaling. As seen in Fig. 4A, following a glucose gavage, the Leprflox/flox Albcre tg+ mice had 1.35-fold increase in plasma insulin relative to the Leprflox/flox Albcre tg− littermate controls (AUC of 252 ± 33 and 186 ± 31, respectively, P = 0.053, n ≥ 13). This increase was not associated with an increase in β-cell mass (Figs. 4B and C). Similarly, a trend for increased glucose-stimulated insulin levels was also seen in Leprflox/flox Albcre tg+ mice on a high-fat diet (supplementary Fig. 4).
FIG. 4.
Attenuation of hepatic leptin signaling increases glucose-stimulated insulin levels. A: Plasma insulin levels were monitored following a gavage of 1.5-mg/g body weight glucose to assess steady-state levels of glucose-stimulated insulin secretion in 16- to 20-week-old male Leprflox/flox Albcre tg+ and tg− mice, n ≥ 13 in each group. B: Insulin-positive area as a function of total pancreas area and (C) total pancreatic β-cell mass, n ≥ 5 mice per genotype and 3 sections measured per pancreas from 22-week-old male mice. All data are mean ± SEM.
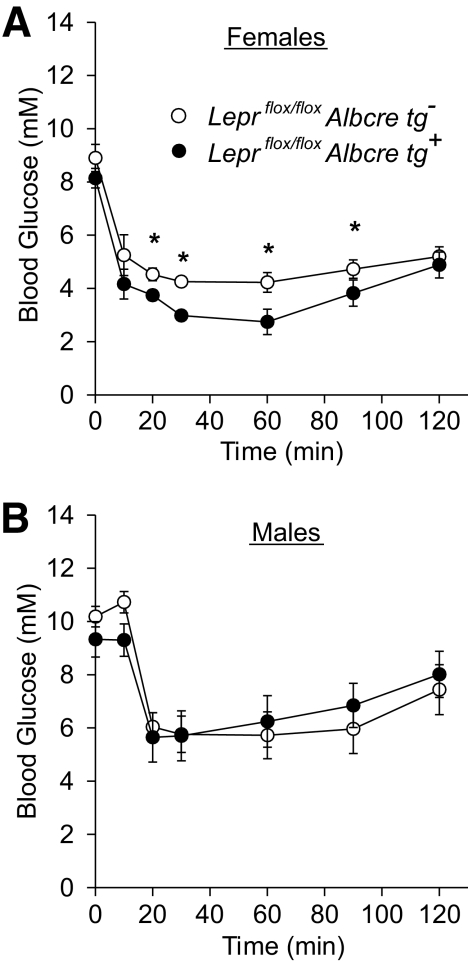
While the increased plasma insulin following a glucose challenge likely contributes to the enhanced glucose tolerance in mice lacking hepatic leptin signaling, we wanted to determine if insulin sensitivity may also contribute to this phenotype. To assess whole-body insulin sensitivity, insulin tolerance tests were performed in male and female mice at 19 weeks of age. The Leprflox/flox Albcre tg+ females displayed increased insulin sensitivity compared with the Leprflox/flox Albcre tg− controls (Fig. 5A), while there was no difference in whole-body insulin sensitivity in the male mice (Fig. 5B).
FIG. 5.
Ablation of hepatic leptin signaling increases insulin sensitivity. Insulin tolerance tests were performed on (A) 19-week-old females (n ≥ 4) and (B) 21-week-old males (n ≥ 6) with and without the Albcre transgene. All data are mean ± SEM. *P ≤ 0.05 vs. littermate controls.
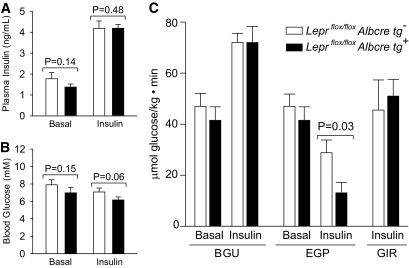
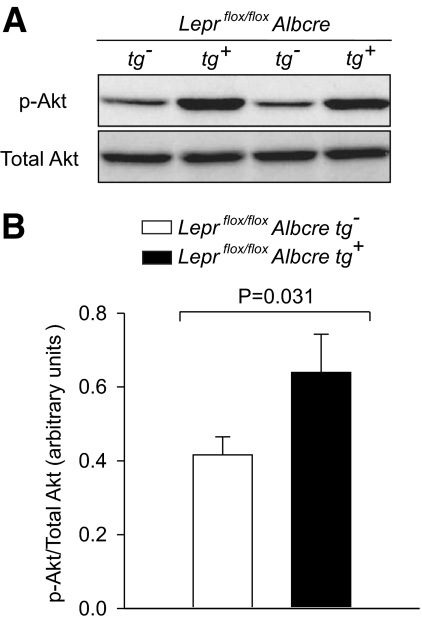
To more specifically explore insulin sensitivity at the level of the liver in male mice, hyperinsulinemic-euglycemic clamp studies were performed. Figure 6A and B shows that hyperinsulinemia was achieved while maintaining euglycemia during the clamp, and that no differences in blood glucose or plasma insulin levels were observed between Leprflox/flox Albcre tg+ and their Leprflox/flox Albcre tg− littermate controls. In the basal (non-hyperinsulinemic) state, whole-body glucose utilization and endogenous glucose production were similar for the Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− littermate controls (Fig. 6C). As expected, in both Leprflox/flox Albcre tg+ and Leprflox/flox Albcre tg− mice, glucose utilization increased and endogenous glucose production decreased in the hyperinsulinemic phase. While whole-body glucose utilization was similar between the mice with and without hepatic leptin signaling, there was a significant difference in insulin-induced suppression of endogenous glucose production (P = 0.03). In the littermate controls, insulin suppressed glucose production by 39%, while in the Leprflox/flox Albcre tg+ mice, insulin suppressed glucose production by 68%. Since the majority of endogenously produced glucose comes from hepatocytes, these data provide evidence that a loss of hepatic leptin signaling mediates enhanced insulin sensitivity in the liver. This notion is supported by our finding that levels of phosphorylated Akt, a key mediator of insulin signaling, was increased in mice lacking hepatic leptin signaling when compared with littermate controls (Fig. 7). Taken together, our data suggest that a loss of hepatic leptin signaling leads to increased insulin sensitivity in the liver.
FIG. 6.
Loss of hepatic leptin signaling enhances liver insulin sensitivity. Male Leprflox/flox Albcre tg+ mice and littermate controls (16 to 20 weeks old) were used in a hyperinsulinemic-euglycemic clamp as described in Research Design and Methods, n ≥ 6. A and B: Plasma insulin and blood glucose levels during basal and hyperinsulinemic states. C: Whole-body glucose utilization (BGU), endogenous glucose production (EGP), and glucose infusion rate (GIR). Data are mean ± SEM.
FIG. 7.
Attenuation of hepatic leptin signaling results in increased insulin-stimulated phosphorylation of Akt in the liver. Following the hyperinsulinemic-euglycemic clamp, liver tissues were harvested and flash-frozen. Liver lysates were prepared and Western blots performed for phosphorylated and total Akt levels. Representative blots from two Leprflox/flox Albcre tg− and two Leprflox/flox Albcre tg+ mice are shown in A. Quantification of all samples by densitometry is shown in B, n ≥ 8. Data are mean ± SEM. p-Akt, phosphorylated Akt.
DISCUSSION
These studies reveal new insights into the role of leptin signaling in the liver. First, we have shown that our mice lacking the leptin receptor signaling domain in the liver have increased hepatic lipid accumulation. This is consistent with the potent lipolytic effects of leptin: db/db mice have substantial hepatic steatosis, and the re-expression of leptin receptors in the livers of leptin receptor-deficient Zucker rats reduces hepatic steatosis (31). Further, our data show that mice lacking hepatic leptin signaling have increased insulin sensitivity in the liver, which given that insulin is a potent promoter of lipogenesis (32) and a suppressor of lipid export from the liver (33), would be expected to contribute to increased hepatic lipid levels. In contrast to our findings, Cohen et al. (20) reported that mice with a lack of the complete leptin receptor in the liver have normal hepatic lipid levels. While our mouse model lacked the leptin receptor signaling domain in the liver but maintained the extracellular and transmembrane domains, mice studied by Cohen et al. had a knockout of the complete receptor. Thus, there may be a role for the extracellular and transmembrane domains of the leptin receptor in liver lipid metabolism. However, we also note that Cohen et al. used different methods to assess hepatic lipid levels, including possibly using mice of different age than in our study (age was unspecified by Cohen et al.) and using mice heterozygous for the floxed leptin receptor gene as controls. These differences in methods could potentially explain the differences in hepatic lipid content seen by us and by Cohen et al. since mice heterozygous for the leptin receptor (db/+) or the leptin gene (ob/+) have been shown to have changes in metabolism when compared with wild-type mice (34), with some changes in ob/+ mice being age-related (35). Under the conditions that we measured hepatic lipid levels, mice with an ablation of the hepatic leptin receptor signaling domain have an increase in hepatic triglyceride and cholesterol levels when compared with littermate controls, suggesting that the ability of leptin to ameliorate hepatic steatosis in rodent models of type 2 diabetes (31) and lipodystrophy (36) may be mediated, in part, through leptin signaling at the level of the liver.
Similar to the work of Cohen et al. (20), we find that the loss of hepatic leptin signaling does not have a major influence on glucose metabolism in the mildly fasting or random-fed state. However, we have extended the work of Cohen et al. to reveal that leptin action on the liver does influence glucose metabolism under certain metabolic stressors. Specifically, as male mice aged, those lacking leptin signaling in the liver performed substantially better during a glucose challenge, remarkably even when fed a high-fat diet. This improved glucose tolerance was likely related to increased hepatic insulin sensitivity in 16- to 20-week-old mice and a trend toward increased glucose-stimulated plasma insulin levels perhaps caused by changes in interorgan communication between the liver and the pancreas, which has been demonstrated by several studies (7,37,38). Taken together, our data show that aged mice lacking hepatic leptin signaling appear to benefit from the combined effect of increased hepatic insulin sensitivity and increased glucose-stimulated insulin levels, resulting in markedly improved glucose tolerance.
Although we did see an improvement in insulin sensitivity as well as reduced fasting insulin levels in female mice lacking hepatic leptin signaling compared with controls, this did not manifest in differences in glucose clearance during an oral glucose challenge. We speculate that this relates to the better insulin sensitivity and glucose tolerance in general of female mice relative to their male counterparts. Since the female controls did not develop glucose intolerance during the time frame we examined, we did not see an improvement in glucose tolerance in female mice lacking hepatic leptin signaling despite the improved insulin sensitivity.
Many studies have investigated the effect of leptin on insulin sensitivity; however there is no clear consensus in the literature if leptin has pro- or anti-insulin sensitizing effects on the liver (39). In agreement with our current data, several other studies have found that leptin can attenuate insulin-induced activities in the liver (17,40). Other studies reveal that leptin can increase insulin-mediated actions in the liver (41–43). Consistent with leptin mediating both pro- and anti-insulin sensitizing effects, we have observed leptin to increase insulin receptor phosphorylation while at the same time increasing expression of protein-tyrosine phosphatase 1B (PTP1B) (41), a negative regulator of insulin receptor signaling (44,45). Reports regarding the effects of leptin on components of the insulin signaling pathway in hepatocytes also differ considerably. For example, leptin has been reported to alter insulin signaling by increasing association of p85 with insulin receptor substrate (IRS)-1 and decreasing IRS-2 tyrosine phosphorylation (17), and alternatively by increasing IRS-2 tyrosine phosphorylation and association with p85 and decreasing IRS-1 tyrosine phosphorylation (42). Other studies have found leptin to reduce insulin-dependent IRS-1/IRS-2 association with p85 (40) while still other studies find that leptin is not able to modify the effects of insulin on IRS-1/IRS-2 phosphorylation (45). Thus, it appears there is a complex relationship between leptin and its effects on hepatic insulin sensitivity and signaling. Chronic versus acute actions of leptin may be one factor dictating if leptin exerts pro- or anti-insulin sensitizing effects on the liver. From our current work, we have assessed the functional significance of a chronic loss of hepatic leptin activity and found that this leads ultimately to increased hepatic insulin sensitivity.
It is interesting that a complete loss of leptin receptor signaling (db/db mice) is associated with a worsening of glucose homeostasis, whereas the loss of leptin action on the liver appears to function in the opposite manner. This observation suggests that leptin has divergent effects on different tissues. While it has been shown that leptin can act directly on the brain to cause a secondary increase in insulin sensitivity in the liver (12,13), our study shows that losing functional leptin receptors in the liver can also increase hepatic insulin sensitivity. Thus, the present study provides evidence that under certain conditions, leptin action on hepatocytes may function to curtail and control the extent of insulin action on the liver. This is consistent with the action of leptin to limit insulin effects in the periphery by directly suppressing insulin secretion from pancreatic β-cells (46–48) and decreasing lipid storage in adipose tissue (49,50). It is possible that leptin plays an important role in keeping insulin effects in check to protect against hypoglycemia after postprandial insulin release. This may explain why the most striking effects on glucose metabolism that we saw as a result of ablated hepatic leptin signaling occurred during the postprandial state (Fig. 3) or during a state of hyperinsulinemia (Figs. 6 and 7) and not when insulin levels were low (supplementary Fig. 2). Therefore, leptin may differentially regulate glucose metabolism by acting either on the brain or the periphery, and the overall effect of leptin may depend on the current metabolic state. Clearly, a complex relationship exists between the effects of leptin on the brain and the periphery, and a disruption of this relationship may result in metabolic abnormalities such as diabetes and obesity. Nonetheless, given the remarkable ability of a loss of hepatic leptin signaling to protect against glucose intolerance during aging and a high-fat diet, which are two of the most prevalent risk factors for type 2 diabetes, hepatic leptin signaling is a candidate therapeutic target and further studies are warranted in this area.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Canadian Institutes of Health Research. F.K.H. is the recipient of scholarships from the Michael Smith Foundation for Health Research (MSFHR) and the Natural Sciences and Engineering Research Council. T.J.K. is the recipient of a Senior Scholarship from MSFHR.
No potential conflicts of interest relevant to this article were reported.
F.K.H., J.L., S.L.G., P.J.V., S.D.C., and T.J.K. designed the research; F.K.H., J.L., H.C.D., S.L.G., P.J.V., U.H.N., M.S., and S.D.C. performed the research; S.C.C. contributed mice; F.K.H., J.L., H.C.D., S.L.G., P.J.V., U.H.N., and S.D.C. analyzed data; and F.K.H., J.L., S.D.C., and T.J.K. wrote the article.
We thank Andrew Yung and Jenny Tso of the University of British Columbia MRI Research Centre for performing measurements of lean-to-lipid mass ratios.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 2.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S: Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995;1:1155–1161 [DOI] [PubMed] [Google Scholar]
- 3.Coleman DL: Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 1978;14:141–148 [DOI] [PubMed] [Google Scholar]
- 4.Flatt PR, Bailey CJ, Cameron AM, Gould BJ: Age effects on glycosylated blood proteins in lean and obese hyperglycaemic (ob/ob) mice. Diabetes Res 1986;3:241–243 [PubMed] [Google Scholar]
- 5.Coleman DL, Hummel KP: Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia 1974;10(Suppl.):607–610 [DOI] [PubMed] [Google Scholar]
- 6.Seufert J, Kieffer TJ, Habener JF: Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci U S A 1999;96:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM: Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 2010;11:11–22 [DOI] [PubMed] [Google Scholar]
- 8.Reitman ML, Arioglu E, Gavrilova O, Taylor SI: Lipoatrophy revisited. Trends Endocrinol Metab 2000;11:410–416 [DOI] [PubMed] [Google Scholar]
- 9.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A: Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–578 [DOI] [PubMed] [Google Scholar]
- 10.Friedman JM, Halaas JL: Leptin and the regulation of body weight in mammals. Nature 1998;395:763–770 [DOI] [PubMed] [Google Scholar]
- 11.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjørbaek C: Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 2009;9:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ: Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, Rossetti L: Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem 1998;273:31160–31167 [DOI] [PubMed] [Google Scholar]
- 14.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI: Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263–1271 [DOI] [PubMed] [Google Scholar]
- 15.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC: Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A 1996;93:6231–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM: Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A 1997;94:7001–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen B, Novick D, Rubinstein M: Modulation of insulin activities by leptin. Science 1996;274:1185–1188 [DOI] [PubMed] [Google Scholar]
- 18.Szanto I, Kahn CR: Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci U S A 2000;97:2355–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam NT, Cheung AT, Riedel MJ, Light PE, Cheeseman CI, Kieffer TJ: Leptin reduces glucose transport and cellular ATP levels in INS-1 β-cells. J Mol Endocrinol 2004;32:415–424 [DOI] [PubMed] [Google Scholar]
- 20.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM: Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 2001;108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Künnecke B, Verry P, Bénardeau A, von Kienlin M: Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res 2004;12:1604–1615 [DOI] [PubMed] [Google Scholar]
- 22.Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V: Lipotoxicity of the pancreatic β-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 2001;50:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voshol PJ, Jong MC, Dahlmans VE, Kratky D, Levak-Frank S, Zechner R, Romijn JA, Havekes LM: In muscle-specific lipoprotein lipase–overexpressing mice, muscle triglyceride content is increased without inhibition of insulin-stimulated whole-body and muscle-specific glucose uptake. Diabetes 2001;50:2585–2590 [DOI] [PubMed] [Google Scholar]
- 24.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB: Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004;42:983–991 [DOI] [PubMed] [Google Scholar]
- 25.McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua SC, Jr: An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome 2004;15:677–685 [DOI] [PubMed] [Google Scholar]
- 26.Banks AS, Davis SM, Bates SH, Myers MG, Jr: Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 2000;275:14563–14572 [DOI] [PubMed] [Google Scholar]
- 27.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK: The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 2005;1:63–72 [DOI] [PubMed] [Google Scholar]
- 28.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB: Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006;49:191–203 [DOI] [PubMed] [Google Scholar]
- 29.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA: Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β-cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999;274:305–315 [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L: RIP-Cre revisited, evidence for impairments of pancreatic β-cell function. J Biol Chem 2006;281:2649–2653 [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH: Liporegulation in diet-induced obesity: the antisteatotic role of hyperleptinemia. J Biol Chem 2001;276:5629–5635 [DOI] [PubMed] [Google Scholar]
- 32.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL: Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A 1999;96:13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grefhorst A, Hoekstra J, Derks TG, Ouwens DM, Baller JF, Havinga R, Havekes LM, Romijn JA, Kuipers F: Acute hepatic steatosis in mice by blocking β-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am J Physiol Gastrointest Liver Physiol 2005;289:G592–G598 [DOI] [PubMed] [Google Scholar]
- 34.Coleman DL: Obesity genes: beneficial effects in heterozygous mice. Science 1979;203:663–665 [DOI] [PubMed] [Google Scholar]
- 35.Haller EW, Wittmers LE, Jr, Haller IV, Regal RR: The obese gene is expressed in lean littermates of the genetically obese mouse (C57BL/6J ob/ob). Am J Physiol 1999;276:E762–E765 [DOI] [PubMed] [Google Scholar]
- 36.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL: Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 1999;401:73–76 [DOI] [PubMed] [Google Scholar]
- 37.Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, Ishihara H, Niijima A, Nakazato M, Asano T, Minokoshi Y, Oka Y: Regulation of pancreatic β-cell mass by neuronal signals from the liver. Science 2008;322:1250–1254 [DOI] [PubMed] [Google Scholar]
- 38.Lautt WW, Ming Z, Legare DJ: Attenuation of age- and sucrose-induced insulin resistance and syndrome X by a synergistic antioxidant cocktail: the AMIS syndrome and HISS hypothesis. Can J Physiol Pharmacol 2010;88:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceddia RB, Koistinen HA, Zierath JR, Sweeney G: Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J 2002;16:1163–1176 [DOI] [PubMed] [Google Scholar]
- 40.Benomar Y, Wetzler S, Larue-Achagiotis C, Djiane J, Tomé D, Taouis M: In vivo leptin infusion impairs insulin and leptin signalling in liver and hypothalamus. Mol Cell Endocrinol 2005;242:59–66 [DOI] [PubMed] [Google Scholar]
- 41.Lam NT, Lewis JT, Cheung AT, Luk CT, Tse J, Wang J, Bryer-Ash M, Kolls JK, Kieffer TJ: Leptin increases hepatic insulin sensitivity and protein tyrosine phosphatase 1B expression. Mol Endocrinol 2004;18:1333–1345 [DOI] [PubMed] [Google Scholar]
- 42.Anderwald C, Müller G, Koca G, Fürnsinn C, Waldhäusl W, Roden M: Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Mol Endocrinol 2002;16:1612–1628 [DOI] [PubMed] [Google Scholar]
- 43.Fishman S, Muzumdar RH, Atzmon G, Ma X, Yang X, Einstein FH, Barzilai N: Resistance to leptin action is the major determinant of hepatic triglyceride accumulation in vivo. FASEB J 2007;21:53–60 [DOI] [PubMed] [Google Scholar]
- 44.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP: Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999;283:1544–1548 [DOI] [PubMed] [Google Scholar]
- 45.Carvalheira JB, Ribeiro EB, Folli F, Velloso LA, Saad MJ: Interaction between leptin and insulin signaling pathways differentially affects JAK-STAT and PI 3-kinase-mediated signaling in rat liver. Biol Chem 2003;384:151–159 [DOI] [PubMed] [Google Scholar]
- 46.Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF: Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic β-cells. Diabetes 1997;46:1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ: The pancreatic β-cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 2006;4:291–302 [DOI] [PubMed] [Google Scholar]
- 48.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN: Disruption of leptin receptor expression in the pancreas directly affects β-cell growth and function in mice. J Clin Invest 2007;117:2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH: Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci U S A 2008;105:6139–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller G, Ertl J, Gerl M, Preibisch G: Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem 1997;272:10585–10593 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.