Abstract
OBJECTIVE
Inducing human β-cell growth while enhancing function is a major goal in the treatment of diabetes. Parathyroid hormone–related protein (PTHrP) enhances rodent β-cell growth and function through the parathyroid hormone-1 receptor (PTH1R). Based on this, we hypothesized that PTH1R is expressed in human β-cells and that PTHrP has the potential to enhance human β-cell proliferation and/or function.
RESEARCH DESIGN AND METHODS
PTH1R expression, β-cell proliferation, glucose-stimulated insulin secretion (GSIS), and expression of differentiation and cell-cycle genes were analyzed in human islets transduced with adenoviral PTHrP constructs or treated with PTHrP peptides. The effect of overexpression of late G1/S cell cycle molecules was also assessed on human β-cell proliferation.
RESULTS
We found that human β-cells express PTH1R. More importantly, overexpression of PTHrP causes a significant approximately threefold increase in human β-cell proliferation. Furthermore, the amino terminus PTHrP(1-36) peptide is sufficient to increase replication as well as expression of the late G1/S cell-cycle proteins cyclin E and cyclin-dependent kinase 2 (cdk2) in human islets. Notably, PTHrP(1-36) also enhances GSIS. Finally, overexpression of cyclin E alone, but not cdk2, augments human β-cell proliferation, and when both molecules are expressed simultaneously there is a further marked synergistic increase in replication.
CONCLUSIONS
PTHrP(1-36) peptide enhances human β-cell proliferation as well as function, with associated upregulation of two specific cell-cycle activators that together can induce human β-cell proliferation several fold. The future therapeutic potential of PTHrP(1-36) for the treatment of diabetes is especially relevant given the complementary therapeutic efficacy of PTHrP(1-36) in postmenopausal osteoporosis.
Loss of functional pancreatic β-cells is the ultimate cause of both type 1 and type 2 diabetes. Therefore, finding ways to maintain or increase pancreatic β-cell growth and function is essential in the long-term treatment of this disease. Many studies over the last decades have shown that adult human β-cells retain the capacity to proliferate, in vivo (1) and in vitro (2,3), providing impetus to search for factors that can further enhance β-cell proliferation without negatively impacting their function.
Although several growth factors enhance rodent β-cell proliferation and function (4), very few have been tested on adult human β-cells. In this regard, PTHrP, an autocrine/paracrine factor that is expressed in almost every tissue in the body, including the endocrine pancreas (5), has beneficial effects on both β-cell growth and function in rodents. PTHrP overexpression in the β-cell of transgenic mice leads to increased β-cell proliferation, β-cell hyperplasia with concomitant hyperinsulinemia and hypoglycemia, improved glucose tolerance, and enhanced GSIS (6–8).
PTHrP is directed into the secretory pathway through its signal peptide (SP) and can be targeted to the nucleus through its nuclear localization signal. It undergoes posttranslational processing to form amino terminus (1-36), mid-region (38-94), and COOH-terminal (107-171) fragments. PTHrP mutants that lack SP fail to be secreted. PTHrP signals through a seven-transmembrane G-protein coupled receptor, PTH1R, which recognizes the amino terminus PTHrP peptide (9). PTH1R is expressed in rodent β-cells and also in rodent β-cell lines (8,10,11). Several in vitro studies have shown that the amino-terminal PTHrP(1-36) peptide, through its receptor, is sufficient to enhance rodent β-cell proliferation as well as insulin expression and GSIS (8,11,12). Thus, PTHrP enhances both replication and function in primary rodent β-cells.
PTHrP is also expressed in human islets and in almost 100% of human insulinomas (5,13,14). However, whether PTH1R is expressed in human islets or in the human β-cell is not known. More importantly, it is unknown whether PTHrP can have beneficial effects on human β-cells. Based on extensive studies in rodent islets, we hypothesize that the PTHrP/PTH1R system has the potential to enhance proliferation and/or function in human islets. Examining the therapeutic potential for PTHrP in human islets is especially significant given that PTHrP(1-36) is well established as a skeletal anabolic agent for the treatment of osteoporosis (15,16), demonstrating the safety of this peptide as a future therapeutic agent.
RESEARCH DESIGN AND METHODS
Human islets, cell culture, and transduction with adenovirus.
Human islets were obtained from donors of both sexes, of ages ranging from ∼5 to 70 years and with a majority being in the 20–50 year range, and with purity ranging from 50–95%, through the Islet Cell Resource Centers (ICRs) and Juvenile Diabetes Research Foundation Basic Science Islet Distribution Programs. Human kidney cell lines, HKC8, and 293 with or without human PTH1R cDNA were used as controls for PTH1R expression. Adenovirus (Ad) constructs with cDNAs were generated (8) including those for β-galactosidase (LacZ), Cre recombinase, and green fluorescent protein, which were used as controls.
Proliferation.
For cell-cycle analysis, human islets transduced with Ad constructs for 72 h were dispersed into single cells and 10,000 cells were analyzed on a BD LSR II flow cytometer (Becton Dickinson Biosciences, San Jose, CA). Data analysis was performed using Modfit LT 3.0 software (Verity Software House, Topsham, ME). β-cell proliferation was analyzed on either Ad-transduced or peptide-treated dispersed human islet cultures stained for DAPI, bromodeoxyuridine (BrdU), and insulin using Olympus Fluoview Confocal or Olympus Provis Fluorescence microscopes (8).
Insulin release and content.
Insulin release from uninfected, PTHrP(1-36) treated, and Ad-transduced islets was measured in triplicate from human islets either transduced with Ad constructs for 24 h or treated with 100 nmol/l of PTHrP(1-36) for 24 h for insulin content or 30 min for GSIS (8). Briefly, uninfected or islets transduced with Ad for 24 h were preincubated in Krebs-Ringer bicarbonate buffer supplemented with 10 mmol/l HEPES, 1% BSA, and 5 mmol/l glucose for 1 h at 37°C in a 5% CO2 incubator. After washing the islets once with the same solution, groups of 15 islet equivalents (IEQs) for each condition were incubated in 1 ml fresh Krebs-Ringer bicarbonate buffer plus 1% BSA and 5.5 or 22 mmol/l glucose. PTHrP peptide or vehicle was added to the islets at the same time as the glucose and incubated for 30 min, after which buffer was removed and frozen at −20°C until insulin measurement by radioimmunoissay (RIA) (Linco Research, St. Louis, MO). Islets were then digested overnight in NaOH at 37°C, and protein was measured by the Bradford method after neutralization with HCl. Results are expressed as a percentage of insulin concentration obtained with uninfected islets incubated at 5.5 mmol/l glucose. Insulin content was measured by radioimmunoassay on acid ethanol extracts of human islets either transduced with Ad-control or Ad-PTHrP or treated with vehicle or 100 nmol/l of PTHrP(1-36) peptide for 24h (8,17).
Gene expression.
Gene expression in whole islets was analyzed by real-time PCR performed on an ABI 7300 System and Western blot analysis on 20–50 μg extracts using the Image J program (NIH) for quantitative densitometry (8). PTHrP(1-36) was quantitated using an immunoradiometric assay or an enzyme immunoassay (Peninsula Laboratories, San Carlos, CA).
Statistical analysis.
The data are expressed as the mean ± SE. Statistical significance considered at P < 0.05 was determined by an unpaired two-tailed Student's t test or a one-way ANOVA with Tukey's honestly significant difference post hoc test. Additional details regarding research design and methods are available in an online appendix (http://diabetes.diabetesjournals.org/cgi/content/full/db09-1796/DC1).
RESULTS
PTH1R and PTHrP expression in human islets.
PTHrP mRNA and protein are expressed in human islets and insulinomas (5,13,14). However, whether the receptor for PTHrP, PTH1R, is expressed in human islets is unknown. We analyzed the expression of PTH1R mRNA by real-time PCR in human islets using HKC8, a human kidney cell line with high PTH1R expression, as a positive control and 293 cells that lack PTH1R expression as a negative control. PTH1R mRNA is clearly detectable in human islets (Fig. 1A), as is PTH1R protein, measured by Western blot (Fig. 1B). Importantly, PTH1R is expressed in human β-cells, demonstrated by costaining of human islet cell cultures for PTH1R and insulin (Fig. 1C).
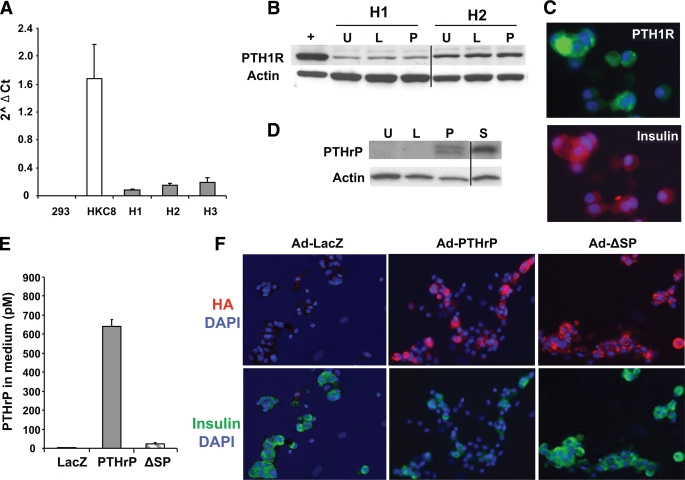
FIG. 1.
PTH1R and PTHrP expression in human islets. A: PTH1R mRNA expression measured by real-time PCR in three human islet preps (H1–H3); HKC8, a human kidney proximal tubule cell line, used as positive control; and the human embryonic kidney cell line 293, used as negative control. Quantitation is shown as log of Ct using actin as the housekeeping control gene. B: Western blot analysis of PTH1R and actin expression in a positive (+) control line (293 cells stably transfected with human PTH1R cDNA) and two human islet preps (H1 and H2) uninfected (U) or transduced with Ad-LacZ (L) or Ad-PTHrP (P). The line divides samples run on two different gels. C: Photomicrograph of human islet cell cultures costained for nuclear DAPI (blue), PTH1R (green), and insulin (red). D: Representative Western blot analysis of PTHrP and actin in human islets either uninfected (U) or transduced with Ad-LacZ (L), Ad-PTHrP (P), or Ad-ΔSP mutant (S). The line divides samples from different regions run on the same gel. E: Quantitation of PTHrP(1-36) by immunoradiometric assay in medium collected after 48 h of transduction of human islets with adenoviral constructs containing LacZ, PTHrP, or ΔSP sequences (n = 2 islet preparations). F: Representative images of human islet cell cultures transduced with Ad-LacZ, Ad-PTHrP, or Ad-ΔSP and costained for DAPI (blue), insulin (green), and HA (red). (A high-quality digital representation of this figure is available in the online issue.)
To overexpress PTHrP in human islets, an Ad construct containing hemagglutinin (HA)-tagged human PTHrP cDNA was transduced into islets. Ad-PTHrP–transduced human islets display increased PTHrP expression relative to uninfected and Ad-LacZ–transduced islets as demonstrated by Western blot using PTHrP antibody (Fig. 1D). Furthermore, PTHrP overexpression did not result in downregulation of PTH1R expression in human islets (Fig. 1B) compared with uninfected or Ad-LacZ–transduced islets (Fig. 1B). Since PTHrP is a secreted protein, we measured its concentration in medium from human islets transduced with Ad-PTHrP or Ad-LacZ. There was significant accumulation of PTHrP, ∼600 pmol/l, in medium from Ad-PTHrP–infected islets versus Ad-LacZ–infected islets (4 pmol/l) (Fig. 1E). Finally, PTHrP is expressed in β-cells of Ad-PTHrP–transduced human islet cell cultures demonstrated by coimmunostaining with HA and insulin antibodies (Fig. 1F).
PTHrP increases human β-cell proliferation without causing dedifferentiation.
We examined the effect of PTHrP overexpression on human β-cell proliferation by costaining human islet cell cultures transduced with Ad-PTHrP or Ad-control with BrdU and insulin antibodies (Fig. 2A). There was a significant, approximately threefold, increase in human β-cell proliferation induced by PTHrP compared with Ad-control–transduced or uninfected human islet cell cultures (Fig. 2B).
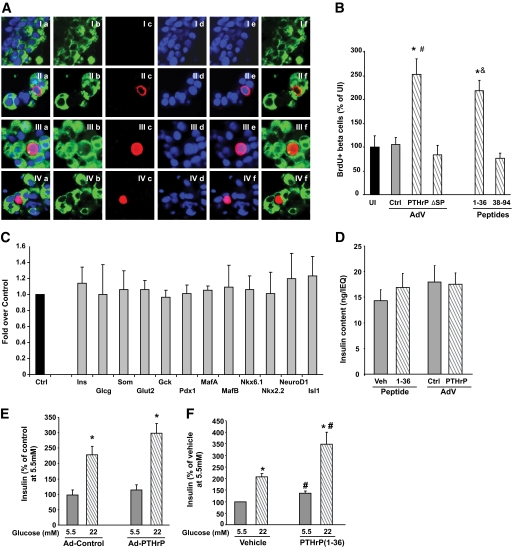
FIG. 2.
PTHrP increases human β-cell proliferation and function without causing dedifferentiation. A: Representative confocal images of human islet cell cultures transduced with Ad-control (I) or Ad-PTHrP (II–IV) and costained for insulin (green, panel b), BrdU (red, panel c), and DAPI (blue, panel d). The merged images of all three stainings are shown in panel a, merge for DAPI and BrdU in panel e, and merge for insulin and BrdU in panel f. B: Quantitation of the percentage of BrdU-positive β-cells from human islet cell cultures uninfected (UI) or transduced with the adenovirus (AdV) constructs control (ctrl), PTHrP, or ΔSP for 72 h or treated with 100 nmol/l of either 1-36 or 38-94 PTHrP peptides for 24 h. There was a significant two- to threefold increase in β-cell proliferation in PTHrP-transduced and 1-36–treated vs. Ad-control–transduced or uninfected control islet cells. Basal rate of β-cell proliferation in uninfected controls was 0.08 ± 0.03% (n = 4–11 individual human islet preps at least in duplicate). *P < 0.05 vs. uninfected or Ad-control by Student's t test; #P < 0.001 vs. Ad-control or Ad-ΔSP and &P < 0.01 vs. UI or 38-94 by one-way ANOVA. C: Expression of differentiation markers by real-time PCR from human islets treated for 24 h with vehicle control (Ctrl) or PTHrP(1-36) peptide (gray bars). PCR cycles for each gene were compared, with actin used as an internal control. The graph is depicted as fold over control, with values from vehicle-treated islets taken as 1 (n = 4–7 human islet preparations in duplicate). D: Insulin content per IEQ in extracts of human islets treated with vehicle (veh) or 100 nmol/l PTHrP(1-36) peptide or transduced with Ad-control (Ctrl) or Ad-PTHrP for 24 h (n = 5 human islet preparations in triplicate). E and F: Insulin secretion measured at 5.5 and 22 mmol/l glucose from human islets transduced with Ad-control or Ad-PTHrP for 24 h (E) and treated with vehicle or 100 nmol/l PTHrP(1-36) peptide for 30 min (F). Insulin secretion is depicted as percentage of vehicle-treated control at 5.5 mmol/l glucose, which was 455.4 ± 109.3 pg/μg protein in 30 min. n = 7–8 human islet preparations in triplicate. *P < 0.05 vs. insulin secretion at 5.5 mmol/l glucose with the same treatment; #P < 0.05 vs. insulin secretion at equivalent glucose concentrations of control. (A high-quality digital representation of this figure is available in the online issue.)
Based on studies in rodent β-cells, we evaluated whether secreted PTHrP is required for human β-cell proliferation by transducing human islets with Ad-ΔSP, a HA-tagged nonsecretory PTHrP mutant that lacks the SP. As expected, secreted PTHrP levels in the medium were much lower in Ad-ΔSP– (22 pmol/l) versus Ad-PTHrP–transduced (600 pmol/l) human islets (Fig. 1E). However, PTHrP levels in islet extracts from Ad-ΔSP were at least comparable with Ad-PTHrP–transduced human islets (Fig. 1D). Moreover, PTHrP was expressed in the β-cells of Ad-ΔSP–transduced human islet cultures as shown by HA and insulin costaining (Fig. 1F). Despite abundant intracellular PTHrP, Ad-ΔSP did not stimulate proliferation of human β-cells relative to uninfected and Ad-control–infected islet cells (Fig. 2B), demonstrating that secreted PTHrP is important for mediating human β-cell proliferation.
To determine which specific secretory form of PTHrP induces human β-cell proliferation, human islet cultures were treated for 24 h with 100 nmol/l of either amino-terminal PTHrP(1-36) or mid-region PTHrP(38-94) peptide. There was a significant twofold increase in human β-cell proliferation only with PTHrP(1-36) and not with PTHrP (38–94) (Fig. 2B). The concentration of PTHrP(1-36) remaining in the culture medium of Ad-transduced and peptide-treated human islet cell cultures was measured at the end of the experiments. Cells treated with vehicle or Ad-control had undetectable PTHrP(1-36), whereas Ad-PTHrP–transduced cells had 469.8 ± 57.4 pg/ml (∼117.5 pmol/l) and PTHrP(1-36)–treated cells had 245.1 ± 20.6 ng/ml (∼61.25 nmol/l) of PTHrP(1-36) in the culture media (n = 4–5 human islet cultures).
Since induction of proliferation of human β-cells can lead to their dedifferentiation (17), we examined mRNA transcripts encoding differentiation markers in human islets treated with vehicle or PTHrP(1-36). There was no change in transcripts encoding islet hormones (insulin, glucagon, and somatostatin), glucose sensors (Glut-2 and glucokinase), or transcription factors important for β-cell differentiation and function (Pdx1, MafA, MafB, Nkx6.1, Nkx2.2, NeuroD, and Isl1) with PTHrP(1-36) treatment (Fig. 2C), suggesting that PTHrP does not cause dedifferentiation of human β-cells. We also examined the effect of PTHrP on insulin content in human islets. Either transduction with Ad-PTHrP or treatment with PTHrP(1-36) for 24 h did not affect the insulin content in islets (Fig. 2D).
PTHrP enhances GSIS in human islets.
Whether PTHrP affects human β-cell function was evaluated by measuring insulin secretion from human islets transduced for 24 h with Ad-PTHrP or treated for 30 min with PTHrP(1-36) peptide. There was no change in basal insulin secretion at 5.5 mmol/l glucose, and there was a small (27%) but not significant increase in insulin secretion at 22 mmol/l glucose from Ad-PTHrP – versus Ad-control–transduced islets (Fig. 2E), suggesting that Ad-PTHrP does not negatively impact human β-cell function. On the other hand, PTHrP(1-36) peptide caused a significant increase in insulin secretion at 5.5 mmol/l glucose (36%), with a further enhancement at 22 mmol/l glucose (67%) over control islets (Fig. 2F). Thus, PTHrP(1-36) enhances insulin secretion in human islets at both physiological and pathophysiological glucose concentrations.
PTHrP increases expression of the late G1/S cell cycle activators cyclin E and cyclin-dependent kinase 2 in human islets at the posttranscriptional level.
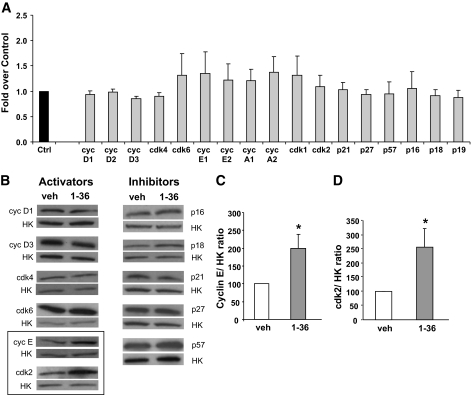
To begin to assess the molecular mechanisms of PTHrP-induced human β-cell proliferation, we measured expression of the G1/S cell cycle regulators. There was no significant change in the level of mRNA expression of any of the cyclins (cyclin D1–3, E1–2, and A1–2), cyclin-dependent kinases (cdks) (1,2,4,6), cyclin inhibitory proteins/kinase inhibitory proteins (CIP/KIP) (p21, p27, and p57) or the inhibitor of kinase (INK) (p16, p18, and p19) family of inhibitors examined with PTHrP(1-36) treatment (Fig. 3A).
FIG. 3.
PTHrP increases expression of cyclin (cyc) E and cdk2 proteins but not mRNA in human islets. A: Expression of G1/S cell cycle regulators by real-time PCR from human islets treated for 24 h with vehicle control (Ctrl) or PTHrP(1-36) peptide (gray bars). PCR cycles for each gene were compared, with actin used as an internal control. The graph is depicted as fold over control, with values from vehicle-treated islets taken as 1 (n = 4–7 human islet preparations in duplicate). B: Representative Western blot analysis of the G1/S cell cycle activators and inhibitors from human islets treated for 24 h with vehicle (veh) or PTHrP(1-36) using actin or tubulin as the internal housekeeping (HK) gene control. Quantitation of the ratio of cyclin E/HK (C) and cdk2/HK protein (D) shows a significant increase in both these proteins in PTHrP(1-36)–treated islets vs. control (veh), depicted as 100%. *P < 0.05 (n = 4–9 human islet preparations).
However, given that cell cycle molecules could be regulated posttranscriptionally, we examined the effect of PTHrP(1-36) on the expression of G1/S cell-cycle proteins by Western blot. There was no change in expression of the early G1/S cell-cycle activators cyclins D1/3 or cdk4/6 or in the INK and CIP/KIP inhibitors p16, p18, p21, p27, and p57 (Fig. 3B). In contrast, the late G1/S cell cycle activator proteins cyclin E (Fig. 3B and C) and cdk2 (Fig. 3B and D) were significantly increased, by twofold, in PTHrP-treated human islets.
Overexpression of cyclin E and cdk2 enhances human β-cell proliferation.
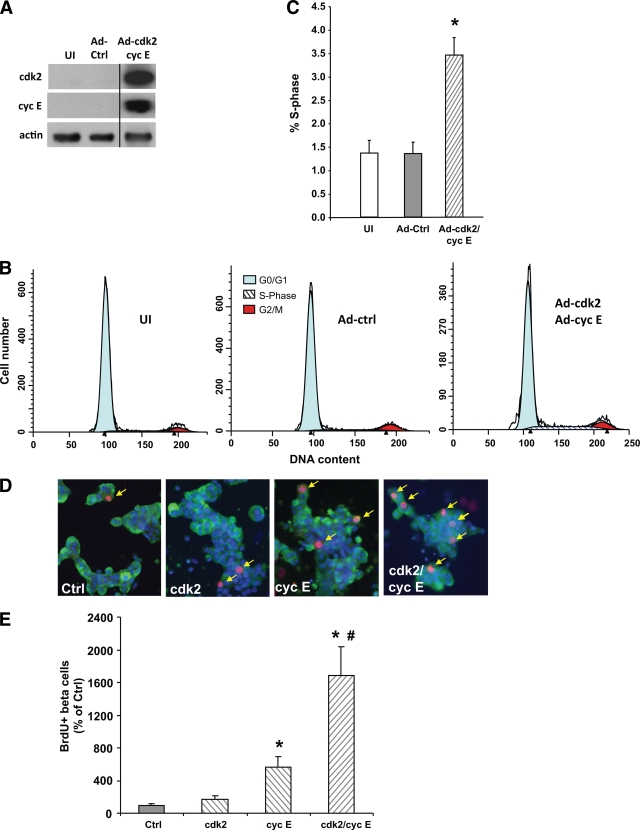
To ascertain whether expression of cyclin E and/or cdk2 per se can drive human β-cell proliferation, these proteins were overexpressed in human islets by transduction with Ad-cdk2/Ad-cyclin E, relative to uninfected and Ad-control–transduced islets (Fig. 4A). Proliferation measured by flow cytometry (Fig. 4B) revealed a significant 2.5-fold increase in the percentage of cells in the S-phase of the cell cycle in Ad-cdk2/Ad–cyclin E–transduced whole human islets compared with uninfected or Ad-control–transduced human islets (Fig. 4C), demonstrating increased islet cell proliferation.
FIG. 4.
Overexpression of cyclin E (cyc E) and cdk2 enhances human β-cell proliferation. A: Western blot analysis of human islets uninfected (UI) or transduced with Ad-control (Ad-ctrl) or a combination of Ad-cdk2/Ad-cyclin E for expression of cdk2, cyclin E, and actin as an internal control. The line divides samples from different regions on the same gel. Representative cell cycle phase distribution profiles (B) and percentage of cells in S-phase (C) of the cell cycle of uninfected, Ad-control, or Ad-cdk2/Ad-cyclin E–transduced human islet cells analyzed using flow cytometry. The percentage of cells in the G1-phase was reduced, although not significantly, in Ad-cdk2/Ad-cyclin E–transduced (G1: 91.1 ± 1.0%, G2/M: 5.4 ± 1.2%) vs. uninfected (G1: 93.0 ± 1.3%, G2/M: 5.4 ± 1.2%) and Ad-control–transduced (G1: 94.0 ± 1.0%, G2/M: 4.6 ± 0.9%) human islets, whereas the percentage of cells in S-phase of the cell cycle in Ad-cdk2/Ad-cyclin E–transduced human islets was significantly greater compared with uninfected or Ad-control–transduced human islets (n = 10 human islet preparations). D: Representative images of human islet cell cultures transduced with 100 MOI of Ad-control, 50 MOI of Ad-control plus 50 MOI of Ad-cdk2, or 50 MOI of Ad-cyclin E and 50 MOI each of Ad-cdk2 and Ad-cyclin E and costained for DAPI (blue), insulin (green), and BrdU (red). Arrows indicate BrdU and insulin-positive cells. E: Quantitation of the percentage of BrdU-positive β-cells from human islet cell cultures transduced with the Ad constructs described for C. There was a significant 5-fold and 16-fold increase in β-cell proliferation in cyclin E– and cdk2/cyclin E– vs. Ad-control–transduced islet cells, respectively. Basal rate of β-cell proliferation in controls was 0.17 ± 0.06%, taken as 100%. n = 9; 3 human islet preparations in triplicate. *P < 0.01 vs. control by Student's t test; #P < 0.001 vs. the other three groups by one-way ANOVA. (A high-quality digital representation of this figure is available in the online issue.)
To independently confirm these observations and to demonstrate whether human β-cell proliferation was induced by cyclin E and/or cdk2, staining for insulin, BrdU, and DAPI was performed on human islet cultures (Fig. 4D). Expression of cdk2 alone did not increase human β-cell proliferation. However, expression of cyclin E alone caused a significant fivefold increase in human β-cell proliferation compared with control cells. Cyclin E and cdk2 together caused a substantive synergistic 16-fold increase in human β-cell proliferation over cells transduced with equivalent multiplicity of infection (MOI) of control virus and also a significant increase over cells transduced with Ad-cdk2 or Ad-cyclin E alone (Fig. 4E).
DISCUSSION
This study documents for the first time that PTHrP can enhance human β-cell growth and function. The major novel findings of this study are as follows: 1) The PTHrP receptor is present in human β-cells. 2) Overexpression of PTHrP causes a significant threefold increase in human β-cell proliferation without negatively impacting function. 3) The secreted amino terminus 1-36 peptide of PTHrP is sufficient to induce replication without causing significant dedifferentiation of human β-cells. 4) PTHrP(1-36) not only increases proliferation but simultaneously enhances human β-cell function, as evidenced by a significant increase in insulin secretion at both physiological (5.5 mmol/l) and pathophysiological (22 mmol/l) glucose concentrations. 5) PTHrP(1-36) specifically increases expression of the late G1/S cell cycle activators cyclin E and cdk2 at the posttranscriptional level in human islets. 6) overexpression of cyclin E but not cdk2 significantly increases human β-cell replication. 7) More importantly, the combination of these two molecules has a substantive synergistic effect on human β-cell proliferation.
The current study, together with previous work (6–8,10–12), establishes PTHrP as a factor that enhances proliferation and function in primary β-cells from both rodent and human islets. Whereas in some instances activation of human β-cell replication may induce dedifferentiation (17), in others—e.g., in this study and with overexpression of cdk6/cyclin D1 or constitutively active Akt (18,19)—induction of human β-cell proliferation maintains or even augments β-cell function. Based on studies in rodent islets (12), it is likely that PTHrP(1-36) may enhance insulin secretion in human islets through activation of the cAMP pathway.
PTHrP enhances proliferation in numerous cell types. This mitogenic activity is associated with changes in cyclin D1 transcription, activation of cyclin E/cdk2 kinase activity, or reduction of p57 or p27 inhibitors (20–22). In human β-cells, we found that PTHrP(1-36) is sufficient to induce proliferation, causing an increase in expression at the posttrancriptional level of two activators of the late phase of the G1/S cell-cycle check point, cdk2 and cyclin E. PTHrP regulates the stability of p27 in smooth-muscle cells, as well as the androgen receptor in prostate cancer cells, through the proteosomal degradation pathway (21,23). Cyclin E protein is also tightly regulated through proteasome-mediated degradation by the E3 ubiquitin ligases (24). Whether PTHrP affects cyclin E stability through this pathway in human β-cells requires further investigation.
There is ample evidence that activators of the early phase of the cell cycle, cyclin Ds and cdk4/cdk6, regulate rodent β-cell proliferation and can induce human β-cell proliferation (18,25). However, whether activators of the late phase of the cell cycle, cyclin E and cdk2, are important for and can induce human β-cell proliferation is not known. Now, we demonstrate for the first time that cyclin E, both alone and together with cdk2, significantly enhances human β-cell proliferation, suggesting that cyclin E may be one of the limiting factors in proliferation of human β-cells.
Understanding the regulation of, and elucidating therapeutic mechanisms to enhance, human β-cell proliferation and function is critical for the long-term treatment of diabetes. This study shows that a small amino terminus peptide of PTHrP is capable of enhancing both proliferation and function in human β-cells in vitro. Further, it identifies two cell-cycle activators as likely downstream targets of PTHrP. Cyclin E and cdk2 together have pronounced effects on human β-cell proliferation and, therefore, have the potential to be harnessed to enhance human β-cell growth ex vivo. The promise for PTHrP(1-36) as a therapeutic agent for diabetes is especially attractive, given that this peptide has been shown to be both safe and effective for the treatment of osteoporosis (15,16) and that PTHrP(1-36) in the 100 pmol/l range, a concentration that is likely transiently induced in humans treated with tolerable doses of PTHrP(1-36) (15,16), is sufficient to enhance human β-cell proliferation in vitro. Future studies will determine whether this peptide can be used either ex vivo or in vivo to improve human islet growth and function.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (DK078060 and DK072264 to R.C.V. and DK055023 and DK07052-32 to A.F.S.) and JDRF Research Awards (1-2008-46 to R.C.V. and 1-2008-39 and 34-2008-630 to A.F.S.).
A.F.S. is a member of Osteotrophin. No other potential conflicts of interest relevant to this article were reported.
N.G.K., S.J.-G., and G.H. researched data, contributed to discussion, and reviewed and edited the manuscript. K.W., X.Y.Z., K.K.T., and P.Z. researched data and contributed to discussion. D.K.S. and A.F.S. contributed to discussion and reviewed and edited the manuscript. A.G.-O. researched data, contributed to discussion, and reviewed and edited the manuscript. R.C.V. researched data, contributed to discussion, and wrote the manuscript.
We are grateful to Drs. Irene Cozar-Castellano and Nathalie Taesch (University of Pittsburgh) for sharing their expertise on the cell cycle and to Darinka Sipula, Taylor Rosa, Jeffrey Kleinberger, and Fatimah Salim (University of Pittsburgh) for superb technical assistance. We acknowledge the ICR and JDRF Basic Science Islet Distribution Programs for supplying human islets.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC: β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS: Growth factor/matrix-induced proliferation of human adult β-cells. Diabetes 1995;44:1458–1460 [DOI] [PubMed] [Google Scholar]
- 3.Efrat S: Ex-vivo expansion of adult human pancreatic beta-cells. Rev Diabet Stud 2008;5:116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocaña A: Growth factors and beta cell replication. Int J Biochem Cell Biol 2006;38:931–950 [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ, Asa SL, Henderson J, Goltzman D: The parathyroid hormone-like peptide gene is expressed in the normal and neoplastic human endocrine pancreas. Mol Endocrinol 1989;3:1589–1595 [DOI] [PubMed] [Google Scholar]
- 6.Vasavada RC, Cavaliere C, D'Ercole AJ, Dann P, Burtis WJ, Madlener AL, Zawalich K, Zawalich W, Philbrick WM, Stewart AF: Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem 1996;271:1200–1208 [DOI] [PubMed] [Google Scholar]
- 7.Porter SE, Sorenson RL, Dann P, Garcia-Ocana A, Stewart AF, Vasavada RC: Progressive pancreatic islet hyperplasia in the islet-targeted, parathyroid hormone-related protein-overexpressing mouse. Endocrinology 1998;139:3743–3751 [DOI] [PubMed] [Google Scholar]
- 8.Vasavada RC, Wang L, Fujinaka Y, Takane KK, Rosa T, Mellado-Gil JM, Garcia-Ocaña A: Protein kinase C-ζ markedly enhances β-cell proliferation: an essential role in growth factor–mediated β-cell mitogenesis. Diabetes 2007;56:2732–2743 [DOI] [PubMed] [Google Scholar]
- 9.Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF: Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev 1996;76:127–173 [DOI] [PubMed] [Google Scholar]
- 10.Fujinaka Y, Sipula D, Garcia-Ocaña A, Vasavada RC: Characterization of mice doubly transgenic for parathyroid hormone–related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic β-cell survival. Diabetes 2004;53:3120–3130 [DOI] [PubMed] [Google Scholar]
- 11.Villanueva-Penacarrillo ML, Cancelas J, de Miguel F, Redondo A, Valin A, Valverde I, Esbrit P: Parathyroid hormone-related peptide stimulates DNA synthesis and insulin secretion in pancreatic islets. J Endocrinol 1999;163:403–408 [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Hosaka M, Sawada Y, Torii S, Mizutani S, Ogata M, Izumi T, Takeuchi T: Parathyroid hormone–related protein induces insulin expression through activation of MAP kinase–specific phosphatase-1 that dephosphorylates c-Jun NH2-terminal kinase in pancreatic β-cells. Diabetes 2003;52:2720–2730 [DOI] [PubMed] [Google Scholar]
- 13.Asa SL, Henderson J, Goltzman D, Drucker DJ: Parathyroid hormone-like peptide in normal and neoplastic human endocrine tissues. J Clin Endocrinol Metab 1990;71:1112–1118 [DOI] [PubMed] [Google Scholar]
- 14.Sawada Y, Kameya T, Aizama T, Izumi T, Takeuchi T: Proprotein-processing endoprotease furin and its substrate parathyroid hormone-related protein are coexpressed in insulinoma cells. Endocr Pathol 2000;11:31–39 [DOI] [PubMed] [Google Scholar]
- 15.Henry JG, Mitnick M, Dann PR, Stewart AF: Parathyroid hormone-related protein-(1-36) is biologically active when administered subcutaneously to humans. J Clin Endocrinol Metab 1997;82:900–906 [DOI] [PubMed] [Google Scholar]
- 16.Horwitz MJ, Tedesco MB, Garcia-Ocaña A, Sereika SM, Prebehala L, Bisello A, Hollis BW, Gundberg CM, Stewart AF: Parathyroid hormone-related protein for the treatment of postmenopausal osteoporosis: defining the maximal tolerable dose. J Clin Endocrinol Metab 2010;95:1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beattie GM, Cirulli V, Lopez AD, Hayek A: Ex vivo expansion of human pancreatic endocrine cells. J Clin Endocrinol Metab 1997;82:1852–1856 [DOI] [PubMed] [Google Scholar]
- 18.Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF: Survey of the human pancreatic β-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human β-cell replication and function in vivo. Diabetes 2009;58:882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao P, Roccisana J, Takane KK, Bottino R, Zhao A, Trucco M, García-Ocaña A: Gene transfer of constitutively active Akt markedly improves human islet transplant outcomes in diabetic severe combined immunodeficient mice. Diabetes 2005;54:1664–1675 [DOI] [PubMed] [Google Scholar]
- 20.Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK: Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J Bone Miner Res 2007;22:951–964 [DOI] [PubMed] [Google Scholar]
- 21.Fiaschi-Taesch N, Sicari BM, Ubriani K, Bigatel T, Takane KK, Cozar-Castellano I, Bisello A, Law B, Stewart AF: Cellular mechanism through which parathyroid hormone-related protein induces proliferation in arterial smooth muscle cells: definition of an arterial smooth muscle PTHrP/p27kip1 pathway. Circ Res 2006;99:933–942 [DOI] [PubMed] [Google Scholar]
- 22.MacLean HE, Guo J, Knight MC, Zhang P, Cobrinik D, Kronenberg HM: The cyclin-dependent kinase inhibitor p57(Kip2) mediates proliferative actions of PTHrP in chondrocytes. J Clin Invest 2004;113:1334–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DaSilva J, Gioeli D, Weber MJ, Parsons SJ: The neuroendocrine-derived peptide parathyroid hormone-related protein promotes prostate cancer cell growth by stabilizing the androgen receptor. Cancer Res 2009;69:7402–7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shumway SD, Xiong Y: Twice primed: Cyclin E is phosphorylated and isomerized before being ubiquitinated. Molecular Cell 2006;23:149–153 [DOI] [PubMed] [Google Scholar]
- 25.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocaña A, Vasavada R, Stewart AF: Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 2006;27:356–370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.