Abstract
OBJECTIVE
In diabetes, retinal vascular basement membrane (BM) undergoes significant thickening and compromises vessel function including increased vascular permeability, a prominent lesion of early diabetic retinopathy. In this study we determined whether altered expression and activity of lysyl oxidase (LOX), a cross-linking enzyme, may compromise vascular basement membrane functional integrity under high-glucose (HG) conditions.
RESEARCH DESIGN AND METHODS
Rat retinal endothelial cells (RRECs) grown in normal (5 mmol/l) or HG (30 mmol/l glucose) medium for 7 days were assessed for expression of LOX and proLOX by Western blot analysis and LOX enzyme activity. To determine whether HG alters cellular distribution patterns of LOX and proLOX, immunostaining with respective antibodies was performed. Similarly, cells grown in normal or HG medium were subjected to both LOX inhibition with β-aminopropionitrile (BAPN) and by small interfering RNA knockdown, and respectively examined for cell monolayer permeability. Additionally, retinas of streptozotocin (STZ)-induced diabetic rats were analyzed to determine if diabetes altered LOX expression.
RESULTS
Western blot analysis revealed significantly increased LOX and proLOX expression in cells grown in HG medium compared with those grown in normal medium. The increased LOX level was strikingly similar to LOX upegulation in the diabetic retinas. In cells grown in HG medium, LOX activity and cell monolayer permeability was significantly increased, as were LOX and proLOX immunostaining. Small interfering RNA- or BAPN–induced-specific blockage of LOX expression or activity, respectively, reduced cell monolayer permeability.
CONCLUSIONS
HG-induced increased LOX expression and activity compromises barrier functional integrity, a prominent lesion of diabetic retinopathy.
The pathogenesis of diabetic microangiopathy is influenced by qualitative and quantitative changes of the capillary basement membrane. Although histologic and functional changes that accompany diabetic microangiopathy have been well documented (1–4), specific intracellular and extracellular mechanisms pertaining to these changes that lead progressively to dysfunction of vessels as seen in diabetic retinopathy remain unclear. The hallmark of diabetic microangiopathy, in particular diabetic retinopathy, is the thickening of the retinal capillary basement membrane (5–7). Although many studies investigating retinal capillary leakage in diabetes have focused on vascular cell abnormalities such as the endothelium (8,9) and on the production of vascular endothelial growth factor (VEGF), regarded as a predominant factor responsible for the development of new dysfunctional vessels, only a few have examined the relationship between biochemical changes of the abnormal accumulation of the extracellular matrix (ECM) and excess permeability. Stabilization, fibril assembly, and polarity, essential components for functional integrity of the basement membrane, depend largely on proper cross-linking of collagen. Cross-linked collagen fibers become insoluble and exhibit progressively increased tensile strength, which is essential for normal connective tissue function.
Lysyl oxidase (LOX) is an extracellular enzyme that is synthesized and secreted as a glycosylated proenzyme (proLOX, 50 kDa), which further undergoes extracellular proteolytic processing into a mature, biologically active 32 kDa form (LOX) (10). LOX enzyme catalyzes oxidative deamination of peptidyl lysine and hydroxylysine residues in secreted collagen precursors, and lysine residues in elastin. These aldehydes spontaneously undergo condensation reactions that result in normal mature and functional extracellular matrices. Excess LOX-dependent cross-linking contributes to excess ECM accumulation in fibrotic diseases (11,12). Although perhaps counter-intuitive, studies have shown that an increase in stiffness of extracellular matrices can enhance cell migration through an ECM in part by altering integrin and cell surface receptor signaling complexes (13). In the present study, we sought to determine whether glucose-dependent regulation of LOX could contribute to increased basement membrane permeability in cultures of retinal endothelial cells. Increased LOX enzyme expression and activity have recently been linked to increased invasiveness of tumor cells, possibly mediated in part by its effects on the structure and physical properties of the ECM (14–16). Studies seem to suggest that the integrity of the basement membrane and the stromal compartment of the ECM require an optimal degree of LOX-dependent cross-linking.
LOX expression has been identified in several tissues, including the skin, aorta, heart, lung, liver, cartilage, bone, kidney, retina, and brain (17–23). Clearly the importance of LOX-mediated cross-linking is significant to tissue integrity and its functionality. Abnormal LOX activity is associated with various pathologies. Reduced LOX activity is known to cause lathyrism (24), whereas its upregulation in tumor cells is associated with metastasis leading to malignancy and cancer (14,25). Importantly, LOX expression is regulated by hypoxia-inducible factors (HIFs), a key player in promoting retinal neovascularization in advanced diabetic retinopathy (14). However, limited information is available on LOX related to the metabolic state of cells grown under high-glucose conditions, and even less is known about the expression of LOX in the diabetic retina.
The underlying mechanism associated with increased vascular permeability in diabetic retinopathy in the context of excess ECM accumulation is still unknown. The present study investigated the effects of HG conditions or diabetes on LOX expression and whether HG-induced changes in LOX activity may contribute to excess permeability.
RESEARCH DESIGN AND METHODS
Cell culture.
Rat retinal endothelial cells (RRECs) ascertained positive for von Willebrand factor were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS (Hyclon, Thermo Scientific, Waltham, MA), antibiotics, and antimycotics. Third to fifth passage cells were used in this study. All experiments were repeated at least four times. To examine the effect of HG on LOX and proLOX expression, RRECs were grown in normal medium (5 mmol/l glucose) or HG medium (30 mmol/l) for 7 days followed by protein isolation and Western blot analysis. In parallel, cells were exposed to 30 mmol/l mannitol as osmotic control and protein analyzed by Western blot analysis. To examine the effect of VEGF stimulation on the expression of LOX, RRECs were exposed to 25 ng/ml of VEGF (Sigma, St. Louis, MO) for 24 or 48 h followed by protein isolation and Western blot analysis for LOX.
Animals.
All animal studies were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Ten Sprague-Dawley male rats, each weighing ∼200 g, were used in this study. Five of the 10 rats were injected intraperitoneally with streptozotocin (STZ) (55 mg/kg body weight) to induce diabetes. The glucose concentration in blood and urine were checked after 2 or 3 days after STZ injection to confirm diabetes status in the animals. The remaining 5 animals served as nondiabetic controls. Blood glucose levels were measured in each animal 2 to 3 times weekly and at the time of death. The diabetic group represented rats with blood glucose levels of ∼350 mg/dl. The diabetic rats received NPH insulin injection as needed to maintain blood glucose levels. After 3 weeks of diabetes, the animals were killed and retinas were isolated and total protein extracted. To examine the effect of diabetes on LOX protein expression, the retinal protein from the diabetic retinas and those of the control nondiabetic retinas were subjected to Western blot analysis for LOX protein expression.
Western blot analysis.
Total protein was isolated from RRECs grown in normal or HG medium and retinas from nondiabetic control and diabetic rats. Briefly, to extract total protein, cells were lysed in buffer containing 1xPBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 3 mol/l urea, pH 7.4. Similarly, retinal protein was isolated by homogenizing the retina in the same ice cold lysis buffer. Cell lysates and the homogenized retinas were centrifuged at 14,000 rpm for 20 min at 4°C. Protein concentration in each sample was determined by the bicinchoninic acid protein assay reagents (bicinchoninic acid protein assay, Pierce, Rockford, IL). Western blot analysis was performed with samples containing equal amounts of protein (25–35 μg) in a 10% SDS–polyacrylamide gel. The separated proteins in the gel were then transferred onto a PVDF membrane. Nonspecific binding sites were blocked by incubating the PVDF membrane in Tris-buffered saline containing 0.1% Tween-20 (TTBS) with 5% nonfat dry milk. Membranes were then incubated overnight at 4°C with rabbit anti-rat mature LOX (Novus, Littleton, CO) and rabbit anti-rat proLOX antibodies (26), washed with TTBS for 20 min three times each for 5 min, and then incubated with the corresponding secondary antibody conjugated with alkaline phosphatase (1:3,000, anti-rabbit IgG, Cell Signaling, Danvers, MA). Experiments presented here were repeated at least four times. After washing with TTBS, Immuno-Star Chemiluminescent Protein Detection System (BioRad, Hercules, CA) was used to detect protein levels of LOX and proLOX. Molecular weights were determined by comparison with prestained protein molecular weight standards (BioRad, Precision Plus Protein Standards). Densitometric analysis of the chemiluminescent signal was performed at nonsaturating exposures using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD).
Immunofluorescence microscopy.
To examine the effect of HG on the localization and distribution of LOX and proLOX in RRECs, immunostaining for LOX and proLOX was performed in cells plated on coverslips. Briefly, the cells were fixed with methanol, blocked with 2% BSA in PBS for 30 min, and incubated overnight in a moist chamber with rabbit anti-rat mature LOX and rabbit anti-rat proLOX antibodies in a PBS-BSA antibody solution (1:100 and 1:100, respectively). Cells were then washed in PBS and incubated with a goat anti-rabbit IgG secondary antibody conjugated with fluorescein isothiocyanate (Jackson Immunoresearch Labs, West Grove, PA) for 1 h at 37°C in a dark chamber. The cells were then washed three times in PBS and mounted in Slow-Fade (Invitrogen, Carlsbad, CA) and examined. Negative control samples were processed in the same manner, except that the primary antibody was omitted. The cells were viewed and photographed with a Nikon Diaphot fluorescence microscope and a Nikon F1 digital camera. The fluorescence intensity of the LOX and proLOX signal was analyzed using ImageJ, image analysis software from the National Institutes of Health.
LOX immunostaining in retinas of normal and diabetic rats.
Retinas from normal and diabetic Sprague-Dawley rats were fixed in 4% formalin, rinsed in PBS, embedded in optimal cutting temperature compound, and frozen in liquid nitrogen. Next, 9-μm sections were cut at −20°C and mounted onto glass slides for immunostaining. Sections were blocked in 2% BSA and incubated overnight with rabbit anti-LOX antibody at 4°C. After washing with PBS, sections were incubated using Rhodamine-conjugated goat anti-rabbit secondary antibody. Sections were analyzed under a microscope equipped with fluorescence and images were digitally photographed.
LOX activity.
To examine the effect of HG on LOX enzyme activity, RRECs were grown in normal or HG medium or in mannitol for 6 days, and at semiconfluence cell culture media was changed to phenol red-free 2% FBS (normal or HG, respectively) media. Cells were grown further for 24 h, resulting in a total of 7 days exposure to HG, or 7 days exposure to mannitol used as osmotic control. On the day of the assay, samples of media from normal and HG cell cultures were collected and LOX enzyme activity was determined using the Amplex Red fluorescence assay as previously described as optimized for LOX (27). Samples were prepared in a final volume of 2 ml containing 1.2 mol/l urea, 0.05 mol/l sodium borate (pH 8.2), 1 unit/ml of horseradish peroxidase (Sigma), and 10 mmol/l 1,5-diaminopentane (Cadaverine; Sigma) and incubated at 37°C for 30 min. Parallel assays were prepared with 0.5 mmol/l β-aminopropionitrile fumarate (BAPN, Sigma), a specific inhibitor of LOX activity (17,28–30). Fluorescence from the samples was measured using a Hitachi F-2000 fluorescence spectrophotometer with excitation and emission wavelengths at 568 and 587 nm, respectively. The amount of hydrogen peroxide (H2O2) produced was determined from a standard curve of nanomoles of H2O2 versus fluorescence in the peroxidase/Amplex Red optimized reaction conditions. The H2O2 solutions were standardized by titration of an acidified dilution of 30% hydrogen peroxide with acidified 0.02 mol/l KMnO4.
Transfection with LOX small interfering RNA.
RRECs were transfected with LOX small interfering RNA (siRNA) (Santa Cruz, Santa Cruz, CA) and nonspecific siRNA (scrambled siRNA, 5′-aauauuggcguuaagauucua-3′, Ambion, Austin, TX) in the presence of 0.2% lipofectamin 2000 (Invitrogen) prepared in Opti-MEM (Invitrogen). The LOX siRNA was targeted against two sequences of the mature rat LOX (5′-CUGAAUCAGACUACAGUA-3′ and 5′-ACAAGTACTCCGACGACAA-3′) that have no homology with the other members of the amine oxidase enzyme family. The optimal concentration of siRNA was determined by testing 16.5, 33, 49.5, and 66 nmol/l of the LOX siRNA in RRECs and harvesting the cells after 2 days, then carrying out Western blot analysis. The concentration of 33 nmol/l LOX siRNA provided ∼42% inhibition of LOX expression in RRECs and was subsequently used in the experiments.
In vitro permeability as a function of LOX activity and as a function of LOX expression.
To examine the effect of HG-induced LOX activity on cell monolayer permeability, RRECs were grown on cell culture inserts (0.4-μm pore size, Falcon, Paramus, NJ) of transwell plates in normal or HG medium for 7 days. HG cells grown in parallel were then incubated with BAPN, an irreversible inhibitor of LOX activity. Cell monolayer permeability was assessed by measuring the diffusion of FITC-dextran (MW 43 kDa, Sigma) from the upper to the lower chamber. The in vitro permeability (IVP) assay was performed as reported earlier (31–33). Briefly, after cells were allowed to reach near confluency, 48 h before the IVP assay measurement, media from both the upper and lower chamber of all groups was replaced with fresh phenol red-free DMEM. Medium in the upper and lower chamber of the BAPN-treated group was substituted with BAPN at a final concentration of 100 μmol/l. After 24 h of incubation with BAPN, media in the upper chamber of all groups was replaced with 600 μl of FITC-dextran solution (0.5 mg/ml) and cells allowed to incubate at 37°C. At the 1-h time point, 200 μl samples from the lower chamber of all transwells were collected and measured at 492 nm using a spectrophotometric microplate reader (SpectraMax Gemini Vmax; Molecular Devices, Sunnyvale, CA). All experiments were performed in triplicate and the solute permeability was calculated based on the following formula: (lower chamber fluorescence/input fluorescence) × 100%.
To examine the effect of HG-induced LOX expression on cell monolayer permeability, RRECs were grown in parallel in similar transwell inserts under normal or HG condition for 7 days. Briefly, cells in four groups (normal, HG, HG+LOX siRNA, and HG+scrambled siRNA) were grown to ∼80% confluency and transfected with LOX siRNA and then in vitro permeability assay performed 24 h after the transfection. Diffusion of FITC-dextran from the upper to the lower chambers was measured from aliquots obtained from the bottom chambers of the transwell plates. All experiments were performed in triplicate and the solute permeability was calculated based on the following formula: (lower chamber fluorescence/input fluorescence) × 100%.
Statistical analysis.
All data are reported as mean ± SD; one-way ANOVA followed by a Student t test was used to analyze all data. Data with values of P < 0.05 were considered significant.
RESULTS
Effect of HG on LOX and proLOX protein expression in RRECs.
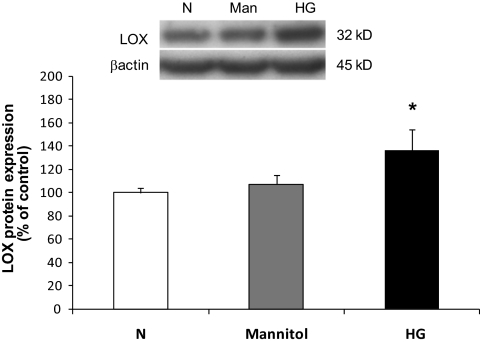
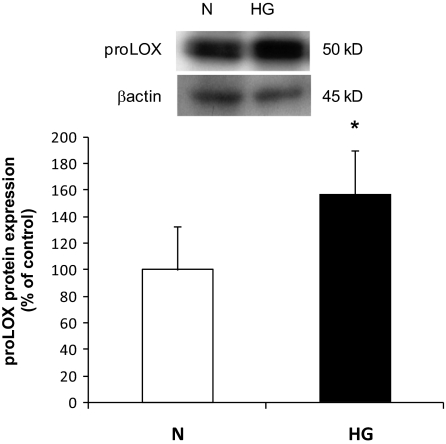
Western blot analysis indicated that RRECs grown in HG medium for 7 days exhibited significant increase in the expression of LOX and proLOX protein levels compared with those of RRECs grown in normal medium (136 ± 18% of control, P < 0.005, n = 9; 157 ± 32% of control, P < 0.0005, n = 9, respectively). Exposure of the cells to 30 mmol/l mannitol for 7 days had no effect on LOX expression. The β-actin protein expression used as an internal control confirmed equal protein loading for all groups (Figs. 1 and 2).
FIG. 1.
Western blot analysis of LOX protein levels in RRECs grown in normal (N) or HG media. Graph shows that the LOX protein level was significantly upregulated in cells grown in HG medium compared with cells grown in normal (N) medium. Cells exposed to mannitol for 7 days exhibited no change in LOX expression. Data are presented as mean ± SD (*P < 0.005; n = 9).
FIG. 2.
Western blot analysis of proLOX protein levels in RRECs grown in normal (N) or HG media. Graph shows proLOX protein level was significantly upregulated in cells grown in HG medium compared with those of cells grown in normal medium. Data are presented as mean ± SD (*P < 0.0005; n = 9).
Effect of HG on localization and distribution of LOX and proLOX in RRECs.
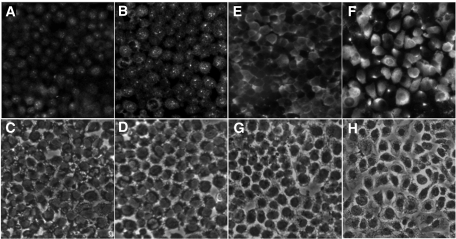
The distribution and localization of LOX and proLOX protein in RRECs was assessed by immunofluorescence microscopy. Immunostaining intensity for LOX and proLOX showed a significant increase in RRECs grown in HG medium (Fig. 3B and F) compared with those of cells grown in normal medium (Fig. 3A and E) LOX immunostaining demonstrated increased intracellular punctate-like staining in the cytoplasm, whereas staining for proLOX revealed a generalized and diffuse cytoplasmic staining. No change was detected with respect to LOX or proLOX distribution under HG condition in RRECs.
FIG. 3.
Representative images of LOX immunostaining in RRECs grown in normal (N) (A) and HG (B) conditions. C and D represent the corresponding brightfield images. Upregulation of LOX under HG shows predominantly focal cytoplasmic staining. Immunostaining of proLOX in RRECs in normal (E) and HG (F) conditions. G and H represent the corresponding brightfield images. Upregulation of proLOX under HG shows diffuse perinuclear distribution of proLOX.
Effect of diabetes on retinal LOX protein expression and localization.
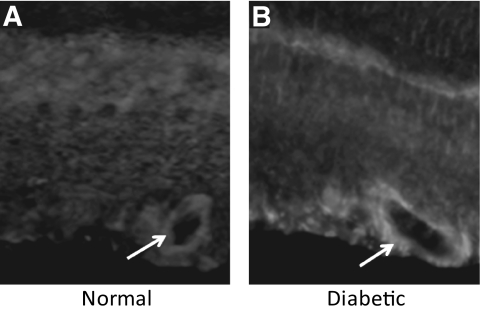
Blood glucose levels measured routinely and at the time of death confirmed the presence of hyperglycemia in these rats compared with control nondiabetic rats (326 ± 16 vs. 104 ± 12, P < 0.01). After 3 weeks of diabetes, retinal LOX protein levels were significantly increased (125 ± 4.8% of control, P < 0.05) compared with those in control nondiabetic rats (n = 5) (Fig. 4). The β-actin protein expression was used as an internal control for protein loading and was similar in all groups. The immunohistochemical analysis of the retinal sections suggests that the perivascular tissue as well as the basement membrane of the retinal capillaries in the diabetic retinas show increased LOX immunostaining compared with blood vessels in the normal retinas (Fig. 5).
FIG. 4.
Western blot analysis of LOX protein levels in diabetic rat retinas. Graph shows LOX protein expression was significantly upregulated in the retinas of diabetic rats compared with control nondiabetic rats. Data are presented as mean ± SD (*P < 0.05, n = 5). DM, diabetes.
FIG. 5.
Cryosections of normal and diabetic rat retinas immunostained with anti-LOX primary antibody and rhodamine-conjugated secondary antibody. Blood vessel in the diabetic retina shows increased LOX immunostaining compared with those of the normal retina.
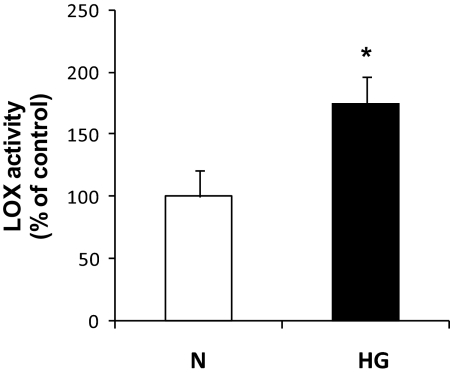
Effect of HG on LOX activity.
LOX activity measurements performed in RRECs grown in HG condition indicated significant upregulation compared with those grown in normal medium (175 ± 21% of control, P < 0.0005, n = 5) (Fig. 6). Cells gown in parallel in 30 mmol/l mannitol showed no change in LOX activity.
FIG. 6.
Graph shows increased LOX activity in the medium in which RRECs were grown in HG condition compared with the activity in medium derived from RRECs grown in normal (N) condition. Data are presented as mean ± SD (*P < 0.0005; n = 5).
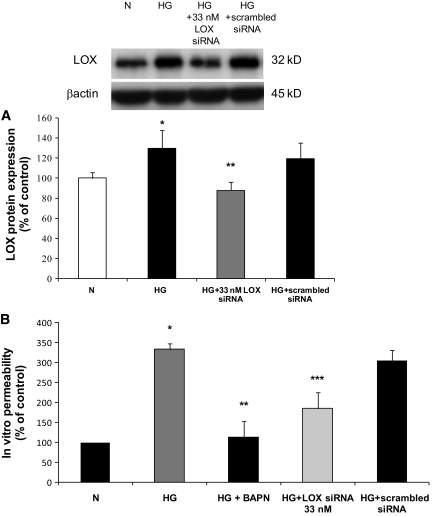
Effect of LOX siRNA in RRECs grown in HG condition and on cell monolayer permeability.
We examined the effect of LOX siRNA in cells grown under HG conditions. In cells grown in HG medium and transfected with LOX siRNA, the LOX expression was significantly reduced compared with that of cells grown in HG medium and transfected with scrambled siRNA (88 ± 8% of control vs. 119 ± 16% of control, P < 0.05, n = 3) (Fig. 7A). When RRECs grown in HG medium were transfected with the LOX siRNA, the permeability of the cell monolayer significantly decreased compared with that of the HG cells transfected with scrambled siRNA (185 ± 35%, P < 0.005; 333 ± 14% of control, P < 0.005; n = 3) (Fig. 7B). Since the LOX siRNA that we used was specifically targeted against the LOX transcript, the data presented here demonstrate that LOX overexpression is involved in increased permeability.
FIG. 7.
A: Graph shows the effect of LOX siRNA on LOX protein level in RRECs. In cells grown in HG medium and transfected with LOX siRNA, the LOX protein expression was significantly decreased compared with that in cells grown in HG medium and transfected with scrambled siRNA. Data are expressed as mean ± SD (*P < 0.005, **P < 0.05). B: Effect of reduced LOX activity on cell monolayer permeability and effect of reduced LOX expression on cell monolayer permeability. The permeability of FITC-conjugated dextran molecules was significantly decreased to near normal level in cells grown in HG medium after incubation with BAPN compared with RRECs grown in HG. Data are expressed as mean ± SD (*P < 0.05, n = 6; **P < 0.05; n = 6). The permeability of FITC-conjugated dextran molecules was also significantly decreased to near normal level in cells grown in HG medium after transfection with LOX siRNA compared with untransfected RRECs grown in HG. Data are expressed as mean ± SD (***P < 0.005; n = 3). N, normal.
BAPN-mediated reduction of HG-induced upregulation of LOX activity on cell monolayer permeability.
The IVP assay was designed to examine the effect of LOX enzyme activity on cell layer permeability. For this we used BAPN that irreversibly blocks the amine oxidase activity of LOX. The IVP assay showing significantly elevated fluorescein permeance was present in the RREC monolayer grown in HG compared to those grown in normal medium (325 ± 17% of control, P < 0.05, n = 6). When RRECs grown in HG medium were incubated with BAPN, the permeability of the cellular monolayer was significantly decreased (141 ± 42%, P < 0.05, n = 6) (Fig. 7B). Since BAPN blocks LOX activity, the data demonstrate that increased LOX activity is, at least in part, involved in increased permeability.
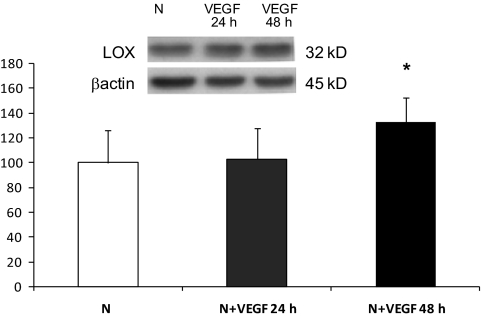
Effect of VEGF on the expression of LOX.
VEGF promotes vascular permeability. To gain insight into whether VEGF could mediate effects of glucose on LOX expression, we determined whether treatment of RRECs with 25 ng/ml VEGF regulate LOX. Western blot analysis indicated that RRECs stimulated with VEGF for 24 h had no effect on LOX expression; however, exposure to VEGF for 48 h modestly increased LOX expression compared with those of RRECs grown in normal medium (132 ± 20% of control, P < 0.05, n = 4) (Fig. 8).
FIG. 8.
Western blot analysis of LOX protein levels in RRECs grown in normal (N) medium and stimulated with VEGF for 24 or 48 h. Graph shows LOX protein level was not significantly changed in cells stimulated with 25 ng/ml of VEGF for 24 h, although after 48 h of VEGF stimulation, LOX expression was significantly increased compared with cells grown in normal medium. Data are presented as mean ± SD (*P < 0.05; n = 4).
DISCUSSION
The results from this study provide evidence that excess synthesis of basement membrane components, such as collagen type IV, and the subsequent thickening of the vascular basement membrane may play a far more critical role during the breakdown of blood retinal barrier as seen in diabetic retinopathy than so far suspected. To our knowledge, this is the first study that shows increased protein expression for both LOX and proLOX under HG condition in RRECs and in diabetic rat retinas and that the HG-induced increase in LOX activity could lead to altered cross-linking of collagen fibrils and contribute to excess permeability. Our data also show increased LOX expression in retinas of diabetic rats that supports a previous study reporting increased LOX-dependent cross-linking in skin collagen in diabetes (34). Although the changes in dermal collagen reported in the earlier study are presumably types I and III, nevertheless, changes in LOX observed in this study suggest that similar biochemical alterations affect different collagen types present in various tissues in diabetes.
A study examining glomerular basement membrane collagen and LOX-mediated cross-links in experimental diabetes reported that the ratio of dihydroxylysinonorleucine to hydroxylysinonorleucine was increased, suggestive of altered cross-linking in diabetes (35). The development of basement membrane thickening in glomerular and retinal capillaries, a histologic hallmark of diabetic microangiopathy, is associated with increased vascular leakage. In this context it seems quite paradoxical that thickened basement membranes are more permissive to permeance; currently a mechanism for this observation is unknown. Data presented here implicate increased LOX expression and activity as mediating, at least in part, increased permeability through retinal basement membranes. To date, only the expression of LOXL2 seems to be implicated in diabetes and specifically in diabetic nephropathy. Microarray analysis of human biopsy samples from patients with DN suggested that LOXL2 was among the HIF target genes that were found to be upregulated, though the increased expression was confirmed only by real-time PCR analysis (36). Furthermore LOXL2 has been reported to have amine oxidase activity, but unlike its family members, its enzyme activity was apparently not inhibited by BAPN (30,37). There are no reports that lysyl oxidase isoforms LOXL1, LOXL3, and LOXL4 are regulated by HG conditions or diabetes. LOXL1 has been reported to play a role in pseudoexfoliation glaucoma and primary open angle glaucoma. LOXL3 and LOX4 have been implicated in breast cancer invasion and head and neck squamous cell carcinoma (15,38). ECM accumulation is now considered to play a more mechanistic role in the development of compromised barrier function. Recent observations point to fibrotic matrices as providing excess ligands for cell surface receptors such as integrins, that in turn modulate cell signaling responses and enhance local synthesis of proteolytic enzymes (13). Similarly, upregulation of LOX in several invasive cancers has provided additional support for the concept that a fibrotic matrix can be more permeable than a normal matrix (39). It is possible that elevation of LOX and consequent increased cross-linking may make fibrotic matrices more conducive for permeability of soluble molecules because of strains and resulting gaps between collagen fibrils. Detailed direct ultrastructural analyses of basement membrane integrity after increased LOX-mediated cross-linking may help us understand LOX dependent effects on basement membranes and increased permeability.
Although collagen fibrillar arrangements are stabilized by covalent cross-links, excessive cross-linking could contribute to disorganized assembly of the collagen fibrils. Electron microscopic investigation has revealed fine structural changes in the collagen fibrillar arrangement in diabetes (40). These differences included increased packing density of collagen fibrils, decreases in fibrillar diameter, and abnormal fibril morphology showing collagen fibrils that appeared twisted, curved, overlapping, and otherwise highly disorganized, suggestive of excess cross-linking that is known to tighten collagen fibrils (40). Diameter measurements on fibrils obtained during a time course of assembly have demonstrated that a fibril diameter distribution are dependent on late-stage assembly of fibrils (41) that are in part regulated by LOX activity. Much is still unknown related to the pathways for the secretion and extracellular assembly of collagen molecules into fibrils and the processing enzymes required for converting the insoluble aggregates into mechanically and chemically stable components of the matrices. The principles governing the self-assembly of collagen fibrils are currently not well understood. Further studies are necessary to understand how cells regulate this process, to learn how the deposition of early collagen fibrils is orchestrated in the basement membrane, and to understand the role of other basement membrane components and their interactions in these processes. Thus, the identification of altered activity of a cross-linking enzyme, which is involved in basement membrane organization and ultrastructural assembly of collagen matrices, may provide new mechanistic insights into the relationship between extracellular matrix accumulation and excess vascular permeability in diabetes.
It is of interest that the LOX family of proteins is multifunctional. Although LOX activity, as already noted, is associated with increased metastatic behavior of tumors, the propeptide region of LOX has tumor-suppressor activity (42–47). The propeptide region of LOX and LOXL1 are each unique in structure, whereas the pro domains of LOXL2 – LOXL4 contain conserved scavenger receptor cystein-rich domains (SRCR) that in other proteins mediate functional protein interactions (48). The biologic activities of prodomains of LOX isoforms have not been explored in the context of vascular biology. The present study clearly identifies LOX expression itself, and LOX activity in particular, as being critical for its effects on endothelial barrier function, but additional activities of LOX and LOXL1-LOXL4 that are independent of enzyme activity may also contribute to its biological roles in vivo.
Morphologic abnormalities of retinal capillary basement membrane of diabetic individuals appear to reflect a poorly known process of structural remodeling. These structural abnormalities may be the result of excessive cross-linking represented by the thickened retinal capillary basement membrane, one of the prominent characteristics of diabetic retinopathy (5,6). Although upregulation of basement membrane components such as fibronectin, collagen IV, and laminin in diabetes has been established, and recent studies indicate its contributory role to excess retinal vascular permeability (2,7,49,50), the exact biochemical changes that modify matrix and promote excess permeability are unclear. However, it is clear that increased vascular permeability requires the passage of solutes through two layers inherent in capillaries, the cellular layer and the ECM layer (basement membrane). Although studies have implicated breakdown of tight junctions and increased vacuolar transport to excess permeability (51), these changes represent abnormalities of the cellular layer. Other mechanisms such as nonenzymatic glycation could also contribute to and potentiate excess permeability (52,53). An increase in collagen solubility under elevated glucose concentrations may lead to unbalanced biosynthesis and processing of collagen precursors (54). This study provides novel information related to the ECM in which biochemical changes of the matrix components may render it more permeable in diabetes.
In this study we observed a modest upregulation of LOX expression by VEGF in retinal endothelial cells. It is, therefore, conceivable that VEGF may partially mediate the regulation of LOX under diabetic conditions, but this requires further study. It is of interest that hypoxia is an upregulator of both VEGF and LOX (55), and it seems likely that hypoxia-mediated LOX upregulation directly or indirectly increases diabetic microvascular retinal permeability. Increased levels of VEGF and thickening of the vascular basement through upregulation of ECM protein expression in diabetes are among the most distinct characteristics of the disease. Such an increase in ECM expression could lead to retinal vascular basement membrane thickening, and in turn, contribute to increased permeability (49).
In summary, our findings indicate that HG-induced increased LOX expression and activity is associated with retinal endothelial cell dysfunction and excess permeability. Successful application of siRNA and BAPN for inhibition of HG-induced upregulation of LOX expression and LOX activity with beneficial outcomes on barrier function opens up the option of reducing LOX overexpression and activity as a potential strategy for preventing increased permeability associated with diabetic retinopathy.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01-EY-14702 (to S.R.), and in part by a departmental grant from the Massachusetts Lions Eye Research Fund, Inc. (to S.R.) and National Institutes of Health grant R01-DE-14066 (to P.T.).
No potential conflicts of interest relevant to this article were reported.
A.C. researched data, wrote the manuscript. A.T. and E.B. researched data. P.C.T. researched data, reviewed/edited the manuscript, and contributed to discussion. S.R. researched data, designed experiments, and wrote the manuscript.
This study was presented in part at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Chavers BM, Mauer SM, Ramsay RC, Steffes MW: Relationship between retinal and glomerular lesions in IDDM patients. Diabetes 1994;43:441–446 [DOI] [PubMed] [Google Scholar]
- 2.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC: Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 1996;44:1469–1479 [DOI] [PubMed] [Google Scholar]
- 3.Merimee TJ, Siperstein MD, Fineberg SE, McKusick VA: The microangiopathic lesions of diabetes mellitus: an evaluation of possible causative factors. Trans Assoc Am Physicians 1970;83:102–112 [PubMed] [Google Scholar]
- 4.Tsilibary EC: Microvascular basement membranes in diabetes mellitus. J Pathol 2003;200:537–546 [DOI] [PubMed] [Google Scholar]
- 5.Roy S, Cagliero E, Lorenzi M: Fibronectin overexpression in retinal microvessels of patients with diabetes. Invest Ophthalmol Vis Sci 1996;37:258–266 [PubMed] [Google Scholar]
- 6.Roth T, Podesta F, Stepp MA, Boeri D, Lorenzi M: Integrin overexpression induced by high glucose and by human diabetes: potential pathway to cell dysfunction in diabetic microangiopathy. Proc Natl Acad Sci U S A 1993;90:9640–9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S, Maiello M, Lorenzi M: Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest 1994;93:438–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd-White J, Williams JC, Jr: Effect of cross-linking on matrix permeability. A model for AGE-modified basement membranes Diabetes 1996;45:348–353 [DOI] [PubMed] [Google Scholar]
- 9.Walton HA, Byrne J, Robinson GB: Studies of the permeation properties of glomerular basement membrane: cross-linking renders glomerular basement membrane permeable to protein. Biochim Biophys Acta 1992;1138:173–183 [DOI] [PubMed] [Google Scholar]
- 10.Trackman PC, Bedell-Hogan D, Tang J, Kagan HM: Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J Biol Chem 1992;267:8666–8671 [PubMed] [Google Scholar]
- 11.Lucero HA, Kagan HM: Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 2006;63:2304–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez C, Rodriguez-Sinovas A, Martinez-Gonzalez J: Lysyl oxidase as a potential therapeutic target. Drug News Perspect 2008;21:218–224 [DOI] [PubMed] [Google Scholar]
- 13.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM: Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8:241–254 [DOI] [PubMed] [Google Scholar]
- 14.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ: Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006;440:1222–1226 [DOI] [PubMed] [Google Scholar]
- 15.Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ: A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res 2002;62:4478–4483 [PubMed] [Google Scholar]
- 16.Payne SL, Hendrix MJ, Kirschmann DA: Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem 2007;101:1338–1354 [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Akiyama H, Iguchi H, Iyama K, Miyamoto M, Ohsawa K, Nakamura T: Molecular cloning and biological activity of a novel lysyl oxidase-related gene expressed in cartilage. J Biol Chem 2001;276:24023–24029 [DOI] [PubMed] [Google Scholar]
- 18.Cardoso WV: Molecular regulation of lung development. Annu Rev Physiol 2001;63:471–494 [DOI] [PubMed] [Google Scholar]
- 19.Gilad GM, Kagan HM, Gilad VH: Evidence for increased lysyl oxidase, the extracellular matrix-forming enzyme, in Alzheimer's disease brain. Neurosci Lett 2005;376:210–214 [DOI] [PubMed] [Google Scholar]
- 20.Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD: Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 2003;278:14387–14393 [DOI] [PubMed] [Google Scholar]
- 21.Kagan HM: Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Pathol Res Pract 1994;190:910–919 [DOI] [PubMed] [Google Scholar]
- 22.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R: Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 2002;106:2503–2509 [DOI] [PubMed] [Google Scholar]
- 23.Hayashi K, Fong KS, Mercier F, Boyd CD, Csiszar K, Hayashi M: Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol 2004;35:845–855 [DOI] [PubMed] [Google Scholar]
- 24.Wilmarth KR, Froines JR: In vitro and in vivo inhibition of lysyl oxidase by aminopropionitriles. J Toxicol Environ Health 1992;37:411–423 [DOI] [PubMed] [Google Scholar]
- 25.Erler JT, Giaccia AJ: Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res 2006;66:10238–10241 [DOI] [PubMed] [Google Scholar]
- 26.Hurtado PA, Vora S, Sume SS, Yang D, St Hilaire C, Guo Y, Palamakumbura AH, Schreiber BM, Ravid K, Trackman PC: Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation. Biochem Biophys Res Commun 2008;366:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palamakumbura AH, Trackman PC: A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem 2002;300:245–251 [DOI] [PubMed] [Google Scholar]
- 28.Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, Sommer P, Font B: Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem 2001;276:48944–48949 [DOI] [PubMed] [Google Scholar]
- 29.Kim MS, Kim SS, Jung ST, Park JY, Yoo HW, Ko J, Csiszar K, Choi S-Y, Kim Y: Expression and purification of enzymatically active forms of the human lysyl oxidase-like protein 4. J Biol Chem 2003;278:52071–52074 [DOI] [PubMed] [Google Scholar]
- 30.Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF: Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer 2009;125:318–327 [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell ME, Brandt JD, Curry FR: Na-K-Cl cotransport regulates intracellular volume and monolayer permeability of trabecular meshwork cells. Am J Physiol 1995;268:C1067–1074 [DOI] [PubMed] [Google Scholar]
- 32.Perkins TW, Alvarado JA, Polansky JR, Stilwell L, Maglio M, Juster R: Trabecular meshwork cells grown on filters. Conductivity and cytochalasin effects Invest Ophthalmol Vis Sci 1988;29:1836–1846 [PubMed] [Google Scholar]
- 33.Li AF, Tane N, Roy S: Fibronectin overexpression inhibits trabecular meshwork cell monolayer permeability. Mol Vis 2004;10:750–757 [PubMed] [Google Scholar]
- 34.Buckingham B, Reiser KM: Relationship between the content of lysyl oxidase-dependent cross-links in skin collagen, nonenzymatic glycosylation, and long-term complications in type I diabetes mellitus. J Clin Invest 1990;86:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Pape A, Guitton JD, Gutman N, Legrand Y, Fauvel F, Muh JP: Nonenzymatic glycosylation of collagen in diabetes: incidence on increased normal platelet aggregation. Haemostasis 1983;13:36–41 [DOI] [PubMed] [Google Scholar]
- 36.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH: Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 2007;117:3810–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB, Neufeld G: Abnormal deposition of collagen around hepatocytes in Wilson's disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol 2005;43:499–507 [DOI] [PubMed] [Google Scholar]
- 38.Görögh T, Weise JB, Holtmeier C, Rudolph P, Hedderich J, Gottschlich S, Hoffmann M, Ambrosch P, Csiszar K: Selective upregulation and amplification of the lysyl oxidase like-4 (LOXL4) gene in head and neck squamous cell carcinoma. J Pathol 2007;212:74–82 [DOI] [PubMed] [Google Scholar]
- 39.Erler JT, Weaver VM: Three-dimensional context regulation of metastasis. Clin Exp Metastasis 2009;26:35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortolan EV, Spadella CT, Caramori C, Machado JL, Gregorio EA, Rabello K: Microscopic, morphometric and ultrastructural analysis of anastomotic healing in the intestine of normal and diabetic rats. Exp Clin Endocrinol Diabetes 2008;116:198–202 [DOI] [PubMed] [Google Scholar]
- 41.Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, Vinik AI: Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg 1997;36:272–278;discussion 330 [DOI] [PubMed] [Google Scholar]
- 42.Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC: The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem 2004;279:40593–40600 [DOI] [PubMed] [Google Scholar]
- 43.Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, Sonenshein GE: The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res 2007;67:1105–1112 [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE: Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res 2007;67:6278–6285 [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Min C, Vora SR, Trackman PC, Sonenshein GE, Kirsch KH: The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells. J Biol Chem 2009;284:1385–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palamakumbura AH, Vora SR, Nugent MA, Kirsch KH, Sonenshein GE, Trackman PC: Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene 2009;28:3390–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, Spicer DB, Rosenberg L, Palmer JR, Sonenshein GE: A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res 2009;69:6685–6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Csiszar K: Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol 2001;70:1–32 [DOI] [PubMed] [Google Scholar]
- 49.Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S: Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes 2006;55:86–92 [PubMed] [Google Scholar]
- 50.Xin X, Khan ZA, Chen S, Chakrabarti S: Glucose-induced Akt1 activation mediates fibronectin synthesis in endothelial cells. Diabetologia 2005;48:2428–2436 [DOI] [PubMed] [Google Scholar]
- 51.Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA: Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol 1999;97:217–228 [DOI] [PubMed] [Google Scholar]
- 52.Brownlee M: Glycation products and the pathogenesis of diabetic complications. Diabetes Care 1992;15:1835–1843 [DOI] [PubMed] [Google Scholar]
- 53.Trueb B, Fluckiger R, Winterhalter KH: Nonenzymatic glycosylation of basement membrane collagen in diabetes mellitus. Coll Relat Res 1984;4:239–251 [DOI] [PubMed] [Google Scholar]
- 54.Ge G, Greenspan DS: Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today 2006;78:47–68 [DOI] [PubMed] [Google Scholar]
- 55.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE: Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 2009;29:4467–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]