Abstract
Retroviral envelope protein p15E and antigenically related proteins have been implicated as potential mediators of immune dysfunction associated with retroviral infections and with neoplasia. Due to its extreme hydrophobicity, purified p15E has not been available in a nondenatured form or in sufficient quantities for detailed studies on the mechanisms of its immunosuppressive effects. Therefore, a plasmid was constructed to direct the synthesis in Escherichia coli of the major hydrophilic region of murine p15E. The purified recombinant p15E derivative, soluble under physiological conditions, inhibited by up to 60% (EC50 = 7.5 nM) the anti-CD3-driven proliferation of human T lymphocytes but had no effect on the proliferation of the transformed T-cell line Jurkat. The recombinant protein also inhibited, by up to an average of 92% (EC50 = 2.1 microM), the proliferation of the murine T-cell line CTLL-2. These data (i) provide direct evidence that a retroviral envelope protein can itself inhibit lymphoproliferative function and (ii) map the inhibitory activity to a specific region of p15E. The availability of soluble, recombinant p15E should facilitate studies of the pathogenesis of the immunosuppression accompanying retroviral infections and neoplastic diseases.
Full text
PDF


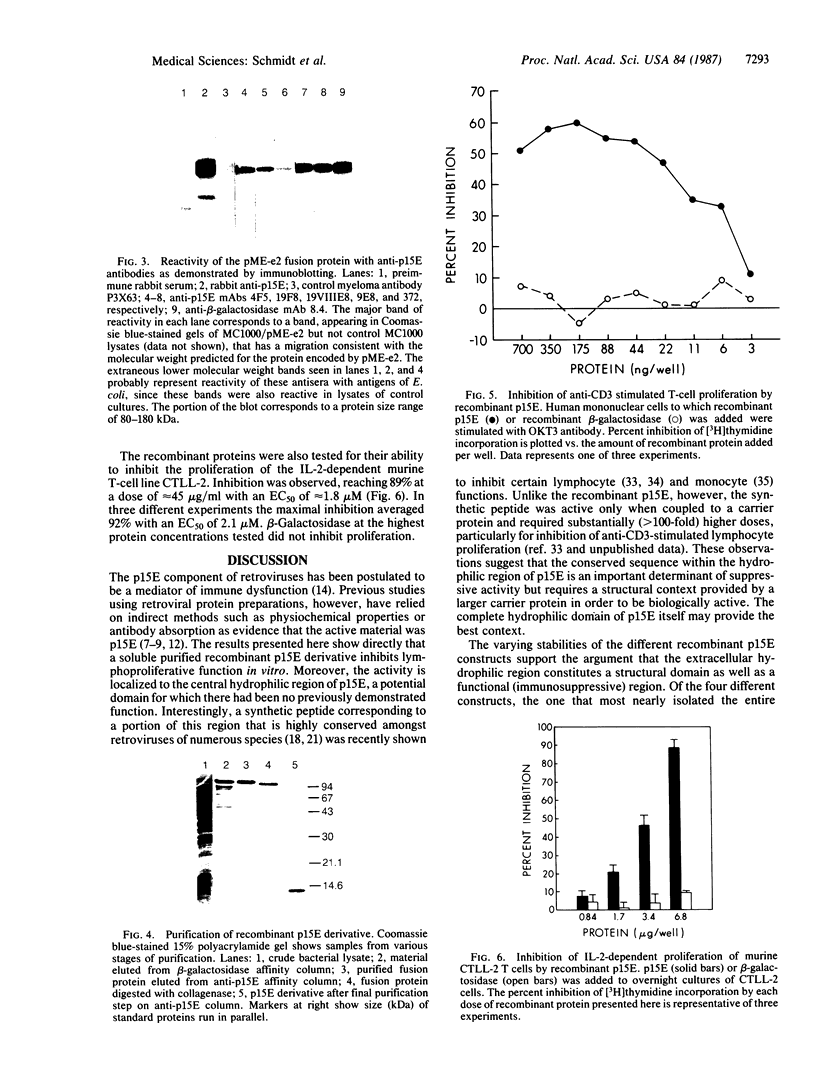

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein K., Lew K. K., Jarvik V., Swanson C. A. Role of antirepressor in the bipartite control of repression and immunity by bacteriophage P22. J Mol Biol. 1975 Feb 5;91(4):439–462. doi: 10.1016/0022-2836(75)90271-5. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Kipnis R. J., Snyderman R. Similarity between p15E of murine and feline leukaemia viruses and p21 of HTLV. Nature. 1984 Oct 11;311(5986):515–515. doi: 10.1038/311515a0. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Lostrom M. E., Tam M., Snyderman R. Murine malignant cells synthesize a 19,000-dalton protein that is physicochemically and antigenically related to the immunosuppressive retroviral protein, P15E. J Exp Med. 1983 Sep 1;158(3):885–900. doi: 10.1084/jem.158.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G. J., Matthews T. J., Bolognesi D. P., Snyderman R. Macrophage accumulation in mice is inhibited by low molecular weight products from murine leukemia viruses. J Immunol. 1980 Jun;124(6):2900–2905. [PubMed] [Google Scholar]
- Cianciolo G., Hunter J., Silva J., Haskill J. S., Snyderman R. Inhibitors of monocyte responses to chemotaxins are present in human cancerous effusions and react with monoclonal antibodies to the P15(E) structural protein of retroviruses. J Clin Invest. 1981 Oct;68(4):831–844. doi: 10.1172/JCI110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A plasmid cloning vector for the direct selection of strains carrying recombinant plasmids. Gene. 1981 Oct;15(1):99–102. doi: 10.1016/0378-1119(81)90108-6. [DOI] [PubMed] [Google Scholar]
- Dent P. B. Immunodepression by oncogenic viruses. Prog Med Virol. 1972;14:1–35. [PubMed] [Google Scholar]
- Engelman R. W., Fulton R. W., Good R. A., Day N. K. Suppression of gamma interferon production by inactivated feline leukemia virus. Science. 1985 Mar 15;227(4692):1368–1370. doi: 10.1126/science.2983424. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Cytotoxic T lymphocyte recognition of transfected cells expressing a cloned retroviral gene. 1983 Oct 27-Nov 2Nature. 305(5937):815–818. doi: 10.1038/305815a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Inhibition of lymphocyte transformation by disrupted murine oncornavirus. Cancer Res. 1977 Dec;37(12):4529–4531. [PubMed] [Google Scholar]
- Germino J., Bastia D. Rapid purification of a cloned gene product by genetic fusion and site-specific proteolysis. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4692–4696. doi: 10.1073/pnas.81.15.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Harrell R. A., Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Suppression of the respiratory burst of human monocytes by a synthetic peptide homologous to envelope proteins of human and animal retroviruses. J Immunol. 1986 May 15;136(10):3517–3520. [PubMed] [Google Scholar]
- Haseltine W. A., Sodroski J. G., Patarca R. Structure and function of the genome of HTLV. Curr Top Microbiol Immunol. 1985;115:177–209. doi: 10.1007/978-3-642-70113-9_12. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Jr, Hanna M. G., Schafer W., Hunsmann G., Bolognesi D. P., HUPER G. Polypeptides of mammalian oncornaviruses. III. Localization of p 15 and reactivity with natural antibody. Virology. 1975 Jan;63(1):60–67. doi: 10.1016/0042-6822(75)90370-0. [DOI] [PubMed] [Google Scholar]
- Laurence J. The immune system in AIDS. Sci Am. 1985 Dec;253(6):84–93. doi: 10.1038/scientificamerican1285-84. [DOI] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P. Abrogation of lymphocyte blastogenesis by a feline leukaemia virus protein. Nature. 1978 Aug 17;274(5672):687–689. doi: 10.1038/274687a0. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P., Adams P. W., Nichols W. S. Immunosuppressive properties of a virion polypeptide, a 15,000-dalton protein, from feline leukemia virus. Cancer Res. 1979 Mar;39(3):950–955. [PubMed] [Google Scholar]
- Mitani M., Cianciolo G. J., Snyderman R., Yasuda M., Good R. A., Day N. K. Suppressive effect on polyclonal B-cell activation of a synthetic peptide homologous to a transmembrane component of oncogenic retroviruses. Proc Natl Acad Sci U S A. 1987 Jan;84(1):237–240. doi: 10.1073/pnas.84.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S., Nelson M., Farram E., Inoue Y. Cancer and subversion of host defences. Aust J Exp Biol Med Sci. 1981 Jun;59(Pt 3):229–262. doi: 10.1038/icb.1981.18. [DOI] [PubMed] [Google Scholar]
- Orosz C. G., Zinn N. E., Olsen R. G., Mathes L. E. Retrovirus-mediated immunosuppression. I. FeLV-UV and specific FeLV proteins alter T lymphocyte behavior by inducing hyporesponsiveness to lymphokines. J Immunol. 1985 May;134(5):3396–3403. [PubMed] [Google Scholar]
- Pahwa S., Pahwa R., Saxinger C., Gallo R. C., Good R. A. Influence of the human T-lymphotropic virus/lymphadenopathy-associated virus on functions of human lymphocytes: evidence for immunosuppressive effects and polyclonal B-cell activation by banded viral preparations. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8198–8202. doi: 10.1073/pnas.82.23.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. Topography of murine leukemia virus envelope proteins: characterization of transmembrane components. J Virol. 1983 Jun;46(3):1056–1060. doi: 10.1128/jvi.46.3.1056-1060.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W., Hunsmann G., Moennig V., Noranha F., Bolognesi D. P., Green R. W., Hüper G. Polypeptides of mammalian oncornaviruses. II Characterization of murine leukemia virus polypeptide (p 15) bearing interspecies reactivity. Virology. 1975 Jan;63(1):48–59. doi: 10.1016/0042-6822(75)90369-4. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Tan I. B., Drexhage H. A., Scheper R. J., von Blomberg-van de Flier B. M., de Haan-Meulman M., Snow G. B., Balm F. J. Immunosuppressive retroviral P15E-related factors in head and neck carcinomas. Arch Otolaryngol Head Neck Surg. 1986 Sep;112(9):942–945. doi: 10.1001/archotol.1986.03780090038006. [DOI] [PubMed] [Google Scholar]
- Ullmann A. One-step purification of hybrid proteins which have beta-galactosidase activity. Gene. 1984 Jul-Aug;29(1-2):27–31. doi: 10.1016/0378-1119(84)90162-8. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Spira B., Boushira M., Margolese R. G. Inhibition by human T-lymphotropic virus (HTLV-I) of T-lymphocyte mitogenesis: failure of exogenous T-cell growth factor to restore responsiveness to lectin. Immunology. 1985 Jan;54(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]