Abstract
OBJECTIVE
Little is known concerning the primary cause(s) of mortality in type 1 diabetes responsible for the excess mortality seen in this population.
RESEARCH DESIGN AND METHODS
The Allegheny County (Pennsylvania) childhood-onset (age <18 years) type 1 diabetes registry (n = 1,075) with diagnosis from 1965 to 1979 was used to explore patterns in cause-specific mortality. Cause of death was determined by a mortality classification committee of at least three physician epidemiologists, based on the death certificate and additional records surrounding the death.
RESULTS
Vital status for 1,043 (97%) participants was ascertained as of 1 January 2008, revealing 279 (26.0%) deaths overall (141 females and 138 males). Within the first 10 years after diagnosis, the leading cause of death was acute diabetes complications (73.6%), while during the next 10 years, deaths were nearly evenly attributed to acute (15%), cardiovascular (22%), renal (20%), or infectious (18%) causes. After 20 years' duration, chronic diabetes complications (cardiovascular, renal, or infectious) accounted for >70% of all deaths, with cardiovascular disease as the leading cause of death (40%). Women (P < 0.05) and African Americans (P < 0.001) have significantly higher diabetes-related mortality rates than men and Caucasians, respectively. Standardized mortality ratios (SMRs) for non–diabetes-related causes do not significantly differ from the general population (violent deaths: SMR 1.2, 95% CI 0.6–1.8; cancer: SMR 1.2, 0.5–2.0).
CONCLUSIONS
The excess mortality seen in type 1 diabetes is almost entirely related to diabetes and its comorbidities but varies by duration of diabetes and particularly affects women and African Americans.
Mortality rates for type 1 diabetes are much higher than in the general population in the U.S. and worldwide (1–3). Understanding the primary cause(s) of this excess mortality is essential for developing interventions to decrease mortality rates in type 1 diabetes. This excess mortality has been attributed both to acute diabetes complications as well as to chronic diabetic renal and cardiovascular disease (CVD). Previous reports have shown that renal disease is the principal cause of mortality in the first 20 years of type 1 diabetes; subsequently, CVD predominates (4–6).
Only a handful of recent reports address cause-specific mortality in population-based type 1 diabetes cohorts (7–12) and even fewer have long-term follow-up (>15 years) (10,11). A recent Norwegian 20-year follow-up report addressed long-term cause-specific mortality in 1,906 individuals with childhood-onset type 1 diabetes (standardized mortality ratio [SMR] = 4.0) (11) and found that, before age 30 years, acute complications was the leading cause of death in this cohort, whereas, after age 30 years, CVD was predominant, consistent with findings from the large Diabetes U.K. Study (12). The Diabetes U.K. Study classified type 1 diabetes as insulin treatment before age 30 years (n = 23,752), and based on death certificate data alone, found that acute metabolic complications predominated before age 30 years, but again thereafter, CVD becomes the leading cause. Aside from the current study, the Norwegian study is the only other long-term type 1 diabetes study to investigate deaths using available clinical data in addition to death certificate data.
Findings from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study suggest that the incidence of end-stage renal disease has declined quite markedly, whereas the incidence of coronary artery disease remains relatively unchanged over 30 years in individuals with type 1 diabetes (13). This is of particular interest, since renal disease is thought to be the major driver of CVD in type 1 diabetes (14).
Herein, we explore cause-specific mortality trends by sex, race, and calendar year of diagnosis to determine how type 1 diabetes mortality is changing over time using a large population-based type 1 diabetes cohort in Allegheny County (Pittsburgh), Pennsylvania (diagnosis 1965–1979). Apart from a race-specific analysis where this cohort was combined with the Pittsburgh EDC study, cause-specific mortality has not been formally evaluated in this population since 1985 (15). We specifically examine whether the percentage of renal-related cardiovascular deaths is changing over time as well as other cause-specific patterns.
RESEARCH DESIGN AND METHODS
Study population.
The Allegheny County Type 1 Diabetes Registry (n = 1,075) has been described in detail (16) and consists of all individuals diagnosed with childhood-onset (age <18 years) type 1 diabetes in Allegheny County between 1 January 1965 and 31 December 1979 and placed on insulin at diagnosis. Individuals were identified via hospital record review and validated by contacting pediatricians throughout the county (ascertainment >95%) (17). Children developing diabetes from a secondary cause (i.e., cystic fibrosis, Down's syndrome, or steroid-induced diabetes) were excluded. This cohort was part of a comparative international study (Diabetes Epidemiology Research International [DERI]) of type 1 diabetes mortality rates (18).
To determine the vital status as of 1 January 2008, all participants were contacted initially by letter, then by telephone if necessary, to complete a brief health update questionnaire. The study protocol was approved by the University of Pittsburgh Institutional Review Board.
Mortality classification procedures.
Deaths were identified by 1) participant contact, 2) the Social Security Death Index, or 3) the National Death Index and then confirmed by death certificate. Attempts were made to obtain the following information, when appropriate: 1) medical records surrounding the death, 2) autopsy/coroner's reports, and 3) interview with next-of-kin regarding the circumstances surrounding the death. Protocols for abstracting standardized data from these records have been comprehensively detailed (15). A Mortality Classification Committee (MCC) consisting of three or more physician epidemiologists used standardized protocols to determine 1) the underlying cause of death and 2) the ordered contribution of all other causes of death. Internal adjudication (A.M.S. and T.J.O.) has been made for all deaths not yet finalized by the MCC (n = 60) based on the same protocols. Causes of death were grouped as shown in Table 1.
TABLE 1.
Cause-of-death groups for classification by the MCC
| Diabetes-related |
| Acute diabetic complications: diabetic ketoacidosis, hyperglycemia, hypoglycemia, hypokalemia, and diabetic coma unspecified |
| Diabetic renal disease: diabetic nephropathy, end-stage renal disease, and dialysis-related complications |
| Cardiovascular: coronary artery disease, myocardial infarction, sudden cardiac death, cerebrovascular disease, peripheral vascular disease, heart failure, arrhythmias, and cardiomyopathy |
| Infection: pneumonia, bronchitis, sepsis, meningitis, encephalitis, osteomyelitis, mycosis, and endocarditis |
| Other diabetes: autonomic neuropathy, angiopathy, uncontrolled diabetes, and post-transplant complications |
| Non–diabetes-related |
| Cancer: any form of cancer |
| Accident or suicide: violent deaths, motor vehicle crashes, alcohol/drug overdose, and other accidental deaths (drowning, fall, fire) |
| Other non-diabetes: other causes not specific to diabetes, e.g., sickle cell trait, bulimia/anorexia, lupus, septic abortion, epilepsy, multiple sclerosis, gastric ulcer, or HIV |
| Unknown, where cause of death could not be determined |
Statistical analysis.
Each individual's contribution to person-years of follow-up was calculated from date of diabetes diagnosis to either 1 January 2008 or date of death or date lost to follow-up. To compare continuous variables between groups, Student t test and one-way ANOVA were used, adjusting for multiple comparisons using the Bonferroni correction. The χ2 or Fisher's exact test was used to compare categorical variables between groups, as appropriate. To evaluate temporal trends in cause-specific mortality, calendar year at diagnosis was categorized into three groups (1965–1969, 1970–1974, and 1975–1979). Also, causes of death were grouped into four broad categories for some analyses (Table 1): 1) acute diabetes-related causes, 2) chronic diabetes-related causes (including the renal, cardiovascular, infection, and other diabetes categories), 3) non-diabetes causes (including accident or suicide, cancer, and other non-diabetes categories), and 4) unknown cause.
Cause-specific mortality rates were calculated by dividing the number of deaths by the person-years of follow-up for individuals at risk during the specified duration interval. Cause-specific expected mortality was estimated using the person-years method based on general population life tables for Allegheny County, Pennsylvania, or when deaths from a particular cause were extremely rare in the age-matched county population (i.e., renal deaths and infections), based on life tables for the state of Pennsylvania (19). Cause-specific SMRs adjusting for age, sex, and race were calculated as the observed divided by the expected number of deaths in each age, sex, and race category, and 95% CIs were determined with the Poisson distribution. Cause-specific mortality rates and SMRs were compared using rate ratio analyses (20). Statistical significance of P < 0.05 was used. Analyses were performed using SPSS 17.0 (SPSS, Chicago, IL).
RESULTS
Demographic characteristics of the Allegheny County Type 1 Diabetes Registry cohort are presented by year of diabetes diagnosis in Table 2. Vital status was confirmed as of 1 January 2008 for 1,043 participants (97.0% ascertainment rate). A total of 279 deaths (26.0%) occurred in this population over 34,363 total person-years of follow-up. Nearly half of the total deaths occurred in the 1965–1969 diagnosis cohort (n = 132), with significantly fewer deaths in the 1970–1974 and 1975–1979 diagnosis cohorts (n = 92 and 55, respectively). No significant sex or racial differences existed by diagnosis cohort; however, age at onset significantly increased across diagnosis cohorts (P = 0.01).
TABLE 2.
Demographic characteristics of Allegheny County Type 1 Diabetes Registry population by diagnosis cohort as of 1 January 2008
| Diagnosis cohort |
Overall | |||
|---|---|---|---|---|
| 1965–1969 | 1970–1974 | 1975–1979 | ||
| n | 355 | 391 | 329 | 1,075 |
| Vital status confirmed | 96.6 (343) | 97.4 (381) | 97.0 (319) | 97.0 (1,043) |
| Deceased | 37.2 (132) | 23.5 (92) | 16.7 (55)* | 26.0 (279) |
| Male | 50.4 (179) | 54.5 (213) | 50.5 (166) | 51.9 (558) |
| Caucasian | 92.4 (328) | 93.6 (366) | 91.8 (302) | 92.7 (996) |
| Age at diabetes diagnosis | 10.5 ± 4.4 | 10.8 ± 4.0 | 11.4 ± 4.0* | 10.9 ± 4.2 |
| Mean diabetes duration† | 34.6 ± 9.0 | 32.2 ± 7.3 | 28.9 ± 4.7* | 32.0 ± 7.6 |
| Mean age† | 45.1 ± 9.2 | 42.9 ± 7.8 | 40.3 ± 5.9* | 42.9 ± 8.0 |
| Person-years of follow-up | 12,277.6 | 12,584.8 | 9,500.7 | 34,363.1 |
Data are % (n) or means ± SD.
*P ≤ 0.01 for either χ2 or ANOVA across diagnosis cohort groups.
†Type 1 diabetes duration and age at death or last follow-up.
Proportions of deaths by cause.
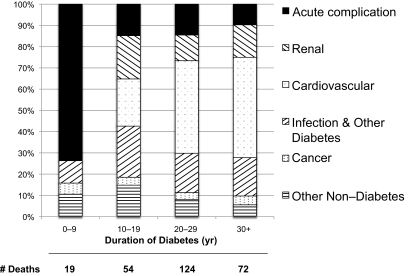
Figure 1 shows the proportions of deaths by underlying cause for 10-year intervals of diabetes duration. Within the first 10 years of diabetes onset, >70% of all deaths were due to acute diabetes complications. After 10 years of diabetes, acute complications become a minor but persistent (9–15%) cause of death in this population. Also, after 10 years of diabetes, CVD becomes the leading cause of death, eventually accounting for 40% of all deaths after 20 years' duration.
FIG. 1.
Distribution of underlying causes of death within 10-year intervals of type 1 diabetes duration. Ten deaths where the cause of death was unknown were excluded.
By separating these data by sex, race, and diabetes duration, some patterns emerge (see supplemental Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0862/DC1). In females, diabetes complications caused >90% of all deaths in the first 30 years after diagnosis compared with 75% of all deaths in males during the same time period. Aside from one death of unknown cause, all deaths in African American participants were due to either acute or chronic diabetes complications, whereas nearly 20% of all deaths in Caucasian participants were not related to diabetes. However, only eight deaths occurred in African Americans in the first 20 years of diabetes, limiting further stratification by year of diagnosis.
Cause-specific mortality rates.
Cause-specific mortality rates were stratified by sex and race (Table 3), as well as by year of diabetes diagnosis (Table 4). Overall, females with type 1 diabetes had a higher mortality rate for diabetes-related deaths (female:male [F:M] mortality rate ratio [RR] = 1.3, P = 0.05), whereas males tended to have a higher mortality rate from non–diabetes-related causes (M:F RR 1.9, P = 0.06). These findings remained significant when deaths not yet finalized by the MCC (n = 60) were excluded from analysis. Of the 16 total deaths caused by accident or suicide, 14 occurred in males (M:F RR 7.2, P = 0.009). When the mortality rates were stratified by both sex and duration of diabetes (data not shown), the only significant difference was that within the first 10 years after diagnosis, 12 of 14 acute complication deaths occurred in women (F:M RR 6.6, P = 0.01).
TABLE 3.
Cause-specific mortality rates by sex and race in the Allegheny County Type 1 Diabetes Registry cohort
| n* | Sex |
Race |
|||
|---|---|---|---|---|---|
| Male (n = 138) | Female (n = 141) | Caucasian (n = 239) | African American (n = 40) | ||
| Diabetes-related | 239 | 613.9 (499.7–728.1) | 786.2 (650.0–922.4)† | 618.0 (532.1–703.8) | 1,851.3 (1,277.6–2,425.1)† |
| Acute complication | 45 | 105.1 (57.8–152.3) | 159.7 (98.3–221.1) | 102.5 (67.5–137.4) | 555.4 (241.2–869.6)† |
| Renal disease | 37 | 88.5 (45.1–131.8) | 129.0 (73.8–184.2) | 86.9 (54.7–119.2) | 416.6 (144.4–688.7)† |
| CVD | 101 | 276.5 (199.9–353.2) | 313.3 (227.3–399.2) | 276.4 (219.0–333.8) | 555.4 (241.2–869.6)† |
| Infection/other | 56 | 143.8 (88.5–199.1) | 184.3 (118.3–250.2) | 152.2 (109.6–194.8) | 324.0 (84.0–564.0) |
| Non–diabetes–related | 40 | 149.3 (93.0–205.6) | 79.8 (36.4–123.3) | 124.2 (85.7–162.7) | 0.0 |
| Accident/violent | 16 | 77.4 (36.9–118.0) | 12.3 (0.0–29.3)† | 49.7 (25.3–74.0) | 0.0 |
| Cancer | 10 | 27.7 (3.4–51.9) | 30.7 (3.8–57.6) | 31.1 (11.8–50.3) | 0.0 |
| Other | 14 | 44.2 (13.6–74.9) | 36.9 (7.4–66.3) | 43.5 (20.7–66.2) | 0.0 |
| Overall | 279 | 763.2 (635.8–890.5) | 866.1 (723.1–1,009.0) | 742.2 (648.1–836.3) | 1,851.3 (1,277.6–2,425.1)‡ |
Data are n or per 100,000 person-years (95% CI).
*Observed deaths.
†P ≤ 0.05 and
‡P ≤ 0.001 for RR compared with male sex or Caucasian race.
TABLE 4.
Cause-specific mortality rates by year of diabetes diagnosis and duration of diabetes censored at 30 years of follow-up
| n* | Diagnosis cohort |
|||
|---|---|---|---|---|
| 1965–1969 (n = 79) | 1970–1974 (n = 74) | 1975–1979 (n = 49) | ||
| Diabetes-related | 171 | 668.7 (507.4–830.1) | 575.7 (433.5–717.8) | 454.9 (317.3–592.5)† |
| Acute complication | 38 | 152.0 (75.1–228.9) | 155.3 (81.5–229.2) | 65.0 (13.0–117.0) |
| Renal disease | 26 | 121.6 (52.8–190.4) | 64.0 (16.6–111.4) | 75.8 (19.6–132.0) |
| CVD | 67 | 263.4 (162.2–364.7) | 219.3 (131.6–307.0) | 184.1 (96.6–271.6) |
| Infection/other | 40 | 131.7 (60.1–203.3) | 137.1 (67.7–206.4) | 130.0 (56.4–203.5) |
| Non–diabetes-related | 31 | 131.7 (60.1–203.3) | 100.5 (41.1–159.9) | 75.8 (19.6–132.0) |
| Accident/violent | 14 | 40.5 (0.8–80.2) | 54.8 (11.0–98.7) | 43.3 (0.9–85.8) |
| Cancer | 7 | 40.5 (0.8–80.2) | 9.1 (0.0–27.1) | 21.7 (0.0–51.7) |
| Other | 10 | 50.7 (6.3–95.1) | 36.6 (0.7–72.4) | 10.8 (0.0–32.1) |
| Overall | 202 | 800.5 (624.0–977.0) | 676.2 (522.1–830.2) | 530.7 (382.1–679.3)† |
Data are n or per 100,000 person-years (95% CI).
*Observed deaths.
†P ≤ 0.05 for RR of 1975–1979 compared with 1965–1969.
When stratified by race, the overall mortality rate was significantly higher in African American participants (RR 2.5, P < 0.001, Table 3). Specifically, African American participants with type 1 diabetes died of diabetes complications at much higher rates than Caucasian participants: acute: RR 4.8, P < 0.001; renal disease: RR 4.6, P < 0.001; CVD: RR 1.9, P = 0.05; and infection: RR 2.8, P = 0.01. All comparisons by race remained significant after excluding deaths not yet finalized by the MCC except for CVD (RR 1.4, P = 0.48). Conversely, all deaths from non–diabetes-related causes occurred in Caucasian participants with type 1 diabetes. Of note, all eight suicides in this cohort occurred in Caucasian males.
Cause-specific mortality rates by diabetes diagnosis cohort were censored at 30 years of follow-up for appropriate comparisons (Table 4). Overall, the 1965–1969 cohort had significantly higher mortality rates from diabetes-related causes compared with the 1975–1979 cohort (RR 1.5, P = 0.05), which remained significant after excluding deaths not yet finalized by the MCC. Mortality rates from non–diabetes-related causes were also higher for the 1965–1969 cohort, but this was not significant (RR 1.7, P = 0.24). While cause-specific mortality rates for nearly all diabetes complications were highest in the oldest cohort (1965–1969) and tended to decrease stepwise to the youngest cohort (1975–1979), none of the mortality rates differed significantly. In addition, further stratification by diabetes duration revealed that, compared with the 1965–1969 cohort, the 1975–1979 cohort had a significantly lower mortality rate over the first 20 years' duration of type 1 diabetes (RR 0.45, P = 0.01) (data not shown).
Cause-specific standardized mortality ratios.
Cause-specific SMRs for this cohort were calculated (Table 5). SMRs for acute complications were not calculated, since these causes of death (hyperglycemia and diabetic ketoacidosis) occur almost exclusively in individuals with type 1 diabetes. Overall, deaths from CVD (SMR 12.9, 95% CI 10.4–15.5), renal disease (104.3, 70.7–137.9), and infection (41.2, 29.3–53.1) all occur at tremendously higher rates than those seen in the comparable local population. However, deaths from non–diabetes-related causes such as accident/suicide (SMR = 1.2, 0.6–1.8) or cancer (SMR = 1.2, 0.5–2.0) occur at similar rates in type 1 diabetes to the age-, sex-, and race-matched general population.
TABLE 5.
Cause-specific standardized mortality ratios by sex, race, and diabetes diagnosis cohort
| n* | CVD | Renal | Infection | Violent† | Cancer | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 138 | 8.8 (6.3–11.2) | 77.8 (39.7–116.0) | 28.1 (16.1–40.1) | 1.4 (0.7–2.1) | 1.2 (0.1–2.2) |
| Female | 141 | 24.7 (17.9–31.6) | 140.8 (80.6–201.1) | 67.8 (41.2–94.4) | 0.7 (0.0–1.6) | 1.4 (0.2–2.5) |
| Race | ||||||
| Caucasian | 239 | 12.4 (9.8–15.0) | 103.7 (65.3–142.1) | 45.0 (31.1–59.0) | 1.5 (0.7–2.2) | 1.3 (0.5–2.2) |
| African American | 40 | 19.4 (8.4–30.3) | 106.3 (36.9–175.8) | 26.2 (5.2–47.2) | — | — |
| Diagnosis cohort | ||||||
| 1965–1969 | 132 | 13.9 (10.0–17.7) | 137.7 (77.4–198.1) | 45.9 (25.8–66.1) | 1.1 (0.1–2.1) | 1.6 (0.3–2.8) |
| 1970–1974 | 92 | 10.9 (7.0–14.8) | 80.1 (30.5–129.7) | 39.7 (20.2–59.2) | 1.2 (0.2–2.2) | 0.7 (0.0–1.8) |
| 1975–1979 | 55 | 14.7 (8.3–21.2) | 82.7 (21.4–144.0) | 35.8 (13.6–58.0) | 1.3 (0.2–2.5) | 1.3 (0.0–3.2) |
| Overall | 279 | 12.9 (10.4–15.5) | 104.3 (70.7–137.9) | 41.2 (29.3–53.1) | 1.2 (0.6–1.8) | 1.2 (0.5–2.0) |
Data are n or cause-specific SMRs (95% CI).
*Observed deaths.
†Accident/suicide.
CVD, renal, and infection SMRs for females in this type 1 diabetes cohort were higher than the respective SMRs for males (3 times higher for CVD and 2.5 times higher for infection), whereas no dramatic differences in SMR were seen by race or by diabetes diagnosis cohort for any cause of death (Table 5).
Contribution of major diabetes complications to death in type 1 diabetes.
Finally, most deaths (65.0%) in this type 1 diabetes cohort had at least one contributing or secondary cause of death. Therefore, to determine the true contribution of each major diabetes complication (acute complications, renal disease, and cardiovascular disease) in the deaths of individuals with type 1 diabetes, each major complication was considered to contribute to a death if it was either the underlying cause of death or a secondary cause of death. For example, an individual develops sepsis in the hospital after a bypass surgery because of a nosocomial infection of a dialysis port. The underlying cause of death might be classified as infection; however, both renal and cardiovascular disease played significant secondary roles in this death.
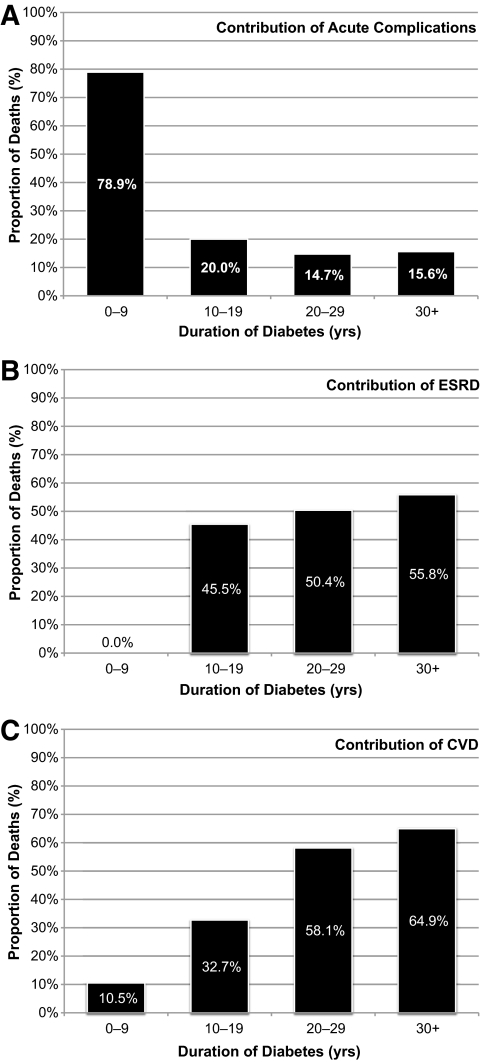
The proportion of deaths to which each major diabetes complication contributed is plotted by diabetes duration in Fig. 2. Of note, at least one of these three major diabetes complications contributed to 82.5% (n = 231) of all deaths in this cohort. Acute complications contributed to 80% of all deaths within the first 10 year of type 1 diabetes, but then dropped to <20% of all deaths thereafter (Fig. 2A). Renal disease does not contribute to any deaths until after 10 years of diabetes duration, where it contributes to about half of all deaths in this cohort (Fig. 2B). Cardiovascular disease contributes to ∼10% of all deaths within the first 10 years of diabetes, but dramatically increases to contribute to 60% of all deaths after 20 years of diabetes (Fig. 2C). The role of renal disease in cardiovascular deaths consistently increased with longer diabetes duration; renal disease contributed to 67% of all CVD deaths before 20 years' duration, but this contribution increases to 85% of all CVD deaths after 30 years' diabetes duration (data not shown).
FIG. 2.
Total contribution (underlying and/or secondary cause of death) of major diabetes complications to deaths in type 1 diabetes. Proportions of death in each diabetes duration interval where the following complications either caused or contributed to death are shown: acute complications (A), end-stage renal disease (ESRD) (B), and CVD (C).
An exploratory analysis of the contribution of these major complications by race, sex, or diabetes duration revealed no significant patterns, except that renal disease contributed to more deaths in African Americans than in Caucasians with type 1 diabetes (62.5 vs. 44.6%, respectively; P = 0.04).
DISCUSSION
These data represent the first attempt to assess cause-specific mortality in a population-based cohort with longstanding (>20 years) type 1 diabetes in the U.S. These results clearly show that the higher mortality seen in type 1 diabetes compared with the general population results almost exclusively from higher rates in diabetes-related acute and chronic complications, since cause-specific SMRs for cancer and for violent deaths do not differ from the age-, sex-, and race-matched local population. Females with type 1 diabetes had higher mortality rates for diabetes-related causes, but males had higher mortality rates for non–diabetes-related causes (especially violent deaths). African Americans with type 1 diabetes have significantly higher mortality rates for all major diabetes-related causes compared with Caucasians. Conversely, no African Americans died of non–diabetes-related causes in this type 1 diabetes cohort.
Causes of early mortality (<10 years' diabetes duration) have been extensively studied in type 1 diabetes across many countries and cohorts. An early report from the Children's Hospital of Pittsburgh cohort diagnosed between 1950 and 1980 reported 64% of all early deaths (compared with 74% in our cohort) after diagnosis were caused by acute complications (all diabetic ketoacidosis) (21). An international study that included early mortality data from the present study showed that acute complications (38%) were a leading cause of death in the first 10 years of type 1 diabetes, followed by accident/suicide (30%), with acute deaths occurring more commonly in Japan and in Allegheny County than in Finland and Israel (15). A more recent report on early mortality in type 1 diabetes from EURODIAB revealed that only 35% of deaths in children diagnosed after 1989 (mean follow-up time 7.6 years) were due to acute diabetes complications (22), with another 53% due to non-diabetes causes (accidents, suicide, etc.).
Cause-specific mortality in longstanding type 1 diabetes (>20 years' duration) has only been explored in a handful of studies. Borch-Johnsen et al. (23) reported cause-specific mortality findings for all individuals diagnosed with type 1 diabetes (age ≤30) before 1943 at Steno Memorial Hospital (Denmark) and followed until 1984. More than 50% of deaths within 35 years of diabetes onset were from renal disease, compared with only 5% of the deaths after 40 years' duration. For comparison, renal disease was determined to be the underlying cause of death in only 17% of individuals who died before 35 years' diabetes duration in our cohort, suggesting that significant improvements in diabetes care between the 1940s and the 1970s have resulted in fewer renal deaths. However, on closer examination, by including deaths where renal disease was a secondary cause of death, renal disease contributed to 47% of all deaths in our cohort, indicating that improvements in care have delayed but not drastically curtailed renal disease in type 1 diabetes over this time period.
A 2006 report from a national Norwegian childhood-onset (age <15 years) type 1 diabetes cohort diagnosed between 1973 and 1982 and followed until 2002 reported findings dramatically different from the present report. Notably, the Norwegian cohort had smaller proportions of both CVD and infections than our cohort (15 vs. 35 and 5 vs. 16%, respectively), but larger proportions of both acute complications and violent deaths (22 vs. 16 and 28 vs. 6%, respectively) (11). While the authors did use a MCC to determine the underlying cause of death, the contribution of any secondary causes of death was not reported, preventing a proper comparison of the contribution of major complications between cohorts.
The cause-specific SMRs reported for diabetes-related causes in our cohort are comparable to other reports (10–12). All reports consistently show that SMRs for diabetes-related causes are significantly higher than the respective general populations.
Little long-term follow-up of African Americans with type 1 diabetes for cause-specific mortality exists outside our cohort. We previously published data on the racial differences in type 1 diabetes mortality combining the present cohort with the Children's Hospital of Pittsburgh cohort (diagnosed 1965–1979) for a 20-year follow-up analysis (24), where African Americans had a significantly higher risk of acute death (hazard ratio 4.9, 95% CI 2.0–11.6). These data are consistent with a retrospective study of death certificates for Chicago residents (age 1–24 years) by Lipton et al. (25) that showed all eight (of 30 total type 1 diabetes deaths) acute complication deaths at onset occurred in either non-Hispanic black or Hispanic patients. Only two deaths occurred in white type 1 diabetic patients during this interval in Chicago, and neither death was due to acute complications.
We now report significantly higher mortality rates for all diabetes-related causes in African Americans compared with Caucasians, which likely explains the dramatically increased overall mortality rates in African Americans with type 1 diabetes in our cohort (2,24,26). It should also be noted that all of the deaths (excluding one unknown cause of death) in African Americans in this cohort were caused by either an acute or a chronic diabetes complication; however, these findings are based on a relatively small number (n = 40) of African American deaths. Young African Americans have the highest violent death rates in the U.S. (27,28), and based on our African American follow-up time (2,161 person-years), we would expect to see 2.5 deaths due to violent causes. However, none were observed, suggesting that young African Americans with type 1 diabetes are “protected” from violent deaths, but are at a much higher risk of diabetes-related deaths than their Caucasian counterparts. The poorer prognosis for African Americans with type 1 diabetes might reflect an underlying racial gap in socioeconomic status or access to and use of health care present in the general U.S. population (27). Regrettably, none of these known factors are available for this cohort. A cause-specific analysis of mortality in the New Jersey 725 (all African Americans with type 1 diabetes) is forthcoming and should help clarify these racial differences (29).
The contribution of major diabetes complications to type 1 diabetes deaths has not been fully explored in a large population-based cohort. Acute complications contribute to 80% of all early (<10 years' duration) deaths, but only contribute to ∼15% of deaths thereafter, consistent with research showing that most early acute deaths in type 1 diabetes result from diabetic ketoacidosis (often at diabetes onset or after an acute illness), whereas later acute deaths tend to result from hypoglycemic episodes (30,31). We also found that renal disease and CVD contribute to 50 and >60% of all deaths, respectively, after 20 years of type 1 diabetes duration. Although the incidence of renal disease appears to be decreasing in the Pittsburgh EDC study (13), the renal effects on cardiovascular disease have yet to diminish in this cohort.
A key limitation of this study is that the cause-specific early mortality rates may not be representative of the current type 1 diabetes experience in the U.S., since study participants have now had diabetes for ≥30 years, and early diagnosis and treatment protocols have improved tremendously since the 1960s and 1970s. In addition, cause-specific analysis is limited to a few basic demographic variables (race, sex, and year of diagnosis), since other socioeconomic and clinical variables were not obtained at study inception (17). Finally, finalizing cause of death in this cohort is an ongoing process. To date, 219 deaths (79%) have been finalized by the international MCC. The remaining deaths have been classified based on the death certificate and all additional information available to date by an internal committee. However, a portion of these deaths might be reclassified as we obtain additional information regarding the circumstances surrounding each death and each death becomes finalized by the MCC. As such, sensitivity analyses were performed by excluding nonfinalized deaths (n = 60), and all comparisons except for the racial difference in CVD deaths remained robust to misclassification.
These results offer the best picture of cause-specific mortality in longstanding (28–43 years' duration) type 1 diabetes in the U.S., since they come from a large (n = 1,075) population-based cohort with near complete ascertainment (97%) of vital status. Also, we now have sufficient follow-up to determine sex, race, and temporal differences in cause-specific mortality.
In summary, mortality rates for all diabetes-related chronic complications show signs of decreasing, but all remain significantly higher than those seen in the age-, sex-, and race-matched local general population. An emphasis on preventing acute complications early after diagnosis as well as preventing or delaying chronic diabetes complications would likely result in major improvements in lifespan for individuals with type 1 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
A.M.S. was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (F30-DK082137).
No potential conflicts of interest relevant to this article were reported.
A.M.S., D.J.B., S.F.K., R.E.L., and T.J.O. created the study concept and design. A.M.S. acquired the study data. A.M.S., D.J.B., S.F.K., R.E.L., and T.J.O. analyzed and interpreted the data. A.M.S. and T.J.O. drafted the manuscript. A.M.S., D.J.B., S.F.K., R.E.L., and T.J.O. critically reviewed the manuscript for important intellectual content. A.M.S. and S.F.K. performed the statistical analysis. A.M.S. and T.J.O. obtained funding. T.J.O. obtained administrative, technical, or material support. T.J.O. supervised the study.
The general population mortality data were provided by the Bureau of Health Statistics and Research, Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
The authors acknowledge the long-term help of the Allegheny County Type 1 Diabetes Registry participants. The authors also acknowledge the continuing assistance of the members of the Mortality Classification Committee: Dr. Naoko Tajima (Jikei University, Tokyo, Japan) and Dr. Jaakko Tuomilehto (University of Helsinki, Helsinki, Finland.)
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 2997.
REFERENCES
- 1.Gu K, Cowie CC, Harris MI: Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 1998;21:1138–1145 [DOI] [PubMed] [Google Scholar]
- 2.Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ: Mortality trends in type 1 diabetes. The Allegheny County (Pennsylvania) Registry 1965–1999. Diabetes Care 2001;24:823–827 [DOI] [PubMed] [Google Scholar]
- 3.Matsushima M, LaPorte RE, Maruyama M, Shimizu K, Nishimura R, Tajima N: Geographic variation in mortality among individuals with youth-onset diabetes mellitus across the world. DERI Mortality Study Group. Diabetes Epidemiology Research International. Diabetologia 1997;40:212–216 [DOI] [PubMed] [Google Scholar]
- 4.Deckert T, Poulsen JE, Larsen M: Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia 1978;14:363–370 [DOI] [PubMed] [Google Scholar]
- 5.Christlieb AR, Warram JH, Królewski AS, Busick EJ, Ganda OP, Asmal AC, Soeldner JS, Bradley RF: Hypertension: the major risk factor in juvenile-onset insulin-dependent diabetics. Diabetes 1981;30:90–96 [DOI] [PubMed] [Google Scholar]
- 6.Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL: The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes 1984;33:271–276 [DOI] [PubMed] [Google Scholar]
- 7.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VMSouth Tees Diabetes Mortality Study Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care 2002;25:43–48 [DOI] [PubMed] [Google Scholar]
- 8.Weiderpass E, Gridley G, Nyrén O, Pennello G, Landström AS, Ekbom A: Cause-specific mortality in a cohort of patients with diabetes mellitus: a population-based study in Sweden. J Clin Epidemiol 2001;54:802–809 [DOI] [PubMed] [Google Scholar]
- 9.Mühlhauser I, Sawicki PT, Blank M, Overmann H, Bender R, Berger M: Prognosis of persons with type 1 diabetes on intensified insulin therapy in relation to nephropathy. J Intern Med 2000;248:333–341 [DOI] [PubMed] [Google Scholar]
- 10.Dawson SI, Willis J, Florkowski CM, Scott RS: Cause-specific mortality in insulin-treated diabetic patients: a 20-year follow-up. Diabetes Res Clin Pract 2008;80:16–23 [DOI] [PubMed] [Google Scholar]
- 11.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G: Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 12.Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, Waugh NR, Smith AW, Hill RD, Bingley PJ, Patterson CC, Qiao Z, Keen H: The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med 1999;16:466–471 [DOI] [PubMed] [Google Scholar]
- 13.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ: The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 14.Borch-Johnsen K, Andersen PK, Deckert T: The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1985;28:590–596 [DOI] [PubMed] [Google Scholar]
- 15.International evaluation of cause-specific mortality and IDDM. Diabetes Epidemiology Research International Mortality Study Group. Diabetes Care 1991;14:55–60 [DOI] [PubMed] [Google Scholar]
- 16.Major cross-country differences in risk of dying for people with IDDM. Diabetes [DOI] [PubMed] [Google Scholar]
- 17.LaPorte RE, Fishbein HA, Drash AL, Kuller LH, Schneider BB, Orchard TJ, Wagener DK: The Pittsburgh insulin-dependent diabetes mellitus (IDDM) registry. The incidence of insulin-dependent diabetes mellitus in Allegheny County, Pennsylvania (1965–1976). Diabetes 1981;30:279–284 [DOI] [PubMed] [Google Scholar]
- 18.International analysis of insulin-dependent diabetes mellitus mortality: a preventable mortality perspective. The Diabetes Epidemiology Research International (DERI) Study. Am J Epidemiol 1995;142:612–618 [PubMed] [Google Scholar]
- 19.Epidemiologic Query and Mapping System (EpiQMS) Bureau of Health Statistics, Pennsylvania Department of Health, Harrisburg, PA: Available from http://www.portal.state.pa.us/portal/server.pt?open=514&objID=596553&mode=2 [Google Scholar]
- 20.Armstrong BG: Comparing standardized mortality ratios. Ann Epidemiol 1995;5:60–64 [DOI] [PubMed] [Google Scholar]
- 21.Scibilia J, Finegold D, Dorman J, Becker D, Drash A: Why do children with diabetes die? Acta Endocrinol Suppl (Copenh) 1986;279:326–333 [DOI] [PubMed] [Google Scholar]
- 22.Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, Svensson J, Schober E, Gyürüs E, Castell C, Urbonaité B, Rosenbauer J, Iotova V, Thorsson AV, Soltész G: Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia 2007;50:2439–2442 [DOI] [PubMed] [Google Scholar]
- 23.Borch-Johnsen K, Nissen H, Henriksen E, Kreiner S, Salling N, Deckert T, Nerup J: The natural history of insulin-dependent diabetes mellitus in Denmark: 1. Long-term survival with and without late diabetic complications. Diabet Med 1987;4:201–210 [DOI] [PubMed] [Google Scholar]
- 24.Bosnyak Z, Nishimura R, Hagan Hughes M, Tajima N, Becker D, Tuomilehto J, Orchard TJ: Excess mortality in black compared with white patients with type 1 diabetes: an examination of underlying causes. Diabet Med 2005;22:1636–1641 [DOI] [PubMed] [Google Scholar]
- 25.Lipton R, Good G, Mikhailov T, Freels S, Donoghue E: Ethnic differences in mortality from insulin-dependent diabetes mellitus among people less than 25 years of age. Pediatrics 1999;103:952–956 [DOI] [PubMed] [Google Scholar]
- 26.Tull ES, Barinas E: A twofold excess mortality among black compared with white IDDM patients in Allegheny county, Pennsylvania. Pittsburgh DERI Mortality Study Group. Diabetes Care 1996;19:1344–1347 [DOI] [PubMed] [Google Scholar]
- 27.Murray CJ, Kulkarni S, Ezzati M: Eight Americas: new perspectives on U.S. health disparities. Am J Prev Med 2005;29:4–10 [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Baker SP: Reducing black/white disparity: changes in injury mortality in the 15–24 year age group, United States, 1999–2005. Inj Prev 2008;14:205–208 [DOI] [PubMed] [Google Scholar]
- 29.Roy M, Rendas-Baum R, Skurnick J: Mortality in African-Americans with type 1 diabetes: the New Jersey 725. Diabet Med 2006;23:698–706 [DOI] [PubMed] [Google Scholar]
- 30.Daneman D: Diabetes-related mortality: a pediatrician's view. Diabetes Care 2001;24:801–802 [DOI] [PubMed] [Google Scholar]
- 31.Dagogo-Jack S: Hypoglycemia in type 1 diabetes mellitus: pathophysiology and prevention. Treat Endocrinol 2004;3:91–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.