Abstract
OBJECTIVE
To determine the effect of the apolipoprotein A-I (ApoA-I) mimetic peptide, D-4F, on atherosclerosis development in a pre-existing diabetic condition.
RESEARCH DESIGN AND METHODS
We induced hyperglycemia in 6-week-old apoE−/− female mice using streptozotocin. Half of the diabetic apoE−/− mice received D-4F in drinking water. Ten weeks later, plasma lipids, glucose, insulin levels, atherosclerotic lesions, and lesion macrophage content were measured.
RESULTS
Diabetic apoE−/− mice developed ∼300% more lesion area, marked dyslipidemia, increased glucose levels, and reduced plasma insulin levels when compared with nondiabetic apoE−/− mice. Atherosclerotic lesions were significantly reduced in the D-4F–treated diabetic apoE−/− mice in whole aorta (1.11 ± 0.73 vs. 0.58 ± 0.44, percentage of whole aorta, P < 0.01) and in aortic roots (36,038 ± 18,467 μm2/section vs. 17,998 ± 12,491 μm2/section, P < 0.01) when compared with diabetic apoE−/− mice that did not receive D-4F. Macrophage content in atherosclerotic lesions from D-4F–treated diabetic apoE−/− mice was significantly reduced when compared with nontreated animals (78.03 ± 26.1 vs. 29.6 ± 15.2 P < 0.001, percentage of whole plaque). There were no differences in glucose, insulin, total cholesterol, HDL cholesterol, and triglyceride levels between the two groups. Arachidonic acid, PGE2, PGD2, 15-HETE, 12-HETE, and 13-HODE concentrations were significantly increased in the liver tissue of diabetic apoE−/− mice compared with nondiabetic apoE−/− mice and significantly reduced by D-4F treatment.
CONCLUSIONS
Our results suggest that oral D-4F can prevent atherosclerosis development in pre-existing diabetic mice and this is associated with a reduction in hepatic arachidonic acid and oxidized fatty acid levels.
Type 1 diabetes is associated with two- to fourfold higher risk of coronary artery disease (CAD) and macrovascular disease (1,2). The excess cardiovascular risk in this population is not entirely explained by traditional risk factors, including hyperglycemia.
Oxidative modification of LDL and the subsequent formation of foam cells are thought to be an initial step in atherogenesis (3). Multiple animal and in vitro studies have supported a role for oxidative processes in all phases of CAD, from foam cell formation to plaque rupture and thrombosis (4–6). Initiation of lipid peroxidation and formation of an array of bioactive fatty acid oxidation products are widely held as critical steps in the atherosclerotic process. It has been suggested that the inflammatory properties of lipoproteins may also be important for the development of the atherosclerotic process in diabetes (7). However, the mechanism by which diabetic dyslipidemia contributes to the development of CAD in type 1 diabetes is not clear.
HDL and apolipoprotein A-I (apoA-I), its major protein, have been efficacious in the treatment of atherosclerosis (8). ApoA-I mimetic peptides 4F (L-4F and D-4F), that form a class A amphipathic helix similar to those found in apoA-I, were found to be efficacious in murine models of atherosclerosis (9) by a mechanism that is independent of plasma cholesterol levels and in part related to its ability to remove oxidized lipids from lipoproteins (9). Moreover, recent studies have shown that apoA-I mimetic peptides increase antioxidants, confer robust vascular protection and improve insulin sensitivity in rodent models of diabetes and obesity (10–12).
In this study, we examined whether oral administration of D-4F can inhibit atherosclerosis development in a pre-existing diabetic condition. Our results show that D-4F is able to decrease atherosclerotic lesion development in diabetic mice, and this is associated with a reduction of hepatic arachidonic acid and hepatic oxidized fatty acids levels.
RESEARCH DESIGN AND METHODS
The Animal Research Committee at University of California Los Angeles approved all the protocols used in these studies. Five-week-old female apoE−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were housed in the Division of Laboratory Animal Medicine at the University of California Los Angeles. The mice were fed normal mouse chow diet and given free access to both food and water throughout the study, except when fasting blood specimens were obtained. After 1 week of acclimatization (at the age of 6 weeks), 40 mice were administrated intraperitoneal injections of streptozotocin ([STZ], Sigma-Aldrich) at a dosage of 65 mg/kg daily for 5 consecutive days. Control animals (n = 20) received vehicle (citrate buffer, pH 4.5) alone. Nine days after the last STZ injection, fasting (6 h) blood glucose levels were measured using a glucose machine (HemoCue 201 AB, Anghelhom, Sweden), and diabetes was verified on the basis of glucose level >250 mg/dl. By random selection, half of the diabetic mice (n = 17) were chosen to receive the apoA-I mimetic peptide, D-4F, in drinking water at a concentration of 0.2 mg/ml for 8 weeks; the remaining mice received regular drinking water. The mice consumed ∼5 ml of water daily (from 3 to 5 ml daily). After 8 weeks of treatment, all of the mice (control, diabetic, and diabetic/D-4F–treated) were fasted for 6 h, after which blood and organs were collected.
Metabolic parameters.
Plasma lipids were determined by enzymatic colorimetric assays as described previously (13). Plasma glucose and insulin levels were determined as previously described (14).
Atherosclerosis quantification.
En face lesions analysis in the entire aorta were performed according to procedures described by Tangirala et al. (15). Briefly, after perfusion-fixation, the aorta was dissected out, opened longitudinally from heart to the iliac arteries, pinned on a black wax pan, and stained with Sudan IV solution. The image of the aorta was captured using a SONY DXC-970MD color video camera, and the image analysis was performed using the Image-Pro plus program (Media Cybernetics, Silver Spring, MD) in a blinded fashion. The area covered by atherosclerotic lesions divided by the area of the entire aorta was calculated for each group, expressed as a percentage, and compared.
Atherosclerotic lesion area in the aortic root was determined as described previously (16). Briefly, the heart and proximal aorta were removed and embedded in optimum cutting temperature compound. Serial 10 μm-thick cryosections from the middle portion of the ventricle to the aortic arch were collected, mounted on precoated slides, and stained with Oil Red O and hematoxylin. The lipid-containing area on each section, centered around the aortic valves, was determined in a blinded fashion, using an ocular piece with a 20 × 20 μm2 grid on a light microscope. The average lesion area per aorta, calculated from 5 to 10 sections of each aorta, was determined.
Macrophage content.
Fresh-frozen aortic root sections were stained for CD-68. Briefly, after fixation in ice-cold acetone, sections were blocked in 4% BSA plus 10% goat serum for 3 h at room temperature. Rat anti-mouse CD-68 antibodies (1:100; Serotec) were used with an overnight incubation at 4°C. Goat anti-rat and goat anti-rabbit alkaline phosphatase secondary antibodies (Jackson Immuno Research) were used at 1:200 for 1-h incubation at room temperature. Immunostaining was visualized using Vector Red substrate plus levamisole to inhibit endogenous alkaline phosphatase activity (Vector Laboratories). Immunostaining was quantified in a blinded fashion using an ocular piece with a 20 × 20 μm2 grid on a light microscope, and the area stained was divided by the area of the entire plaque and the ratios obtained were compared between groups.
Hepatic free fatty acid levels
Chemicals.
Arachidonic acid (AA); 9-oxo-11a,15S-dihydroxy-prosta-5Z,13E-dien-1-oic acid (prostaglandin E2; PGE2), 9a,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid (prostaglandin D2; PGD2), (±)12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-HETE), (±)15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (15-HETE), (±)13-hydroxy-9Z,11E-octadecadienoic acid (13-HODE), (±)9-hydroxy-10E,12E-octadecadienoic acid (9-HODE), 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic-3,3,4,4-day4 acid (prostaglandin D2-d4;PGD2-d4), 12[S]-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-day8 acid (12(S)-HETE-d8), 15(S)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic-5,6,8,9,11,12,14,15-day8 acid (15(S)-HETE-d8), 13(S)-hydroxy-9Z,11E-octadecadienoic-9,10,12,13-day4 acid (13[S]-HODE-d4) were purchased from Cayman Chemicals (Ann Arbor, MI).
Sample preparation.
We performed lipid extraction as described previously with slight modification (17). Briefly, a piece of liver, previously dissected and weighed on dry ice, after the addition of methanol (0.02% BHT), internal standard (15[S]-HETE-d8), 12[S]-HETE-d8, 13[S]-HODE-d4, PGD2-d4), formic acid and water, was homogenized, vortexed, and incubated for 1 h on ice. The homogenate was centrifuged and the supernatant was loaded onto an Oasis HLB (60 mg, 30 μm) solid-phase extraction cartridge (Waters) and equilibrated with 2-ml methanol and 2-ml water, and then washed with 2-ml 0.5% methanol, and eluted with 3-ml 0.5% methanol. The solvent was evaporated under argon and reconstituted with 50 μl of methanol, vortexed, and transferred to an auto sampler vial for liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis.
LC/MS/MS analysis.
LC/MS/MS analysis was performed as previously described (18). The transitions monitored were mass-to-charge ratio (m/z): 303.1→259.2 for AA; 351.1→271.2 for PGD2; 351.1 →271.2 for PGE2; 319.1→179.0 for 12-HETE; 319.1→219.0 for 15-HETE; 295.0→194.8 for 13-HODE; 295.0→171.0 for 9-HODE; 327.1→226.1 for 15(S)-HETE-d8; 327.1→184.0 for 12(S)-HETE-d8; 299.0 →197.9 for 13(S)-HODE-d4, 355.0→237.0 for PGD2-d4.
Statistical analysis.
All data were expressed as mean ± SD. Differences between groups were determined by ANOVA or Wilcoxon/Kruskal-Wallis for nonparametric analysis.
RESULTS
Metabolic parameters were not affected by D-4F treatment.
Diabetic apoE−/− mice were found to have a significant reduction in body weight and decreased serum insulin levels and HDL cholesterol levels. The diabetic apoE−/− mice developed significant elevations in plasma total cholesterol, LDL cholesterol, and serum glucose levels compared with nondiabetic apoE−/− mice. Treatment with D-4F in drinking water at 0.2 mg/ml for 8 weeks had no effect on body weight, serum glucose, insulin levels, and lipoprotein cholesterol levels compared with untreated diabetic apoE−/− mice (Table 1).
TABLE 1.
Characteristics of control apoE−/− mice, diabetic apoE−/− mice, and diabetic apoE−/− mice treated with D-4F
| Controls | Diabetic | Diabetic + D-4F | |
|---|---|---|---|
| Total cholesterol (mg/dl) | 493 ± 124 | 993 ± 521* | 1,082 ± 417 |
| LDL cholesterol (mg/dl) | 467 ± 120 | 954 ± 526* | 1,027 ± 410 |
| HDL cholesterol (mg/dl) | 18 ± 4 | 14 ± 4* | 14 ± 3 |
| Triglyceride (mg/dl) | 41 ± 17 | 59 ± 65 | 62 ± 35 |
| Glucose (mg/dl) | 181 ± 32 | 366 ± 123* | 420 ± 89 |
| Insulin (pg/ml) | 281 ± 113 | 132 ± 68* | 181 ± 114 |
| Weight (g) | 20.1 ± 1.4 | 16.9 ± 3.7* | 16.2 ± 2.6 |
Values are expressed as average ±SD. Mice were treated with 8 weeks of D-4F treatment in drinking water; 0.2 mg/ml as described in methods.
*P < 0.01 diabetic vs. control.
D-4F treatment reduced atherosclerotic lesions in diabetic apoE−/− mice.
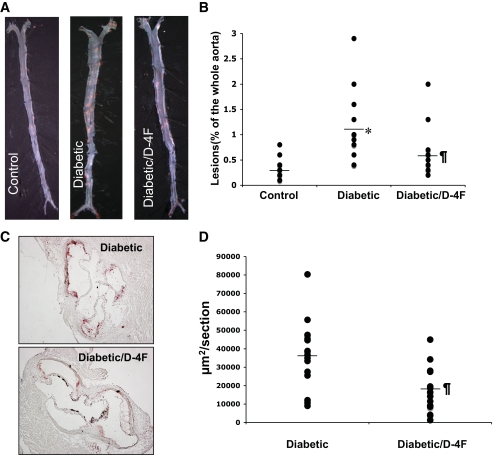
Total atherosclerotic lesion area was quantified in control apoE−/− mice (n = 20), untreated diabetic apoE−/− mice (n = 17), and D-4F–treated diabetic apoE−/− mice (n = 17). Diabetic apoE−/− mice were found to have a 300% increase in whole aortic atherosclerotic lesion area compared with nondiabetic apoE−/− mice. Atherosclerotic lesion area was significantly reduced in D-4F–treated diabetic apoE−/− mice (0.58 ± 0.44 vs. 1.11 ± 0.73, percentage of the whole aorta, P < 0.01) (Fig. 1A and B), when compared with untreated diabetic apoE−/− mice. The average lesion area as measured by Oil-Red-O staining of the aortic sinus sections was significantly reduced in D-4F–treated diabetic apoE−/− mice when compared with untreated diabetic apoE−/− mice (36,038 ± 18,467 vs. 17,998 ± 12,491 μm2/section, P < 0.01, n = 15 per group) (Fig. 1C and D).
FIG. 1.
Decreased atherosclerotic lesion formation and lipid content in the diabetic d4F- treated mice. A: Representative images of the whole aorta by en face method in control (apoE−/− mice that were not diabetic), in diabetic apoE−/− mice (diabetic), and in diabetic apoE−/− mice treated with D-4F (diabetic/D-4F). B: Quantitative analysis of en face lesions in female apoE−/− mice on a chow diet; n = 20 for control; n = 17 for diabetic mice; and n = 17 for diabetic mice treated with D-4F (diabetic/D-4F). C: Representative sections of mouse aortic roots, stained with Oil red O. D: Quantitative analysis of lesion area in female diabetic apoE−/− mice (diabetic) and in diabetic apoE−/− mice treated with D-4F (diabetic/D-4F); n = 15 for both groups; *P < 0.01 diabetic versus control; ¶P < 0.01 diabetic versus diabetic/D-4F. (A high-quality digital representation of this figure is available in the online issue.)
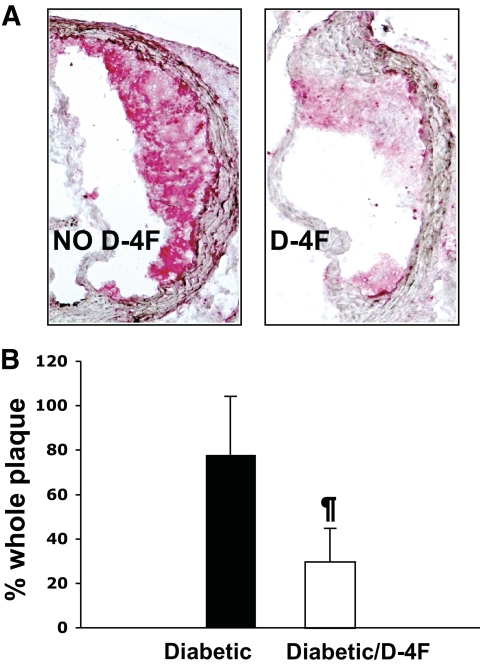
D-4F treatment causes a reduction in the macrophage content of atherosclerotic lesions in diabetic apoE−/− mice.
Quantification for macrophages in the aortic sections using CD-68 immunohistochemistry demonstrated a dramatic reduction in macrophage content in D-4F–treated diabetic apoE−/− mice when compared with untreated diabetic apoE−/− mice (78.03 ± 26.1 vs. 29.6 ± 15.2, expressed as a percentage of the whole plaque, P < 0.001) (Fig. 2A and B).
FIG. 2.
The D-4F–treated mice demonstrated significantly decreased macrophage content. Sections from mouse aorta were analyzed by immunohistochemistry for the presence of macrophages using CD-68 antibody (as described in research design and methods). A: Representative image of macrophage content in atherosclerotic plaques. B: Quantitative analysis of macrophage content in diabetic female apoE−/− mice (diabetic) on a chow diet (n = 10) and in diabetic female apoE−/− mice treated with D-4F (diabetic/D-4F) (n = 10). ¶P < 0.01 diabetic/D-4F versus diabetic. (A high-quality digital representation of this figure is available in the online issue.)
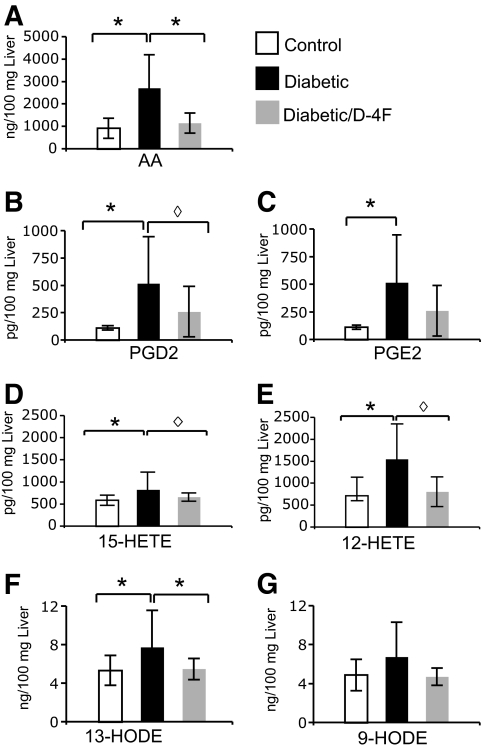
D-4F treatment results in the reduction of arachidonic acid and oxidized fatty acid content in the livers of diabetic apoE−/− mice.
Binding and removal of bioactive lipids, including oxidized fatty acids, has been identified as one of the mechanisms by which apoA-I mimetic peptides prevent the development of atherosclerosis (19,20). Therefore, we determined 1) whether the levels of these lipids were elevated in the livers of diabetic apoE−/− mice compared with the livers of control apoE−/− mice, and 2) whether D-4F treatment altered these levels. Liver tissue extracts from control apoE−/−, diabetic apoE−/−, and D-4F–treated diabetic apoE−/− mice (n = 5 per group) were analyzed by LC/MS/MS as described in methods (20). Arachidonic acid, prostaglandin E2 (PGE2), PGD2, 15-hydroxyeicosatetraenoic acid (15-HETE), 12-HETE, and 13-hydroxyoctadecadienoic acid (13-HODE) were significantly increased in the livers of diabetic apoE−/− mice compared with control apoE−/− mice. The D-4F–treated diabetic apoE−/− mice showed a significant decrease in hepatic tissue levels of these lipids compared with diabetic apoE−/− mice that did not receive D-4F (Fig. 3). The levels of these lipids in the diabetic apoE−/− mice that were not treated with D-4F compared with those that were treated with D-4F were significantly greater for arachidonic acid (26,97.5 ± 1,489.3 vs. 1,151.0 ± 452.25 ng/100 mg of liver, P < 0.05), PGD2 (470 ± 330 vs. 130 ± 110 pg/100 mg of liver, P = 0.05), 15-HETE (825 ± 398 vs. 660 ± 95 pg/100 mg of liver, P = 0.06), 12-HETE (1,550 ± 805 vs. 805 ± 340 pg/100 mg of liver, P = 0.05) and 13-HODE (7.75 ± 3.81 vs. 5.5 ± 1.1 ng/100 mg of liver, P < 0.05).
FIG. 3.
Effect of D-4F on hepatic lipid levels. As described in methods, lipid levels were determined by LC/MS/MS in the livers of apoE−/− mice (control), diabetic apoE−/− mice (diabetic), and diabetic apoE−/− mice treated with D-4F (diabetic/D-4F). A: Arachidonic acid (AA); B. PGD2; C: PGE2; D: 15-HETE; E: 12-HETE; F: 13-HODE; G: 9-HODE; (n = 5 for each group); *P < 0.05; ◊P ≤ 0.06.
DISCUSSION
The major finding of the present study is that the apoA-I mimetic peptide D-4F prevented the acceleration of atherosclerosis in STZ-induced diabetic apoE−/− mice. We recognize that diabetic apoE−/− mice may not perfectly mimic human type 1 diabetes; however, this is a well accepted animal model for studying hyperglycemia-induced atherosclerosis. Induction of diabetes by STZ in apoE−/− mice has been previously used to establish a role for both the advanced glycation end products (21) and the renin-angiotensin system (22) in the attenuation of atherosclerosis under conditions of hyperglycemia. Renard et al. (23) used a virally induced pancreatic destruction method to induce diabetes and concluded that diabetic conditions accelerate atherosclerosis similar to the STZ method.
The finding that D-4F drastically reduced atherosclerosis development with no improvement in serum levels of glucose, insulin, or lipoprotein cholesterol levels (Table 1 and Fig. 1) confirms that the effect of the peptide on atherosclerotic lesions is not related to plasma cholesterol levels or alteration in severity of diabetes. D-4F mediated prevention of atherosclerosis development in diabetic apoE−/− mice was associated with a reduction in lipid and macrophage content of the atherosclerotic lesions (Fig. 2), indicating that D-4F treatment significantly altered the structure and composition of the plaque without altering plasma glucose or cholesterol levels.
The antiatherogenic effect of D-4F in the absence of any changes in hyperglycemia or plasma lipoprotein or lipid levels suggests that lipoprotein oxidation may have a role in the accelerated atherosclerotic process in apoE−/− diabetic mice. Atherosclerosis is the result of complex interactions between oxidized-lipoproteins, monocytes/macrophages, injured endothelium, and smooth muscle cells. Biologic oxidation products of arachidonic and linoleic acid, including prostaglandins, HETEs, and HODEs, play a role in LDL oxidation, one of the first steps in atherosclerosis (3). LDL oxidation is a complex process influenced by a multitude of oxidation pathways (24) including the lipoxygenase pathway which can generate potent lipid oxidants that include hydroperoxyoctadecadienoic acid (HPODE) and hydroperoxyeicosatetraenoic acid (HPETE) (3,25). A number of previous studies have shown that products from the arachidonic acid and linoleic acid pathways are contained in oxLDL. Sevanian and colleagues noted that a subpopulation of freshly isolated LDL that they described as LDL(−) contains lipid hydroperoxides (26). Parthasarathy (27), Witzum and Steinberg (28), Thomas and Jackson (29), Frei and colleagues (30), and Thomas, Kalyanaraman, and Girotti (31) studied metal ion-dependent LDL oxidation in vitro and hypothesized that LDL must be “seeded” with reactive oxygen species before it can be oxidized. Thomas and Jackson (29) and Parthasarathy (27) suggested a role for lipoxygenases in the seeding of LDL. We previously showed that the seeding molecules present in freshly isolated LDL are derived in part from the cellular metabolism of linoleic acid (HPODE) and arachidonic acid (HPETE) (3). The products resulting from the action of fatty acid hydroperoxides on LDL account for the ability of LDL to induce endothelial cells to bind monocytes and secrete the potent monocyte chemoattractant monocyte chemotactic protein-1 (MCP-1), which is one of the first steps in the development of atherosclerosis. It has been reported that in vitro apoA-I and apoA-I mimetic peptides bound nonoxidized fatty acids such as arachidonic acid and linoleic acid similarly, but the 4F peptides bound oxidized fatty acids derived from arachidonic acid or linoleic acid with a remarkable higher affinity than apoA-I (19). More recently, we reported that plasma oxidized fatty acids levels were significantly reduced within a few hours of L-4F administration in mice (18).
Lipid oxidation products are continuously produced in the tissues and enter the circulation. Because of the amount of plasma required for determining specific plasma-oxidized fatty acid levels, we did not make such measurements in these studies. Instead we determined the hepatic levels of arachidonic acid and oxidized fatty acids. Our results show that the reduction in atherosclerosis with D-4F treatment was associated with a significant reduction of arachidonic acid and oxidized fatty acids in liver.
Liver is the organ with the highest accumulation of oxidized lipids. Previous studies have shown that there is a selective hepatic uptake of oxidized LDL (ox-LDL) and oxidized cholesterol esters (32,33) mediated by the selective binding by scavenger receptors on liver endothelial and Kupfer cells for modified LDL (34). More recently it was shown that there is a selective uptake by hepatocytes of oxidized lipids from ox-LDL-loaded macrophages (35). Shaish et al. (36) reported the organ distribution of 125I-LDL, 125I-HDL, and 125I-oxLDL in apoE−/− mice and demonstrated that the highest accumulation of ox-LDL 24 h after administration was in liver (sixfold more than unoxidized or normal LDL).
Our finding of an increase in arachidonic acid and oxidized fatty acid levels in the livers of diabetic mice is particularly interesting in light of the hypothesis that hyperglycemia may directly contribute to the generation of oxidative stress (37). Enhanced lipid peroxidation in diabetes has been demonstrated in STZ-induced diabetic rats that showed a marked increase in plasma levels and urinary excretion rates of F2-isoprostanes (a free radical oxidation product derived from the oxidation of arachidonic acid) (38). Enhanced lipid peroxidation has been shown to be one of the early events in the development of type 1 diabetes (39). Arachidonic acid peroxidation products have been proposed as a noninvasive index in type 1 diabetes that may be useful for monitoring pharmacologic interventions aimed at interfering with disease development and progression (40).
The studies detailed here used only female apoE−/− mice. We chose to use female apoE−/− mice because on a chow diet they develop larger atherosclerotic lesions associated with lower cholesterol and triglyceride levels when compared with male apoE−/− mice (41). Additionally, we previously reported that the effect of D-4F was not different in female and male apoE−/− mice (42).
In conclusion, our results suggest that the apoA-I mimetic peptide, D-4F, was effective in preventing the development of accelerated atherosclerosis in mice with pre-existing diabetes. The beneficial effects of D-4F were associated with a significant reduction in hepatic arachidonic acid and oxidized fatty acid levels, which supports the current literature suggesting that diabetes is characterized by glucose-mediated oxidative stress. The relevance of these findings to humans with diabetes remains to be determined.
ACKNOWLEDGMENTS
This work was supported in part by U.S. Public Health Service grants HL-30568, HL-34343, HL-082823, and the Laubisch, Castera, and M.K. Gray Funds at University of California Los Angeles and by an Internal Medicine School of Specialty Fellowship to C.M. from the University of Pisa.
M.N., A.M.F., and S.T.R. are principals in Bruin Pharma, and A.M.F. is an officer in Bruin Pharma.
No other potential conflicts of interest relevant to this article were reported.
C.M. researched data, contributed to discussion, and wrote and reviewed the manuscript. S.I. researched data and contributed to discussion. V.G. and M.N. researched data. A.M.F. reviewed the manuscript. S.T.R. contributed to discussion and reviewed the manuscript.
Parts of this study were presented in oral form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soedamah-Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, Manes C, Fuller JH: Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care 2004;27:530–537 [DOI] [PubMed] [Google Scholar]
- 3.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, Fogelman AM: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res 2000;41:1481–1494 [PubMed] [Google Scholar]
- 4.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ: Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation 1995;91:2488–2496 [DOI] [PubMed] [Google Scholar]
- 5.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M: Oxidized lipids in atherogenesis: formation, destruction and action. Thromb Haemost 1997;78:195–199 [PubMed] [Google Scholar]
- 6.Harrison D: Oxidative stress and coronary artery disease. Can J Cardiol 1998;14 Suppl D:30D–32D [PubMed] [Google Scholar]
- 7.Krauss RM: Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004;27:1496–1504 [DOI] [PubMed] [Google Scholar]
- 8.Kontush A, Chapman MJ: Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 2006;58:342–374 [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM: Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 2002;105:290–292 [DOI] [PubMed] [Google Scholar]
- 10.Peterson SJ, Husney D, Kruger AL, Olszanecki R, Ricci F, Rodella LF, Stacchiotti A, Rezzani R, McClung JA, Aronow WS, Ikehara S, Abraham NG: Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J Pharmacol Exp Ther 2007;322:514–520 [DOI] [PubMed] [Google Scholar]
- 11.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG: D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 2005;111:3126–3134 [DOI] [PubMed] [Google Scholar]
- 12.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L'Abbate A, Abraham NG: The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res 2009;50:1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrick CC, Castellani LW, Warden CH, Puppione DL, Lusis AJ: Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. J Biol Chem 1993;268:20676–20682 [PubMed] [Google Scholar]
- 14.Castellani LW, Goto AM, Lusis AJ: Studies with apolipoprotein A-II transgenic mice indicate a role for HDLs in adiposity and insulin resistance. Diabetes 2001;50:643–651 [DOI] [PubMed] [Google Scholar]
- 15.Tangirala RK, Rubin EM, Palinski W: Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res 1995;36:2320–2328 [PubMed] [Google Scholar]
- 16.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA: Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 1987;68:231–240 [DOI] [PubMed] [Google Scholar]
- 17.Yue H, Jansen SA, Strauss KI, Borenstein MR, Barbe MF, Rossi LJ, Murphy E: A liquid chromatography/mass spectrometric method for simultaneous analysis of arachidonic acid and its endogenous eicosanoid metabolites prostaglandins, dihydroxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and epoxyeicosatrienoic acids in rat brain tissue. J Pharm Biomed Anal 2007;43:1122–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamiah GM, Fogelman AM, Reddy ST: L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett 2010;4:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM: Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res 2008;49:2302–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imaizumi S, Victor G, Navab M, Van Lenten BJ, Wagner AC, Anantharamaiah GM, Fogelman AM, Reddy ST: L-4F Diffrentially Alters Plasma Levels of Oxidized Fatty Acids Resulting in more anti-inflammatory HPL in mice. Drug Metab Lett 2010;4:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4:1025–1031 [DOI] [PubMed] [Google Scholar]
- 22.Candido R, Allen TJ, Lassila M, Cao Z, Thallas V, Cooper ME, Jandeleit-Dahm KA: Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation 2004;109:1536–1542 [DOI] [PubMed] [Google Scholar]
- 23.Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE: Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest 2004;114:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parthasarathy S, Litvinov D, Selvarajan K, Garelnabi M: Lipid peroxidation and decomposition–conflicting roles in plaque vulnerability and stability. Biochim Biophys Acta 2008;1781:221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res 2000;41:1495–1508 [PubMed] [Google Scholar]
- 26.Sevanian A, Bittolo-Bon G, Cazzolato G, Hodis H, Hwang J, Zamburlini A, Maiorino M, Ursini F: LDL- is a lipid hydroperoxide-enriched circulating lipoprotein. J Lipid Res 1997;38:419–428 [PubMed] [Google Scholar]
- 27.Parthasarathy S: Mechanism(s) of Cell-Mediated Oxidation of Low Density Lipoprotein. London, Richelieu Press, 1994 [Google Scholar]
- 28.Witztum JL, Steinberg D: Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991;88:1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas CE, Jackson RL: Lipid hydroperoxide involvement in copper-dependent and independent oxidation of low density lipoproteins. J Pharmacol Exp Ther 1991;256:1182–1188 [PubMed] [Google Scholar]
- 30.Shwaery GT, Mowri H, Keaney JF, Jr, Frei B: Preparation of lipid hydroperoxide-free low-density lipoproteins. Methods Enzymol 1999;300:17–23 [DOI] [PubMed] [Google Scholar]
- 31.Thomas JP, Kalyanaraman B, Girotti AW: Involvement of pre-existing lipid hydroperoxides in Cu(2+)-stimulated oxidation of low-density lipoprotein. Arch Biochem Biophys 1994;315:244–254 [DOI] [PubMed] [Google Scholar]
- 32.Fluiter K, Sattler W, De Beer MC, Connell PM, van der Westhuyzen DR, van Berkel TJ: Scavenger receptor BI mediates the selective uptake of oxidized cholesterol esters by rat liver. J Biol Chem 1999;274:8893–8899 [DOI] [PubMed] [Google Scholar]
- 33.Fluiter K, van der Westhuijzen DR, van Berkel TJ: In vivo regulation of scavenger receptor BI and the selective uptake of high density lipoprotein cholesteryl esters in rat liver parenchymal and Kupffer cells. J Biol Chem 1998;273:8434–8438 [DOI] [PubMed] [Google Scholar]
- 34.De Rijke YB, Biessen EA, Vogelezang CJ, van Berkel TJ: Binding characteristics of scavenger receptors on liver endothelial and Kupffer cells for modified low-density lipoproteins. Biochem J 1994;304(Pt 1):69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M, Zhou H, Tan KC, Guo R, Shiu SW, Wong Y: ABCG1 mediated oxidized LDL-derived oxysterol efflux from macrophages. Biochem Biophys Res Commun 2009;390:1349–1354 [DOI] [PubMed] [Google Scholar]
- 36.Shaish A, Keren G, Chouraqui P, Levkovitz H, Harats D: Imaging of aortic atherosclerotic lesions by (125)I-LDL, (125)I-oxidized-LDL, (125)I-HDL and (125)I-BSA. Pathobiology 2001;69:225–229 [DOI] [PubMed] [Google Scholar]
- 37.Maritim AC, Sanders RA, Watkins JB, 3rd: Effects of α-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem 2003;14:288–294 [DOI] [PubMed] [Google Scholar]
- 38.Morrow JD, Minton TA, Roberts LJ, 2nd: The F2-isoprostane, 8-epi-prostaglandin F2α, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins 1992;44:155–163 [DOI] [PubMed] [Google Scholar]
- 39.Davi G, Chiarelli F, Santilli F, Pomilio M, Vigneri S, Falco A, Basili S, Ciabattoni G, Patrono C: Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation 2003;107:3199–3203 [DOI] [PubMed] [Google Scholar]
- 40.Davi G, Falco A, Patrono C: Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal 2005;7:256–268 [DOI] [PubMed] [Google Scholar]
- 41.Maeda N, Johnson L, Kim S, Hagaman J, Friedman M, Reddick R: Anatomical differences and atherosclerosis in apolipoprotein E-deficient mice with 129/SvEv and C57BL/6 genetic backgrounds. Atherosclerosis 2007;195:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navab M, Anantharamaiah GM, Hama S, Hough G, Reddy ST, Frank JS, Garber DW, Handattu S, Fogelman AM: D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 2005;25:1426–1432 [DOI] [PubMed] [Google Scholar]