Abstract
Purpose
To better understand how sex differences in anterior knee joint laxity (AKL) impact knee joint biomechanics, we examined the consequence of greater absolute baseline (males and females) and cyclic increases in AKL during the menstrual cycle (females) on anterior tibial translation (ATT) as the knee transitioned from non-weight bearing (NWB) to weight bearing (WB) conditions, while also controlling for genu recurvatum (GR).
Methods
Males and females (71F,48M;18-30 years) were measured for AKL and GR, and underwent measurement of ATT. Females were tested on the days of their cycle when AKL was at its minimum (T1) and maximum (T2); males were matched in time to a female with similar AKL. Linear regressions examined relationships between absolute baseline (AKLT1, GRT1) and cyclic changes (Δ=T2-T1; AKLΔ, GRΔ)(females only) in knee laxity with ATT as measured at T1 and T2, and Δ (T2-T1) (females only).
Results
AKL and GR increased in females, but not males, from T1 to T2. Greater AKLT1 and GRT1 predicted greater ATTT1 and ATTT2 in males (R2=21.0, P<.007). The combination of greater AKLT1, AKLΔ and less GRΔ predicted greater ATTT1 and ATTT2 in females (R2=12.5-13.1, P<.05), with AKLΔ being a stronger predictor (coefficient, P-value) of ATTT2 (0.864, P=.027) compared to ATTT1 (0.333, P=.370). AKLΔ was the sole predictor of ATTΔ (R2=.104; 0.740, P=.042).
Conclusions
Greater absolute baseline and cyclic increases in AKL were consistently associated with greater ATT produced by transition of the knee from NWB to WB. As the ACL is the primary restraint to ATT, these findings provide insight into possible mechanisms by which greater AKL may be associated with at risk knee biomechanics during the weight acceptance phase of dynamic tasks.
Keywords: Injury Mechanism, anterior knee laxity, genu recurvatum, knee biomechanics, hormone response, menstrual cycle
INTRODUCTION
Serious knee trauma, such as anterior cruciate ligament (ACL) injury, most often occurs when the knee transitions from non-weight bearing (NWB) to weight bearing (WB) during sudden deceleration or change-of-direction with the knee relatively extended (12, 20). Studies have shown that as the knee transitions to WB at low knee flexion angles (e.g. 15-30°), there is anterior translation of the tibia relative to the femur (ATT) (8, 37) that is restrained by the ACL in the normal knee (8). The ACL's role in maintaining normal knee biomechanics during weight acceptance is further supported by evidence of substantial increases in ATT with ACL-sectioning in the cadaveric knee (37), and increased ATT in subjects with ACL-deficient knees (1). Yet despite the role of the ACL in controlling ATT, the impact of anterior knee laxity (AKL) on knee biomechanics during the transition from NWB to WB is not well understood. Understanding this relationship is important as greater magnitudes of AKL have been associated with an increased risk of suffering ACL injury in females (39), and females are reported to have greater AKL values than males (24, 28, 39), with these differences being more pronounced at certain times of the menstrual cycle compared to others (28).
AKL is a measure of the displacement of the tibia relative to the femur in response to an anterior directed load applied to the tibia while the knee is non-weight bearing with the muscles relaxed, and is typically considered a biomechanical assessment of the ACL which is the primary ligament restraint to this motion (2). As the ACL is the primary ligament restraint to both AKL and ATT, the greater AKL observed in females suggest they may experience greater ATT in response to weight bearing loads that act at the knee. However, it is well accepted that additional factors may contribute to control of anterior tibial motion during weight acceptance, such as knee flexion angle, joint geometry and congruency (e.g., condylar depth, the slope of the tibial plateau), the extensor moment arm, and the sequence and magnitude of muscle co-activation. This is supported by Shultz et al (34) who examined the relationship between AKL, produced by a 133N anterior directed load applied to the tibia, and the magnitude of ATT produced during the transition from non-weight bearing to a weight bearing load (40% of body weight) applied to the limb. While a strong relationship between AKL and ATT (R2=35.5%) was reported, 65% of the variance remained unexplained by AKL alone. Moreover, males and females were examined collectively, and given known sex differences in knee joint geometry (10) and muscle activation patterns (31), it is possible the relationship between AKL and ATT is not the same for males and females. Further, cyclic variations in AKL across the female's cycle were not controlled. Individual variations in hormone profiles among females makes this later assessment difficult, as not all women experience cyclic changes in AKL, and the time during the menstrual cycle when these cyclic changes occur is quite variable (21, 32). This inter-subject variability also raises the question whether some women may have greater difficulty with neuromuscular control of the tibiofemoral joint during dynamic activities such as weight acceptance if their cyclic variations in AKL are of sufficient magnitude to disrupt normal knee joint biomechanics.
In an effort to better understand how sex differences in AKL may impact knee joint biomechanics when transitioning from non-weight bearing to weight bearing (for example, during the initial application and transmission of the joint compressive loads that are produced during activities that involve foot strike with the ground), our purpose was to examine the consequence of both greater absolute baseline (males and females) and cyclic increases in AKL during the menstrual cycle (females only) on ATT as measured at two time points; the days of minimum and maximum AKL in females. ATT was measured as the knee transitioned from NWB to WB while also accounting for thigh muscle strength, the magnitude of thigh muscle activation during the measurement of ATT, and the magnitude of genu recurvatum. Thigh muscle strength and activation levels are often observed to be different between males and females (31), and both the position of the tibia relative to the femur and the absolute magnitude of anterior tibial displacement can be altered by muscle forces near full knee extension (37). Examining the contributions of genu recurvatum to ATT is also important, given its association with ACL injury risk (11, 18, 23), the greater values reported in females compared to males (19), its potential to also vary across the menstrual cycle (29) and its potential to alter the neutral position of the tibiofemoral joint and the posterior tibial slope (5, 16, 17) (the later of which can in turn influence ATT (37)). Our expectations were that once controlling for these factors, strong associations would be noted between AKL and ATT in both males and females, and that greater cyclic changes in AKL would predict greater cyclic changes in ATT in females.
METHODS
Subjects initially included 74 females and 50 males between 18 and 30 years of age, who were recreationally active (2.5- 10 hrs/week) for the past 3 months and non-smokers, and had a body mass index (weight/height2) ≤ 30 and no history of ligament or cartilage injury to the knee. We chose to exclude individuals categorized as obese (those with a body mass index > 30) to limit the potential for abnormal hormone levels and menstrual cycle irregularities (40), and to control for excessive spatial filtering of the EMG signals which can occur with excess tissue between the surface electrode and muscle (6). Additional criteria for females were self-reported menstrual cycles lasting 26-32 days (varying no more than ±1day month to month), no use of exogenous hormones for the past 6 months, and no history of or plans to become pregnant. Potential subjects were prescreened to obtain a wide distribution of baseline AKL values so that we could 1) include more subjects with a wide range of AKL values found to be predictive of ACL injury in females (39), and 2) determine if the effects of cyclic changes in AKL vary according to the subject's baseline AKL values. We then used a prospective study design to evaluate the relationship between absolute baseline and cyclic changes in AKL with ATT produced by the transition of the knee from NWB to WB. All subjects were informed of study procedures and risk, and signed an informed consent approved by the University's institutional review board for the protection of human subjects.
Because there is no uniform day in the cycle when AKL is known to be at its minimum or maximum, females were measured each morning (7:00-9:00a.m., within ± 30 minutes between test days) for AKL (and genu recurvatum) during the first 6 days following the onset of menses (per self report) and the first 8 days of the early luteal phase following evidence of ovulation [CVS One Step Ovulation Predictor (sensitivity 20 mIU/ml LH, accuracy 99%); CVS Corporation, Woonsocket, RI] for two consecutive cycles. In the following month, males and females were tested on all variables at two time points (T1 and T2; within ± 1 hour of the same time of day). For females, T1 and T2 corresponded to the estimated days of minimum AKL (AKLT1) during menses and maximum AKL (AKLT2) during the early luteal phase, respectively, based on the patterns of AKL observed during the two prior months. For males, the time between tests was matched to a female with a similar baseline AKL value (within ± 0.5mm). Subjects were familiarized to all procedures approximately two weeks prior to testing, and asked to refrain from any physical activity prior to testing. All measures were obtained on the dominant stance limb (preferred limb when kicking a ball).
AKL was measured as the anterior displacement of the tibia relative to the femur when a 133N anterior directed load was applied with the KT2000™ Knee Arthrometer (MEDmetric Corp; San Diego, CA) while the knee was positioned in 25° of flexion. Genu recurvatum (GR) was measured in supine (with a 10.2cm bolster under the distal tibia) as the amount of knee hyperextension while the subject performed a maximal active knee extension (30). A single tester with establish reliability [ICC(2,3)(SEM) = 0.96(0.3mm) for AKL, 0.97(0.5°) for GR] (26) obtained all laxity measures.
To obtain thigh muscle torques and to normalize muscle responses recorded with surface electromyography (sEMG) during ATT testing, participants were positioned in a dynamometer (Biodex Medical Systems Inc., Shirley, NY) at 20° of knee flexion (this duplicated positioning of the knee during measurement of ATT) and asked to complete three 5s maximal effort isometric knee extension and knee flexion contractions (MVIC). This was done after first warming up on a bicycle ergometer and completing practice trials at 25%, 50%, 75%, and 100% of maximal effort. Peak knee flexor and knee extensor torques (Nm) were recorded for each trial. Maximum sEMG signals were recorded with a 16 channel Myopac telemetric system (Run Technologies, Mission Viejo, CA: amplification 1 mV / V, frequency bandwidth 10-1000 Hz, CMRR 90 dB min at 60 Hz, input resistance 1 MΩ, and internal sampling rate 8 KHz) via 10mm bipolar Ag–AgCl surface electrodes (Medicotest Blue Sensor Model #N-00-S; Ambu Products, Germany) applied over prepared skin areas of the vastus medialis and lateralis, the medial hamstring and biceps femoris, with a 2.5cm center-to-center distance. The reference electrode was placed on the anterior tibial shaft. Manual muscle testing confirmed signal fidelity and absence of cross talk.
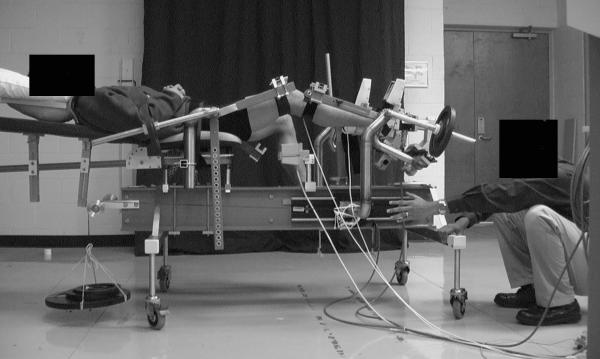
With the sEMG electrodes still attached, subjects were positioned supine in the Vermont Knee Laxity Device (VKLD, University of Vermont, Burlington, VT) to measure ATT as the knee transitioned from NWB to a WB load equal to 40% body weight as previously described (Figure 1) (26, 34). It is appreciated that muscular strategies used to control knee joint motion upon weight acceptance using this supine model may differ from what occurs during upright weight bearing postures. We chose this model to accurately control the magnitude, direction and rate of application of the external loads applied to the limb, and to minimize the extraneous factors that may limit measurement precision of ATT during upright weight bearing postures in order to test our hypothesis. These factors include the ability to control the knee position upon weight acceptance, trunk position relative to the knee, the tibiofemoral shear loads acting at the knee prior to application of the weight bearing loads, and the magnitude and direction of the weight bearing load acting in a reproducible manner through the ankle and hip joint centers of rotation. Specifically, this approach allowed us to: (1) established a repeatable, initial zero-shear load reference position of the tibia relative to the femur through counterbalanced weighting, and (2) apply a consistent axial compression load that acts through the axes of rotation of the ankle and hip joints (34). The 40% weight bearing load utilized in this study is consistent with what would be experienced during double leg stance (assuming 50% of bodyweight applied to each leg, and 10% of bodyweight distributed below the knee) (34), and is intended to represent the early loading period as one transitions to full weight bearing.
Figure 1.
Subject Positioned in the Vermont Knee Laxity Device
Six-degree of freedom position sensors (Mini Birds Ascension Technologies, Burlington, VT, USA) were secured to the lateral thigh, patella and the anteromedial aspect of the proximal tibia. Hip, knee, and ankle joint centers were determined using the centroid method (34). Initial anatomical position was established with the knee fully extended and the 2nd metatarsal aligned with the vertical axis of the VKLD. A counter weight system was applied to the thigh and shank to offset gravitational loads acting on the lower extremity, creating an initial zero anterior-posterior directed shear load across the tibiofemoral joint while it was unweighted (38). The ankle and knee were flexed to 90° and 20° respectively, and the subjects were instructed to relax their leg muscles. Prior to each loading trial, 3 anterior-to-posterior directed forces were applied to the proximal tibia to obtain a reproducible initial position of the tibia relative to the femur, and knee flexion angle was confirmed within ±5°. Data collection began with the subjects’ knee in the initial NWB position and continued as a compressive force equal to 40% of the subject's bodyweight was applied under the control of gravity through the ankle and hip joint axes of rotation to load the tibiofemoral joint (34). A six degree-of-freedom load transducer (Model MC3A, Advanced Medical Technology, Inc; Watertown, MA) measured compressive loads at the foot (500Hz), and initiation of weight acceptance was defined as when the compressive force exceeded 10 N. Subjects were not told when the weight would be released, but were asked to try and maintain the 20° knee position during load acceptance. Three practice trials were followed by 6 test trials. Kinematic data were collected (100Hz) during trials 1-3 and analyzed using the Motion Monitor (Innovative Sports Training, Chicago, IL, USA) electromagnetic tracking system and software. SEMG data were collected during trials 4-6 due to noise interference from the electromagnetic system. Pilot testing prior to commencement of the study confirmed that muscle activation amplitudes obtained in trials 4-6 were quite consistent with those obtained in trials 1-3 (All ICC(2,3) >.98) (26).
Data Reduction
For each time point (T1, T2), 3 trials of AKL and GR were measured and averaged. Quadriceps and hamstring torques were recorded as the mean of the peak torques obtained over the 3 MVIC trials, and normalized to the subject's body mass (Nm/kg). ATT was calculated as the change in displacement of the proximal shank sensor relative to the patella sensor in the A-P plane between the onset of weight acceptance (NWB) and peak axial compressive load (WB). During this same time frame, knee flexion excursion in degrees was also recorded. SEMG data (1000 Hz) were synchronized with the kinematic data using the same foot contact force threshold of 10N. SEMG signals were band pass filtered from 10 Hz to 350 Hz, using a fourth-order, zero-lag Butterworth filter(31), and MVIC (100ms) and weight acceptance (5ms) trials were processed using a centered root mean square algorithm, and ensemble averaged over their respective trials. Muscle reflex activation amplitude was defined as the mean normalized RMS amplitude (%MVIC) over the duration from muscle onset to peak WB load. The medial and lateral aspects of each muscle were averaged to represent a single quadriceps and hamstring normalized reflex amplitude, respectively.
Statistical Analyses
Separate 2 × 2 repeated measures ANOVAs examined mean differences in AKL, GR and ATT by sex and time (T1, T2). To further explore potential relationships between absolute baseline AKL as measured at T1 (herein called AKLT1) and cyclic changes in AKL as measured from T1 to T2 (herein called AKLΔ) with ATT in females, separate multiple linear regressions examined these relationships when ATT was measured at T1 (ATTT1), at T2 (ATTT2), and the cyclic change from T1 to T2 (ATTΔ). This was done while also accounting for GR as measured at T1 and the change from T1 to T2 (herein called GRT1 and GRΔ), as well as 5 supressor variables [quadriceps and hamstring strength (QTrq, HTrq) and activation (Q%MIVC, H%MVIC), and changes in knee flexion angle (KFLEX) during load acceptance] as measured at each time point, given their potential to confound the measurement of ATT (34, 37). The three full models examined for females were:
ATTT1=α0+β1AKLT1+β2GRT1+β3AKLΔ+β4GRΔ+β5QTrqT1+β6HTrqT1+β7Q%MVICT1+β8H%MVICT1+β9KFLEX T1+ε
ATTT2=α0+β1AKLT1+β2GRT1+β3AKLΔ+β4GRΔ+β5QTrqT2+β6HTrqT2+β7Q%MVICT2+β8H%MVICT2 + β9KFLEX T2+ε
ATTΔ=α0+β1AKLT1+β2GRT1+β3AKLΔ+βGRΔ+β5QTrqΔ+β6HTrqΔ+β7Q%MVICΔ+β8H%MVICΔ+β9KFLEXΔ+ε
Because none of these variables vary cyclically in males, T1 and T2 values for all variables would be expected to differ by random error only, and, consequently, all delta variables would be expected to have a mean value of zero. For this reason, and to better control for variables highly correlated with sex, males were examined separately. We present model results for males for both ATTT1 and ATTT2 in order to demonstrate whether the expected similarity of findings across the two time points is indeed observed. Model results for ATTΔ are not presented, because this variable in males represents essentially random error. The two full models for males were:
ATTT1=α0+β1AKL T1+β2GRT1+β3QTrqT1+β4HTrqT1+β5Q%MVICT1+β6H%MVICT1+β7KFLEX T1+ξ
ATTT2=α0+β1AKLT1+β2GRT1+β3QTrqT2+β4HTrqT2+β5Q%MVICT2+β6H%MVICT2+β7KFLEX T2+ε
For each analysis, we used a two step modeling procedure. On the first step, we examined how much variance in the ATT variables was accounted for by the five suppressor variables. On the second step, we added AKL and GR to the model (AKLT1, GRT1, AKLΔ, GRΔ for females; AKLT1, GRT1 for males) to examine their contribution and parameter estimates relative to ATT while accounting for the suppressor variables. All analyses were performed with PASW Statistics 17.0 (release 17.0.2; SPSS Inc., Chicago, IL). Alpha-level was set a priori at p<.05.
RESULTS
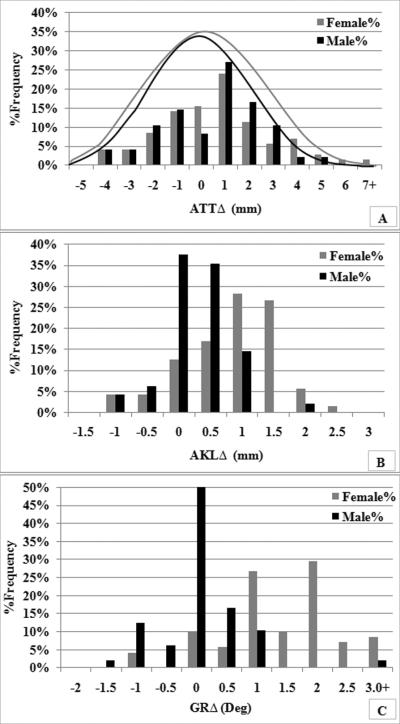
Three females did not complete the study and were therefore excluded from the data analysis. One male subject was excluded due to mechanical errors in the acquisition of ATTT1. A second male was excluded due to noncompliance with refraining from exercise prior to the 2nd test session that resulted in a 2mm increase in AKL at T2 compared to T1. Table 1 list the means ± standard deviations for each dependent and independent variable by sex for the remaining 71 females and 48 males at T1, T2 and Δ change from T1 to T2 (95% confidence intervals are also presented for Δ values). Significant sex by time interactions were observed for both AKL and GR (P<.001). As expected, AKL and GR increased in females but not males from T1 to T2, and this resulted in greater values in females compared to males at T2 (Table 1). Sex differences in AKLΔ and GRΔ are further described in figure 2. These frequency distributions confirm that the majority of males changed less than ±0.5mm for AKLΔ (73% of cases) and ±0.5° for GRΔ (79% of the cases) between T1 and T2, while only 34% and 15% of the females changed less than ±0.5mm(or ±0.5°) for AKLΔ and GRΔ, respectively. In 34% and 54% of the female cases, respectively, AKL changed more than 1.5mm, and GR changed more than 1.5° (values that exceed 3-5 times what would be expected simply due to measurement error). Despite these sex differences in AKLΔ and GRΔ from T1 to T2, no mean differences were observed in ATT between sex (P=.841), time (P=.713) or the sex by time interaction (P=.550). However, there was considerable dispersion in the absolute (T1 and T2) and delta values for ATT (figure 2) and many of the suppressor variables (see delta means and standard deviations reported in Table 1).
Table 1.
Means ± Standard Deviations (and 95% Confidence Intervals for Delta Values) for Each Independent and Dependent Variables by Sex and Test Session (T1, T2)
| Variable | Sex | Test Session | Delta | |
|---|---|---|---|---|
| T1 | T2 | (Δ = T2 - T1) | ||
| Anterior Tibial Translation (mm) | F | 6.8±2.3 | 7.0±2.4 | 0.2±2.3 (-0.34-0.74) |
| M | 6.8±2.9 | 6.8±2.9 | -0.0±2.0 (-0.55-0.55) | |
| Anterior Knee Laxity (mm) | F | 6.7±2.0 | 7.4±2.1* | 0.6±0.8 (0.42-0.78) |
| M | 6.6±1.8 | 6.6±1.8 | 0.1±0.5 (-0.05-0.25) | |
| Genu Recurvatum (°) | F | 3.7±3.6 | 5.0±3.6* | 1.3±1.0 (1.07-1.53) |
| M | 3.5±3.7 | 3.5±3.6 | -0.1±0.7 (-0.29-0.09) | |
| Quadriceps Muscle Torque (Nm/kg)† | F | 2.3±0.4 | 2.3±0.4 | -0.0±0.3 (-0.07-0.07) |
| M | 2.6±0.4 | 2.7±0.6 | 0.0±0.4 (-0.12-0.12) | |
| Hamstring Muscle Torque (Nm/Kg)† | F | 1.7±0.2 | 1.7±0.3 | -0.0±0.1 (-0.03-0.03) |
| M | 2.1±0.3 | 2.2±0.3 | 0.1±0.2 (0.06-0.14) | |
| Quadriceps Activation (%MVIC)† | F | 13.6±5.8 | 13.9±5.0 | 0.3±5.0 (-0.88-1.48) |
| M | 11.5±5.7 | 11.2±4.5 | -0.3±3.3 (-1.24-0.64) | |
| Hamstrings Activation (%MVIC)† | F | 4.9±3.2 | 5.1±3.2 | 0.3±2.9 (-0.36-0.96) |
| M | 4.2±3.4 | 4.3±3.2 | 0.1±2.2 (-0.51-0.71) | |
| Knee Flexion Change @ Peak ATT (°) | F | 8.3±3.4 | 7.7±3.3 | -0.6±3.1 (-1.33-0.13) |
| M | 8.5±3.3 | 8.1±3.2 | -0.4±2.9 (-1.22-0.42) | |
N= 71F, 48M
Female T2 > female T1 and male T1 and T2 values
Female ≠ male for both T1 and T2 (sex main effect)
Figure 2.
Comparative frequency distribution of a) ATTΔ, b) AKLΔ, and c) GRΔ and for males and females (note a normal curve distribution has been superimposed over the dispersion in ATTΔ given the considerable overlap between males and females).
Table 2 presents the regression model summary results. When examining the results for females, the 5 suppressor variables explained 11.3% and 11.1% of the variance in ATTT1 and ATTT2, respectively (P>.159), and 29.6% of the variance for ATTΔ (P<.001). When AKL(T1 and Δ) and GR(T1 and Δ) were added to the model, R2 values increased by comparable amounts for ATTT1 (0.125, P=.052), ATTT2 (0.131, P=.042) and ATTΔ (0.104, P=.042). While greater AKLT1 and less GRΔ were consistent predictors of ATTT1 and ATTT2 (both in terms of the magnitude of their coefficients and p-values), AKLΔ was a strong predictor of ATTT2 than ATTT1, and the sole predictor of ATTΔ. Thus, the major difference between the model results for ATTT1, ATTT2, and ATTΔ in females appears to be the substantially larger coefficients for AKLΔ when predicting ATTT2 (0.864, P=.027) and ATTΔ (0.740, P=.028), than when predicting ATTT1 (0.333, P=.135).
Table 2.
Regression summary results of the association between AKL and ATT, stratified by sex.
| R2 (P-Value) | R2Change (P-Value) | Unstandardized Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Sex | QTRQ | HTRQ | Q%MVIC | H%MVIC | KFLEX | AKLT1 | AKLΔ | GRT1 | GRΔ | ||
| ATTT1 | F | 0.113 (.159) | --- | -.928‡ | .794 | -1.720 | 23.152* | .041 | --- | --- | --- | --- |
| 0.238 (.042) | 0.125 (.052) | -1.075‡ | 1.019 | -1.516 | 20.498* | .066 | .234‡ | .333 | .048 | -.591* | ||
| M | 0.209 (.070) | --- | -1.655‡ | -2.180 | 10.202‡ | -3.667 | -.050 | --- | --- | --- | --- | |
| 0.417 (.002) | 0.208 (.002) | -2.937* | -1.080 | .216 | -6.615 | -.023 | .252 | --- | .335* | --- | ||
| ATTT2 | F | 0.111 (.168) | --- | -1.436† | 2.630† | 3.816 | 4.059 | -.006 | --- | --- | --- | --- |
| 0.242 (.037) | 0.131 (.042) | -2.006* | 2.425* | .859 | -.738 | .007 | .245‡ | .864* | .056 | -.473‡ | ||
| M | 0.046 (.840) | --- | -.378 | -.188 | 3.507 | -15.799 | .076 | --- | --- | --- | --- | |
| 0.259 (.080) | 0.210 (.007) | 1.371‡ | .471 | -10.034 | -20.055‡ | .063 | .358‡ | --- | .297* | --- | ||
| ATTΔ | F | 0.296 (<.001) | --- | -.346 | -3.451‡ | 2.128 | -10.370 | .393* | --- | --- | --- | --- |
| 0.400 (<.001) | 0.104 (.042) | -.043 | -4.221* | 1.052 | -9.375 | .443* | .007 | .740* | -.078 | .244 | ||
significant at p<.05
significant at P<.10
significant at P<.20
ATT = anterior tibial translation, AKLT1 and GRT1 = anterior knee laxity and genu recurvatum as measured at T1, AKLΔ and GRΔ = change in anterior knee laxity and genu recurvatum from T1 to T2, QTRQ & HTRQ = quadriceps and hamstring torque (Nm/kg), Q%MVIC & H%MVIC = quadriceps and hamstring reflex amplitude, KFLEX = knee flexion excursion during ATT.
When examining the results for males, the 5 suppressor variables explained 20.9% (P=.070) and 4.9% (P=.826) of the variance in ATTT1 and ATTT2, respectively. When AKL and GR were entered on the next step, they explained an additional 21% of the variance in ATTT1 and ATTT2 (P<.018). In both models, greater AKL and GR were associated with greater ATT, with GR being the stronger predictor. As expected, there did not appear to be any noteworthy or consistent differences for any of the modeling steps given for ATTT1 and ATTT2 in males. (When using AKLT2 and GRT2 in the model for predicting ATTT2, the results were essentially the same, supporting the consistency in these values across time in males).
DISCUSSION
Our primary findings were that AKL and GR were significant predictors of ATT in both males and females, accounting for a larger percentage of the variance in ATT in men (R2 change ~21%) than women (R2 change ~10-13%). This was also apparent when comparing the strength of the coefficients for AKLT1 and GRT1 between males and females, where their associations with ATT were generally smaller in magnitude for women than for men. While the strength of the association of AKLT1 with ATT did not differ markedly between males and females for either T1 or T2, GRT1 was only a significant positive predictor of ATT in males, and GRΔ was a significant negative predictor of ATT in females. As expected, the model results for ATT as measured at T1 (when AKL was at its minimum in females) vs. T2 (when AKL was at its maximum in females) were essentially the same for males, but were substantially different in females relative to the larger coefficients and stronger associations between AKLΔ with ATTT2 compared to ATTT1. When examining the change in ATT from T1 to T2 in females (ATTΔ), AKLΔ was the sole significant predictor once differences in thigh strength and activation and knee flexion angles between T1 and T2 were accounted for. Therefore, our expectations that greater baseline AKL would positively predict greater ATT in both males and females, and that greater AKLΔ would independently predict greater ATTΔ in females was in large part supported. However, the difference in model results between males and females suggests that ATT may be further increased in females who experience large cyclic changes in AKL across their cycle, and that the relationship between AKL and ATT may be somewhat dependent on the magnitude of GR.
AKL represents the anterior displacement of the tibia relative to the femur once the neutral or initial reference position of the tibia relative to the femur is obtained, where the ACL is the primary restraint to this motion (2). Conversely, posterior ligament structures (i.e. oblique popliteal, posterior cruciate and the posterior lateral corner) are the primary restraints to GR (16). As such, the combined measurements of AKL and GR may be a reflection of how the overall anterior-posterior alignment of the tibiofemoral joint surfaces is controlled when the knee transitions from NWB to WB, thus the overall magnitude of ATT that occurs. The positive relationships between both AKL and GR with ATT in males suggest that the tibia may initially be positioned more posterior in individuals with greater GR (owing to the greater posterior laxity), allowing greater anterior displacement of the tibia from the initial NWB condition until the motion is restrained by the ACL during WB. However, these results are based on a controlled load applied through the centers of the hip and ankle joints, and may in part be attributed to the testing protocol. Specifically, we standardized initial tibiofemoral joint position prior to each ATT measurement by placing anterior-posterior directed loads on the tibia relative to the femur and then allowing the counter weight system to create a zero shear load across the knee prior to application of the compressive, weight bearing load to the foot. The implication of the balance in magnitude between AKL and GR and their influence on tibiofemoral joint position in upright weight bearing deserves further study.
But while GRT1 (measured when AKL was at its minimum in females) along with AKL was often an important predictor in males, GRT1 had little impact on the prediction of ATT in females. We considered three possible explanations for these findings. First, AKL and GR were more highly correlated in females (.528) compared to males (.361), thus potentially explaining less overall variance in ATT in females due to a greater shared variance. Secondly, sex differences in lower extremity and knee joint anatomy may also modify the relationships between AKL, GR and ATT. For example, sex differences have been noted in the magnitude and variability of the lateral and medial posterior inferior tibial slopes as well as the coronal tibial slope (10, 36). These slope differences along with the depth of the concavity of the medial compartment of the tibiofemoral joint (tibiofemoral joint congruence) may impact articular contact and joint biomechanics, and contribute more or less resistance to ATT upon joint loading (9). Known sex differences in other aspects of lower extremity anatomy, such as lower extremity alignment, the length of the knee extensor moment arm, or the size and material properties of the ACL, may also play a role (3, 4, 7, 30). Work is ongoing to examine these potential anatomical contributions, and how they may interact with AKL and GR to impact joint biomechanics as the knee transitions from NWB to WB.
A third consideration is that cyclic variation in GR across the female menstrual cycle may have impacted the relationship between GRT1 with ATT. Although GRT1 did not add substantially to the model in females, greater ATT at both T1 and T2 was generally observed in females who had greater AKLT1 and AKLΔ values but who experienced less cyclic changes in GRΔ. The reciprocal relationship between AKLΔ and GRΔ when predicting ATT is not entirely clear. Although significant low to moderate correlations were noted between GRΔ and AKLΔ in females (r=.379, P=.001), there were considerable variations across women as to how much of an increase in GR they experienced relative to the change in AKL from T1 to T2. Because GR was only measured on the days when AKL was at minimum and maximum values, and the timing of changes in GR and AKL are not always consistent across the cycle (29) the true magnitude of baseline and cyclic changes in GR within an individual may not always have been reflected in GRT1 and GRΔ. As such, we considered whether GRΔ may represent somewhat of an adjustment for GRT1, together providing a better estimate of the magnitude of GR within that individual. However, when we removed GRΔ from the model, this resulted in little to no change in the coefficients for AKLT1 (.264) or GRT1 (.044) and the amount of variance explained in ATT decreased by 5%. Perhaps a more likely explanation for this reciprocal relationship is that the balance between cyclic variations in AKL and GR (thus the extent to which they are correlated) may be influenced by various anatomical contributions to GR. While the ACL is the primary restraint to AKL, the magnitude of GR can be influenced by multiple factors, including the bony alignment of the proximal tibia (e.g. a reduced / reversed posterior slope of the tibial plateau has been associated with greater GR), excessive capsuloligamentous laxity, or a combination of both (5, 13, 16, 22). If the cause is more structural in nature (e.g. associated with a reduced posterior slope of the tibial plateau rather than posterior ligament laxity), this form of GR may be less likely to produce cyclic variations across the cycle, and in turn, be less likely to produce ATT with axial compression (37) as compared to someone with a normal or increased posterior directed slope of the tibial plateau, and greater total anterior-posterior laxity. This is further supported by the model results when predicting the change in ATT from T1 to T2; while greater increases in both AKL and GR predicted greater increases ATT from T1 to T2, the change in GR was a much weaker predictor than the change in AKL.
AKLΔ (the cyclic increases in AKL from T1 to T2) was the strongest predictor for ATTT2 and the sole significant predictor of ATTΔ. Although AKLΔ explained only 10% additional variance in ATTΔ once all suppressor variables were accounted for, the regression coefficient indicates that for every 1.00 mm increase in AKL from T1 to T2, there is a predicted 0.74 mm increase in ATT (see Table 2). These findings suggests that if a female's AKL changes as much as 3mm across her cycle, she may experience an increase in ATT over 2 mm, a change that represents approximately 30% of the mean magnitude in ATT we observed at T1. Of interest, the magnitude of change in ATT in response to changes in AKL was not dependent on the individual's minimum AKL, suggesting that biomechanical effects associated with these cyclic variations may be additive to those associated with one's baseline laxity values.
Clinical Implications
When considering the biomechanical implications of these findings and how they are related to the risk of suffering ACL trauma, there are a number of considerations that require further study. First, it is well appreciated that the ACL is the primary restraint controlling ATT (1, 37). As such, it is possible that during the initial weight acceptance of the limb during dynamic tasks (e.g. landing from a jump) that greater magnitudes of AKL, thus greater magnitudes of ATT, may be associated with greater acceleration and movement of the tibia before the ACL restrains the motion. This may be of particular concern when combined with unopposed quadriceps contractions (14, 15, 37) and higher axial directed compressive loads (25, 37) at lower knee flexion angles, which have also been associated with greater anterior directed shear forces and tibial translation. Second, greater anterior tibial displacement may also impact tibiofemoral joint biomechanics upon full weight acceptance. While there is minimal tibiofemoral translation once full axial compression is applied to the joint, greater ATT during the transition to full weight bearing may result in starting subsequent full weight bearing motions with the tibia in a more anterior position relative to the femur, which may potentially lead to abnormal joint kinematics and muscle function (37, 38). While this contention is supported by studies noting altered lateral hamstring activation in females who have greater magnitudes of AKL (24, 27), the actual impact this altered tibiofemoral joint position may have on ACL strain biomechanics has yet to be determined.
Finally, greater ATT may be coupled with frontal and transverse plane rotational biomechanics of the knee, and this may also have an important impact on ACL strain biomechanics. Previous research indicates that females who had greater AKL also had greater varus-valgus and internal-external rotational laxity of the knee (35), and these laxity values have been associated with altered transverse and frontal plane hip and knee joint kinematics and kinetics during landing (33). Other work (21) suggests that small cyclic increases in AKL as little as 1.3-mm may increase knee adduction moment during a stop jump task as much as 20%. Still other work has noted an association between increased AKL with greater peak lateral tibial acceleration immediately after foot strike during gait in ACL deficient knees compared to normal knees and ACL reconstructed knees (42). They attributed this finding to greater adduction moment during walking, potentially causing an increase in the shear and compression loading of the medial compartment, and accelerating the development of osteoarthritis. Based on these collective findings, and the observed association between increased AKL and increased ATT, further research is needed to examine the implications of ATT (both absolute and cyclic increases) on coupled frontal and transverse plane knee biomechanics, and their combined influence on ACL strain.
In summary, greater magnitudes of AKL are associated with greater magnitudes of ATT and these relationships were often influenced by the amount of baseline (males) or cyclic changes (females) in GR. Moreover, females who experience greater AKLΔ across the cycle also experienced greater ATTΔ, further supporting a link between AKL and ATT. Although this study accounted for more factors than previously examined (34), approximately 60-75% of the variance in ATT remained unexplained by these factors, indicating that other important factors that regulate ATT remain unaccounted for. As previously noted, factors such as condylar geometry, extensor moment arm, and lower extremity structural alignment should be examined in future studies as they relate to the association between knee joint laxity and ATT. Further, it is important to note that for the weight bearing conditions used in this study, the muscles were relaxed prior to the application of the weight bearing load, and the weight bearing load was substantially lower than what one would experience during high demand activities associated with sport. While we believe this research model represents an important first step in understanding the relationship between knee laxity (a risk factor for injury to the ACL) and tibiofemoral joint biomechanics during the transition from NWB to WB (a very important event which includes application and transmission of large compression and shear loads across the knee and is associated with a majority of non-contact ACL injuries), it is well accepted that muscle pre-activation occurs in anticipation of ground contact during common athletic tasks such as landing from a jump. Research has shown that muscle pre-activation may in part be modulated by the amount of AKL an individual possesses (24, 27) and consequently, it is possible that an individual may effectively compensate for the increased AKL through appropriate timing and amplitude of contraction of their dynamic restraints when the action is anticipated.(41) However, should a mismatch occur between what is anticipated and what actually occurs (i.e. so that the thigh muscles are not able to respond in an appropriate manner to an unanticipated external load), the current findings suggest that greater joint laxity may lead to greater joint displacement prior to any restraint provided by reactive muscle action. This may be particularly true with the higher magnitudes of axial compressive loads and loading rates associated with physical activity, as ATT has been shown to increase with increasing magnitudes of axial loads despite faster and stronger reactive muscle response times and activation amplitudes (25).
ACKNOWLEDGEMENT
The project described was supported by Grant Number R01- AR53172 NIH-NIAMS, and through a U54 Center grant as part of the Specialized Cooperative Centers Program in Reproductive Research [NICHD (SCCPRR) Grant U54 HD28934, University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core]. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Funding Source: Supported by Grant Number R01- AR53172 NIH-NIAMS, and through a U54 Center grant as part of the Specialized Cooperative Centers Program in Reproductive Research [NICHD (SCCPRR) Grant U54 HD28934]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Beynnon BD, Fleming BC, Labovitch R, et al. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20:332–337. doi: 10.1016/S0736-0266(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 2.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. J Bone Joint Surg. 1980;62-A(2):259–270. [PubMed] [Google Scholar]
- 3.Chandrashekar N, Mansour JM, Slauterbeck J, et al. Sex-based differences in the tensile proerties of the human anterior cruciate ligament. J Biomech. 2006;39:2943–2950. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Chandrashekar N, Slauterbeck J, Hashemi J. Sex-Based Differences in the Anthropometric Characteristics of the Anterior Cruciate Ligament and Its Relation to Intercondylar Notch Geometry. Am J Sports Med. 2005;33(10):1492–1498. doi: 10.1177/0363546504274149. [DOI] [PubMed] [Google Scholar]
- 5.Dejour D, Bonin N, Locatelli E. Tibial antirecurvatum osteotomies. Oper Tech Sports Med. 2000;8(1):67–70. [Google Scholar]
- 6.DeLuca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- 7.Fayad LM, Rosenthal EH, Morrison WB, et al. Anterior cruciate ligament volume: Analysis of gender differences. J Magnetic Res Imag. 2008;27:218–223. doi: 10.1002/jmri.21239. [DOI] [PubMed] [Google Scholar]
- 8.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(3):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 9.Giffin JR, Vogrin TM, Zantop T, et al. Effects of increasing tibial slope on the biomechanics of the knee. Am J Sports Med. 2004;32(2):376–382. doi: 10.1177/0363546503258880. [DOI] [PubMed] [Google Scholar]
- 10.Hashemi J, Chandrashekar N, Gill B, et al. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90(12):2724–2734. doi: 10.2106/JBJS.G.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer LC, Denegar CR, Buckley WE, et al. Factors associated with anterior cruciate ligament injury: history in female athletes. J Sports Med Phys Fit. 2007;47:446–454. [PubMed] [Google Scholar]
- 12.Krosshaug T, Slauterbeck JR, Engebretsen L, et al. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007;17(5):508–519. doi: 10.1111/j.1600-0838.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 13.Laura G, Berruto M, Bianchi M. Genu recurvatum following distal epiphysiodesis of the femur: X-ray evaluation and therapeutical approach. Ital J Orthop Traumatol. 1992;18(4):505–514. [PubMed] [Google Scholar]
- 14.Li G, Rudy TW, Sakane M, et al. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 15.Markolf KL, O'Neill G, Jackson SR, et al. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32(5):1144–1149. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- 16.Morgan PM, LaPrade RF, Wentorf FA, et al. The role of the oblique popliteal ligament and other structures in preventing knee hyperextension. Am J Sports Med. 2010;38(3):550–557. doi: 10.1177/0363546509348742. [DOI] [PubMed] [Google Scholar]
- 17.Moroni A, Pezzuto V, Pompili M, et al. Proximal osteotomy of the tibia for treatment of genu recurvatum in adults. J Bone Jt Surg [Am] 1992;74A(4):577–586. [PubMed] [Google Scholar]
- 18.Myer GD, Ford KR, Paterno MV, et al. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36(6):1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen AD, Shultz SJ. Sex Differences in Lower Extremity Posture. J Orthop Sports Phys Ther. 2007;37(7):389–398. doi: 10.2519/jospt.2007.2487. [DOI] [PubMed] [Google Scholar]
- 20.Olsen O, Myklebust G, Engebretsen L, et al. Injury mechanisms for anterior cruciate ligament injuries in team handball. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 21.Park SK, Stefanyshyn DJ, Ramage B, et al. Alterations in Knee Joint Laxity During the Menstrual Cycle in Healthy Women Leads to Increases in Joint Loads During Selected Athletic Movements. Am J Sports Med. 2009;37(6):1169–1177. doi: 10.1177/0363546508330146. [DOI] [PubMed] [Google Scholar]
- 22.Piriou P, Garreau C, Combelles F, et al. Original technique for the treatment of ligament-related genu recurvatum: preliminary results. Knee Surg Sports Traumatol Arthrosc. 2002;10(4):260–264. doi: 10.1007/s001670100243. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh R, VonArx O, Azzopardi T, et al. The risk of anterior cruciate ligament rupture with generalised joint laxity. J Bone Jt Surg. 2005;87-B:800–803. doi: 10.1302/0301-620X.87B6.15833. [DOI] [PubMed] [Google Scholar]
- 24.Rozzi SL, Lephart SM, Gear WS, et al. Knee joint laxity and neuromuscular characteristics of male and female soccer and basketball players. Am J Sports Med. 1999;27(3):312–319. doi: 10.1177/03635465990270030801. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz RJ, Kim HS, Shultz SJ. Effect of Axial Load on Anterior Tibial Translation when Transitioning from Non-Weight Bearing to Weight Bearing. Clin Biomech. 2010;25(1):77–82. doi: 10.1016/j.clinbiomech.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shultz SJ, Beynnon BD, Schmitz RJ. Sex Differences in Coupled Knee Motions During the Transition from Non-Weight Bearing to Weight Bearing. J Orthop Res. 2009;27(6):717–723. doi: 10.1002/jor.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shultz SJ, Carcia CR, Perrin DH. Knee joint laxity affects muscle activation patterns in the healthy knee. J Electromyogr Kinesiol. 2004;14:475–483. doi: 10.1016/j.jelekin.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Shultz SJ, Kirk SE, Sander TC, et al. Sex differences in knee laxity change across the female menstrual cycle. J Sports Med Phys Fit. 2005;45(4):594–603. [PMC free article] [PubMed] [Google Scholar]
- 29.Shultz SJ, Levine BJ, Nguyen AD, et al. A Comparison of Cyclic Variations in Anterior Knee Laxity, Genu Recurvatum and General Joint Laxity Across the Menstrual Cycle. J Orth Res. doi: 10.1002/jor.21145. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shultz SJ, Nguyen A, Levine BJ. The Relationship Between Lower Extremity Alignment Characteristics and Anterior Knee Joint Laxity. J Sports Health. 2009;1(1):54–60. doi: 10.1177/1941738108326702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shultz SJ, Nguyen AD, Leonard MD, et al. Thigh Strength and Activation as Predictors of Knee Biomechanics During a Drop Jump Task. Med Sci Sports Exer. 2009;41:857–66. doi: 10.1249/MSS.0b013e3181e3b3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shultz SJ, Sander TC, Kirk SE, et al. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shultz SJ, Schmitz RJ. Effects of Transverse and Frontal Plane Knee Laxity on Hip and Knee Neuromechanics During Drop Landings. Am J Sports Med. 2009;37(9):1821–1830. doi: 10.1177/0363546509334225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz SJ, Shimokochi Y, Nguyen A, et al. Non-Weight Bearing Anterior Knee Laxity is Related to Anterior Tibial Translation During Transition from Non-Weight Bearing to Weight Bearing. J Orthop Res. 2006;24(3):516–523. doi: 10.1002/jor.20040. [DOI] [PubMed] [Google Scholar]
- 35.Shultz SJ, Shimokochi Y, Nguyen A, et al. Measurement of Varus-Valgus and Internal-External Rotational Knee Laxities In-Vivo Part II: Relationship with Anterior-Posterior and General Joint Laxity in Males and Females. J Orthop Res. 2007;25(8):989–996. doi: 10.1002/jor.20398. [DOI] [PubMed] [Google Scholar]
- 36.Todd MS, Lalliss S, Garcia S, et al. The Relationship Between Posterior Tibial Slope and Anterior Cruciate Ligament Injuries. Am J Sports Med. 2010;38(1):63–67. doi: 10.1177/0363546509343198. [DOI] [PubMed] [Google Scholar]
- 37.Torzilli PA, Deng X, Warren RF. The effect of joint-compressive load and quadriceps muscle force on knee motion in the intact and anterior cruciate ligament-sectioned knee. Am J Sports Med. 1994;22(1):105–112. doi: 10.1177/036354659402200117. [DOI] [PubMed] [Google Scholar]
- 38.Uh BS, Beynnon BD, Churchill DL, et al. A new device to measure knee laxity during weightbearing and non-weight bearing conditions. J Orthop Res. 2001;19:1185–1191. doi: 10.1016/S0736-0266(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 39.Uhorchak JM, Scoville CR, Williams GN, et al. Risk factors associated with non-contact injury of the anterior cruciate ligament. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 40.Wei S, Schmidt MD, Dwyer T, et al. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity. 2009;17(5):1070–1076. doi: 10.1038/oby.2008.641. [DOI] [PubMed] [Google Scholar]
- 41.Yack HJ, Riley LM, Whieldon TR. Anterior tibial translation during progressive loading of the ACL-deficient knee during weight-bearing and nonweight-bearing isometric exercise. J Orthop Sports Phys. 1994;20:247–253. doi: 10.2519/jospt.1994.20.5.247. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura I, Naito M, Hara M, et al. Analysis of the significance of the measurement of acceleration with respect to lateral laxity of the anterior cruciate ligament insufficient knee. Int Orthop. 2000;24(5):276–278. doi: 10.1007/s002640000171. [DOI] [PMC free article] [PubMed] [Google Scholar]