Abstract
Protopanaxadiol (PPD), an aglycon of ginseng saponins, has shown anticancer activity in previous studies. Here we report the semi-synthesis of 9 PPD derivatives with acetyl substitutions. Subsequently, the antiproliferative effects of these 9 analogs on different human cancer cell lines were investigated. Compounds 1 and 3 showed more significant and more potent antiproliferative activity compared to PPD and other derivatives. A flow cytometric assay indicated that Compounds 1 and 3 arrested cell cycle progression in the G1 phase and significantly induced apoptosis of cancer cells.
Keywords: protopanaxadiol derivatives, semi-synthesis, apoptosis, cell cycle, colon cancer
Introduction
Cancer is the second-leading cause of human death in the U.S. The clinical management of cancer invariably involves diverse conventional modalities, including surgery, radiation, and chemotherapy[1]. Novel therapeutic agents and ethanopharmacological screenings provide scientists with an alternative avenue to discover active components and compounds to treat cancer with traditional medicines like botanicals. In recent years, botanicals have become an important source of biologically active natural products.
Botanicals contain effective anticancer compounds that could potentially be used alone or as adjuncts to existing chemotherapy to improve efficacy and reduce drug-induced adverse events. A series of analyses has demonstrated the continuing and valuable contributions of botanicals as sources not only of potential chemotherapeutic agents, but also of lead compounds for the semi-synthesis or total synthesis of new drugs. In current cancer treatments, approximately 80% of novel drugs originated from natural products [5].
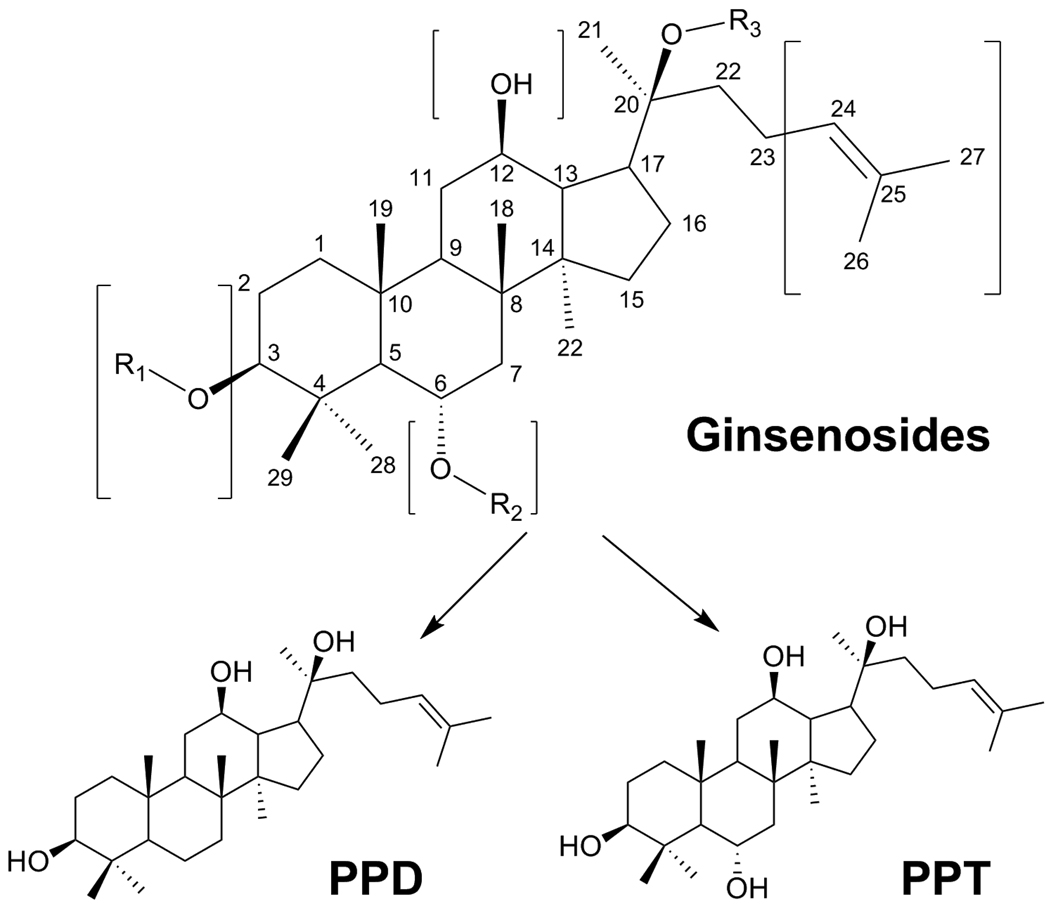
Ginsengs, including Asian ginseng (Panax ginseng C. A. Meyer) and American ginseng (Panax quinquefolius L.), are very commonly used herbal medicines in many countries [6–7]. Ginseng extracts, especially steamed ginseng extracts, possess significant anticancer activities [8–9], and confer a radio-protective effect against radiation-induced damage in DNA [10]. The major active components of ginseng are ginsenosides, a diverse group of triterpenoid saponins (Fig. 1) [11]. The antiproliferative effects of ginseng saponin aglycons, such as protopanaxadiol (PPD), are even greater than those of ginsenosides. Thus, protopanaxadiol could serve as a lead compound, since its derivatives may exert better anticancer activity [12]. To date, most ginseng studies have focused on the natural compounds isolated from ginseng. Limited work has been done on the synthesis or semi-synthesis of PPD derivatives and their anticancer activity evaluation.
Figure 1.
Chemical structure of ginsenosides, PPD and PPT.
We designed and synthesized a series of PPD derivatives using semi-synthetic methods. Our structure modification was mainly based on reactive sites of PPD. The antiproliferative effects of the PPD derivatives on different cancer cell lines were subsequently evaluated. The mechanisms of action of selected derivatives were also investigated. Using chemical and biological data, we also explored the structural-functional relationship of the PPD derivatives.
Materials and methods
Materials
All cell culture plastic wares were obtained from Falcon Labware (Franklin Lakes, NJ) and Techno Plastic Products (Trasadingen, Switzerland). Trypsin, McCoy’s 5A medium, and phosphate buffered saline were obtained from Mediatech, Inc. (Herndon, VA). DMEM/F12 medium was obtained from Lonza (Conshohocken, PA). Penicillin G/streptomycin was obtained from Sigma (St. Louis, MO). An MTS assay kit, CellTiter 96 Aqueous One Solution Cell Proliferation Assay, was obtained from Promega (Madison, WI). An Annexin V-FITC Apoptosis Detection Kit was obtained from BD Biosciences (Rockville, MD). PI/RNase staining buffer was obtained from BD Biosciences Pharmingen (San Diego, CA). Human cell lines, HCT-116, SW-480, MCF-7, and CRL-1831, were obtained from ATCC.
Preparation of protopanaxadiol (PPD)
Total ginsenosides (2.0 g), n-BuOH (250 mL), and sodium hydroxide (10 g) were added to a 500 mL round bottom flask. The mixture was heated to 130°C while stirring under argon for 2 days and allowed to cool to room temperature. The reaction mixture was then washed with water (2×100 mL), 1% HCl (2×100 mL), 5% NaHCO3 and brine. The organic phase was dried over MgSO4. The removal of the solvent under reduced pressure resulted in a sticky oil, which was purified by silica gel column to give PPD (120 mg) and protopanaxatriol (PPT) (110 mg). Since the PPD and PPT were obtained from a group of mixed ginsenosides, the yield information was unavailable.
Synthesis of PPD derivatives
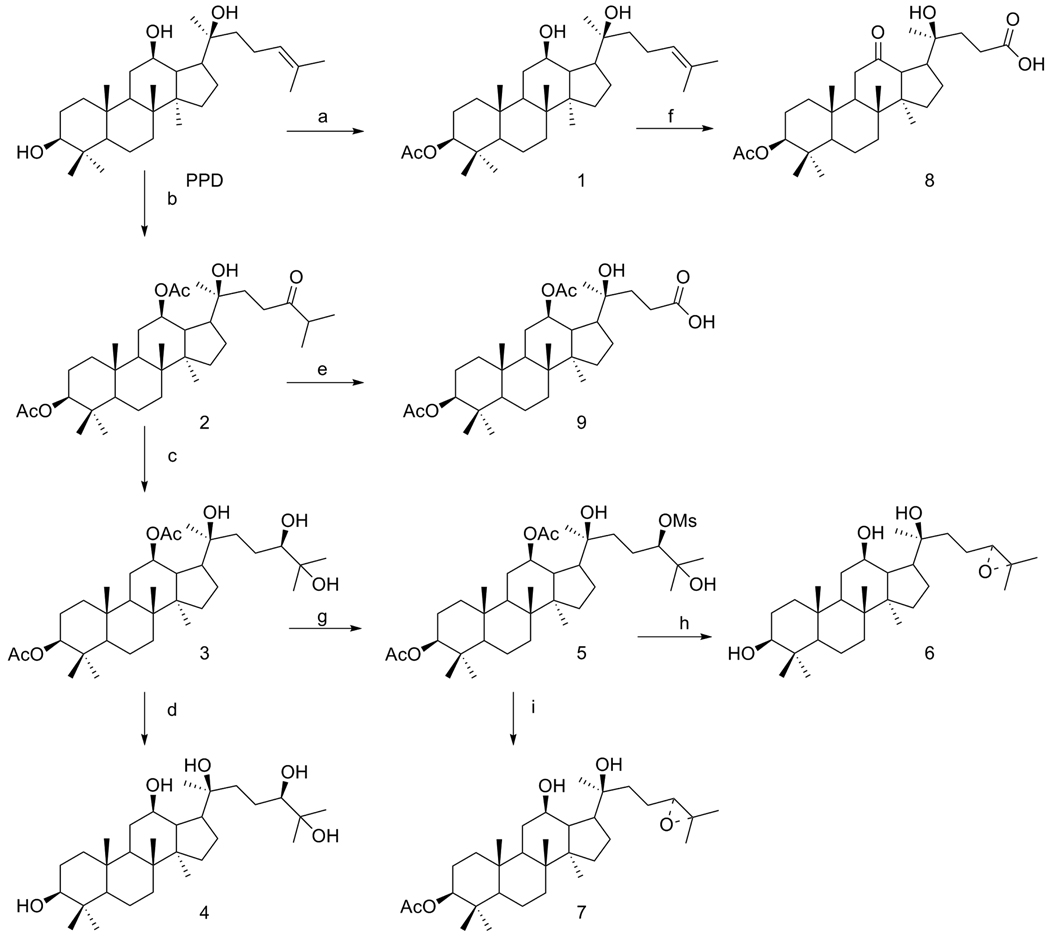
Compounds 1–9 were semi-synthesized from PPD (Fig. 2), and their structure and purity (>90%) were confirmed by 1H, 13C, nuclear magnetic resonance spectroscopy and TLC.
Figure 2.
Semi-synthesis routes of PPD derivatives.
Compounds 1 and 2
Pyridine (10 mL) and acetic anhydride (0.5 mL) were added to a 100 mL round bottom flask containing PPD (120 mg). The mixture was heated to 50°C overnight while stirring under argon. After the reaction mixture cooled to room temperature, methanol (1 mL) was added to quench the reaction. Then, all of the solvents were evaporated under reduced pressure and the residue was co-evaporated with toluene (2×20 mL). The resulting oil was purified by silica gel column producing a 3-acetyl PPD derivative, Compound 1, (3-acetate protopanaxadiol) in a 30% yield and a 3,12-diacetyl PPD derivative, Compound 2, (3, 12-diacetate, 24-oxoheptan protopanaxadiol) in a 45% yield.
Compound 3
T-butanol (10 mL), water (10 mL), AD-mix-β (500 mg) and methanesulfonamide (40 mg) were added to a 100 mL round bottom flask containing the 3, 12-diacetyl PPD derivative (Compound 2, 260 mg). The mixture was stirred at room temperature for 2 days and then cooled to 0°C. Na2SO3 (400 mg) was added and the mixture was stirred for 1 h. Then, water (50 mL) was added and the mixture was extracted with ethyl acetate (50 mL×2). The combined organic phase was washed with brine and dried over MgSO4 before all the solvents were evaporated under reduced pressure. The residue was purified by silica gel column to give Compound 3, (3, 12-diacetate, 24,25-dihydroxyl protopanaxadiol) in an 85% yield.
Compound 4
Ethanol and sodium methoxide were added to a 50 mL round bottom flask containing 3, 12-diacetyl, 24-beta, 25-dihydroxyl-PPD derivative (Compound 3). The mixture was stirred at room temperature for 1 day before acetic acid was added to adjust the pH to 7.0. Removal of all the solvents under reduced pressure resulted in an oil residue, which was purified using a silica gel column to produce Compound 4; (24,25-dihydroxyl protopanaxadiol) in a 65% yield.
Compound 5
Dichloromethane (10 mL), pyridine (0.3 mL) and methanesulfonyl chloride (0.1 mL) were added to a 50 mL round bottom flask containing 3, 12-diacetyl, 24-beta, 25-dihydroxyl-PPD derivative (Compound 3, 100 mg). The mixture was stirred at room temperature for 24 h. Another portion of methanesulfonyl chloride (0.1 mL) was added and the mixture was stirred for another 8 h. Dichloromethane (50 mL) was added and the mixture was washed with NaHCO3 (5%, 50 mL) and brine, and was dried over MgSO4 before the solvent was removed under reduced pressure. The resulting oil residue was purified by running a silica gel column to produce Compound 5; (3,12-diacetate, 24-OMS, 25-hydroxyl protopanaxadiol) in a 68% yield.
Compound 6
Methanol (5 mL), water (0.7 mL) and K2CO3 (150 mg) were added to a 50 mL round bottom flask containing 25-hydroxyl-PPD 24-beta-mesylate derivative (Compound 5, 50 mg) and then the mixture was stirred at room temperature for 72 h. The solvents were removed under reduced pressure and the residue was added to dichloromethane (50 mL). The mixture was washed with water and brine, and dried over MgSO4. The resulting oil residue was purified by running a silica gel column to produce Compound 6; (24-cyclopropane protopanaxadiol) in a 42% yield.
Compound 7
Methanol (5 mL), water (0.7 mL) and K2CO3 (150 mg) were added to a 50 mL round bottom flask containing 25-hydroxyl-PPD 24-beta-mesylate derivative (Compound 5, 32 mg) before the mixture was stirred at room temperature for 3 h. Then, dichloromethane (50 mL) was added. The mixture was washed with water and brine, and dried over MgSO4. The resulting oil residue was purified by running a silica gel column to produce Compound 7; (3- acetate 24-cyclopropane protopanaxadiol) in a 55% yield.
Compound 8
A pre-mixed solution of CrO3 (150 mg), pyridine (0.25 mL) and acetic anhydride (0.15 mL) in dichloromethane (5 mL) was added to a 50 mL round bottom flask containing Compound 1 (32 mg) before the reaction mixture was stirred at room temperature for 1 h. Ethyl acetate (100 mL) was then added and the mixture was filtered to eliminate the solid. The filtrate was concentrated under reduce pressure and the residue was purified by a silica gel column to produce Compound 8; (3-acetate, 12- oxoheptan protopanaxadiol acid) in a 65% yield.
Compound 9
A pre-mixed solution of CrO3 (150 mg), pyridine (0.25 mL), and acetic anhydride (0.15 mL) in dichloromethane (5 mL) was added to a 50 mL round bottom flask containing Compound 2 (30 mg) and the reaction mixture was stirred at room temperature for 1 h. Ethyl acetate (100 mL) was then added and the mixture was filtered to eliminate the solid. The filtrate was concentrated under reduce pressure and the residue was purified by silica gel column to produce Compound 9; (3, 12 diacetate protopanaxadiol acid) in a 70% yield.
Cell culture
The human colorectal cancer cell lines HCT-116 and SW-480, human breast cancer cell line MCF-7 and normal human colon epithelial cell line CRL-1831 (ATCC, Manassas, VA) were routinely grown in McCoy's 5A medium (for HCT-116), Leibovitz's L-15 medium (for SW-480), DMEM medium (for MCF-7), or DMEM/F12 medium (for CRL-1831). All the media were supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (50 units/ml). Cells were maintained in a tissue culture flask and kept in a humidified incubator (5% CO2 in air at 37°C). The medium was changed every 2–3 days. When the cells reached 70%–80% confluence, they were trypsinized, harvested, and seeded into a new tissue culture flask.
Antiproliferative assay
The effect of PPD and its derivatives on the proliferation of HCT-116, SW-480, and MCF-7 cell lines was determined by the modified trichrome stain (MTS) assay. Cancer cells were plated into a 96-well plate at a density of 1×104 cells/well. After seeding for 24 h, the cells were treated with one of the different concentrations of PPD or its derivatives. All experiments were performed in triplicate. At the end of the sample exposure period, either 24 h or 48 h, the medium of each well was discarded and 100 µl of fresh medium and 20 µl of CellTiter 96 aqueous solution were added. The plate was returned to the incubator where it remained for 1–4 h in a humidiWed atmosphere at 37°C. Then, 60 µl of medium from each well was transferred to an ELISA 96-well plate, and the absorbance of the formazan product was measured at 490 nm. The blank control was recorded by measuring the absorbance at 490 nm with wells containing medium mixed with CellTiter 96 aqueous solution, but not cells. Results were expressed as a percentage of control (vehicle set at 100%).
Cell cycle analysis using flow cytometry
HCT-116 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed and cells were treated with PPD and its derivatives at different concentrations. Cells were incubated for 48 h before harvesting. The cells were fixed gently with 80% ethanol before being placed in a freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 30 µl of phosphate buffered saline (PBS) containing 40 µg/ml propidium iodide and 0.1 mg/ml RNase. Cells were incubated in a dark room for 20 min at room temperature before cell cycle analysis with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). For each measurement, at least 10,000 cells were counted.
Apoptotic analysis after staining by annexin V/propidium iodide
HCT-116 cells were seeded in 24-well tissue culture plates. After 24 h, the medium was changed and PPD or its derivatives was added. After treatment for 48 h, cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then, culture medium containing 10% FBS (and floating cells) was added to inactivate the trypsin. When gentle pipetting was completed, the cells were centrifuged for 5 min at 1500 g. The supernatant was removed and cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer's instructions. Untreated cells were used as control for double staining. Immediately after staining, the cells were analyzed by a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted.
Statistical analysis
The data are presented as mean ± standard error (SE). A one-way ANOVA determined whether the results had statistical significance. In some cases, the Student's t-test was used for comparing two groups. The level of statistical significance was set at P<0.05.
Results
Route of semi-synthesis and identification of compounds
As shown in Fig. 1, PPD and PPT were prepared from ginsenosides by alkaline hydrolysis. The routes of semi-syntheses of Compounds 1–9 are shown in Fig. 2. Compounds 1 and 2 were acetylated from PPD and isolated using column chromatography. Compound 8 was synthesized from Compound 1. Other compounds were synthesized from Compound 2.
Protopanaxadiol (PPD)
1H NMR: (CDCCl3, 500 MHz): δ 5.11 (T, J=5.0 Hz, 1H), 3.16 (dd, J=5.0, 11.0 Hz, 1H), 2.12 (m, 1H), 2.00 (m, 2H), 1.80 (m, 1H), 1.70 (m, 1H), 1.66 (s, 3H), 1.59 (s, 3H), 1.23–1.50 (m, 16H), 1.14(s, 3H), 0.95 (s, 3H), 0.94 (s, 3H), 0.84 (s, 3H), 0.83 (s, 3H), 0.74 (s, 3H); 13C NMR: (CDCCl3, 500 MHz): δ 131.58 125.02, 78.82, 74.25, 70.88, 55.84, 53.45, 51.58, 50.06, 47.57, 39.70, 38.99, 38.91, 37.06, 34.75, 34.58, 31.19, 31.00, 28.03, 27.29, 26.70, 26.45, 25.76, 22.31, 18.26, 17.74, 16.81, 16.13, 15.67, 15.41;
Protopanaxatriol (PPT)
1H NMR: (CDCCl3, 500 MHz): δ 5.14 (T, J=7.0 Hz, 1H), 4.09 (m, 1H), 3.57 (m, 1H), 3.17 (dd, J=5.0, 7.0 Hz, 1H), 2.15 (m, 1H), 2.03 (m, 2H), 1.72 (m, 1H), 1.69 (s, 3H), 1.63 (s, 3H), 1.31(s, 3H), 1.18 (s, 3H), 1.05(s, 3H), 0.98 (s, 3H), 0.92 (s, 3H), 0.90 (s, 3H); 13C NMR: (CDCCl3, 500 MHz): δ 131.83, 124.88, 78.53, 74.36, 70.62, 68.59, 61.07, 53.40, 51.37, 49.50, 47.32, 46.88, 40.92, 39.27, 39.11, 38.78, 34.42, 31.02, 30.95, 30.88, 26.96, 26.88, 26.42, 25.78, 22.32, 17.77, 17.22, 17.18, 16.82, 15.53;
Compound 1
1H NMR: (CDCCl3, 500 MHz): δ 5.16 (t, J=7.0 Hz, 1H), 4.48 (d, J=5.5, 6.0 Hz, 1H), 3.60 (m, 1H), 2.17 (m, 1H), 2.05 (s, 1H), 1.85 (m, 2H), 1.7 (s, 3H), 1.64 (s, 3H), 1.2–1.6(mm, 10H), 1.19 (s, 3H), 0.99(s, 3H), 0.91 (s, 3H), 0.89 (s, 3H), 0.89 (s, 6H); 13C NMR: (CDCCl3, 500 MHz): δ 171.03, 131.81, 124.96, 80.81, 74.38, 70.82, 55.92, 53.47, 51.57, 49.96, 47.76, 39.75, 38.63, 37.85, 37.01, 34.69, 34.44, 31.21, 30.96, 27.98, 26.98, 25.77, 23.66, 22.35, 21.31, 18.15, 17.75, 16.81, 16.19, 15.70;
Compound 2
1H NMR: (CDCCl3, 500 MHz): δ 5.15 (t, J=7.0 Hz, 1H), 4.72 (m, 1H), 4.48 (d, J1=4.5, 6.5 Hz, 1H), 3.01 (s, 1H), 2.20 (m, 1H), 2.03 (s, 9H), 1.70 (s, 3H), 1.63 (s, 3H), 1.12 (s, 3H), 1.00(s, 3H), 0.94 (s, 3H), 0.87(s, 3H), 0.84 (s, 6H); 13C NMR: (CDCCl3, 500 MHz): δ 170.81, 169.56, 131.27, 125.18, 80.51, 76.53, 73.63, 55.85, 52.86, 52.66, 49.88, 44.83, 39.70, 38.48, 37.85, 37.04, 36.06, 34.48, 31.43, 28.22, 27.95, 27.14, 26.19, 25.75, 23.53, 22.23, 21.50, 21.26, 18.09, 17.67, 17.24, 16.46, 16.22, 15.56;
Compound 3
1H NMR: (CDCCl3, 500 MHz): δ 4.74 (m, 1H), 4.48 (m, 1H), 3.43 (d, J=10.0 Hz, 1H), 2.04 (d, J=2.0 Hz, 1H), 1.25–2.0 (m, 15H), 1.25 (s, 3H), 1.18 (s, 3H), 1.14 (s, 3H), 1.01 (s, 3H), 0.95 (s, 3H), 0.882 (s, 3H), 0.85 (s, 6H); 13C NMR: (CDCCl3, 500 MHz): δ 170.92, 169.56, 80.56, 79.06, 76.58, 73.90, 73.29, 55.86, 52.84, 52.72, 49.95, 44.91, 39.73, 38.49, 37.86, 37.06, 34.45, 32.77, 31.56, 28.26, 27.96, 27.27, 26.66, 26.46, 25.70, 23.53, 23.19, 21.53, 21.29, 18.09, 17.38, 16.46, 16.24, 15.56;
Compound 4
1H NMR: (CDCCl3, 500 MHz): δ 3.57(m, 1H), 3.32(d, J=1.5 Hz, 1H), 2.70 (dd, J=1.5, 11.0 Hz, 1H), 2.08 (m, 3H), 1.20 (s, 3H), 1.19 (s, 3H), 1.18 (s, 3H), 1.04 (s, 3H), 0.98 (s, 3H), 0.95 (s, 3H), 0.80 (s, 3H); 13C NMR: (CDCCl3, 500 MHz): δ 79.22, 78.12, 73.22, 72.56, 70.75, 55.90, 53.95, 51.23, 50.01, 48.13, 47.11, 39.58, 38.89, 38.64, 36.82, 34.58, 32.12, 30.63, 27.26, 26.62, 26.01, 25.27, 24.86, 24.02, 23.89, 18.04, 15.77, 15.38, 14.83, 14.75;
Compound 5
1H NMR: (CDCCl3, 500 MHz): δ 4.72 (m, 1H), 4.65 (dd, J=10.0, 2.0 Hz, 1H), 4.47 (dd, J=11.5, 4.5 Hz, 1H), 3.36 (s, 1H), 3.13 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H), 1.30 (s, 3H), 1.26 (s, 3H), 1.11 (s, 3H), 1.03 (s, 3H), 0.95 (s, 3H), 0.84 (s, 3H); 13C NMR: (CDCCl3, 500 MHz): δ 170.89, 169.46, 91.66, 80.58, 76.67, 73.57, 72.65, 55.92, 52.88, 52.41, 50.14, 44.82, 39.75, 38.80, 37.08, 34.43, 32.77, 31.82, 28.28, 27.95, 27.77, 27.36, 26.11, 24.60, 23.58, 23.54, 21.50, 21.28, 18.08, 17.44, 16.44, 16.27, 15.29;
Compound 6
1H NMR: (CDCCl3, 500 MHz): δ 3.85 (dd, J=7.0, 9.0 Hz, 1H), 3.52 (m, 1H), 3.19 (dd, J=4.5, 10.5 Hz, 1H), 2.19 (m, 1H), 2.02 (m, 1H), 1.88 (m, 3H), 1.39–1.72 (mm, 9H), 1.29 (s, 3H), 1.27 (s, 3H), 1.11 (s, 3H), 0.99 (s, 1H), 0.97 (s, 3H), 0.91 (s, 3H), 0.86 (s, 3H), 0.73 (d, J=9.0, 1H); 13C NMR: (CDCCl3, 500 MHz): δ 86.52, 85.43, 78.86, 71.01, 70.10, 56.01, 52.04, 50.53, 49.43, 47.98, 39.75, 38.97, 38.94, 37.16, 34.84, 32.63, 31.35, 31.23, 28.61, 28.00, 27.94, 27.61, 27.46, 26.14, 25.03, 18.30, 18.18, 16.33, 15.39, 15.28;
Compound 7
1H NMR: (CDCCl3, 500 MHz): δ 4.34(dd, J=5.5, 11.0 Hz, 1H), 3.72(dd, J=6.5, 9.0 Hz), 3.39 (m, 1H), 2.06 (m, 1H), 1.92 (s, 3H), 1.16–1.80 (mm, 16H), 1.15 (s, 3H), 1.14 (s, 3H), 0.97 (s, 3H), 0.86 (s, 3H), 0.77 (s, 3H), 0.75 (s, 3H), 0.72 s, 6H); 13C NMR: (CDCCl3, 500 MHz): δ 170.53, 86.07, 84.98, 80.34, 70.51, 69.65, 55.63, 51.57, 49.99, 48.96, 47.52, 39.33, 38.21, 37.41, 36.64, 34.33, 32.17, 30.89, 30.76, 28.15, 27.50, 27.47, 27.16, 25.70, 24.56, 23.25, 20.86, 17.73, 17.71, 15.96, 14.95;
Compound 8
1H NMR: (CDCCl3, 500 MHz): δ 4.47 (dd, J=5.0, 6.5 Hz, 1H), 2.90 (d, J=9.5 Hz, 1H), 2.61 (m, 1H), 2.21 (m, 3H), 2.05 (s, 3H), 1.39–1.93 (mm, 15H), 1.25 (s, 3H), 1.21 (s, 3H), 0.96 (s, 3H), 0.88 (s, 3H), 0.87 (s, 3H), 0.77 (s, 3H); 13C NMR: (CDCCl3, 500 MHz): δ 210.50, 176.96, 170.86, 88.73, 80.30, 56.81, 55.96, 55.79, 54.12, 42.72, 40.41, 39.58, 38.25, 37.86, 37.54, 34.09, 32.45, 31.53, 28.95, 27.95, 24.89, 24.27, 23.45, 21.26, 18.21, 16.41, 16.11, 15.75;
Compound 9
1H NMR: (CDCCl3, 500 MHz): δ 4.89 (m, 1H), 4.50 (d, J=5.0 Hz, 1H), 2.59 (m, 2H), 2.34 (m, 1H), 2.14 (s, 3H), 2.08 (s, 3H), 1.40 (s, 1H), 1.01 (s, 3H), 0.98 (s, 3H), 0.87 (s, 3H), 0.85(s, 6H); 13C NMR: (CDCCl3, 500 MHz): δ 176.03, 170.44, 170.42, 89.11, 80.08, 74.05, 55.38, 52.03, 49.30, 48.70, 46.38, 39.16, 38.05, 37.43, 36.59, 33.94, 32.03, 30.91, 28.65, 27.52, 25.99, 23.80, 23.11, 21.45, 20.84, 17.65, 17.16, 16.04, 15.67, 15.05;
Antiproliferative effects of PPD derivatives on three human cancer cell lines
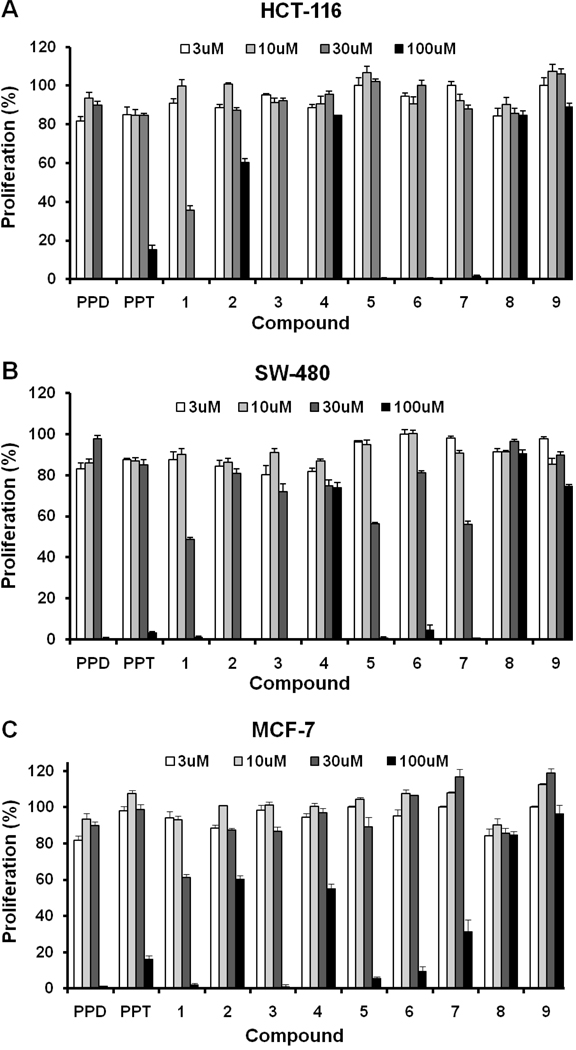
After treatment with PPD, PPT, or Compounds 1–9 for 48 h, the proliferation of HCT-116, SW-480 and MCF-7 cells was markedly suppressed after exposure to Compounds 1, 3, and PPD dose dependently between concentrations of 30 µM and 100 µM (Fig. 3). In the HCT-116 cell line, the IC50 was more than 60 µM for PPD, 57.8 µM for Compound 3, 54.7 µM for Compound 5, and 43.3 µM for Compound 1, while it was more than 60 µM for all other compounds. In the SW-480 cell line, the IC50 was 59.6 µM for Compound 3 and 30.0 µM for Compound 1, while it was more than 60 µM for all of the other compounds. In the MCF-7 cell line, the IC50 was 54.7 µM for of Compound 3 and 37.3 µM for Compound 1, while it was more than 60 µM for all of the other compounds. The leading compound PPD showed an antiproliferative effect at 100 µM, but no effect at 30 µM. Compound 1 significantly suppressed the proliferation of all three cell lines at 30 µM (P<0.01). Although the antiproliferative effect of Compounds 3 and 5 was less than the effect of Compound 1, the compounds had a significantly stronger antiproliferative effect than PPD (Fig. 3).
Figure 3.
Antiproliferative effects of PPD, PPT, and Compounds 1–9 on human cancer cell lines HCT-116, SW-480, and MCF-7. Cells were treated with 3, 10, 30, and 100 µM of compound for 48 h.
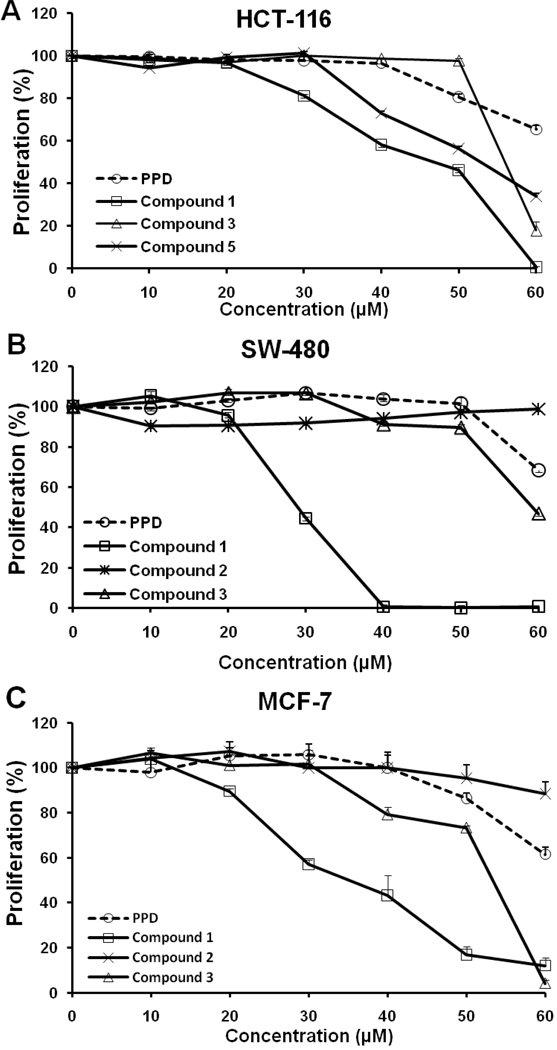
Based on structure and biological activity, we selected PPD and Compounds 1, 2, 3, and 5 for further testing on different cancer cell lines in the dose-sensitive range. The antiproliferative effects of PPD and Compounds 1, 2, 3, and 5 in the three cell lines at 10, 20, 30, 40, 50, or 60 µM after treatment for 48 h are shown in Fig. 4. For the cell line SW-480, Compound 1 inhibited growth significantly at 40 µM (P<0.01) and for the HCT-116 and the MCF-7 cell lines, its antiproliferative activity was most potent (Fig. 3 and Fig. 4).
Figure 4.
Antiproliferative effects of PPD and Compounds 1, 2, 3, and 5 on human cancer cell lines HCT-116, SW-480, and MCF-7. Cells were treated with 10, 20, 30, 40, 50, and 60 µM of compound for 48 h.
In addition, we also selected Compound 1 to evaluate its effects on normal human colon epithelial cells (CRL-1831). At a concentration of 20 µM, the proliferation of the CRL-1831cells was 96.0%, while it was 81.9% for the HCT-116 cells (P<0.01). At a concentration of 40 µM, the proliferation of the CRL-1831 cells was 80.9%, while it was 58.7% for the HCT-116 cells (P<0.01). Thus, compared to normal colon cells, Compound 1 has a significant cytotoxic effect only on cancer cells.
Effects of PPD derivatives on cell cycle distribution in HCT-116 cells
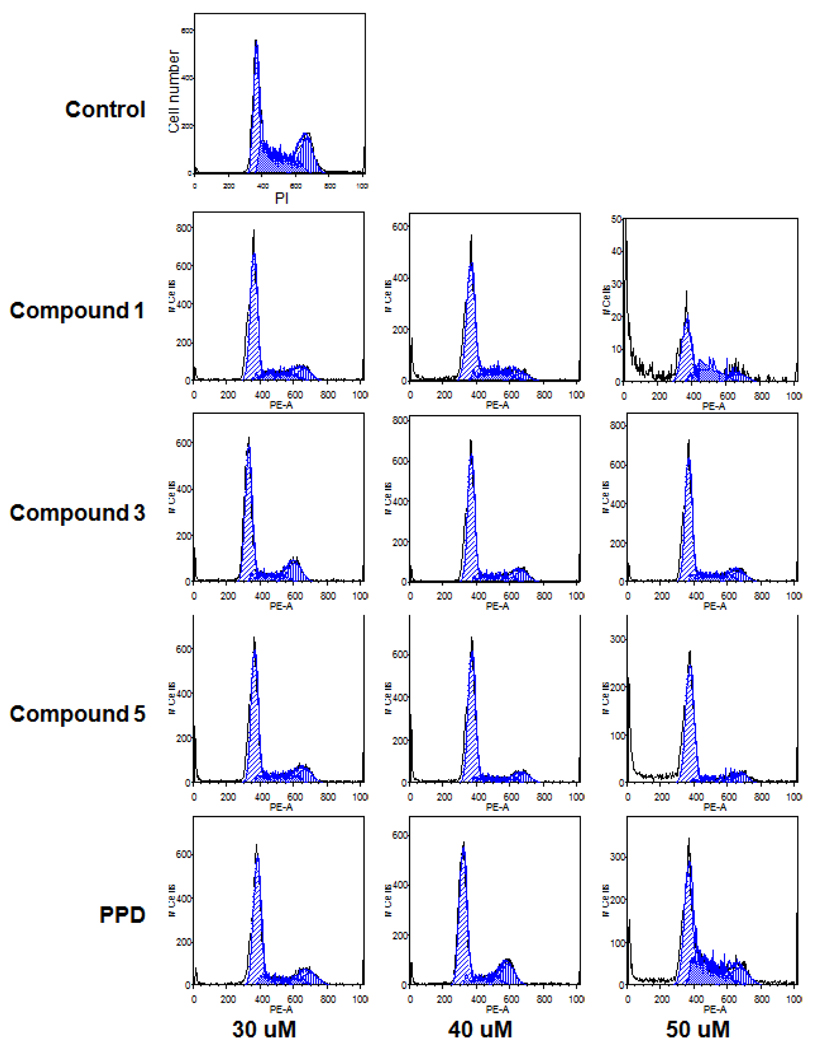
Since PPD and its derivatives had an antiproliferative effect on cancer cells, we explored the potential mechanisms through which cell growth was inhibited. The cell cycle profile was assayed by flow cytometry after staining with PI, and the assay data from PPD derivatives and PPD were compared. As shown in Fig. 5, compared to the control (33.3% of G1 phase, 33.7% of S phase, and 26% of G2/M phase) treatment with 30–40 µM PPD did not change the cell cycle profile of HCT-116 cells. At a 50 µM concentration of PPD, the fractions of cells in different cell cycle phases were 41.5% (G1 phase), 29.5% (S phase), and 14.5% (G2/M phase). With the treatment of 50 µM of Compound 3, the cell cycle profile was 65.3% (G1 phase), 19.6% (S phase), and 12.7% (G2/M phase). For the treatment with 40 µM of Compound 5, the cell cycle profile was 68.9% (G1 phase), 12.3% (S phase), and 10.7 (G2/M phase). For the treatment with 40 µM of Compound 1, the cell cycle profile was 63.2% (G1 phase), 22.2% (S phase), and 8.2% (G2/M phase). These results suggest that PPD and its derivatives arrest HCT-116 cells in the G1-phase.
Figure 5.
Effects of PPD and Compounds 1, 3, and 5 on the cell cycle. After treatment with compound for 48 h, the HCT-116 cells were stained with PI and assayed using flow cytometry.
Apoptotic effects of PPD derivatives on HCT-116 cells
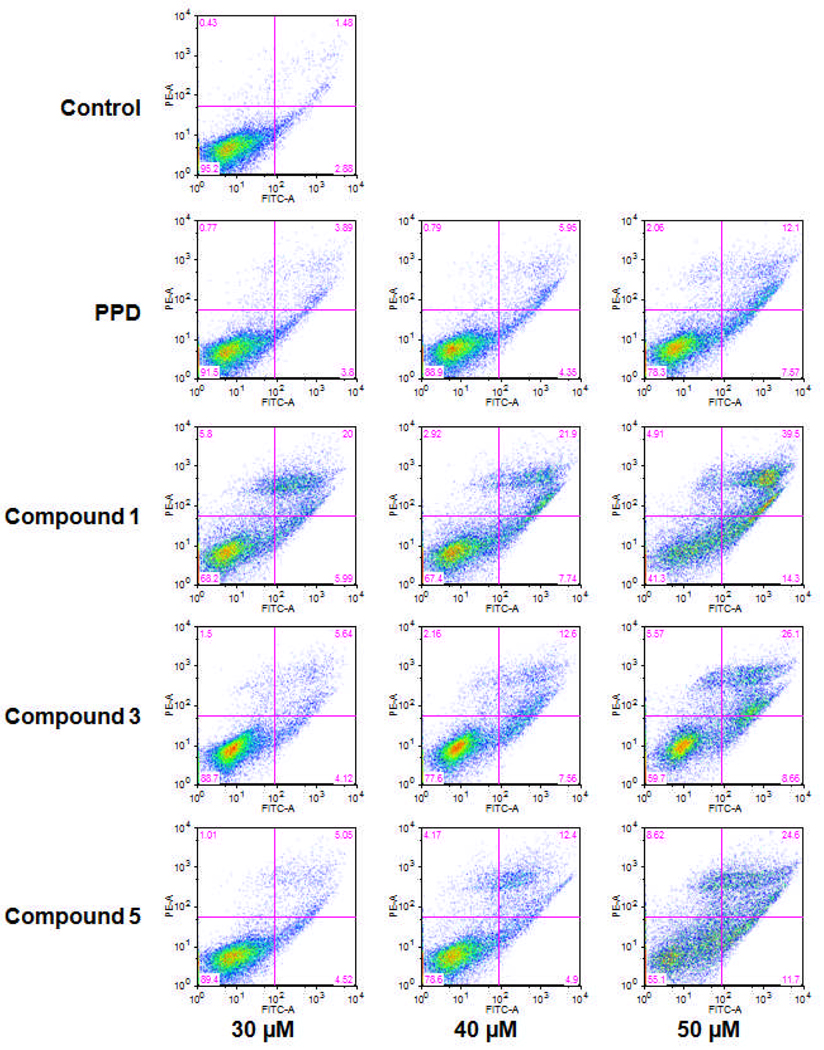
After treatment with PPD derivatives, especially Compound 1, the viable cell proportions decreased in a dose-dependent manner. In the cell cycle assay, we also observed that the number of viable cells decreased as the concentration of PPD derivatives increased. To further characterize the potential mechanism of the anticancer effect of PPD derivatives, we performed an apoptotic assay by flow cytometry after staining with annexin V and PI. Annexin V can be detected in both the early and late stages of apoptosis. PI enters the cell in late apoptosis or necrosis. Viable cells were negative for both annexin V and PI (lower left quadrant); early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant); late apoptotic or necrotic cells displayed both positive annexin V and PI (upper right quadrant); non-viable cells which underwent necrosis were positive for PI and negative for annexin V (upper left quadrant). As an intermediate between Compounds 1 and 3 (Fig. 2), Compound 2 did not induce apoptosis. Compared to the control, it was shown that PPD induced cell apoptosis in HCT-116 cells. Interestingly, Compounds 1, 3, and 5 were more potent for inducing apoptosis than PPD. After treatment for 48 h, the percentage of apoptotic cells induced by Compound 1 at 30, 40, and 50 µM was 26.0%, 29.6%, and 53.8%, respectively; by Compound 3, the percentages were 9.8%, 20.2%, and 34.8%, respectively; by Compound 5, the percentages were 9.6%, 17.3%, and 36.3%, respectively, compared to PPD, which was 19.7% at a concentration of 50 µM (Fig. 6). These results suggested that the antiproliferative effect of PPD derivatives could be mediated by the induction of apoptosis.
Figure 6.
Apoptosis assay using flow cytometry after annexin V/PI staining. HCT-116 cells were treated with 30, 40, 50 µM of Compounds 1, 3, and 5 for 48 h.
Discussion
Although botanicals are bioactive, structural modification may be necessary to obtain effective drugs from them. Through synthetic chemistry modifications, structural diversity is possible. Structure optimization frequently entails modification, removal, or introduction of functional groups to improve bioactivities [13].
Data has shown that one of the health benefits of ginseng root is its potential for anticancer activities [14–16]. In an epidemiological study of over a thousand subjects in Korea, those who used Asian ginseng were at a decreased risk for cancers compared to those who did not [17–18]. However, the preventive effect against cancer was not organ specific [18].
The bioactive constituents in Asian ginseng and American ginseng are dammarane saponins, commonly referred to as ginsenosides. Shibata et al. performed the first isolation and structural characterization studies on ginsenosides and their corresponding sapogenins [19–20]. The major ginsenosides can be divided into two classes on the basis of the aglycons, i.e., a PPD group and a PPT group. The structural difference between the two lies on the R2 group (Fig. 1). The total assignment of 1H-NMR spectra on these two compounds was demonstrated recently [21].
In previous cancer chemoprevention studies using ginseng constituents, the aglycon of ginsenosides had a stronger antiproliferative effect than the ginsenosides [22]. Compared to PPT, PPD showed more potent anticancer activity [23]. We hypothesized that the anticancer potential of PPD could be enhanced by structural modification. To synthesize PPD derivatives, we prepared the leading compound PPD by hydrolyzing ginsenosides. Taking PPD as the leading compound, we synthesized a series of 9 compounds, most with acetyl substitutions (Fig. 2). The reaction conditions were moderate and the synthesis rates were reasonable. Among the 9 compounds, 5 (Compounds 5–9) are novel.
We determined the antiproliferative effects of all the PPD derivatives on three human cancer cell lines. In our in vitro bioassay, PPD and its derivatives had an antiproliferative effect on three cancer cell lines. Compounds 1, 3, and 5 showed significantly higher activity than the other compounds (Fig. 3 and Fig. 4). Previous studies reported anticancer activities and structure–activity relationships of ginsenosides [24]. However, no pharmacological evaluation was performed using semi-synthesis compounds of ginsenoside derivatives, especially PPT derivatives. Our study was the first to evaluate the anticancer effects of a series of novel synthesized PPD derivatives in addition to Compound 1.
Although the mechanism by which ginsenosides and aglycons exert their inhibitive activity on cancer cell growth is largely unknown, several mechanisms are possible: antioxidant properties, regulation of carcinogen metabolism, anti-inflammatory properties cell cycle regulation, and induction of apoptosis [2; 25–26]. Among these mechanisms, cell cycle regulation and apoptosis are important pathways for the inhibition of cancer cells by many anticancer agents. According to our preliminary morphological observations, the inhibitory effects of PPD and its derivatives may not be due to a direct killing of the cancer cells. Rather, they may be due to cell cycle regulation or induction of apoptosis.
With the cell cycle and apoptotic assays we showed that PPD derivatives arrested cancer cells in the G1-phase. Compounds 1, 3, and 5 reduced the fraction of cells in the G2/M-phase. After staining with annexin V/PI, apoptosis of HCT-116 cells by PPD derivatives was also evaluated. Compounds 1, 3, and 5 induced apoptosis at concentrations of 40–50 µM for 48 h, the percentage of apoptotic cells was over 30%, and the ratio of early to late apoptosis was approximately 1:3. The antiproliferative effect of PPD derivatives was mediated by cell cycle arrest and the induction of apoptosis.
Based on the chemical structures and observed biological activities, we explored the structure-activity relationships of these compounds. First, compared to PPT, PPD exerted stronger antiproliferative activity. Hydroxylation at the C6 position negatively influenced its antiproliferative potential. Second, structural changes at the C23 did not significantly improve bioactivity. Compounds 6 and 7 both have structure modification at this position, and they also showed improved activity but less than Compounds 1 and 3. Additionally, this data showed that the acid group would reduce anticancer activity, while a substituent at this position with an epoxy group and hydroxyl group did not affect the activity of Compound 1. When comparing Compounds 1, 2, and 3, it is shown that not only the modification at the C23 position, but also acetylation at C12 position would provide negative effects to the activity (Fig. 2). Finally, since Compound 1 had the strongest activity, future work should focus on structural modifications at the C3 position while keeping the hydroxyl group at C12.
In this study, we also evaluated cytotoxic effects of the test compounds on normal human colon cells. We selected Compound 1, since it has the most potent antiproliferative effect. We observed that after treating with Compound 1, the growth inhibition was significantly higher in cancer cells compared to the normal cells, suggesting that our test compounds have selective cytotoxic effects on cancer cells. This toxicity evaluation is consistent with previously reported studies [27].
In summary, we prepared 9 PPD derivatives using semi-synthetic method. Of these 9 compounds, 5 compounds, i.e., Compounds 5–9, are novel. The antiproliferative activities of all 9 compounds on different human cancer cell lines were evaluated. Compounds 1 and 3 had significant antiproliferative and apoptotic induction activities compared to PPD and other derivatives. Among the 5 novel compounds, Compound 5 possesses the best anticancer potential.
Acknowledgements
This work was supported in part by the NIH/NCCAM grants P01 AT004418, R21 AT003255, and K01 AT005362.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Society AC. Cancer Facts and Figures 2009. Atlanta, GA: American Cancer society; 2010. [Google Scholar]
- 2.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 3.Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efferth T, Fu YJ, Zu YG, Schwarz G, Konkimalla VSB, Wink M. Molecular target-guided tumor therapy with natural products derived from traditional Chinese medicine. Curr Med Chem. 2007;14:2024–2032. doi: 10.2174/092986707781368441. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 6.Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286:208–216. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 7.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 8.Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, et al. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 9.Xie JT, Wang CZ, Li XL, Ni M, Fishbein A, Yuan CS. Anti-diabetic effect of American ginseng may not be linked to antioxidant activity: comparison between American ginseng and Scutellaria baicalensis using an ob/ob mice model. Fitoterapia. 2009;80:306–311. doi: 10.1016/j.fitote.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim TH, Lee YS, Cho CK, Park S, Choi SY, Yool SY. Protective effect of ginseng on radiation-induced DNA double strand breaks and repair in murine lymphocytes. Cancer Biother Radiopharm. 1996;11:267–272. doi: 10.1089/cbr.1996.11.267. [DOI] [PubMed] [Google Scholar]
- 11.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Seo GS, Ko G, Kim JB, Sohn DH. Anti-inflammatory activity of 20(S)-protopanaxadiol: enhanced heme oxygenase 1 expression in RAW 264.7 cells. Planta Med. 2005;71:1167–1170. doi: 10.1055/s-2005-873147. [DOI] [PubMed] [Google Scholar]
- 13.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 14.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang CZ, Aung HH, Zhang B, Sun S, Li XL, He H, et al. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008;28:2545–2551. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev. 1995;4:401–408. [PubMed] [Google Scholar]
- 18.Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol. 1998;27:359–364. doi: 10.1093/ije/27.3.359. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S, Tanaka O, Ando T, Sado M, Tsushima S, Ohsawa T. Chemical studies on oriental plant drugs. XIV. Protopanaxadiol, a genuine sapogenin of ginseng saponins. Chem Pharm Bull (Tokyo) 1966;14:595–600. doi: 10.1248/cpb.14.595. [DOI] [PubMed] [Google Scholar]
- 20.Nagai OTaSS Y. Chemical studies on the oriental plant drugs—XXIV : Structure of ginsenoside-Rg1, a neutral saponin of ginseng root. Tetrahedron. 1971;27:12. [Google Scholar]
- 21.Usami Y, Liu YN, Lin AS, Shibano M, Akiyama T, Itokawa H, et al. Antitumor agents. 261. 20(S)-protopanaxadiol and 20(s)-protopanaxatriol as antiangiogenic agents and total assignment of (1)H NMR spectra. J Nat Prod. 2008;71:478–481. doi: 10.1021/np070613q. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, Zhao L, Qu C, Meng X, Zhang H. Microwave degradation of floatation-enriched ginsenoside extract from Panax quinquefolium L. leaf. J Agric Food Chem. 2009;57:10252–10260. doi: 10.1021/jf902153a. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Rayburn ER, Zhao Y, Wang H, Zhang R. Novel ginsenosides 25-OH-PPD and 25-OCH3-PPD as experimental therapy for pancreatic cancer: anticancer activity and mechanisms of action. Cancer Lett. 2009;278:241–248. doi: 10.1016/j.canlet.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 25.Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo HN, Jeong HJ, Choi IY, An HJ, Moon PD, Kim SJ, et al. Mountain grown ginseng induces apoptosis in HL-60 cells and its mechanism have little relation with TNF-alpha production. Am J Chin Med. 2007;35:169–182. doi: 10.1142/S0192415X07004710. [DOI] [PubMed] [Google Scholar]
- 27.Ho YS, Wu CH, Chou HM, Wang YJ, Tseng H, Chen CH, et al. Molecular mechanisms of econazole-induced toxicity on human colon cancer cells: G0/G1 cell cycle arrest and caspase 8-independent apoptotic signaling pathways. Food Chem Toxicol. 2005;43:1483–1495. doi: 10.1016/j.fct.2005.04.002. [DOI] [PubMed] [Google Scholar]