Abstract
Stromal derived factor-1 (SDF-1) is a chemokine signaling molecule that binds to its transmembrane receptor CXC chemokine receptor-4 (CXCR4). While we previously detected that SDF-1 was co-required with bone morphogenetic protein 2 (BMP2) for differentiating mesenchymal C2C12 cells into osteoblastic cells, it is unknown whether SDF-1 is similarly involved in the osteogenic differentiation of mesenchymal stem cells (MSCs). Therefore, here we examined the role of SDF-1 signaling during BMP2-induced osteogenic differentiation of primary MSCs that were derived from human and mouse bone marrow. Our data showed that blocking of the SDF-1/CXCR4 signal axis or adding SDF-1 protein to MSCs significantly affected BMP2-induced alkaline phosphatase (ALP) activity and osteocalcin (OCN) synthesis, markers of preosteoblasts and mature osteoblasts, respectively. Moreover, disrupting the SDF-1 signaling impaired bone nodule mineralization during terminal differentiation of MSCs. Furthermore, we detected that blocking of the SDF-1 signaling inhibited the BMP2-induced early expression of Runt-related factor-2 (Runx2) and osterix (Osx), two “master” regulators of osteogenesis, and the SDF-1 effect was mediated via intracellular Smad and Erk activation. In conclusion, our results demonstrated a regulatory role of SDF-1 in BMP2-induced osteogenic differentiation of MSCs, as perturbing the SDF-1 signaling affected the differentiation of MSCs towards osteoblastic cells in response to BMP2 stimulation. These data provide novel insights into molecular mechanisms underlying MSC osteogenesis, and will contribute to the development of MSC therapies for enhancing bone formation and regeneration in broad orthopaedic situations.
Keywords: Bone morphogenetic protein 2, CXC chemokine receptor-4, Mesenchymal stem cell, Osteogenic differentiation, Stromal derived factor-1
1. Introduction
Non-union and delayed healing are major complications in orthopaedic patients who receive spine fusion or fracture fixation. As one of the novel biological means to enhance bone formation, mesenchymal stem cells (MSCs) are receiving attentions, because these cells, under osteoinductive conditions, will give rise to cells of osteoblast lineages and therefore form bone. Although a number of experimental and clinical studies have attempted to repair or regenerate bones with MSCs (Bruder et al., 1998; Kørbling and Estrov, 2003; Marcacci et al., 2007), comprehensive molecular mechanisms that control MSC osteogenic differentiation have not been fully elucidated.
Stromal derived factor-1 (SDF-1, also named CXC chemokine ligand-12 (CXCL12)) is a member of the CXC chemokine family (Shirozu et al., 1995; Zlotnik and Yoshie, 2000), with several isoforms (SDF-1α, -β, -γ, -δ, -ε, and -ϕ) that vary in the number of amino acid extensions at the carboxyl (C) terminus (Yu et al., 2006). SDF-1 signals through its G-protein-coupled trans-membrane receptor CXC chemokine receptor-4 (CXCR4) (D’Apuzzo et al., 1997; Zlotnik and Yoshie, 2000); and a second receptor, CXCR7, was recently identified in several types of cells (Hartmann et al., 2008; Levoye et al., 2009). Both SDF-1 and CXCR4 are widely expressed in many types of tissues, and are essential for embryonic organ development, since mice lacking either of them die in uterus or perinatally with severe defects in developing nervous, haematopoietic and cardiovascular systems (Ma et al., 1998; Nagasawa et al., 1996; Zou et al., 1998). SDF-1 signaling is also important for maintaining postnatal tissue homeostasis, such as cellular inflammatory and immune response (Aiuti et al., 1999), blood homeostasis (Baggiolini, 1998), and bone remodeling (Yu et al., 2003). At the level of cell function, the binding of SDF-1 to CXCR4 leads to cytoskeleton rearrangements and integrin activation, eventually resulting in the directional migration of CXCR4-expressing cells towards high gradients of SDF-1 (Dar et al., 2006; Gronthos et al., 2001; Peled et al., 2000). This SDF-1/CXCR4-orientated chemotaxis regulates the homing into and retention of hematopoietic stem cells and MSCs within marrow microenvironments, as well as the metastatic colonization of bone and bone marrow by breast and prostate cancer cells (Lataillade et al., 2000; Majka et al., 2000). Increasing evidence suggest that SDF-1 signaling is also required for MSC-mediated tissue repair and regeneration. In injuries of murine brain (Ji et al., 2004), heart (Hill et al., 2004), muscle (Ratajczak et al., 2003), liver (Hatch et al., 2002), kidney (Tögel et al., 2005) and skin (Ceradini et al., 2004), and during bone healing (Kitaori et al., 2009; Otsuru et al., 2008), up-regulation of SDF-1 at injury sites is thought to serve as a potent chemoattractant to recruit circulating or residing CXCR4-expressing stem/progenitor cells. The interactions between SDF-1 and CXCR4 are responsible for maintaining the cellular growth and survival of those stem/progenitor cells until a tissue-specific differentiation is elicited by local environmental stimuli.
One group of stimuli known to induce MSC or progenitors to undergo osteogenic differentiation and therefore promote bone formation are several members of the bone morphogenetic protein (BMP) family, such as BMP2, −4, −6, −7, and −9 (Cei et al., 2006; Chen et al., 2004; Fujii et al., 1999; Gu et al., 2004; Katagiri et al., 1994; Yeh et al., 2002; Zachos et al., 2006; Zhu et al., 2006). BMP binds to its serine/threonine kinase receptors and activates intracellular receptor-regulated Smad proteins (R-Smads, Smad1/5/8) and the mitogen-activated protein kinase (MAPK) components Erk1/2, which subsequently transmit the BMP signal to the nucleus where the transcription of osteoblast genes is regulated (Chen et al., 2004; Fujii et al., 1999). Previously, in C2C12 cells, we reported that blocking of the SDF-1 signaling inhibited the progression of these cells towards osteoblastic cells in response to BMP2 stimulation (Zhu et al., 2007), suggesting a role for SDF-1 in BMP2-induced osteogenic differentiation of mesenchymal precursor cells. Based on this data, we further hypothesized that SDF-1 signaling was required for osteogenic differentiation of MSCs. We therefore tested the role of SDF-1 during the BMP2-induced differentiation of primary MSCs originating from human and mouse bone marrow, which retain some complexities of in vivo environments for bone generation. Our results suggested that SDF-1 regulated BMP2-induced differentiation of primary MSCs into osteoblastic cells, and that the regulatory effect of SDF-1 was mediated via intracellular Smad and Erk activation.
2. Material and methods
2.1. Reagents and antibodies
Recombinant (r) BMP2 protein, rSDF-1 protein and anti-SDF-1 neutralizing antibody were purchased from R&D Systems (Minneapolis, MN). Anti-CXCR4 neutralizing antibody (clone 12G5) was purchased from BD PharMingen (San Jose, CA), while anti-CXCR4 antibody for Western blotting detection was obtained from eBioscience (San Diego, CA). The CXCR4 antagonist AMD3100 and all other chemicals were purchased from Sigma (St. Louis, MO). Anti-Smad1/5/8, anti-phosphorylated Smad1/5, anti-total Erk, anti-phosphorylated Erk1/2, and anti-β-tubulin antibodies were purchased from Cell Signaling (Danvers, MA). The secondary goat and rabbit Igs were obtained from Santa Cruz (Santa Cruz, CA). All cell culture media and supplements were from Gibco (Invitrogen, Carlsbad, CA).
2.2. Animals
CXCR4flox/flox homozygous mice, in which the CXCR4 gene is flanked by loxP sequences (Zou et al., 1998), were kindly provided by Yong-Rui Zou PhD(Columbia University,NewYork, NY). C57BL/6 wild-type mice were purchased from Jackson Laboratory (Bar Harbor, MN). Both male and female mice at 6–8 weeks old were used for bone marrow collection.
2.3. Primary mouse and human MSC cultures
Following a protocol approved by the Institutional Animal Care and Utilization Committee (IACUC) at Hospital for Special Surgery (HSS), mouse bone marrow were obtained from tibia and femora of CXCR4flox/flox mice or C57BL/6 wild-type mice by flushing the marrow cavity using a 23G needle attached to a syringe filled with α-MEM. Marrow stromal stem cells were washed twice with α-MEM plus 1% penicillin–streptomycin, and then plated in α-MEM supplemented with 20% FBS and 1% penicillin-streptomycin at a density of ~2 × 107 cells per 100mm culture dish.
Human bone marrow was collected from iliac crests of patients who underwent spine fusion surgery, based on a protocol approved by the Institutional Review Board (IRB) at HSS. Mononucleated cells were isolated from hematopoietic cells or blood-forming cells by Ficoll density centrifugation separation following the manufacturer’s protocol (Amersham Biosciences, Piscataway, NJ), and plated at ~2 × 107 cells per 100mm culture dish in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. For both mouse and human cultures, the non-adherent cells were removed after 48–72 h, and adherent cells (MSCs) were replenished with fresh medium every 2–3 days until confluence. Cells in passage ≤ 3 were used in the experiments below.
2.4. Perturbing SDF-1 signaling in MSCs
To block the SDF-1 signaling in primary MSCs, cells were incubated for 2 h at 37 °C with either anti-SDF-1 or anti-CXCR4 neutralizing antibody, which recognize a specific epitope on the protein domain of SDF-1 or CXCR4, respectively (Baribaud et al., 2001; Bleul et al., 1996; Shirozu et al., 1995; Strizki et al., 1997). In other experiments, cells were incubated with the CXCR4 antagonist AMD3100, which selectively binds to CXCR4 and therefore prevents CXCR4 from binding to SDF-1 in a concentration-dependent manner (Donzella et al., 1998; Hatse et al., 2005; Schols et al., 1997). Anti-SDF-1 was used at 100 µg/ml, anti-CXCR4 12G5 was used at concentrations ranging from 10 to 100 ng/ml, and AMD3100 was used at concentrations ranging from 50 to 400 µM. These concentrations have been shown to effectively inhibit the SDF-1 signaling without cellular toxicity (Baribaud et al., 2001; Hatse et al., 2005; Kortesidis et al., 2005; Ratajczak et al., 2003; Zhu et al., 2007).
To enhance the SDF-1 signaling in MSCs, cells were incubated at 37 °C with rSDF-1 at 300 ng/ml for 2 h (Kortesidis et al., 2005; Ratajczak et al., 2003; Zhu et al., 2007).
2.5. Adenovirus transfection
To decrease the CXCR4 expression in MSCs, MSCs derived from bone marrow of CXCR4flox/flox mice were incubated with adenovirus (Ad) vectors carrying a gene encoding for Cre recombinase fused with green fluorescent protein (GFP) (AdCre/GFP, Vector Development Laboratory, Houston, TX) in serum-free medium for ~4 h at 37 °C. The concentrations of Ad transfection ranged from 1 × 103 to 1 × 104 viral particles per cell, as suggested by the manufacturer. After rinsing with phosphate buffered saline (PBS), Ad-transfected cells were further cultured in serum-containing full medium until 70–80% of cells were observed GFP positive under a fluorescent microscope (Nikon TE2000, Melville, NY).
2.6. Quantitative real time PCR
Total RNA was extracted from cells using RNeasy Minikit (Qiagen, Valencia, CA). One microgram of total RNA was reverse transcribed (Applied Biosystems, Foster City, CA), and quantitative real time PCR was performed in triplicate using iQ Syber-Green Supermix and an iCycler iQ thermal cycler following the manufacturer’s protocols (all from Bio-Rad, Hercules, CA). Expression of CXCR4 in AdCre/GFP-transfected mouse MSCs, expression of Runt-related factor-2 (Runx2) and osterix (Osx) in BMP2-stimulated mouse and human MSCs were detected using previously published primer sequences (Zhu et al., 2007), and were normalized relative to levels of glyceraldehydes phosphate dehydrogenase (GAPDH).
2.7. Western blotting
After Ad transfection or BMP2 stimulation, whole cell lysates were obtained as described previously (Zhu et al., 2007), and the amount of total cellular protein was determined using bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). To detect the level of CXCR4 protein relative to β-tubulin, or the phosphorylated intracellular Smad1/5 and Erk1/2 compared with total Smad and Erk proteins, equal loading of 20 µg aliquots of total protein from each sample were fractionated on 12% Bis–Tris gels following manufacturer’s protocol (Invitrogen). After incubating with appropriate antibodies, immunoreactive bands were visualized with enhanced chemiluminescence (ECL) detection reagents (Amersham Biosciences) on autoradiography films (Denville, Metuchen, NJ), as described previously (Zhu et al., 2007). The intensity of immunoreactive bands was quantified by gel image analysis software (ImageJ_1.32J, NIH).
2.8. Alkaline phosphatase (ALP) activity and osteocalcin (OCN) synthesis assays
MSCs were stimulated with BMP2 at 100 ng/ml to induce osteogenic differentiation, and ALP activity and OCN synthesis were evaluated as markers for preosteoblasts and mature osteoblasts, respectively (Kortesidis et al., 2005; Zhu et al., 2007). ALP activity was measured in cell lysates 5–7 days after BMP2 stimulation, using a colorimetric assay (Sigma) and normalized to the level of total cellular protein (Pierce). OCN synthesized by MSCs was measured in culture medium 10–12 days after BMP2 stimulation, using a commercially available ELISA kit (Biomedical Technologies, Stoughton, MA). To detect the BMP2 stimulation of ALP and OCN, cells were maintained in culture medium with reduced serum (2–3%) to lower cell proliferation and serum-associated influences on differentiation (Zhu et al., 2007).
2.9. Calcium deposition assay
A proportion of MSCs were maintained in culture medium supplemented with 25 µg/ml ascorbic acid, 5 mM β-glycerophosphate (all from Sigma) and 100 ng/ml rBMP2. After 2 weeks of BMP2 stimulation, cultured cells were rinsed briefly with PBS and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. To detect the mineralization of bone nodules in culture, cells were stained with 2% (wt/vol, pH 4.1–4.3) Alizarin red S dye solution (Sigma) for 10–30 min at room temperature until the red color was fully developed. Excessive dyes were washed away with distilled water, and color intensities of stained cells were quantified by Bioquant OsteoII software (Bioquant, Nashville, TN).
3. Statistical analysis
Experiments were repeated two to three times independently, with four to six samples included in each experimental group. Measurements in each experiment were run in triplicate. Results were reported as the mean ± standard deviation (S.D.). Differences between two treatment groups were compared by unpaired Student’s t-test, with significance accepted at p < 0.05.
4. Results
4.1. Lowering CXCR4 expression in MSCs decreased the ALP activity induced by BMP2
As an initial step to determine whether SDF-1 signaling is directly involved in osteogenic differentiation of MSCs, we derived MSCs from bone marrow of CXCR4flox/flox mice, and transfected these cells with AdCre/GFP followed by BMP2 stimulation. The differentiation of MSCs towards osteoblastic cells was monitored by ALP activity, a marker for preosteoblasts.
Approximately 70–80% of cells were GFP positive after 5 days of viral transfection (data not shown). Real time PCR results suggested that AdCre/GFP concentration-dependently decreased CXCR4 mRNA expression (Fig. 1A). When compared to the control cells maintained in culture medium only, a 42% and a 72% decrease in CXCR4 gene were detected in cells transfected with AdCre/GFP at 5 × 103 and 1 × 104 viral particles per cell, respectively (Fig. 1A). At these concentrations of AdCre/GFP transfection, Western blot analysis further confirmed reduced CXCR4 protein levels than that of medium control cells, while levels of the β-tubulin control remained similar in all cells independent of treatment (Fig. 1B).
Fig. 1.
Lowering CXCR4 expression in MSCs decreased the ALP activities induced by BMP2. MSCs that were derived from bone marrow of CXCR4flox/flox mice were pre-transfected with AdCre/GFP at concentrations ranging from 1 × 103 to 1 × 104 viral particles per cell for 5 days, and then followed with BMP2 stimulation at 100 ng/ml for another 5 days. As controls, cells were maintained in culture medium without viral transfection. (A) Real time PCR detection of CXCR4 gene expression normalized to levels of GAPDH. (B) Western blotting detection of CXCR4 protein expression relative to β-tubulin control. (C) Enzymic activity of ALP in cell lysates normalized to the level of total protein. N= 6 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
Subsequently, AdCre/GFP-transfected cells were stimulated with BMP2 for 5 days. While BMP2 stimulation increased ALP activity in control cells maintained in culture medium only, pretreatments with AdCre/GFP at 5 × 103 and 1 × 104 viral particles per cell led to a 36% and a 73% decrease in BMP2-induced ALP activities, respectively, suggesting that lowering the SDF-1 receptor CXCR4 decreased the differentiation of MSCs towards osteoblastic cells in response to BMP2 stimulation (Fig. 1C). Transfection with AdCre/GFP alone did not affect ALP activities over the baseline level in medium control cells (Fig. 1C).
4.2. Perturbing the SDF-1/CXCR4 signal axis affected the BMP2-induced osteogenic differentiation in mouse bone marrow-derived MSCs
To confirm the role of SDF-1 signaling in osteogenic differentiation of MSCs, in addition to lowering the CXCR4 expression in MSCs of CXCR4flox/flox mice, we further tested how perturbing the SDF-1/CXCR4 signal axis in MSCs derived from bone marrow of wild-type mice would affect the BMP2-induced differentiation of these cells towards osteoblastic cells. We measured ALP activity and OCN synthesis, marker proteins for preosteoblasts and mature osteoblasts, respectively, as well as calcium deposition at the terminal stages of differentiation.
Our data showed that stimulation with BMP2 for 5 days significantly increased ALP activity over control cells maintained in culture medium only (Fig. 2A). Pretreatment with anti-SDF-1 neutralizing antibody and CXCR4 antagonist AMD3100 reduced the BMP2-induced ALP activities by 47% and 65%, respectively, while the anti-SDF-1 blockade was reversed by the addition of SDF-1 protein (Fig. 2A). Moreover, adding exogenous SDF-1 protein prior to BMP2 stimulation further enhanced the BMP2-induced ALP activity by 43% in those primary mouse MSCs (Fig. 2A).
Fig. 2.
Perturbing the SDF-1 signaling affected the BMP2-induced ALP activity, OCN synthesis, and calcium deposition in mouse MSCs. MSCs derived from bone marrow of wild-type mice were stimulated with rBMP2 at 100 ng/ml, or as controls, maintained in culture medium only. In parallel, cells were pretreated with anti-SDF-1 neutralizing antibody at 100 µg/ml, CXCR4 antagonist AMD3100 at 400 µM, and/or rSDF-1 protein at 300 ng/ml, for 2 h at 37 °C prior to BMP2 stimulation. Enzymic activity of ALP (A) in cell lysates was measured 5 days after BMP2 stimulation and normalized to the level of total protein. The synthesis of OCN protein (B) was measured in cell medium 10 days after BMP2 stimulation. The calcium deposition of bone nodules in a proportion of cells cultured in medium supplemented with 25 µg/ml of ascorbic acid and 5mM of β-glycerophosphate was detected by Alizarin red staining (C) after 12 days of BMP2 stimulation, the color intensities of stained cells (D) were quantified by BioQuant software and compared to that of medium control cells. Length of scale bar equals to 100 µm. N = 4–6 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
After 10 days of BMP2 stimulation, we detected increased OCN synthesis relative to that of medium control cells (Fig. 2B). However, prior blocking of the SDF-1/CXCR4 signal axis by anti-SDF-1 and AMD3100 decreased the BMP2-increased OCN synthesis by 40% and 52%, respectively, and the anti-SDF-1-decreased OCN level was restored by the addition of SDF-1 protein (Fig. 2B). Moreover, the OCN synthesis induced by BMP2 was increased 47% by pretreatment with exogenous SDF-1 protein (Fig. 2B). Treatments with anti-SDF-1 neutralizing antibody, AMD3100, or SDF-1 protein alone did not affect ALP or OCN levels over control cells maintained in culture medium (data not shown). Collectively, these data suggested that perturbing the SDF-1 signaling in mouse MSCs affected their osteogenic differentiation in response to BMP2 stimulation.
When compared to control cells maintained in culture medium only, BMP2 stimulation increased the Alizarin red staining (Fig. 2C) for calcium by 5.7-fold (Fig. 2D). However, the BMP2-stimulated calcium-staining was reduced 75% by the pretreatment of AMD3100 (Fig. 2C and D). The addition of exogenous SDF-1 protein prior to BMP2 stimulation did not further enhance the level of calcium deposition relative to that of cells stimulated with BMP2 alone (Fig. 2C and D). Alizarin red staining of cells treated with AMD3100 or SDF-1 alone showed no difference relative to that of medium control cells (Fig. 2C and D). Taken together, these data suggested that SDF-1 signaling played a role in regulating the BMP2-induced osteogenic differentiation of primary mouse MSCs.
4.3. Blocking of the SDF-1 signaling inhibited the BMP2-induced osteogenic differentiation in human bone marrow-derived MSCs
To determine whether SDF-1 signaling is involved in osteogenic differentiation of human MSCs as it is in mouse cells, we examined how blocking the SDF-1 receptor CXCR4 would affect ALP activity and OCN synthesis in human bone marrow-derived MSCs after BMP2 stimulation.
In MSCs derived from a 55-year-old female patient, we detected the up-regulation of ALP activity after 7 days of BMP2 stimulation when compared to cells maintained in culture medium (Fig. 3A); pretreatment with CXCR4 antagonist AMD3100 decreased the BMP2-induced ALP activity in a concentration-dependent manner (Fig. 3A). While AMD3100 at 50 µM did not affect the ALP activity induced by BMP2, a 22% decrease was observed with pretreatment of AMD3100 at 100 µM, a 44% decrease was further detected in cells pretreated with AMD3100 at 200 µM, and 400 µM of AMD3100 reduced the BMP2-induced ALP activity to a level comparable to that of medium control cells (Fig. 3A). Similarly, pretreatment with anti-CXCR4 neutralizing antibody 12G5 at 100 ng/ml versus lower concentrations reduced the BMP2-induced ALP activity by 43% (Fig. 3A), further suggesting that blocking CXCR4 inhibited the ALP activity of human MSCs induced by BMP2.
Fig. 3.
Blocking of the CXCR4 inhibited the BMP2-induced ALP activity and OCN synthesis in human bone marrow-derived MSCs. MSCs were derived from a 55-year-old female patient and stimulated with rBMP2 at 100 ng/ml, or as controls, maintained in culture medium only. In parallel, cells were pretreated with CXCR4 antagonist AMD3100 at concentrations ranging from 50 to 400 µM, or anti-CXCR4 neutralizing antibody clone 12G5 at concentrations ranging from 10 to 100 ng/ml, for 2 h at 37 °C prior to BMP2 stimulation. Enzymic activity of ALP (A) in cell lysates was measured 7 days after BMP2 stimulation and normalized to the level of total protein. The synthesis of OCN protein (B) was measured in cell medium 12 days after BMP2 stimulation. N = 4 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
In this patient’s MSCs, we also detected that OCN synthesis induced by BMP2 stimulation for 12 days was decreased 29–49% by pretreatments with AMD3100 from 50 to 200 µM, and was further decreased to a level similar to that of medium control cells by AMD3100 at 400 µM (Fig. 3B). More evidence was shown in cells pretreated with anti-CXCR4 12G5 neutralizing antibodies, which also reduced the BMP2-induced OCN synthesis in a concentration-dependent manner (35%, 43% and 58% decreases by 10, 50 and 100 ng/ml of 12G5, respectively) (Fig. 3B). Treatment with AMD3100 or 12G5 alone did not affect ALP activity or OCN synthesis over medium control cells (data not shown).
To confirm the role of SDF-1 signaling in human MSC osteogenic differentiation, we further examined MSCs derived from a 45-year-old male patient. Similarly, we detected that the BMP2-induced ALP activity was decreased 56% by the blockade of CXCR4 antagonist AMD3100, and that could not be restored by adding exogenous SDF-1 protein (Fig. 4A). Moreover, we detected in these MSCs that the addition of SDF-1 protein prior to BMP2 stimulation further enhanced the BMP2-induced ALP activity by 74% (Fig. 4A). Treatment with AMD3100 or SDF-1 alone did not affect ALP levels over the control cells maintained in culture medium only (Fig. 4A).
Fig. 4.
Perturbing the SDF-1/CXCR4 signal axis affected the BMP2-induced ALP activity and calcium deposition in human bone marrow-derived MSCs. MSCs were derived from a 45-year-old male patient and stimulated with rBMP2 at 100 ng/ml, or as controls, maintained in culture medium only. In parallel, cells were pretreated with CXCR4 antagonist AMD3100 at 400 µM, and/or rSDF-1 protein at 300 ng/ml for 2 h at 37 °C prior to BMP2 stimulation. Enzymic activity of ALP (A) in cell lysates was measured 7 days after BMP2 stimulation and normalized to the level of total protein. Fourteen days after BMP2 stimulation, the mineral deposition of bone nodules in a proportion of cells cultured in medium supplemented with 25 µg/ml of ascorbic acid and 5mM of µ-glycerophosphate was detected by Alizarin red staining (B), the color intensities of stained cells (C) were quantified by BioQuant software and compared to that of medium control cells. Length of scale bar equals to 100 µm. N = 4 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
To detect the calcium deposition by mature osteoblastic cells when forming nodules, Alizarin red staining (Fig. 4B) was performed on a proportion of this MSC culture, and there was a 8.2-fold increase in staining of cells stimulated with BMP2 for 14 days versus control cells maintained in culture medium only (Fig. 4C). However, this BMP2-increased bone nodule calcification was almost abolished by pretreatment with AMD3100 (a 81% decrease in Alizarin red staining), while adding exogenous SDF-1 protein prior to BMP2 stimulation led to a similar level of calcium deposition as that of cells stimulated with BMP2 alone (Fig. 4C). Alizarin red staining on cells treated with AMD3100 or SDF-1 alone showed no difference from that of medium control cells (Fig. 4B and C). Taken together, these data suggested that SDF-1 signaling played a role in regulating the BMP2-induced osteogenic differentiation of primary human MSCs.
In a third MSC line derived from bone marrow of a 35-year-old female patient, BMP2 stimulation for 7 days significantly increased ALP activity by 2.3-fold when compared to control cells maintained in culture medium only (Fig. 5A). However, pretreatment with anti-SDF-1 neutralizing antibody or CXCR4 antagonist AMD3100 reduced the BMP2-induced ALP activities to levels similar to that of medium control cells, and the anti-SDF-1 blockade was rescued by the addition of SDF-1 protein (Fig. 5A). Moreover, adding exogenous SDF-1 protein prior to BMP2 stimulation further enhanced the BMP2-induced ALP activity by 35% in cells (Fig. 5A).
Fig. 5.
Perturbing the SDF-1 signaling affected the BMP2-induced ALP activity and OCN synthesis in human bone marrow-derived MSCs. MSCs were derived from a 35-year-old female patient and stimulated with rBMP2 at 100 ng/ml, or as controls, maintained in culture medium only. In parallel, cells were pretreated with CXCR4 antagonist AMD3100 at 400 µM, or anti-CXCR4 neutralizing antibody clone 12G5 at 100 ng/ml, for 2 h at 37 °C prior to BMP2 stimulation. Enzymic activity of ALP (A) in cell lysates was measured 7 days after BMP2 stimulation and normalized to the level of total protein. The synthesis of OCN protein (B) was measured in cell medium 12 days after BMP2 stimulation. N = 4 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
In this patient’s cells, we also detected that BMP2 stimulation for 12 days increased OCN synthesis by 2.2-fold when compared to that of medium control cells (Fig. 5B). However, prior blocking of the SDF-1/CXCR4 signal axis by anti-SDF-1 antibody or AMD3100 decreased the BMP2-increased OCN synthesis to similar levels of that of medium control cells, and the anti-SDF-1 effect was reversed by the addition of SDF-1 protein (Fig. 5B). Moreover, the OCN synthesis induced by BMP2 was enhanced 41% by the pretreatment of exogenous SDF-1 protein (Fig. 5B). Treatments with anti-SDF-1 neutralizing antibody, AMD3100, or SDF-1 protein alone did not affect ALP or OCN levels over control cells maintained in culture medium (data not shown). Collectively, data from three independent human MSC lines suggested that perturbing the SDF-1 signaling affected the osteogenic differentiation of human MSCs in response to BMP2 stimulation.
4.4. Blocking of the SDF-1/CXCR4 signal axis inhibited the Runx2 and Osx expression induced by BMP2
The transcriptional regulation of osteoblast genes, such as ALP, OCN and bone sialoprotein (BSP), takes place at their promoter regions via interacting with crucial transcription factors, Runx2 and Osx, which are expressed by MSCs at earliest stages of osteogenic differentiation (Chen et al., 2004). To begin to understand mechanisms underlying the SDF-1 function in BMP2-induced osteogenic differentiation, we blocked SDF-1 signaling and monitored expression of the master genes Runx2 and Osx in primary MSCs derived from both human and mouse bone marrow.
After 6 h of BMP2 stimulation, the time of peak expression of Runx2 and Osx in C2C12 cells (Zhu et al., 2007), we detected up-regulated Runx2 expression in both human (Fig. 6A) and mouse MSCs (Fig. 6C) as compared to control cells maintained in culture medium only. Pretreatments with anti-SDF-1 neutralizing antibody and CXCR4 antagonist AMD3100 decreased the BMP2-induced Runx2 expression by 59% and 70%, respectively, in human MSCs (Fig. 6A); and by 42% and 53%, respectively, in mouse MSCs (Fig. 6C).
Fig. 6.
Blocking of the SDF-1/CXCR4 signal axis inhibited the BMP2-induced Runx2 and Osx expression. MSCs derived from human (A and B) and mouse (C and D) bone marrow were stimulated with rBMP2 at 100 ng/ml for 6 h, or as controls, maintained in culture medium only. In parallel, cells were pretreated with anti-SDF-1 neutralizing antibody at 100 µg/ml or CXCR4 antagonist AMD3100 at 400 µM for 2 h at 37 °C prior to BMP2 stimulation. One microgram of total RNA was collected from cells after BMP2 stimulation, and reversed transcribed. Levels of Runx2 (A and C) and Osx (B and D) expression were quantified by real time PCR and normalized to the level of GAPDH. N = 4–6 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
BMP2 stimulation for 6 h compared to medium control also significantly increased Osx expression in both human (Fig. 6B) and mouse MSCs (Fig. 6D). However, the Osx expression induced by BMP2 was decreased 43% or 74% in human MSCs with prior treatment of anti-SDF-1 or AMD3100 (Fig. 6B). Similarly, pretreatments with anti-SDF-1 and AMD3100 inhibited the BMP2-induced Osx expression by 38% and 62%, respectively, in mouse MSCs (Fig. 6D). Treatment with antibody or antagonist alone did not change the expression of Runx2 or Osx over medium control cells (data not shown). Collectively, these data provided additional evidence for the involvement of SDF-1 signaling in osteogenic differentiation of MSCs by showing that blocking of the SDF-1/CXCR4 signal axis inhibited the BMP2-induced early expression of Runx2 and Osx.
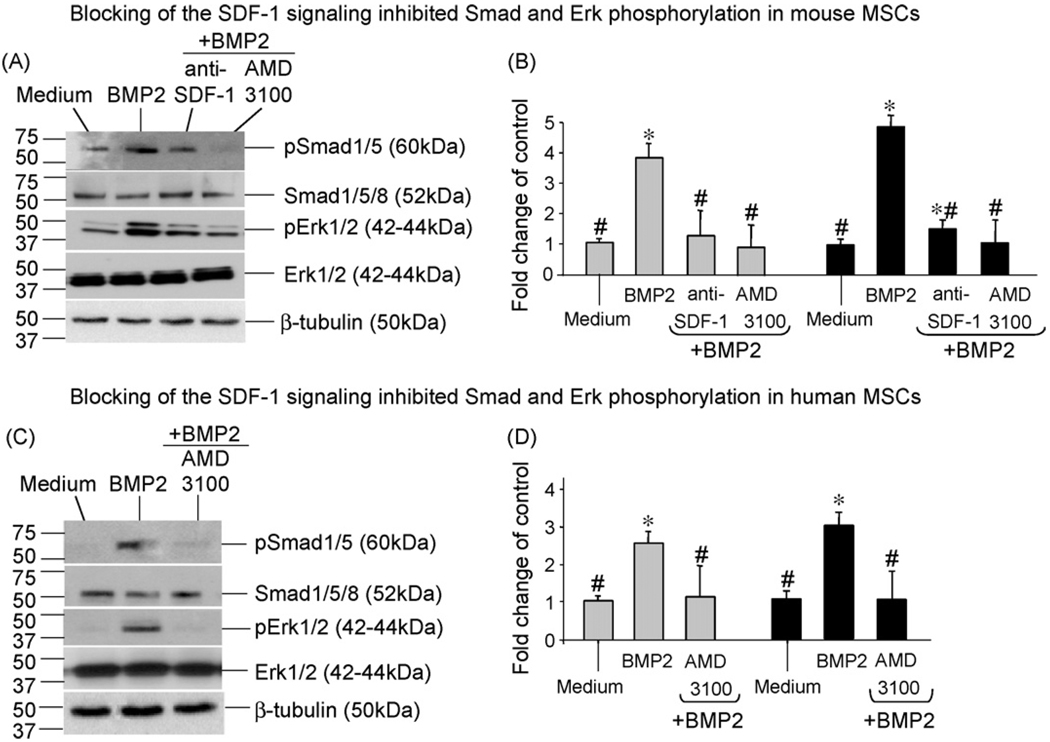
4.5. Blocking of the SDF-1/CXCR4 signal axis inhibited the BMP2-induced intracellular Smad and Erk phosphorylation
The transduction of BMP osteogenic signal from cell surface to nucleus is mainly controlled via intracellular Smad and Erk pathways (Chen et al., 2004; Fujii et al., 1999). To further understand mechanisms underlying the SDF-1 regulatory effect, we examined how perturbing the SDF-1 signaling would affect the activation of Smad and Erk proteins in mouse and human bone marrow-derived MSCs immediately after 15 min of BMP2 induction.
In mouse MSCs, Western blotting results (Fig. 7A) showed that BMP2 stimulation increased the phosphorylation of Smad1/5 and Erk1/2 proteins by 4-fold and 5-fold, respectively, relative to medium control cells (Fig. 7B). Pretreatment with anti-SDF-1 neutralizing antibody and CXCR4 antagonist AMD3100 almost abolished the BMP2-induced Smad1/5 phosphorylation; and largely decreased the BMP2-induced Erk1/2 phosphorylation by 70% and 80%, respectively (Fig. 7A and B).
Fig. 7.
Blocking of the SDF-1 signaling inhibited the BMP2-dependent intracellular Smad and Erk activation. MSCs derived from mouse (A and B) or human (C and D) bone marrow were stimulated with rBMP2 at 100 ng/ml for 15 min, or as controls, maintained in culture medium only. In parallel, cells were pretreated with anti-SDF-1 neutralizing antibody at 100 µg/ml or CXCR4 antagonist AMD3100 at 400 µM for 2 h at 37 °C prior to BMP2 stimulation. Total Smad1/5/8 and Erk1/2 proteins, the phosphorylated (p) Smad1/5 and Erk1/2, and µ-tubulin were detected in cell lysates by Western blotting using appropriate antibodies (A and C). Levels of pSmad1/5 (bars in gray) and pErk1/2 (bars in dark) were measured by densitometry of immunoreactive bands using ImageJ software, and compared to that of medium control cells (B and D). N = 4 samples were included in each treatment group. Data were presented as mean ± SD. *,# p < 0.05 versus medium control and BMP2 stimulation, respectively.
Western detection in human MSCs (Fig. 7C) also showed that the BMP2-induced phosphorylation of Smad1/5 (2.6-fold of medium control) and Erk1/2 (3-fold of medium control) were decreased 62% and 67%, respectively, by the blockade of AMD3100 (Fig. 7D). Similar levels of total Smad, Erk and β-tubulin were detected in mouse or human cells regardless of different treatments (Fig. 7A and C). Treatment with anti-SDF-1 or AMD3100 alone did not alter Smad and Erk phosphorylation over medium control cells (data not shown). These data suggested that the SDF-1 regulation on BMP2-osteoinduction was mediated via intracellular Smad and Erk activation.
5. Discussion
While MSCs have been scrutinized and studied for over forty years, attention was focused on SDF-1 originally only for its function as a chemotaxis regulator in MSC engraftment and in tissue engineering (Wong and Korz, 2008). In this study, we demonstrated that SDF-1 also directly played a role in regulating osteogenic differentiation of MSCs induced by BMP2, since lowering endogenous CXCR4 levels in MSCs of CXCR4flox/flox mice or perturbing the SDF-1/CXCR4 signal axis in primary MSCs derived from human and mouse bone marrow significantly affected the differentiation of MSCs towards osteoblastic cells in response to BMP2 stimulation. These results are in agreement with our previous findings of SDF-1 in osteogenic differentiation of mesenchymal C2C12 cells (Zhu et al., 2007), and provided further insights into potential roles of SDF-1 signaling in MSC-mediated tissue repair and regeneration, not only for recruiting MSCs to injury sites, but also for regulating a specific lineage differentiation of MSCs in cooperation with local stimuli.
The high expression of SDF-1 by MSCs and osteoprogenitors indicates the intimacy between SDF-1 signaling and the initiation of osteogenesis. High levels of SDF-1 have been detected in human (Kortesidis et al., 2005) and mouse (Kitaori et al., 2009; Ratajczak et al., 2003) MSCs at early stages of differentiation induced by dexamethasone or BMP2, while SDF1’s expression declines with cell maturation. Concomitantly, histology evaluations revealed abundant SDF-1 in periosteum of injured or embryonic bones, a region rich in MSCs and other osteoprogenitors (Jung et al., 2006; Petit et al., 2002; Ponomaryov et al., 2000; Salvucci et al., 2002). In this context, our detection that blocking of the SDF-1/CXCR4 signal axis inhibited BMP2-induced Runx2 and Osx expression further suggested the involvement of SDF-1 signaling in the osteogenic fate determination of MSCs, since Runx2 and Osx are two “master” regulators expressed by MSCs at their earliest phases of differentiation when committing into an osteogenic pathway (Chen et al., 2004; Nakashima et al., 2002; Otto et al., 1997). While we focused on examining the SDF-1 regulation on osteogenic aspect of MSCs, recent studies in human periodontal tissue-derived MSCs suggested an up-regulation of SDF-1 during adipogenic differentiation as opposed to down-regulated SDF-1 expression in osteogenic differentiation (Trubiani et al., 2008), indicating distinct roles of SDF-1 in differential lineage development of MSCs.
The condensation and differentiation of MSCs into matrix-synthesizing osteoblasts lay foundations for embryonic bone development and postnatal bone healing. While the role of SDF-1 signaling in the complexities of in vivo bone formation remains to be fully understood, we provided ex vivo evidence that blocking of the SDF-1 signaling in primary MSCs inhibited their differentiation towards functional bone-forming osteoblasts that produced ALP, OCN, and deposited calcium in the matrix. In support of this notion, decreased endochondral bone healing was recently observed in mice with systemic blockade for SDF-1 or CXCR4 (Kitaori et al., 2009). Further in agreement with our finding that adding exogenous SDF-1 protein enhanced the BMP2’s activities on inducing ALP in human and mouse MSCs, over expression of SDF-1 in human bone marrow-derived MSCs versus control MSCs was found to induce a greater level of ectopic bone in nude mice (Kortesidis et al., 2005). Since we did not detect increased bone nodule mineralization in MSC cultures by adding SDF-1 with BMP2, other than limited methodology sensitivities, this could suggest that SDF-1 was less effective on osteoblast maturation when compared to its role in regulating osteogenic differentiation of MSCs at relatively early stages. To more precisely determine the role of SDF-1 in MSC-based bone formation, future studies will use transgenic mouse models to examine skeletal aberrations associated with genetic interruption of SDF-1 signaling in MSCs or in developing osteoprogenitors.
The finding of the SDF-1 regulation on BMP2-induced osteogenic differentiation led us to examine potential crosstalk between these two signaling pathways in the context of promoting osteogenesis. Intracellular Smad and Erk pathways are major sub pathways engaged in BMP2 signal transduction, our results that blocking of the SDF-1/CXCR4 axis inhibited the BMP2-induced phosphorylation of Smad1/5 and Erk1/2 suggested the involvement of these two intracellular elements in mediating the SDF-1 effects. To further understand the upstream mechanisms underlying the SDF-1 function on BMP2-osteoinduction, future studies are required to examine the receptor interactions between SDF-1 and BMP2 and how that affects the onset of osteogenic signal transduction. When compared to C2C12 cell line, the use of primary MSCs is essential for demonstrating the osteogenic role of SDF-1 signaling in MSC and potentially the MSC-based bone formation. However, we are aware that MSCs form a heterogeneous population consisting of mixed cell types particularly in their early passages (Guo et al., 2006; Kitaori et al., 2009; Kortesidis et al., 2005) which could vary the response of cells to BMP2 stimulation or inhibition of neutralizing antibodies and antagonist, and therefore hamper the accuracy of analysis. Moreover, due to the limited source and amount of patient bone marrow available to this study, we have yet to examine more MSC lines to minimize individual discrepancies and verify the role of SDF-1 signaling in human cells. Given the development of markers for identifying “true” stem cells, future experiments using relatively purified MSCs based on CXCR4-immunosorting may help elucidate the regulatory mechanisms of SDF-1 in MSC osteogenic differentiation.
In conclusion, our results demonstrated for the first time an important role of SDF-1 signaling in osteogenic differentiation of bone marrow-derived primary MSCs, and showed that the regulatory effect of SDF-1 on BMP2-osteoinduction was mediated via intracellular Smad and Erk pathways. These findings provide novel insights into comprehensive mechanisms governing the differentiation of MSCs into osteoblastic cells, and will potentially lead to the development of MSC therapies for enhancing bone formation or regeneration in a wide range of orthopaedic situations.
Acknowledgements
This work was supported by grants from Arthritis Foundation and Beatrice and Samuel A. Seaver Foundation.
Footnotes
Conflict of interest
All authors have no conflicts of interest.
References
- Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lymphohematopoietic progenitors. Eur J Immunol. 1999;29:1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Baribaud F, Edwards TG, Sharron M, Brelot A, Heveker N, Price K, et al. Antigenically distinct conformations of CXCR4. J Virol. 2001;75:8957–8967. doi: 10.1128/JVI.75.19.8957-8967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Cei S, Kandler B, Fugl A, Gabriele M, Hollinger JO, Watzek G, et al. Bone marrow stromal cells of young and adult rats respond similarly to platelet-released supernatant and bone morphogenetic protein-6 in vitro. J Periodontol. 2006;77:699–706. doi: 10.1902/jop.2006.050155. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- D’Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, et al. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Donzella GA, Schols D, Lin SW, Esté JA, Nagashima KA, Maddon PJ, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, et al. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–181. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- Gu K, Zhang L, Jin T, Rutherford RB. Identification of potential modifiers of Runx2/Cbfa1 activity in C2C12 cells in response to bone morphogenetic protein-7. Cells Tissues Organs. 2004;176:28–40. doi: 10.1159/000075025. [DOI] [PubMed] [Google Scholar]
- Guo Z, Li H, Li X, Yu X, Wang H, Tang P, et al. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 2006;24:992–1000. doi: 10.1634/stemcells.2005-0224. [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A, et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84:1130–1140. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- Hatse S, Princen K, De Clercq E, Rosenkilde MM, Schwartz TW, Hernandez-Abad PE, et al. AMD3465, a monomacrocyclic CXCR4 antagonist and potent HIV entry inhibitor. Biochem Pharmacol. 2005;70:752–761. doi: 10.1016/j.bcp.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts: a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- Kørbling M, Estrov Z. Adult stem cells for tissue repair–a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Dupuy C, Rigal S, Jasmin C, Bourin P, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka M, Ratajczak J, Lee B, Honczarenko M, Douglas R, Kowalska MA, et al. The role of HIV-related chemokine receptors and chemokines in human erythropoiesis in vitro. Stem Cells. 2000;18:128–138. doi: 10.1634/stemcells.18-2-128. [DOI] [PubMed] [Google Scholar]
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, et al. Cbfa1 a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- Schols D, Struyf S, Van Damme J, Esté JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Turner JD, Collman RG, Hoxie J, González-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with the dual-tropic human immunodeficiency virus type 1 isolate HIV-1(89.6) but not the T-tropic isolate HIV-1(HxB) J Virol. 1997;71:5678–5683. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Trubiani O, Isgro A, Zini N, Antonucci I, Aiuti F, Di Primio R, et al. Functional interleukin-7/interleukin-7Ralpha, and SDF-1alpha/CXCR4 are expressed by human periodontal ligament derived mesenchymal stem cells. J Cell Physiol. 2008;214:706–713. doi: 10.1002/jcp.21266. [DOI] [PubMed] [Google Scholar]
- Wong D, Korz W. Translating an antagonist of chemokine receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Tsai AD, Lee JC. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem. 2002;87:292–304. doi: 10.1002/jcb.10315. [DOI] [PubMed] [Google Scholar]
- Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, et al. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–179. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Zachos TA, Shields KM, Bertone AL. Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or -6. J Orthop Res. 2006;24:1279–1291. doi: 10.1002/jor.20068. [DOI] [PubMed] [Google Scholar]
- Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG, et al. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Boachie-Adjei O, Rawlins BA, Frenkel B, Boskey AL, Ivashkiv LB, et al. A novel regulatory role for stromal-derived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem. 2007;282:18676–18685. doi: 10.1074/jbc.M610232200. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]