Abstract
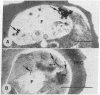
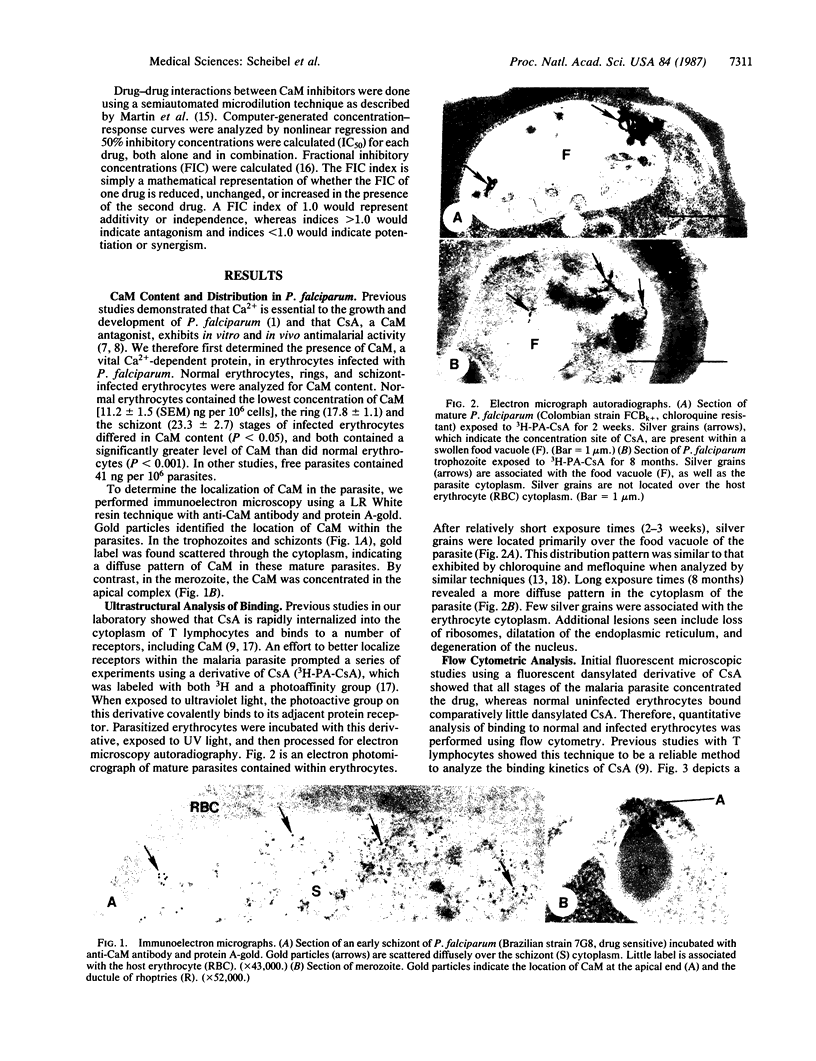
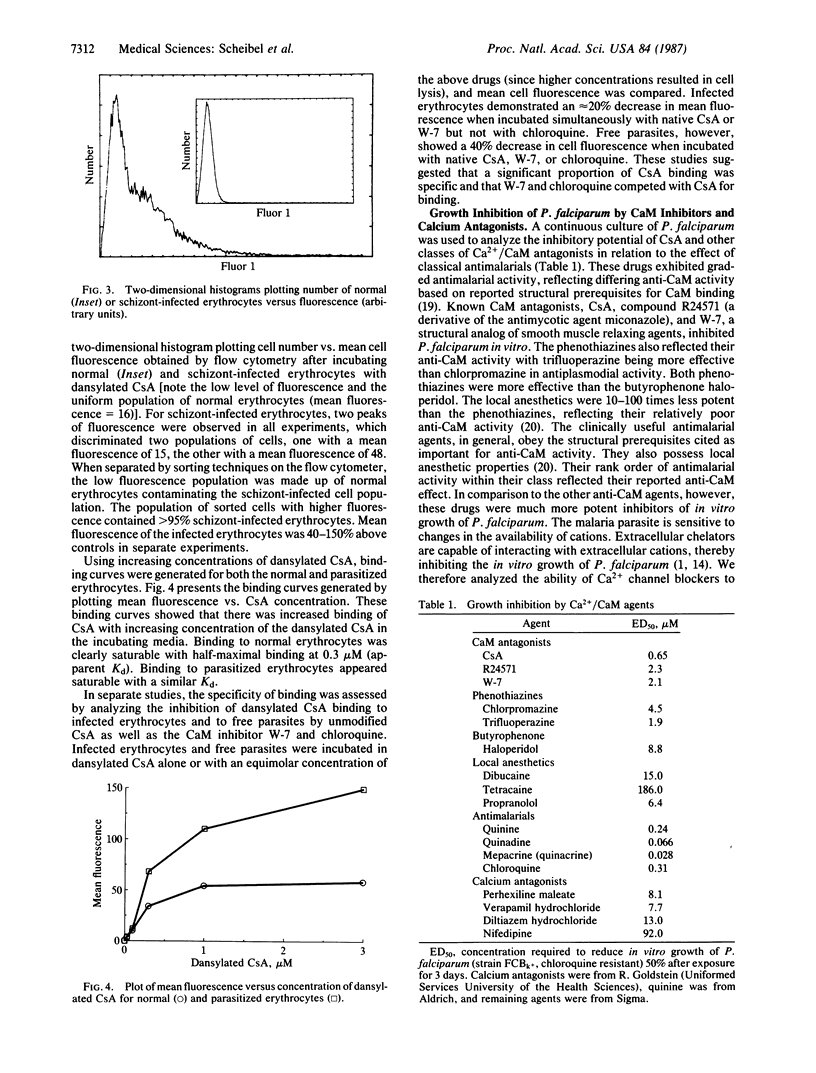
The malaria parasite has an obligate calcium requirement for normal intracellular growth and invasion of host erythrocytes. Calmodulin (CaM) is a vital calcium-dependent protein present in eukaryotes. We found by radioimmunoassay that free parasites contain CaM. Schizont-infected erythrocytes had CaM levels of 23.3 +/- 2.7 ng per 10(6) cells compared to normals (11.2 +/- 1.5 ng per 10(6) cells). CaM levels were proportional to parasite maturity. Immunoelectron microscopy identified CaM diffusely within the cytoplasm of mature parasites and at the apical end of merozoites within the ductule of rhoptries, which may explain the calcium requirement for invasion. Cyclosporin A (CsA) was also found by electron microscopic autoradiography to concentrate in the food vacuole, as do chloroquine and mefloquine, and to distribute within the cytoplasm of mature parasites. The binding of dansylated CsA to schizont-infected erythrocytes was higher than to normal erythrocytes as analyzed by flow cytometry. Kinetic analysis revealed that binding was saturable for normal and infected erythrocytes and possibly free parasites. Competition for binding existed between dansylated CsA and native CsA as well as the CaM inhibitor W-7 and the classic antimalarial chloroquine. The in vitro growth of Plasmodium falciparum was sensitive to CaM antagonists, and in large part inhibition of the parasite was proportional to known anti-CaM potency. Antagonism existed between combinations of these drugs in multi-drug-resistant strains of P. falciparum, suggesting possible competition for the same binding site. In addition, the malaria parasite was also susceptible to calcium antagonists.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. High-resolution autoradiography of malarial parasites treated with 3 H-chloroquine. Am J Pathol. 1972 May;67(2):277–284. [PMC free article] [PubMed] [Google Scholar]
- Ardeshir F., Flint J. E., Matsumoto Y., Aikawa M., Reese R. T., Stanley H. cDNA sequence encoding a Plasmodium falciparum protein associated with knobs and localization of the protein to electron-dense regions in membranes of infected erythrocytes. EMBO J. 1987 May;6(5):1421–1427. doi: 10.1002/j.1460-2075.1987.tb02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani P. M., Robb A., Hess A. D. Cyclosporin A binding to calmodulin: a possible site of action on T lymphocytes. Science. 1985 Apr 19;228(4697):337–339. doi: 10.1126/science.3885394. [DOI] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Jacobs G. H., Aikawa M., Milhous W. K., Rabbege J. R. An ultrastructural study of the effects of mefloquine on malaria parasites. Am J Trop Med Hyg. 1987 Jan;36(1):9–14. doi: 10.4269/ajtmh.1987.36.9. [DOI] [PubMed] [Google Scholar]
- Krungkrai J., Yuthavong Y. Enhanced Ca2+ uptake by mouse erythrocytes in malarial (Plasmodium berghei) infection. Mol Biochem Parasitol. 1983 Mar;7(3):227–235. doi: 10.1016/0166-6851(83)90023-3. [DOI] [PubMed] [Google Scholar]
- LeGrue S. J., Turner R., Weisbrodt N., Dedman J. R. Does the binding of cyclosporine to calmodulin result in immunosuppression? Science. 1986 Oct 3;234(4772):68–71. doi: 10.1126/science.3749892. [DOI] [PubMed] [Google Scholar]
- Leida M. N., Mahoney J. R., Eaton J. W. Intraerythrocytic plasmodial calcium metabolism. Biochem Biophys Res Commun. 1981 Nov 30;103(2):402–406. doi: 10.1016/0006-291x(81)90466-6. [DOI] [PubMed] [Google Scholar]
- Löffler B. M., Bohn E., Hesse B., Kunze H. Effects of antimalarial drugs on phospholipase A and lysophospholipase activities in plasma membrane, mitochondrial, microsomal and cytosolic subcellular fractions of rat liver. Biochim Biophys Acta. 1985 Jul 31;835(3):448–455. doi: 10.1016/0005-2760(85)90114-6. [DOI] [PubMed] [Google Scholar]
- Martin S. K., Oduola A. M., Milhous W. K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987 Feb 20;235(4791):899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- Masaracchia R. A., Hassell T. C., Donahue M. J. Structural analysis of the calcium-binding protein calmodulin from Ascaris suum obliquely striated muscle. J Parasitol. 1986 Apr;72(2):299–305. [PubMed] [Google Scholar]
- Nickell S. P., Scheibel L. W., Cole G. A. Inhibition by cyclosporin A of rodent malaria in vivo and human malaria in vitro. Infect Immun. 1982 Sep;37(3):1093–1100. doi: 10.1128/iai.37.3.1093-1100.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck W. C., Weiss B. Inhibition of calmodulin by phenothiazines and related drugs: structure-activity relationships. J Pharmacol Exp Ther. 1982 Sep;222(3):509–516. [PubMed] [Google Scholar]
- Scheibel L. W., Stanton G. G. Antimalarial activity of selected aromatic chelators. IV. Cation uptake by Plasmodium falciparum in the presence of oxines and siderochromes. Mol Pharmacol. 1986 Oct;30(4):364–369. [PubMed] [Google Scholar]
- Schlondorff D., Satriano J. Interactions with calmodulin: potential mechanism for some inhibitory actions of tetracyclines and calcium channel blockers. Biochem Pharmacol. 1985 Sep 15;34(18):3391–3393. doi: 10.1016/0006-2952(85)90366-1. [DOI] [PubMed] [Google Scholar]
- Seebeck T., Gehr P. Trypanocidal action of neuroleptic phenothiazines in Trypanosoma brucei. Mol Biochem Parasitol. 1983 Nov;9(3):197–208. doi: 10.1016/0166-6851(83)90097-x. [DOI] [PubMed] [Google Scholar]
- Slater L. M., Sweet P., Stupecky M., Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest. 1986 Apr;77(4):1405–1408. doi: 10.1172/JCI112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Reynolds I. J. Calcium-antagonist drugs. Receptor interactions that clarify therapeutic effects. N Engl J Med. 1985 Oct 17;313(16):995–1002. doi: 10.1056/NEJM198510173131606. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mikkelsen R. B., Wallach D. F. Calcium transport of Plasmodium chabaudi-infected erythrocytes. J Cell Biol. 1982 Jun;93(3):680–684. doi: 10.1083/jcb.93.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson S., MacNeil S., Walker S. W., Ollis C. A., Merritt J. E., Brown B. L. Calmodulin and cell function. Clin Sci (Lond) 1984 May;66(5):497–507. doi: 10.1042/cs0660497. [DOI] [PubMed] [Google Scholar]
- Van der Straeten C., Klippel J. H. Antimalarials and pneumococcal immunization. N Engl J Med. 1986 Sep 11;315(11):712–713. doi: 10.1056/NEJM198609113151116. [DOI] [PubMed] [Google Scholar]
- Volpi M., Sha'afi R. I., Feinstein M. B. Antagonism of calmodulin by local anesthetics. Inhibition of calmodulin-stimulated calcium transport of erythrocyte inside-out membrane vesicles. Mol Pharmacol. 1981 Sep;20(2):363–370. [PubMed] [Google Scholar]
- Wasserman M., Alarcón C., Mendoza P. M. Effects of Ca++ depletion on the asexual cell cycle of Plasmodium falciparum. Am J Trop Med Hyg. 1982 Jul;31(4):711–717. doi: 10.4269/ajtmh.1982.31.711. [DOI] [PubMed] [Google Scholar]