SUMMARY
Apolipoprotein (apo) E has important and diverse functions in neurobiology, and apoE4 is the major known genetic risk factor for Alzheimer’s disease. Here we report that adult neural stem/progenitor cells (NSCs) express apoE. In apoE knockout mice, neurogenesis in the hippocampus was ~60% lower than in wildtype mice, and most newborn cells developed into astrocytes rather than into neurons as in wildtype mice. This impairment was not observed in human apoE3 knock-in mice. In apoE4 knock-in mice, however, the maturation and dendritic development of newborn hippocampal neurons was significantly impaired as a result of apoE4 and its fragment-caused GABAergic interneuron dysfunction. This impairment was fully rescued by treatment with a GABAA receptor potentiator. These findings demonstrate the importance of apoE in adult hippocampal neurogenesis and show that apoE4 inhibits hippocampal neurogenesis by impairing neuronal maturation mediated by GABA signaling.
INTRODUCTION
In the mammalian central nervous system, new neurons are generated throughout life. In adults, active neurogenesis occurs in two brain regions. One is the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Aimone et al., 2006; Altman and Dasq, 1965; Cameron et al., 1993; Christie and Cameron, 2006; Eriksson et al., 1998; Kaplan and Bell, 1984; Kempermann et al., 2003; Ming and Song, 2005; Zhao et al., 2008; Suh et al., 2009), where newly generated neurons may participate in learning and memory formation (Aimone et al., 2006; Christie and Cameron, 2006; Ming and Song, 2005). The other is the subventricular zone (SVZ) of the lateral ventricle, where new neurons migrate to the olfactory bulb (Alvarez-Buylla and García-Verdugo, 2002; Carleton et al., 2003; Rochefort et al., 2002). Generally, adult neurogenesis proceeds through four developmental stages (Christie and Cameron, 2006; Lie et al., 2004; Ming and Song, 2005; Zhao et al., 2008; Suh et al., 2009): (1) proliferation of neural stem/progenitor cells (NSCs), (2) neuronal fate determination of NSCs, (3) maturation and migration of new neurons, and (4) functional integration of new neurons into existing neuronal circuits. Adult neurogenesis is regulated by several factors, including transcription factors, hormones, neurotransmitters, cell niches, exercise, and specific molecules (Alvarez-Buylla and Lim, 2004; Lie et al., 2004; Ming and Song, 2005; Zhao et al., 2008; Suh et al., 2009), although the detailed mechanisms are still poorly understood.
Apolipoprotein (apo) E, a polymorphic protein with 299 amino acids, has important and diverse functions in neurobiology. In the brain, apoE is mainly synthesized and secreted by astrocytes, but neurons under stress also express apoE (Aoki et al., 2003; Xu et al., 1999; Xu et al., 2006; Xu et al., 2008). ApoE distributes lipids among cells in the central nervous system for normal lipid homeostasis and participates in neuronal repair and remodeling (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). However, the three major human isoforms (apoE2, apoE3, and apoE4) differ in their ability to accomplish these tasks (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). ApoE3 is the most common and considered to be the normal form (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). ApoE4, the major known genetic risk factor for Alzheimer’s disease (AD), is associated with an earlier onset of AD in a gene dose-dependent manner (Corder et al., 1993; Saunders et al., 1993). It may also contribute to age-related shrinking of the hippocampus and memory deficits in humans (Cohen et al., 2001; Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006; Moffat et al., 2000; Caselli et al., 2009). The hippocampus is one of the first regions of the brain damaged in AD, and memory deficits and disorientation are among the early symptoms (Selkoe, 1991; Tanzi and Bertram, 2001). In apoE transgenic and knock-in (KI) mice, apoE4 impairs hippocampus-dependent learning and memory (Grootendorst et al., 2005; Harris et al., 2003; Raber et al., 1998; Raber et al., 2000; Villasana et al., 2006; Bour et al., 2008). Although many hypotheses have been advanced (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006), the molecular mechanisms underlying the pathogenic actions of apoE4 in AD are still unclear.
In this study, we assessed the role of apoE in adult hippocampal neurogenesis in mice. Our results reveal an important role for apoE in this process and suggest that apoE4 inhibits neurogenesis by impairing neuronal maturation mediated by GABA signaling, which might contribute to AD pathogenesis.
RESULTS
Adult NSCs Express ApoE
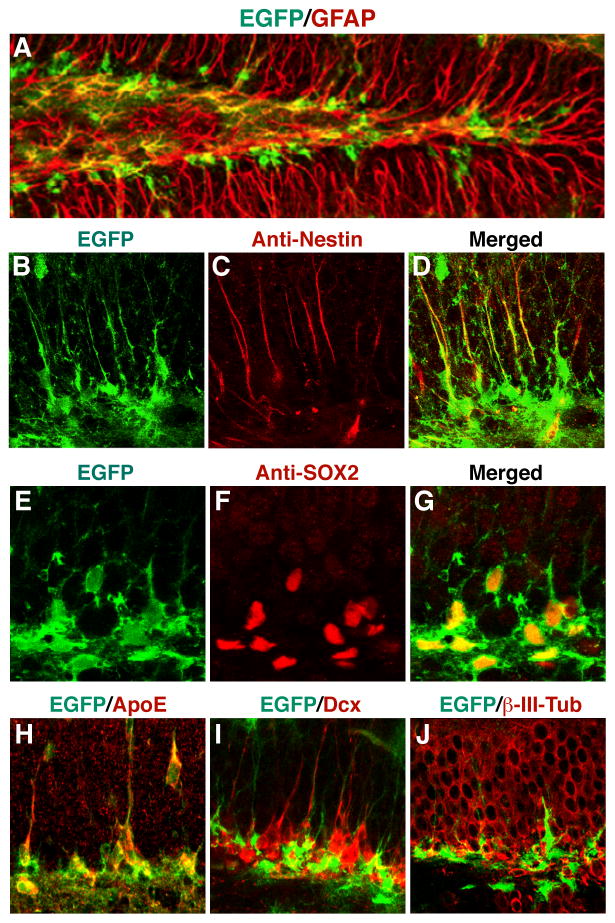
To study the regulation of apoE expression in the central nervous system, we generated mice in which a cDNA encoding enhanced green fluorescent protein (EGFP) with a stop codon was inserted by gene targeting into the apoE gene locus immediately after the translation initiation site (Xu et al., 2006). In heterozygous EGFPapoE reporter mice, in which one apoE allele is sufficient to maintain normal lipid metabolism, confocal imaging revealed many EGFP-positive cells (representing apoE-expressing cells) along the SGZ (Figure 1A). Their location suggested that these apoE-positive cells were NSCs. To test this possibility, we immunostained brain sections with antibodies against nestin, a cytoskeletal protein localized predominantly in the processes of NSCs. The EGFP-positive cells were positive for nestin, particularly in their radial processes (Figures 1B–1D). These cells were also positive for Sox2 (Figures 1E–1G), another NSC marker in the brain (Suh et al., 2007), and expressed apoE (Figure 1H). Triple immunostaining for apoE, nestin, and Sox2 revealed that NSCs in the SGZ of wildtype mice express apoE (Figures S1A). The nestin- and Sox2-positive NSCs in the SVZ and rostral migratory stream (RMS) of wildtype and EGFPapoE reporter mice also expressed apoE (Figures S1B–1K). However, EGFP expression was turned off when NSCs developed into immature neurons expressing doublecortin (Dcx) (Figures 1I, S1L, and S1M) or β-III-tubulin (Figure 1J). The apoE expression in NSCs was confirmed by western blot analysis of in vitro cultured NSCs from brains of wildtype, apoE3-KI, and apoE4-KI mice (Figure S2I, note that mouse apoE is 5 amino acids shorter than human apoE). These findings suggest that apoE is important in adult neurogenesis.
Figure 1. ApoE Is Expressed in Hippocampal NSCs.
(A–J)Confocal images of the dentate gyrus of EGFPapoE reporter mice. Green indicates EGFP representing apoE (A, B, D, E, G–J). Red indicates immunostaining positive for anti-GFAP (A), anti-nestin (C, D), anti-Sox2 (F, G), anti-apoE (H), anti-Dcx (I), or anti-β-III-tubulin (J).
ApoE Deficiency Inhibits Neurogenesis but Stimulates Astrogenesis in the Hippocampus
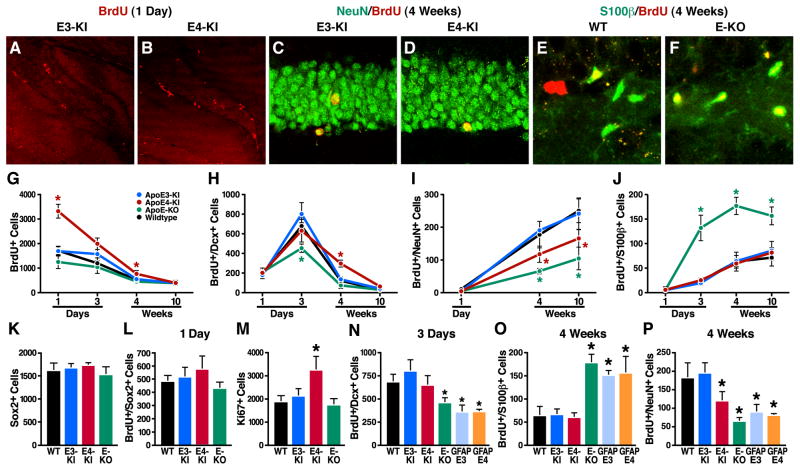
To analyze hippocampal neurogenesis and astrogenesis, we followed the survival and neural differentiation of newborn cells in 6–7-month-old mice after intraperitoneal injection of 5-bromo-2′-deoxyuridine (BrdU). At 3 days and 4 and 10 weeks after the injection, apoE knockout (KO) mice had many more newly generated astrocytes (BrdU+/S100β+) than wildtype mice (Figures 2E, 2F, and 2J); however, they had many fewer newly generated immature neurons (BrdU+/Dcx+) at 3 days (Figure 2H) and fewer mature neurons (BrdU+/NeuN+) at 4 and 10 weeks (Figure 2I). The stimulatory effect of apoE deficiency on astrogenesis was confirmed by the significantly greater number of astrocytes (S100β+) in the hippocampal hilus of apoE-KO mice than wildtype mice (Figure S3).
Figure 2. Effects of ApoE Deficiency and ApoE Isoforms on Hippocampal Neurogenesis and Astrogenesis.
(A, B) Representative confocal images of the BrdU-positive cells in the SGZ of female apoE3-KI (A) and apoE4-KI (B) mice at 6–7 months of age were collected 1 day after BrdU injection.
(C, D) Representative confocal images of the BrdU and NeuN double positive cells in the SGZ of female apoE3-KI (C) and apoE4-KI (D) mice at 6–7 months of age were collected 4 weeks after BrdU injection.
(E, F) Representative confocal images of the BrdU and S100β double positive cells in the SGZ of female wildtype (E) and apoE-KO (F) mice at 6–7 months of age were collected 4 weeks after BrdU injection.
(G–J) Numbers of newborn cells (BrdU+) (G), immature neurons (BrdU+/Dcx+) (H), mature neurons (BrdU+/NeuN+) (I), and astrocytes (BrdU+/S100β+) (J) in the SGZ of female mice of various apoE genotypes at 6–7 months of age were determined 1 and 3 days and 4 and 10 weeks after BrdU injection. Values are mean ± SD (n = 4–6 mice per genotype). * p < 0.05 vs. other groups (t test).M
(K) Total numbers of Sox2-positive cells in the SGZ of female wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice at 6–7 months of age. Values are mean ± SD (n = 4 mice per genotype).M
(L) Numbers of BrdU and Sox2 double-positive cells in the SGZ of female wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice at 6–7 months of age were determined 1 day after BrdU injection. Values are mean ± SD (n = 4 mice per genotype).
(M) Total numbers of Ki67-positive cells in the SGZ of female wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice at 6–7 months of age. Values are mean ± SD (n = 4 mice per genotype).
(N–P) Numbers of immature neurons (BrdU+/Dcx+) (N), astrocytes (BrdU+/S100β+) (O), and mature neurons (BrdU+/NeuN+) (P) in the SGZ of female mice of various apoE genotypes at 6–7 months of age were determined at 3 days and 4 weeks after BrdU injection. Values are mean ± SD (n = 4–6 mice per genotype). * p < 0.05 vs. wildtype and apoE3-KI mice (t test).
ApoE Deficiency Does Not Alter the Number or Proliferation of NSCs
ApoE deficiency did not significantly affect the number of newborn cells (BrdU+) (Figure 2G) or Sox2-positive cells (Figure 2K). The latter cells represent total NSCs. Nor did apoE deficiency affect the number of BrdU and Sox2 double-positive cells (Figure 2L), which reflect proliferation of NSCs, in the SGZ.
Human ApoE3, but Not ApoE4, Rescues the Impaired Hippocampal Neurogenesis in ApoE-Deficient Mice
Next, we compared the effects of human apoE isoforms and mouse apoE on adult hippocampal neurogenesis in 6–7-month-old apoE3-KI, apoE4-KI, and wildtype mice. Like mouse apoE, human apoE3 supported hippocampal neurogenesis, including NSC proliferation (Figure 2G) and the generation of immature neurons (Figure 2H), mature neurons (Figure 2I), and astrocytes (Figures 2J and S3). Thus, like mouse apoE, human apoE3 evidently rescues both the impaired hippocampal neurogenesis and aberrant astrogenesis in apoE-deficient mice. In contrast, human apoE4 rescued only the aberrant astrogenesis (Figures 2G–2J and S3).
Human ApoE4 Stimulates Proliferation of Hippocampal NSCs
One day after BrdU injection, 6–7-month old apoE4-KI mice had twofold more BrdU-positive cells in the SGZ than apoE3-KI mice (Figures 2A, 2B, and 2G). ApoE4 affected neither the total number of Sox2-positive cells (Figure 2K) nor the number of BrdU/Sox2 double-positive cells (Figure 2L) in the SGZ. Thus, the number of NSCs and their proliferation are normal in apoE4-KI mice. However, in the SGZ, apoE4-KI mice had ~60% more proliferating cells, shown by Ki67 immunostaining, than apoE3-KI, wildtype, and apoE-KO mice (Figure 2M).
ApoE4 Inhibits Maturation of Newborn Neurons in the Hippocampus
At 4 weeks after BrdU injection, apoE4-KI mice had ~50% fewer mature neurons in the SGZ than apoE3-KI mice (Figures 2C, 2D, and 2I) but threefold more immature neurons (Figure 2H) and similar numbers of astrocytes (Figure 2J). These results suggest that apoE4 inhibits the maturation of newborn neurons in the hippocampus. At 10 weeks after injection, apoE4-KI mice still had significantly fewer mature neurons (Figure 2I), although the number of immature neurons had decreased nearly to baseline levels in both groups (Figure 2H). Furthermore, at 4 weeks after BrdU injection, apoE4-KI mice also had ~25% fewer mature neurons in the olfactory bulb (OB), which originated from the SVZ, than apoE3-KI mice (Figure S4). Thus, apoE4 inhibits neurogenesis in both the SGZ and the SVZ/OB, with a more pronounced effect in the SGZ.
Effect of ApoE Deficiency on Hippocampal Neurogenesis Is Cell-Autonomous but Effect of ApoE4 Is Non-Cell-Autonomous
To determine whether apoE deficiency has a cell-autonomous effect on hippocampal neurogenesis, we studied GFAP-apoE3 and GFAP-apoE4 transgenic mice on a mouse apoE-KO background. In these mice, human apoE is expressed only in adult astrocytes and is secreted into the intercellular space in the brain (Brecht et al., 2004). Immunofluorescence staining revealed that nestin-positive NSCs in the SGZ and SVZ did not express apoE in GFAP-apoE4 (Figures S5A–5F) and GFAP-apoE3 (data not shown) transgenic mice, although mature astrocytes in the hippocampus (Figure S5G) or other brain regions (data not shown) did express apoE. At 3 days after BrdU injection, GFAP-apoE3, GFAP-apoE4, and apoE-KO mice had similar numbers of newly generated immature neurons (BrdU+/Dcx+) but significantly fewer than wildtype, apoE3-KI, and apoE4-KI mice (Figure 2N). At 4 weeks, GFAP-apoE3, GFAP-apoE4, and apoE-KO mice had similar numbers of newly generated astrocytes (BrdU+/S100β+) but significantly more than wildtype, apoE3-KI, and apoE4-KI mice (Figure 2O). GFAP-apoE3, GFAP-apoE4, apoE-KO, and apoE4-KI mice also had similar numbers of mature neurons (BrdU+/NeuN+) but significantly fewer than wildtype and apoE3-KI mice (Figure 2P). Thus, astrocyte-secreted apoE does not effectively support hippocampal neurogenesis or suppress astrogenesis.
To further test the effects of apoE deficiency and apoE4 on neurogenesis, we analyzed neural differentiation of cultured NSCs in vitro (Figures S2A–2H). At 7 days in culture, double immunostaining for MAP2 and GFAP revealed a significantly lower percentage of neurons, but a much higher percentage of astrocytes, generated from NSCs of apoE-KO mice than from those of wildtype mice (Figures S2G, S2H, S2J, and S2K). The percentages of neurons and astrocytes generated from NSCs of apoE3-KI, apoE4-KI, and wildtype mice were similar (Figures S2E–2G, S2J, and S2K). Thus, the effect of apoE deficiency on hippocampal neurogenesis from NSCs is cell-autonomous but the effect of apoE4 is non-cell-autonomous.
ApoE Deficiency Decreases Noggin Expression in NSCs, Leading to Stimulation of Astrogenesis and Inhibition of Neurogenesis
Expression of Noggin in the SGZ and SVZ was reported previously in mice and rats (Fan et al., 2003; Lim et al., 2000; Tang et al., 2009). Noggin inhibits astrogenesis and stimulates neurogenesis by antagonizing the function of bone-morphogenetic protein (BMP) (Lim et al., 2000). We found that Sox2 and apoE double positive NSCs in the SGZ and SVZ expressed Noggin (Figures S6A–S6H). Interestingly, NSCs cultured from apoE-KO mice had an ~80% lower Noggin protein levels, as determined by anti-Noggin western blot, than NSCs cultured from wildtype mice (Figures S7E and S7F). Addition of recombinant mouse Noggin into the culture of NSCs from apoE-KO mice under conditions of neuronal differentiation inhibited astrogenesis and stimulated neurogenesis to levels similar to those of NSCs from wildtype mice (Figures S7A–S7D, S7G, S7H). Thus, apoE deficiency decreases Noggin expression in NSCs, leading to stimulation of astrogenesis and inhibition of neurogenesis.
Exercise Stimulates Hippocampal Neurogenesis to a Greater Extent in ApoE3-KI Than in ApoE4-KI Mice
In apoE3-KI mice, voluntary exercise for 4 weeks on a running wheel in the home cage significantly increased the number of surviving newborn cells (Figure S8A) and mature neurons (Figure S8C) at 4 weeks after BrdU injection. Exercise did not alter the number of immature neurons (Figure S8B). In apoE4-KI mice, however, voluntary exercise did not stimulate the survival of newborn cells (Figure S8A) and increased the number of mature neurons only moderately (Figure S8C).
ApoE4 Impairs Dendritic Development of Newborn Hippocampal Neurons
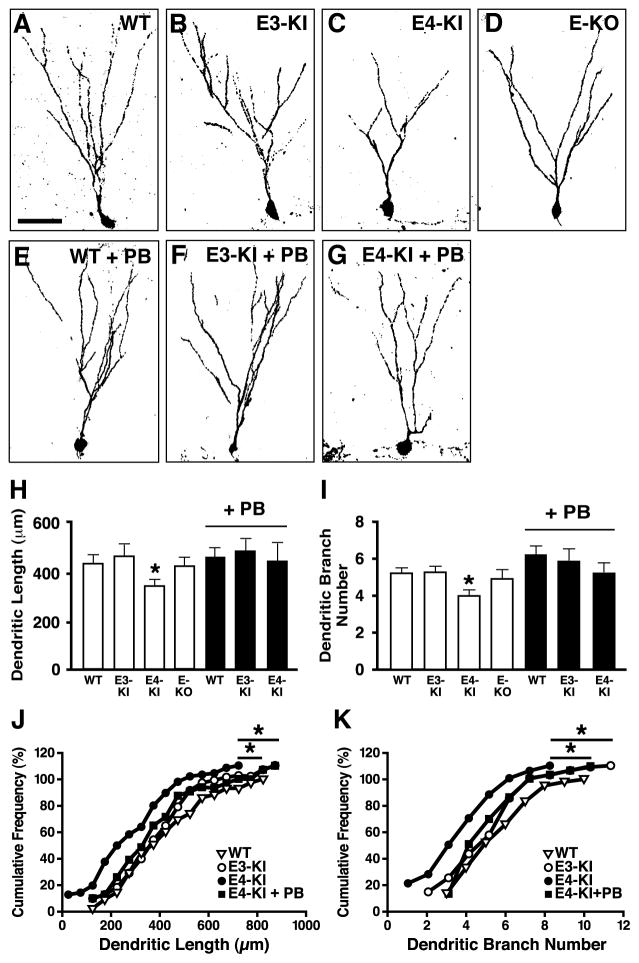
Functional neurogenesis requires dendritic and synaptic integration of newly generated neurons into the existing neuronal circuitry (Duan et al., 2007; Ge et al., 2006a; Lie et al., 2004; Ming and Song, 2005). To determine if apoE4 affects dendritic development, we reconstructed the dendritic arbors of newborn hippocampal neurons from confocal microscopic images. Newborn neurons were labeled by stereotaxically injecting retrovirus expressing GFP into the dentate gyrus (Ge et al., 2006a; Zhao et al., 2006). Four weeks after injection, when the newborn neurons were fully developed and integrated (Aimone et al., 2006; Ge et al., 2006a; Lie et al., 2004; Ming and Song, 2005), GFP+ newborn neurons had much less elaborate dendrites in apoE4-KI mice than in apoE3-KI, wildtype, and apoE-KO mice (Figures 3A–3D). The total dendritic length and branch number of newborn neurons were significantly lower in apoE4-KI mice (Figures 3H–K).
Figure 3. ApoE4 Impairs Dendritic Development of Newborn Neurons in the Hippocampus.
(A–G) Confocal three-dimensional reconstruction of dendrites (inverted images) of newborn neurons (4 weeks after retrovirus-GFP injection) in the dentate gyrus of wildtype (A), apoE3-KI (B), apoE4-KI (C), and apoE-KO (D) mice, wildtype mice treated with PB (E), apoE3-KI mice treated with PB (F), and apoE4-KI mice treated with PB (G). Scale bar, 50 µm.
(H–K) Total dendritic length (H, J) and dendritic branch number (I, K) of newborn neurons. * p < 0.05 (t test in H and I; Kolmogorov-Smirnov test in J and K). WT, n = 43; E3-KI, n = 84; E4-KI, n = 73; E-KO, n =35; WT + PB, n =52; E3-KI + PB, n =42; E4-KI + PB, n = 31. Values in panels H and I are mean ± SEM.
ApoE4 Decreases the Number of GABAergic Interneurons in the Dentate Gyrus
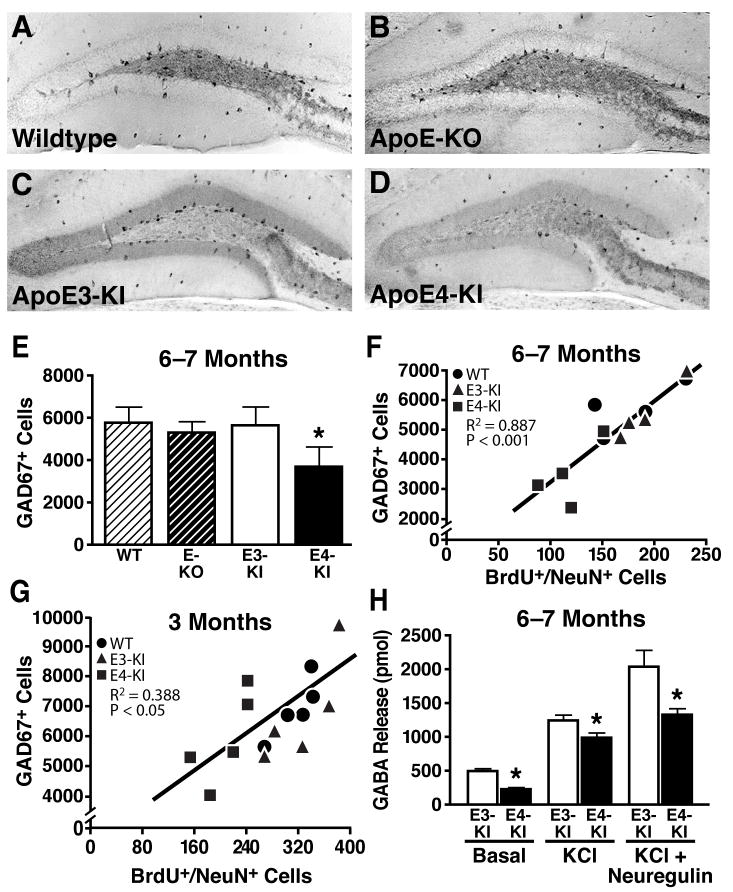
The phenotype of abnormal hippocampal neurogenesis in apoE4-KI mice—increased NSC proliferation and impaired neuronal maturation and dendritic development—mirrors that in mice with GABA signaling inhibition (Earnheart et al., 2007; Ge et al., 2006a; Ge et al., 2006b; Liu et al., 2006; Tozuka et al., 2005). To determine whether apoE4 impairs GABAergic interneurons in the hilus of the hippocampus, we performed anti-GAD67 immunostaining for GABAergic interneurons in wildtype, apoE-KO, apoE3-KI, and apoE4-KI mice (Figures 4A–4D). At 6–7 months of age, apoE4-KI mice had ~30% fewer GAD67-positive interneurons in the hilus than wildtype, apoE-KO, and apoE3-KI mice (Figure 4E). Importantly, the number of GAD67-positive GABAergic interneurons correlated positively with the number of newly generated mature neurons (BrdU+/NeuN+) in the SGZ of wildtype, apoE3-KI, and apoE4-KI mice (Figure 4F). A similar positive correlation was also observed among different groups of mice at 3 months of age (Figure 4G). Similar results were obtained by anti-somatostatin immunostaining for GABAergic interneurons in mice at 6 months of age (Figures S9A–S9F). Thus, apoE4 decreases the numbers of GABAergic interneurons in the hilus, which correlates with the level of neurogenesis. Consistent with the finding of GABAergic interneuron reduction, both basal and KCl- or neuregulin-evoked GABA release in hippocampal slices were significantly lower in apoE4-KI than apoE3-KI mice (Figure 4H), as determined by mass spectrometry. Furthermore, the axonal termini of GABAergic interneurons on granule cells in the dentate gyrus were also significantly decreased at both the absolute level and relative to a general presynaptic marker, synaptophysin (Figures S10). Interestingly, apoE3-KI, wildtype, and apoE-KO mice had similar numbers of GABAergic interneurons (Figures 4E and S9E) and axonal termini onto dentate gyrus granule cells (Figures S10), suggesting that apoE deficiency does not decrease the number of GABAergic interneurons or their axonal termini.
Figure 4. ApoE4 Impairs GABAergic Interneurons and GABA Release in the Hippocampus.
(A–D) Immunostaining of GAD67-positive GABAergic interneurons in the hilus of female wildtype (A), apoE-KO (B), apoE3-KI (C), and apoE4-KI (D) mice at 6–7 months of age.
(E) Numbers of GAD67-positive GABAergic interneurons in different mice at 6–7 months of age. Values are mean ± SD (n = 4–7 mice per genotype). * p < 0.05 vs. wildtype and apoE3-KI mice (t test).
(F, G) Positive correlation between the number of GAD67-positive interneurons and the number of BrdU+/NeuN+ neurons among female wildtype, apoE3-KI, and apoE4-KI mice at 6–7 months of age (F, n = 12 mice) and at 3 months of age (G, n = 15 mice).
(H) GABA release in hippocampal slices, determined by mass spectrometry. Values are mean ± SD (n = 4–7 mice per genotype). * p < 0.05 vs. wildtype and apoE3-KI mice (t test).
At 12–13 months of age, apoE4-KI mice had ~40% fewer GAD67-positive interneurons in the hilus than apoE3-KI mice (Figure S11A). Newly generated mature neurons in the SGZ similarly decreased in apoE4-KI mice (Figure S11B). However, at 1 month of age, apoE4-KI, apoE3-KI, wildtype, and apoE-KO mice had similar numbers of GAD67-positive GABAergic interneurons in the hilus (7527±593, 8320±804, 7740±1751, 7256±1545, n=4, p>0.05), suggesting that the effect of apoE4 on GABAergic interneurons is not due to an early developmental impairment.
ApoE4 Generates More Neurotoxic Fragments, Increases Tau Phosphorylation, and Decreases GABAergic Interneuron Survival in Primary Hippocampal Neuronal Cultures
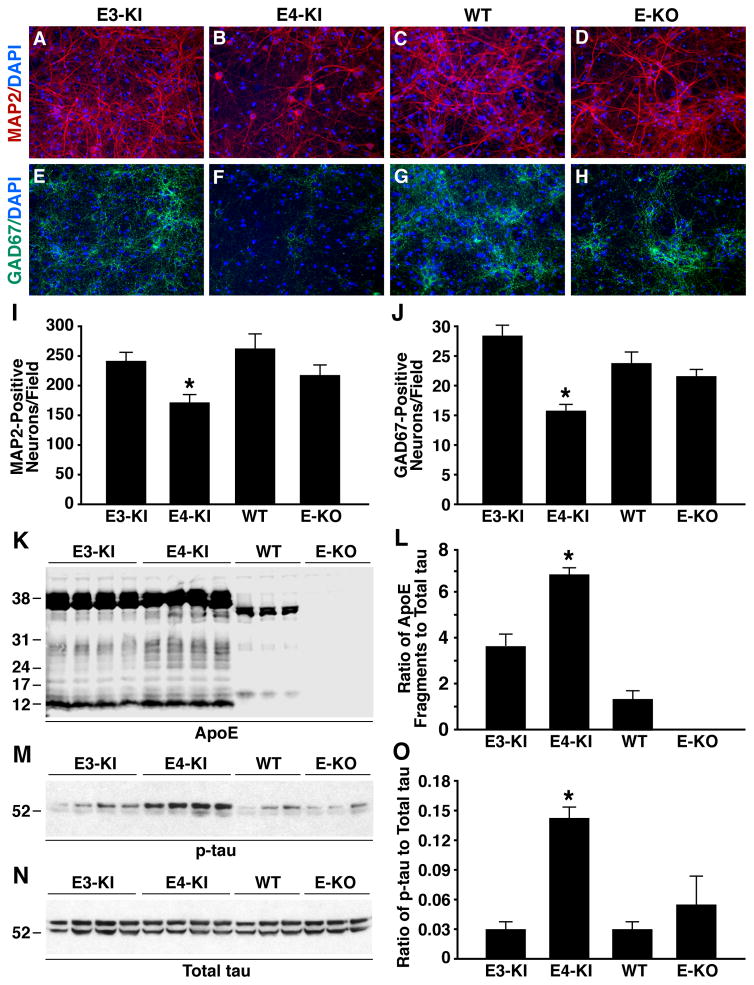
To determine the mechanisms of the detrimental effects of apoE4 on GABAergic interneurons, we analyzed primary hippocampal neurons from apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice. After 14 days of in vitro culture, immunostaining for MAP2 (a neuronal marker) and GAD67 (a GABAergic neuronal marker) revealed ~25% and ~45% lower survival of total and GABAergic neurons, respectively, from apoE4-KI mice than from apoE3-KI mice (Figures 5A–5J). We reported previously that neurons under stress, including neurons cultured in vitro (Harris et al., 2004; Xu et al., 2008), express apoE and that neuronal apoE undergoes proteolytic cleavage to generate neurotoxic fragments in vitro and in vivo, with apoE4 being more susceptible to the cleavage than apoE3 (Brecht et al., 2004; Chang et al., 2005; Harris et al., 2003; Huang et al., 2001). In the current study, significantly more apoE fragments were generated in neurons from apoE4-KI mice than in those from apoE3-KI and wildtype mice, as shown by western blot with anti-apoE (Figures 5K and 5L). The apoE4 fragmentation pattern was very similar to that in the brains of neuron-specific apoE4 transgenic mice and humans with AD (Brecht et al., 2004; Harris et al., 2003; Huang et al., 2001).
Figure 5. ApoE4 Generates More Neurotoxic Fragments, Increases Tau Phosphorylation, and Decreases GABAergic Neuron Survival in Primary Hippocampal Neuronal Cultures.
(A–H) Primary hippocampal neuron cultures were prepared from P0 pups of apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice, cultured for 14 days in vitro (14 DIV), and stained with anti-MAP2 (red) and DAPI (blue) (A–D) or anti-GAD67 (green) and DAPI (blue) (E–H). Shown are representative images from five coverslips of each genotype and five fields per coverslip (magnification, 200x).
(I, J) MAP2-positive (I) and GAD67-positive (J) neurons were quantified. Values are mean ± SEM (five images per coverslip and five coverslips per genotype). * p < 0.05 versus other groups (t test).
(K) Anti-apoE western blot of primary neuron lysates from apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice. Note that mouse apoE is 5 amino acids shorter than human apoE.
(L) ApoE fragmentation, reported as the ratio of total apoE fragments to total tau. Values are mean ± SD (n = 3–4 mice per genotype). * p < 0.001 versus other groups (t test).
(M, N) Anti-p-tau (M, AT8 monoclonal antibody) and anti-total tau (N, tau-5 monoclonal antibody) western blots of primary neuron lysates from apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice.
(O) The level of tau phosphorylation, reported as the ratio of p-tau to total tau. Values are mean ± SD (n = 3–4 mice per genotype). * p < 0.001 versus other groups (t test).
Since apoE4 fragments generated in neurons can increase tau phosphorylation, which is one of the major pathological hallmarks of AD (Tanzi and Bertram, 2001), leading to neuronal cell death in vitro and in vivo (Brecht et al., 2004; Chang et al., 2005; Harris et al., 2003; Huang et al., 2001), we determined the levels of phosphorylated tau (p-tau) in neurons from mice with different apoE genotypes. Clearly, neurons from apoE4-KI mice had significantly higher levels of p-tau than in those from apoE3-KI, wildtype, and apoE-KO mice, as shown by western blot with anti-p-tau (Figures 5M–5O). Anti-GAD67 and anti-p-tau double immunostaining revealed ~fourfold more p-tau positive GABAergic neurons from apoE4-KI mice than from apoE3-KI mice (Figures S12A–S12G), although the total numbers of GABAergic neurons were ~45% less from apoE4-KI mice than from apoE3-KI mice (Figures 5A–5J). Thus, over 70% of GABAergic neurons from apoE4-KI mice were positive for p-tau, as compared to ~10% of them from apoE3-KI mice.
To further determine the relationship of apoE4-induced tau pathology and GABAergic neuron death, we knocked down tau (~70%) in primary hippocampal neurons from apoE3-KI and apoE4-KI mice using a lentiviral tau-shRNA approach (Figures S13A–S13C). Clearly, knocking down tau significantly increased the survival of total and GABAergic neurons from apoE4-KI mice, reaching levels similar to those of neurons from apoE3-KI mice (Figures S13D–S13M). Knocking down tau did not significantly alter the survival of total and GABAergic neurons from apoE3-KI mice (Figures S13D–S13M). Thus, apoE4 impairs the survival of GABAergic interneurons by generating more neurotoxic apoE fragments and increasing p-tau levels, leading to GABAergic interneuron death, which can be fully rescued by lowering the endogenous tau.
ApoE4 Impairs GABAergic Interneuron Functions in the Dentate Gyrus
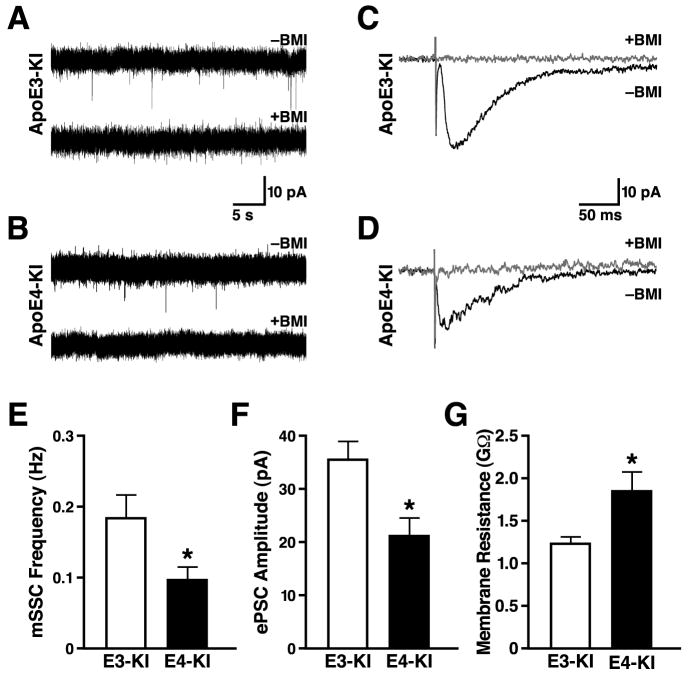
To assess the functional consequence of the decreased number of GABAergic interneurons in the hilus, we performed whole-cell patch-clamp recordings from newborn granule cells in acute slices of hippocampus from retrovirus-GFP-injected apoE3-KI and apoE4-KI mice. Two weeks after stereotaxical viral injection, when GABAergic input is critical for neuronal maturation of newborn cells (Ge et al., 2006a; Ge et al., 2006b; Liu et al., 2006; Tozuka et al., 2005), about 90% of GFP+ newborn neurons in both groups had active GABAergic spontaneous synaptic currents (SSCs). However, GFP+ neurons in apoE4-KI mice had significantly higher input resistance (Figure 6G). Since the input resistance of newborn neurons decreases as they mature (Duan et al., 2007; Espósito et al., 2005), these results suggest a delayed maturation of newborn neurons in apoE4-KI mice.
Figure 6. ApoE4 Impairs the GABAergic Electrophysiological Inputs in Newborn Neurons in the Hippocampus.
(A, B) Sample traces of mSSCs in a GFP+ neuron 2 weeks after retrovirus-GFP injection from an apoE3-KI (A) or an apoE4-KI (B) mouse during whole-cell voltage clamp recording in the presence of DNQX (20 μM), D-AP5 (50 μM), and TTX (1 μM). The mSSCs were blocked by bath application of BMI (100 μM). Scale bars, 10 pA and 5 s.
(C, D) Sample traces of ePSCs in a GFP+ neuron at 2 weeks after retrovirus-GFP injection from an apoE3-KI (C) or an apoE4-KI (D) mouse during whole-cell voltage clamp recording in the presence of DNQX (20 μM) and D-AP5 (50 μM). Currents were blocked by bath application of BMI (100 μM). Scale bars: 10 pA and 50 ms.
(E) Average mSSC frequency in GFP+ neurons was lower in apoE4-KI mice than apoE3-KI mice. Values are mean ± SD (n = 21–28 cells per genotype). * p < 0.05 vs. apoE3-KI mice (t test).
(F) Average ePSC amplitude in GFP+ neurons was lower in apoE4-KI mice than in apoE3-KI mice. Values are mean ± SD (n = 21–28 cells per genotype). * p < 0.05 vs. apoE3-KI mice (t test).
(G) Average membrane resistance of GFP+ neurons in apoE3-KI and apoE4-KI mice 2 weeks after retrovirus-GFP injection. Values are mean ± SD (n = 40 cells per genotype). * p < 0.005 vs. apoE3-KI mice (t test).
Next we assessed the level of GABAergic synaptic innervation, which is critical for neuronal development of newborn hippocampal cells (Ge et al., 2006a; Ge et al., 2006b; Liu et al., 2006; Tozuka et al., 2005). GABAergic miniature SSCs (mSSCs) were recorded from GFP+ neurons (resting membrane potential Vm = −65 mV) 2 weeks after injection of the retrovirus-GFP construct. These studies were conducted in the presence of 6,7-dinitroquinoxaline-2,3-dione (DNQX) (20 μM) and D-(−)-2-amino-5-phosphonovaleric acid (D-AP5) (50 μM) to block glutamate-mediated currents and tetrodotoxin (TTX) (1 μM) to block action potential–mediated GABA release. Bicuculline methoiodide (BMI, 100 μM) abolished the mSSCs, confirming the contribution of GABAA receptors to mSSCs (Figures 6A and 6B). mSSCs were detected in almost 90% of GFP+ neurons in apoE3-KI and apoE4-KI mice, and the mean amplitudes of mSSCs were almost identical (Figure S14D); however, the mSSC frequency was ~50% lower in apoE4-KI mice (Figure 6E), consistent with the ~50% decrease in basal GABA release in apoE4-KI mice (Figure 4H), suggesting a significant reduction of functional presynaptic GABAergic inputs on newborn neurons in apoE4-KI mice.
In the presence of DNQX and D-AP5, GABA-evoked postsynaptic currents (ePSCs) recorded from GFP+ neurons were generated by electrical stimulation of GABAergic axons in the molecular layer of the dentate gyrus. This indicates a functional coupling between hilar GABAergic interneurons and newborn neurons in both apoE3-KI and apoE4-KI mice. However, the ePSC peak amplitude was ~40% lower in apoE4-KI mice (Figures 6C, 6D, 6F). This finding is consistent with a reduction of presynaptic GABAergic inputs onto newborn neurons available for electrical activation in apoE4-KI mice. However, no significant difference in the 10–90% rise time or the ePSC decay time was observed (Figures S14A and S14B), suggesting that the GABAA receptor composition and number are not significantly altered in newborn neurons in either group. Indeed, focal application of exogenous GABA (500 μM), which bypasses presynaptic elements and allows assessment of postsynaptic GABAA receptor function, evoked similar current amplitudes in GFP+ neurons from apoE3-KI and apoE4-KI mice (Figure S14C). This finding reconfirms the intact GABAA receptor function in newborn neurons of apoE4-KI mice. These results suggest that apoE4 impairs the maturation of newborn neurons by causing GABAergic interneuron loss, which leads to impaired GABA signaling on newborn neurons.
GABAA Receptor Antagonist Inhibits Hippocampal Neurogenesis in ApoE3-KI mice and Its Potentiator Rescues the Impairment in ApoE4-KI Mice
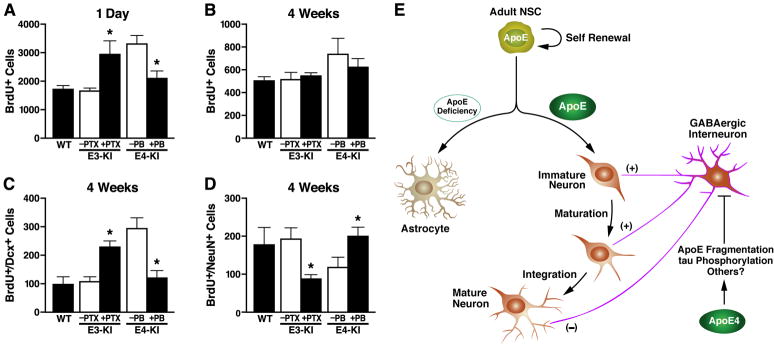
Treatment of apoE3-KI mice for 3 days with picrotoxin (PTX), a GABAA receptor antagonist (Tozuka et al., 2005), increased the number of newborn cells 1 day after BrdU injection to a level similar to that in untreated apoE4-KI mice (Figure 7A). Conversely, treatment of apoE4-KI mice with pentobarbital (PB), a GABAA receptor potentiator (Tozuka et al., 2005), decreased the number of newborn cells to levels similar to those of wildtype and untreated apoE3-KI mice (Figure 7A). Thus, the increased NSC proliferation in apoE4-KI mice likely reflects impaired GABA signaling (Liu et al., 2005; Owens and Kriegstein, 2002; Tozuka et al., 2005).
Figure 7. GABAA Receptor Potentiator Rescues ApoE4-Induced Impairment of Hippocampal Neurogenesis and A Working Model for the Roles of ApoE and Its Isoforms in Adult Hippocampal Neurogenesis.
Female apoE3-KI and apoE4-KI mice at 6–7 months of age were treated with a GABAA receptor potentiator (PB, 50 mg/kg) or antagonist (PTX, 4 mg/kg) as described in the text. BrdU-positive cells in the SGZ were counted at 1 day (A) and 4 weeks (B), and immature neurons (C) and mature neurons (D) were counted at 4 weeks. Untreated wildtype mice at 6–7 months of age served as controls. Values are mean ± SD (n = 4–6 mice per genotype). * p < 0.01 vs. untreated mice of the same apoE genotype (t test).
(E) A working model for the roles of apoE and its isoforms in adult hippocampal neurogenesis. Adult hippocampal NSCs express apoE, which plays an important role in cell fate determination of NSCs toward neuronal development. ApoE deficiency stimulates astrogenesis and inhibits neurogenesis. ApoE4 decreases hippocampal neurogenesis by inhibiting neuronal maturation of NSCs through impairing GABAergic input onto newborn neurons.
Next, apoE3-KI mice that had received BrdU injections were treated with daily injections of PTX for 7 days to inhibit GABA signaling. At 4 weeks after BrdU injection, the number of immature neurons was increased (Figure 7C) and the number of mature neurons was decreased (Figure 7D) in the hippocampus of apoE3-KI mice to levels similar to those in untreated apoE4-KI mice; the survival of newborn cells was unaltered (Figure 7B). Conversely, stimulation of GABA signaling with daily injections of PB for 4 weeks decreased the number of immature neurons and increased the number of mature neurons in apoE4-KI mice, to levels similar to those in wildtype and untreated apoE3-KI mice (Figures 7C and 7D). Importantly, this stimulation also improved the dendritic development of newborn neurons of apoE4-KI mice, as reflected by increases in both dendritic length and branch number to levels similar to those in wildtype and apoE3-KI mice (Figures 3G–3K). These results further support a non-cell-autonomous effect of apoE4 on hippocampal neurogenesis from NSCs. Interestingly, stimulation of GABA signaling with PB in wildtype mice also showed a trend toward significant increase (p = 0.053) in the number of dendritic branches of newborn neurons (Figure 3E and 3I) although the same treatment did not alter the length or number of dendritic branches of newborn neurons in apoE3-KI mice (Figure 3F, 3H, 3I). Thus, treatment with a GABAA receptor potentiator rescues the impairment of hippocampal neurogenesis associated with GABAergic interneuron dysfunction due to apoE4.
DISCUSSION
In this study, we demonstrate that adult NSCs in mice express apoE. Adult apoE-KO mice had significantly less hippocampal neurogenesis, but significantly more astrogenesis, than wildtype mice due to decreased Noggin expression in NSCs. Further, apoE4 inhibited neuronal maturation by impairing the survival and function of GABAergic interneurons in the hilus of the hippocampus, and a GABAA receptor potentiator rescued the apoE4-associated impairment of hippocampal neurogenesis. Thus, apoE plays an important role in adult hippocampal neurogenesis, and apoE4 inhibits this process by impairing GABAergic input to newborn neurons (Figure 7E). A recent gene expression microarray analysis also showed expression of apoE in NSCs in mouse brains (Zhang et al., 2008). Knockdown of orphan nuclear receptor TLX, which reduces NSC proliferation, increased apoE expression in NSCs more than fourfold (Zhang et al., 2008); treatment with retinoic acid, which promotes the early stage of neurogenesis, upregulated apoE expression in NSCs two- to fivefold (Jacobs et al., 2006), again suggesting the importance of apoE in neurogenesis.
Although both apoE deficiency and apoE4 impaired hippocampal neurogenesis, they acted in different ways (Figure 7E). ApoE deficiency decreased the BMP antagonist Noggin expression in NSCs, leading to stimulation of astrogenesis and inhibition of neurogenesis, but did not affect NSC number and proliferation or GABA signaling–mediated neuronal maturation. Thus, apoE is important in directing NSCs toward neuronal development. Importantly, the effect of apoE on cell fate determination of NSCs was cell autonomous. On the other hand, apoE4 impaired the survival of GABAergic interneurons, leading to decreased GABAergic input onto newborn neurons and thereby inhibiting neuronal maturation of NSCs and neurogenesis; however, it did not affect the cell fate determination of NSCs. Thus, the effect of apoE4 on neuronal maturation of NSCs was non-cell-autonomous.
GABAergic interneurons synthesize and release the neurotransmitter GABA and contribute substantially to the inhibitory regulation of the adult neuronal network (Owens and Kriegstein, 2002). However, in adult hippocampus, as in the embryonic nervous system, GABAergic neurotransmission in NSCs and immature neurons is excitatory, owing to their high intracellular concentration of chloride (Ganguly et al., 2001; Ge et al., 2006a; Ge et al., 2006b; Liu et al., 2006; Tozuka et al., 2005). GABAergic excitation is critical for the maturation of newborn neurons and for their dendritic and synaptic integration into existing neuronal circuits (Ge et al., 2006a; Ge et al., 2006b; Liu et al., 2006; Tozuka et al., 2005). In genetic and pharmacological studies in mice, inhibition of GABAergic interneuron function greatly reduced the maturation and functional integration of newborn granule neurons in the hippocampus (Ge et al., 2006a; Ge et al., 2006b; Liu et al., 2006; Tozuka et al., 2005), as in apoE4-KI mice. Conceivably, the maturation and functional integration of newborn neurons in apoE4-KI mice are impaired by insufficient GABAergic stimulation resulting from GABAergic interneuron loss and dysfunction.
In the brain, apoE has been implicated in lipid metabolism and neuronal repair and remodeling (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). However, the apoE isoforms differ in their ability to fulfill these functions (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). In general, apoE3 is neurotrophic or neuroprotective, while apoE4 and its fragments are neurotoxic (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). Accumulating evidence suggests that this neurotoxicity causes neurodegeneration, contributing to AD pathogenesis (Huang, 2006a, b; Huang et al., 2004; Mahley et al., 2006). Our current study shows that apoE4, the major known genetic risk factor for AD (Corder et al., 1993; Saunders et al., 1993), can also cause GABAergic interneuron dysfunction, leading to impaired hippocampal neurogenesis. The neurotoxicity induced by apoE4 fragments increases tau phosphorylation, leading to GABAergic interneuron impairment, as demonstrated by the current study (Figure 7E). The major function of tau, which is abundant in the axons of neurons, is to bind and stabilize microtubules (Lee et al., 2001). Phosphorylation of tau prevents it from binding with microtubules, leading to microtubule destabilization, neurofibrillary tangle formation, and neurodegeneration, as shown in tau transgenic mice and in AD brains (Götz et al., 1995; Ishihara et al., 1999; Lee et al., 2001). In addition, apoE4 or its fragments could also impair the receptor-mediated cellular signaling pathways and/or alter lipid metabolism in the brain, contributing to GABAergic interneuron impairment. Thus, besides causing neurodegeneration, apoE4 impairs the regeneration of new neurons in the adult hippocampus, which may also contribute to the pathogenesis of AD.
EXPERIMENTAL PROCEDURES
Animals
Wildtype and apoE-KO mice were from the Jackson Laboratory (Bar Harbor, Maine). Human apoE3-KI and apoE4-KI mice, which were reported previously (Sullivan et al., 1997; Sullivan et al., 2004), were from Taconic (Hudson, NY). GFAP-apoE3 and GFAP-apoE4 transgenic mice were generated at Gladstone (Brecht et al., 2004). The EGFPapoE reporter mice were reported previously (Xu et al., 2006) and described in detail in Supplemental Data.
BrdU Injection and Collection of Mouse Brains
Female mice (3, 6–7, or 12–13 months of age) received two intraperitoneal injections of BrdU (Sigma, 100 mg/kg body weight) 6 h apart. One day, 3 days, and 4 and 10 weeks after the second injection, the brains were perfused with phosphate-buffered saline and collected.
Treatment of Mice with GABAA Receptor Potentiator or Antagonist
To investigate the effect of GABAA receptor potentiator or antagonist treatment on NSC proliferation, we treated female mice (6–7 months of age) with once-daily intraperitoneal injections of PB (Sigma, 50 mg/kg; apoE4-KI mice) or PTX (Sigma, 4 mg/kg; apoE3-KI mice). Twenty-four hours after the last treatment, mice received two injections of BrdU (100 mg/kg) 6 h apart and were sacrificed 1 day later. To investigate the effect of GABAA receptor potentiator or antagonist treatment on neuronal maturation, female mice (6–7 months of age) received two injections of BrdU 6 h apart. Twenty-four hours later, apoE3-KI mice received daily injections of PTX for 7 consecutive days, and apoE4-KI mice received daily injections of PB for 28 consecutive days. Mice were sacrificed 4 weeks after the second BrdU injection.
Stereotaxic Surgery of Engineered Retrovirus Expressing GFP
Engineered self-inactivating murine retrovirus expressing GFP was used to specifically label proliferating cells and their progeny (van Pragg et al., 2002). Virus was prepared by cotransfecting three constructs (encoding GFP, vesicular stomatitis virus-glycoprotein (VSV-G), and gag/pol) into HEK293T cells, purified by centrifugation, and stereotaxically injected into the dentate gyrus of wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice as described in Supplemental Data. Mice were sacrificed 2 weeks after injection for electrophysiologic studies and 4 weeks after injection for studies of dendritic development.
Immunostaining and Quantification of Neurogenesis and Dendritic Development of Newborn Neurons and GABAergic Interneurons
Mouse brains were fixed in 4% paraformaldehyde for 3 days, and coronal sections (40 m) were cut continuously throughout the entire hippocampus with a vibratome. Every eighth section was immunostained with various primary and secondary antibodies and examined with a laser-scanning confocal system as described in Supplemental Data. Single- or double-immunostained cells on both sides of the hippocampus of all stained sections were counted and calculated as described in Supplemental Data. Numbers of newborn cells (BrdU+) and immature neurons (BrdU+ and Dcx+) in the SGZ were determined 1 or 3 days after BrdU injection; numbers of surviving newborn cells (BrdU+) and mature neurons (BrdU+ and NeuN+) in the SGZ were determined 4 or 10 weeks after injection. Newborn neurons that had migrated from the SVZ to the OB were quantified 4 weeks after BrdU injection as reported (Galvão et al., 2008) and described in Supplemental Data. The number of GABAergic interneurons in the hilus of the dentate gyrus was determined by counting GAD67- and somatostatin-positive cells as described in Supplemental Data.
The dendritic structure of GFP+ newborn neurons was imaged with a confocal microscope, and the dendritic processes were reconstructed in three dimensions by merging Z-series stacks of 10–18 sections as described in Supplemental Data. All GFP+ neurons with largely intact dendritic trees were analyzed for total dendritic length and branch number (Duan et al., 2007; Ge et al., 2006a).
Primary Hippocampal Neuronal Culture and Quantification of Neuronal Survival
Primary hippocampal neuronal cultures were prepared from P0 pups of homozygous apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice (Chen et al., 2005) as described in Supplemental Data. In some experiments, primary neurons were transduced with lenti-tau-shRNA viruses (Open Biosystems) at 5 days in culture to knock down tau expression. After 14 days in vitro, the cultures were fixed in 4% paraformaldehyde and immunostained for anti-MAP2 and anti-GAD67 as described in Supplemental Data. To measure neuronal survival in hippocampal neuron cultures, MAP2- and GAD67-positive neurons were counted in 15–30 random fields under a fluorescence microscope (200x magnification) (Chen et al., 2005). In parallel experiments, human full-length apoE, p-tau, and total tau in cell lysates were analyzed by western blotting (Brecht et al., 2004; Huang et al., 2001) and quantified as described in Supplemental Data.
NSC Culture and Neural Differentiation in vitro
NSCs were isolated from brains of apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice at postnatal day 1 (P1) using a modified neurosphere method (Brewer and Torricelli, 2007; Ray and Gage, 2006). The expression of apoE and Noggin in NSCs with different apoE genotypes was assessed by anti-apoE and anti-Noggin (R&D Systems) western blots of cell lysates. Neural differentiation of the established NSCs with different apoE genotypes was induced in vitro as reported (Song et al., 2002). In some experiments, recombinant mouse Noggin (R&D Systems) was added at 500 ng/ml into the medium during neural differentiation. Neuronal and astrocytic differentiation of NSCs at 7 days was determined and quantified as described in Supplemental Data.
Mass Spectrometry Analysis of GABA Release
Female apoE3-KI and apoE4-KI mice (6–7 months of age) were decapitated, and the hippocampi were isolated and sliced. The slices were preincubated for 10 min at 37°C in 200 μl of 95% O2- and 5% CO2-saturated basal medium with Eagle’s salts (BME). The basal and depolarization-evoked GABA release were determined by matrix-assisted laser desorption/ionization mass spectrometry (Bolteus and Bordey, 2004) as described in Supplemental Data.
Electrophysiology
ApoE3-KI and apoE4-KI mice (2–3 months) were sacrificed 2 weeks after retrovirus-GFP injection and processed for slice preparation as described (Duan et al., 2007; Ge et al., 2006a). More details are described in Supplemental Data. Whole-cell voltage-clamp recordings from visually identified GFP+ neurons (2 weeks after injection) were obtained with an infrared differential interference contrast video microscopy system as described in Supplemental Data.
Statistical Analysis
Values are expressed as mean ± SD. The statistical significance of the difference between means was assessed with unpaired, two-sample t tests. The statistical significance of the difference in total dendritic length or dendritic branch number was assessed by the Kolmogorov-Smirnov test. p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the J. David Gladstone Institutes, grants T2-00003 and RN2-00952-1 from the California Institute of Regenerative Medicine, and grant P01 AG022074 from the National Institutes of Health. The retroviral construct encoding GFP was a generous gift of Dr. Fred H. Gage (Salk Institute for Biological Studies, La Jolla, CA). We thank John Carroll and Alisha Wilson for graphics, Linda Turney for manuscript preparation, and Stephen Ordway and Gary Howard for editorial assistance. The authors declare there are no competing financial interests in relation to this work. The study was designed by G.L., N.B., Y.A., and Y.H. Experiments were performed by G.L, N.B., Y.A., Q.X., A.B., K.R., C.D., and Y.H.. Data were analyzed and interpreted by G.L., N.B., Y.A., Q.X., B.H., R.M., and Y.H.. G.L., N.B., and Y.H. wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Altman J, Dasq GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, Wakayama Y. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34:875–880. doi: 10.1161/01.STR.0000064320.73388.C6. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retentioin deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Fish JD, Wyss-Coray T, Buttini M, Mucke L, et al. Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protocols. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DEC, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related momory decline and the APOE 4 effect. N Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Ma TR, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid- toxicity through inhibiting NF-κB signaling. j Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Lüscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Erikkson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Xu H, Cai W, Yang Z, Zhang J. Spatial and temporal patterns of expression of Noggin and BMP4 in embryonic and postnatal rat hippocampus. Developmental Brain Res. 2003;146:51–58. doi: 10.1016/j.devbrainres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo MM. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006a;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2006b;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Götz J, Probst A, Spillantini MG, Schäfer T, Jakes R, Bürki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 1995;14:1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Tesseur I, Brecht WJ, Xu Q, Mullendorff K, Chang S, Wyss-Coray T, Mahley RW, Huang Y. Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- Huang Y. Apolipoprotein E and Alzheimer disease. Neurology. 2006a;66(Suppl 1):S79–S85. doi: 10.1212/01.wnl.0000192102.41141.9e. [DOI] [PubMed] [Google Scholar]
- Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr Opin Drug Discov Dev. 2006b;9:627–641. [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E. Diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VMY. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev PharmacolToxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylia A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55:134–136. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: Increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31:560–573. doi: 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Liedo PM. Enriched odor exposure inceases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hppocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in Adult Neurogenesis. Annu Rev Cell Dev Biol. 2009;25:11.1–11.23. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Tang J, Song M, Wang Y, Fan X, Xu H, Bai Y. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APPswe/PSIΔE9 transgenic mouse model of Alzheimer's disease. Biochemical & Biophysical Res Communications. 2009;385:341–345. doi: 10.1016/j.bbrc.2009.05.067. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32:181–184. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- van Pragg H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiation Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Xu PT, Gilbert JR, Qiu HL, Ervin J, Rothrock-Christian TR, Hulette C, Schmechel DE. Specific regional transcription of apolipoprotein E in human brain neurons. Am J Pathol. 1999;154:601–611. doi: 10.1016/S0002-9440(10)65305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (apoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the apoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Walker D, Bernardo A, Brodbeck J, Balestra ME, Huang Y. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci. 2008;28:1452–1459. doi: 10.1523/JNEUROSCI.3253-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Naure. 2008;451:1004–1009. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.