Abstract
Purpose
We evaluated the chemotherapeutic effect of water extract of white cocoa tea (WCTE) against human prostate cancer (PCa) in vitro and in vivo.
Methods
Cell viability and cell cycle distribution were determined by MTT assay and flow cytometry, respectively. Western blotting was performed to determine changes in levels of various proteins. Effect of WCTE was determined in athymic nude mice implanted with PC-3 cells.
Results
Treatment with WCTE (100–150 μg/ml) inhibited cell proliferation, which correlated with G2/M phase arrest in PC-3 cells. WCTE treatment to PC-3 cells resulted in (1) induction of WAF1/p21 and KIP1/p27, (2) decrease in cyclins D1, D2 and E, (3) decrease in cyclin-dependent kinase (cdk) 2, 4 and 6, (4) induction of Bax and down-regulation of Bcl-2, (5) decrease in procaspase-3, −8, (6) inhibition of nuclear translocation and phosphorylation of NF-κB and activation of IKKα, and (7) inhibition of phosphorylation and degradation of IκBα. Oral administration of WCTE (0.1 and 0.2%, wt/vol) to athymic nude mice resulted in greater than 50% inhibition of tumor growth. There was a decrease in expressions of cyclin D1, Bcl-2 and p-NF- κB and an increase in WAF1/p21 and Bax in tumor tissues of mice.
Conclusion
WCTE can be a useful chemotherapeutic agent against human PCa.
Keywords: apoptosis, cell cycle arrest, prostate cancer, white cocoa tea
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy and the second leading cause of male death in the U.S. and many European countries. The American Cancer Society has estimated that 192,280 men in the U.S. will be diagnosed with prostate cancer and 27,360 men will die of the disease in 2009, and about 1 in 6 men will develop the disease during his lifetime (1). Although Asians have the lowest incidence and mortality rates of PCa in the world, the rates have risen rapidly in the past two decades in many Asian countries (2,3). It is therefore necessary to intensify our efforts to better understand this disease and develop novel approaches for its prevention and treatment. In recent years, many dietary agents have been described that show a wide range of anti-cancer effects against PCa. One such agent is tea (Camellia sinensis), which is the most widely consumed beverage in the world, only next to water (4–8).
Cocoa tea (Camellia ptilophylla) is a naturally decaffeinated tea plant which grows in southern China. The local people have habitually drank cocoa tea for a long time, not only as a healthy beverage but also as a traditional remedy for ailments. An earlier study showed that cocoa tea had significant cytotoxic effect on Hela, CNE2 and MGC-803 cell lines and had an inhibitory effect against Ehrlich solid carcinoma in mice (9). It also had an inhibitory effect on DNA polymerase of Ehrlich ascite tumor cells (10). A recent study showed cocoa tea had hypolipemic activity and changed the ambulatory behaviors when administered in combination with green tea (11,12). Cocoa tea belongs to Camellia Sect. Thea. Dyer and has a similar chemical profile as tea (Camellia sinensis). However, cocoa tea contains theobromine instead of caffeine, and the major catechin is gallocatechin gallate (GCG) instead of EGCG (13,14). We have also found that cocoa tea contained more tea polyphenols and its antioxidant activity was higher than other teas (data not shown).
We hypothesized that cocoa tea may afford cancer-chemotherapeutic effects against PCa. We selected three main types of cocoa for our initial study: white cocoa tea exhibited strong inhibition of cell growth in PCa PC-3 cells in the MTT assay, compared to green cocoa tea and black cocoa tea. Therefore, we selected white cocoa tea for further studies.
In this study, we show that water extract of white cocoa tea (WCTE) derived from Camellia ptilophylla, through modulations in the cyclin kinase inhibitor-cyclin-cyclin-dependent kinase (cdk) machinery, resulted in the inhibition of cell growth followed by apoptosis of highly aggressive human PCa PC-3 cells. These events were found to be associated with alterations in the levels of Bax and Bcl-2, shifting the Bax/Bcl-2 ratio in favor of apoptosis. Next, we determined the chemotherapeutic potential of WCTE on phosphorylation and activation of NF-κB in PCa PC-3 cells. In the next series of experiments, we show that oral administration of WCTE to athymic nude mice implanted with PC-3 cells resulted in significant inhibition of tumor growth.
MATERIALS AND METHODS
Materials
Bax, Bcl-2, IκBα, IκBα (phospho), anticyclins D1, D2 and E cdks 2, 4 and 6, WAF1/p21 and KIP1/p27, p-NF-κB/p65 antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). The mono and polyclonal antibodies IKKα, Procaspase 3, 8 and NF-κB/p65 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-mouse and anti-rabbit secondary antibody horseradish peroxidase (HRP) conjugate was obtained from Amersham Life Science Inc. (Arlington Height, IL, USA). BCA Protein assay kit was obtained from Pierce (Rockford, IL, USA). Novex precast Tris–glycine gels were obtained from Invitrogen (Carlsbad, CA, USA). The Apo-Direct kit for flow cytometry was purchased from Phoenix Flow Systems (San Diego, CA, USA). Matrigel was procured from BD Bioscience.
Preparation and Composition of Cocoa Tea
White cocoa tea leaves (12.5 g) were added to 500 ml of boiling water and were steeped for 15 min. The infusion was cooled to room temperature and then filtered. The tea leaves were extracted a second time with 500 ml of boiling water and filtered, and the two filtrates were combined to obtain a 1.25% white tea water extract (1.25 g of tea leaves/100 ml of water) (15). Then the aqueous tea extracts were concentrated under vacuum and freeze–dried. The dark-yellow solid matter was called WCTE powder. Catechins and alkaloids in the tea extracts were determined as described (14). WCTE was found to mainly contain (−)-gallocatechin gallate, (−)-gallocatechin, (+)-catechin, (−)-epigallocatechin gallate and theobromine. No caffeine was detected, and the content of GCG was 20% (data not shown). Furthermore, WCTE contained more catechins than green cocoa tea and black cocoa tea (date not shown). Since the content of GCG in WCTE was 20%, the concentration of WCTE used in our experiments at 100, 125 and 150 μg/ml is equivalent to 44, 55 and 66 μM of GCG, respectively.
Cell Culture and Treatment
Human PCa PC-3 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotics. The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment. WCTE was dissolved in hot water and was used for the treatment of cells. A total of 50–60% confluent cells were treated with WCTE (100–150 μg/ml) for 72 h in complete growth medium.
Cell Viability
The effect of WCTE on the viability of cells was determined by the MTT assay. The cells were plated at 1×104 cells per well in 200 μl of complete culture medium containing 100–150 μg/ml concentrations of WCTE in 96-well microlitre plates for 72 h. After incubation for specified times at 37°C in a humidified incubator, 3-[4,5-dimethylth-iazol-2-yl]-2,5-diphenyl tetrazoliumbromide (5 mg/ml in PBS) was added to each well and incubated for 2 h, after which the plate was centrifuged at 1,000 rpm for 5 min at 4°C. After careful removal of the medium, 0.1 ml buffered DMSO was added to each well. The absorbance was recorded on a microplate reader at the wavelength of 540 nm. The effect of WCTE on growth inhibition was assessed as percent cell viability where DMSO-treated cells were taken as 100% viable. DMSO at the concentrations used was without any effect on cell viability.
DNA Cell Cycle Analysis
The cells (50–60% confluent) were treated with WCTE (100–150 μg/ml) for 72 h in complete medium. The cells were trypsinized thereafter, washed twice with cold PBS and centrifuged. The cell pellet was resuspended in 50 μl cold PBS to which cold methanol (450 μl) was added, and the cells were incubated for 1 h at 4°C. The cells were centrifuged at 1,000 rpm for 5 min, pellet washed twice with cold PBS, suspended in 500 μl PBS and incubated with 5 μl RNase (20 μg/ml final concentration) for 30 min. The cells were chilled over ice for 10 min and incubated with propidium iodide (50 μg/ml final concentration) for 1 h for analysis by flow cytometry. Flow cytometry was performed with a FACScan (Becton Dickinson, Germany). A minimum of 10,000 cells per sample were collected, and the DNA histograms were further analyzed by using ModiFitLT software (Verily Software House, Topsham, ME, USA) for cell cycle analysis.
Preparation of Cytosolic and Nuclear Lysates
Following treatment of cells with WCTE (100–150 μg/ ml;72 h), the medium was aspirated, and the cells were washed twice in PBS (10 mM, pH 7.4). The cells were incubated in 0.2 ml ice-cold lysis buffer (HEPES (10 mM, pH 7.9), KCl (10 mM), EDTA (0.1 mM), EGTA (0.1 mM), DTT (1 mM), PMSF (1 mM) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA, USA) for 15 min, after which 12.5 μl of 10% Nonidet P-40 was added and the contents were mixed on a vortex and then centrifuged for 1 min (14,000 g) at 4°C. The supernatant was saved as cytosolic lysate and stored at −80°C. The nuclear pellet was resuspended in 50 μl of ice-cold nuclear extraction buffer [HEPES (20 mM, pH 7.9), NaCl (0.4 M), EDTA (1 mM), EGTA (1 mM), DTT (1 mM), PMSF (1 mM)] with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem) for 30 min with intermittent mixing. The tubes were centrifuged for 5 min (14,000 g) at 4°C, and the supernatant (nuclear extract) was stored at −80°C.
Protein Extraction and Western Blotting
Following the treatment of cells as described above, the media were aspirated, the cells were washed with cold PBS (pH 7.4) and ice-cold lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1%Triton X-100, 1 mM PMSF (pH 7.4) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem) over ice for 30 min. The cells were scraped, and the lysate was collected in a microfuge tube and passed through needle to break up the cell aggregates. The lysate was cleared by centrifugation at 14,000 g for 15 min at 4°C, and the supernatant (whole cell lysate) was used or immediately stored at −80°C.
For western blotting, 30–50 μg protein was resolved over 12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% non-fat dry milk/1% Tween 20; in 20 mM TBS, pH 7.6) for 1 h at room temperature, incubated with appropriate monoclonal or polyclonal primary antibody in blocking buffer for 1.5 h to overnight at 4°C, followed by incubation with anti-mouse or anti-rabbit secondary antibody HRP conjugate obtained from Amersham Life Science Inc. and detected by chemiluminescence and autoradiography using XAR-5 film obtained from Eastman Kodak Co. (Rochester, NY, USA). Densitometric measurements of the band in western blotting analysis were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT, USA).
In Vivo Tumor Xenograft Model
Athymic (nu/nu) male nude mice were obtained from NxGen Biosciences (San Diego), housed under pathogen-free conditions with a 12 h light/12 h dark schedule, and fed with an autoclaved diet ad libitum. To establish tumor xenografts in mice, PC-3 (1×106) cells were suspended in 1:1 medium mixed with Matrigel and were s.c. inoculated on the left and right flanks of each mouse. Eighteen animals were then randomly divided into three groups consisting of six animals each. The first group of animals received drinking water and served as controls. The animals of Groups 2 and 3 received the same drinking water supplemented with 0.1% and 0.2% white cocoa tea (wt/ vol), respectively. Freshly prepared solution of cocoa tea in water was supplied every Monday, Wednesday, and Friday. This feeding regimen is well tolerated by animals and has been used in mice in various studies (16,17). Body weight, diet and water consumption were recorded twice weekly throughout the study. Tumor sizes were measured twice weekly, and tumor volume was calculated by the formula 0.5238×L1×L2×H, where L1 is the long diameter, L2 is the short diameter and H is the height of the tumor (18). Tumors were collected after the animals were sacrificed and stored at −80°C until further study.
Statistical Analysis
Results were analyzed using a two-tailed Student’s t-test to assess statistical significance, and P-values <0.05 were considered significant. The Kaplan–Meier method was used to estimate survival, and differences were analyzed by the log-rank test.
RESULTS
Inhibition of Cell Growth and Induction of Apoptosis by WCTE in PC-3 Cells
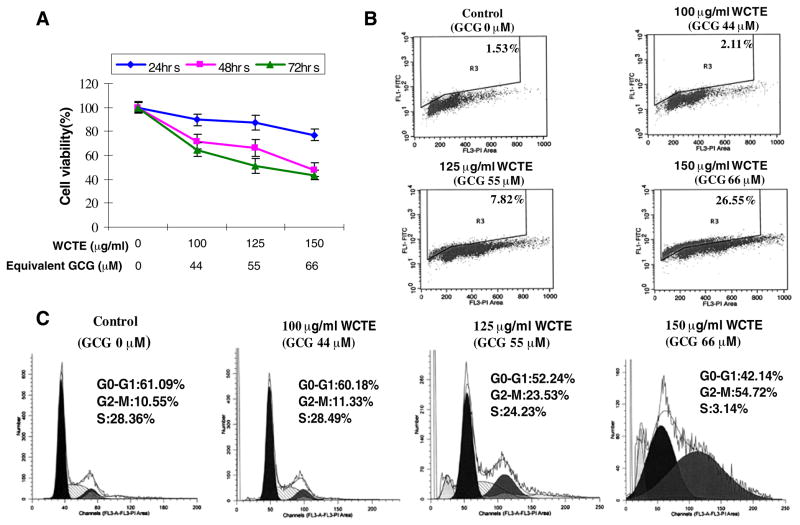
To evaluate the effect of WCTE on cell viability of human PCa cells, we performed MTT assay. The cells were treated with varying concentrations of WCTE at 100, 125 and 150 μg/ml for 24, 48 and 72 h. WCTE treatment to PC-3 cells resulted in a significant dose-dependent and time-dependent inhibition of cell growth. As shown in Fig. 1A, WCTE treatment of PC-3 cells resulted in a 35, 49 and 57% decrease in cell viability at the dose of 100–150 μg/ml of WCTE after 72 h, respectively. The IC50 value at 24, 48 and 72 h post-treatment with WCTE for PC-3 cells was 214, 153 and 131 μg/ml, respectively.
Fig. 1.
(A) Effect of WCTE on cell growth. As detailed in Materials and Methods, PC-3 cells were treated with WCTE and the viablility of cells was determined by the MTT assay. The data are expressed as the percentage of cell viability and represent the means ± SE of three experiment in which each treatment was performed in multiple wells. (B) Effect of WCTE on apoptosis in PC-3 cells. (C) Effect of WCTE on cell cycle distribution in PC-3 cells. The cells treated with WCTE were collected and stained with PI by using an apoptosis APO-DIRECT kit obtained from Phoenix Flow systems as per vendor’s protocol followed by flow cytometry. Following FACS analysis, cellular DNA histograms were further analyzed by ModfitLT V3.0. The data are representative example for duplicate tests. The details are described in Materials and Methods.
To test whether WCTE-mediated decrease in cell growth is due to induction of apoptosis, we conducted Annexin V and propidium iodide stainings in WCTE-treated cells. As shown by data in Fig. 1B, WCTE treatment of PC-3 cells resulted in 2, 8 and 26% increases in TUNEL-positive cells at 100, 125 and 150 μg/ml WCTE, respectively, suggesting a significant induction of apoptosis by WCTE at dose of 100–150 μg/ml in PC-3 cells.
G2-M Phase Cell Cycle Arrest by WCTE in PC-3 Cells
Several studies have shown that the induction of apoptosis might be due to cell cycle arrest (19, 20). Inhibition of the cell cycle has been appreciated as a target for the management of cancer. To assess the effect of WCTE on the distribution of cells in the cell cycle, we preformed DNA cell cycle analysis. As shown in Fig. 1C, WCTE treatment resulted in a dose-dependent accumulation of cells in the G2-M phase of the cell cycle by 11%, 23% and 55% at 100, 125 and 150 μg/ml concentration of WCTE, respectively. These data suggest that WCTE induces cell cycle arrest in G2-M phase.
Inhibition of Cyclins, Cdks and Induction of WAF1/p21 and KIP1/p27 by WCTE in PC-3 Cells
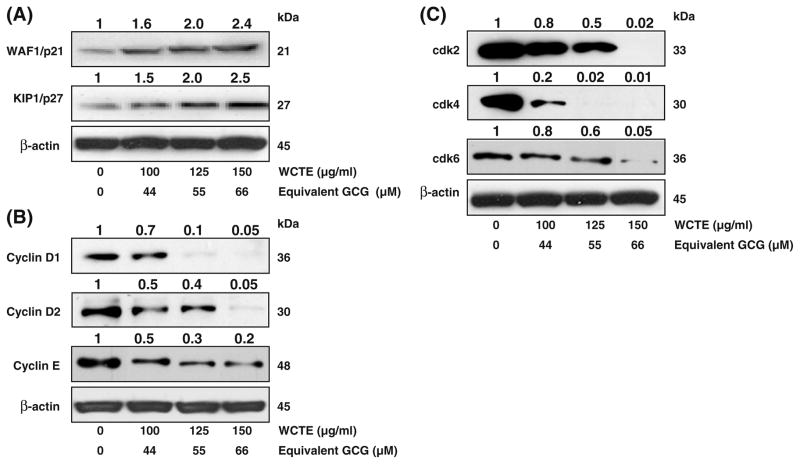
Molecular analysis of human cancers has revealed that cell cycle regulators are frequently mutated in most common malignancies. We examined the effect of WCTE on cell cycle inhibitory proteins KIP1/p27 and WAF1/p21, which are involved in cell cycle progression. Western blotting analysis showed a significant induction of these proteins in a dose-dependent manner (Fig. 2A). We next evaluated the effect of WCTE on the protein levels of cyclins and cdks, which are known to be regulated by KIP1/p27 and WAF1/p21. WCTE treatment of cells resulted in a significant dose-dependent decrease in the protein levels of cyclin D1, D2 and E as well as cdk 2, 4 and 6 (Fig. 2B, C). These results suggested that WCTE restored proper checkpoint control via modulation of the cyclins, cdks and the expression of their inhibitors.
Fig. 2.
(A) Effect of WCTE on protein expression of WAF1/p21 and KIP1/p27 in PC-3 cells. (B) Effect of WCTE on protein expression of cyclin D1, cyclin D2 and E in PC-3 cells. (C) Effect of WCTE on protein expression of cdk2, cdk4 and cdk6 in PC-3 cells. As detailed in Materials and Methods, the cells were treated with WCTE (100–150 μg/ml) and then harvested. Total cell lysates were prepared and 50 μg protein was subjected to SDS-PAGE followed by western blotting analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the western blotting and reprobing it for β-actin. The western blotting shown here are representative of three independent experiments with similar results. The values above the figures represent relative density of the bands normalized to β-actin.
Induction of Bax and Inhibition of Bcl-2 and Procaspases in PC-3 Cells
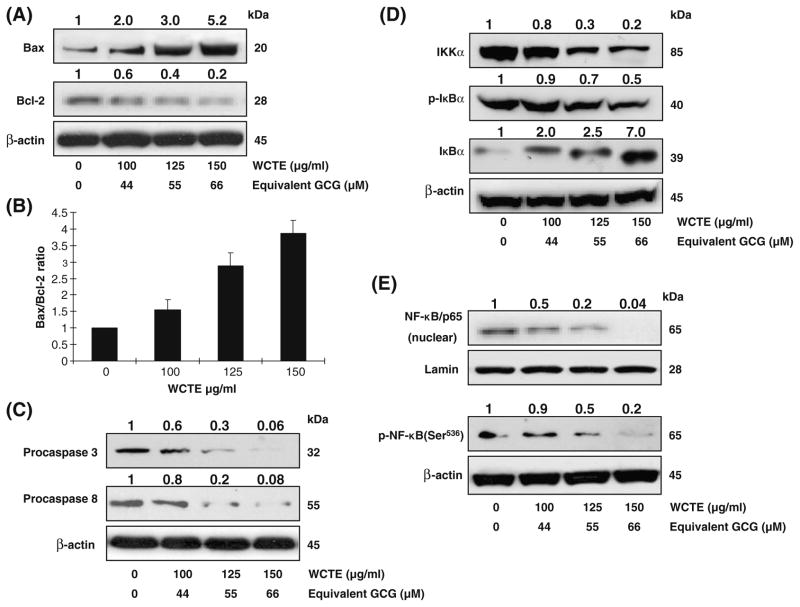
Because Bax and Bcl-2 play crucial roles in apoptosis, we studied the dose-dependent effect of WCTE on the protein levels of Bax and Bcl-2 in PC-3 cells. As shown in Fig. 3A, WCTE caused an increase in the protein expression of proapoptotic Bax and a decrease in the antiapoptotic Bcl-2 in PC-3 cells. Modulations in the expressions of Bax and Bcl-2 by WCTE resulted in change in the ratio of these molecules in a way that favored apoptosis (Fig. 3B). Using western blotting analysis, we also observed a significant decrease in the expression of procaspase-3 and −8 on treatment of PC-3 cells with WCTE (Fig. 3C).
Fig. 3.
(A) Effect of WCTE on protein expression of Bax, Bcl-2 in PC-3 cells. (B) Bax to Bcl-2 ratio. (C) Effect of WCTE on protein expression of caspase-3 and -8 in PC-3 cells. (D) Effect of WCTE on IKKα and phosphorylation and degradation of IκBα in PCa PC-3 cells. (E) Effect of WCTE on activation of NF-κB PCa PC-3 cells by western blotting analysis. As detailed in Materials and Methods, the cells were treated with WCTE (100–150 μg/ml) and then harvested. Total cell lysates were prepared and 50 μg protein was subjected to SDS-PAGE followed by western blotting analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the western blot and reprobing it for β-actin. The western blots shown here are representative of three independent experiments with similar results. The values above the figures represent relative density of the bands normalized to β-actin.
Inhibition of NF-κB Pathway by WCTE in PC-3 Cells
It has been shown that activation of NF-κB blocks apoptosis and promotes cell proliferation. One of the critical events in NF-κB activation is its dissociation with subsequent degradation of inhibitory protein IκBα via phosphorylation and ubiquitination. The treatment of PC-3 cells with WCTE (100–150 μg/ml) resulted in a significant inhibition in the phosphorylation of IκBα protein (Fig. 3D). To evaluate the possible inhibitory mechanism of WCTE on IκBα protein degradation, we measured IKKα protein level. Western blotting analysis showed that pre-treatment of PC-3 cells with WCTE inhibited IKKa in a dose-dependent manner (Fig. 3D). Then, we tested whether WCTE treatment inhibits constitutive NF-κB activation. Using Western blotting analysis, we observed that WCTE treatment of cells resulted in decreased phospho-NF-κB/p65 at Ser536 and inhibition of translocation of NF-κB/p65 in the nuclear fraction (Fig. 3E).
Inhibition on the Growth of Human PCa PC-3 Cells by WCTE in Athymic Nude Mice
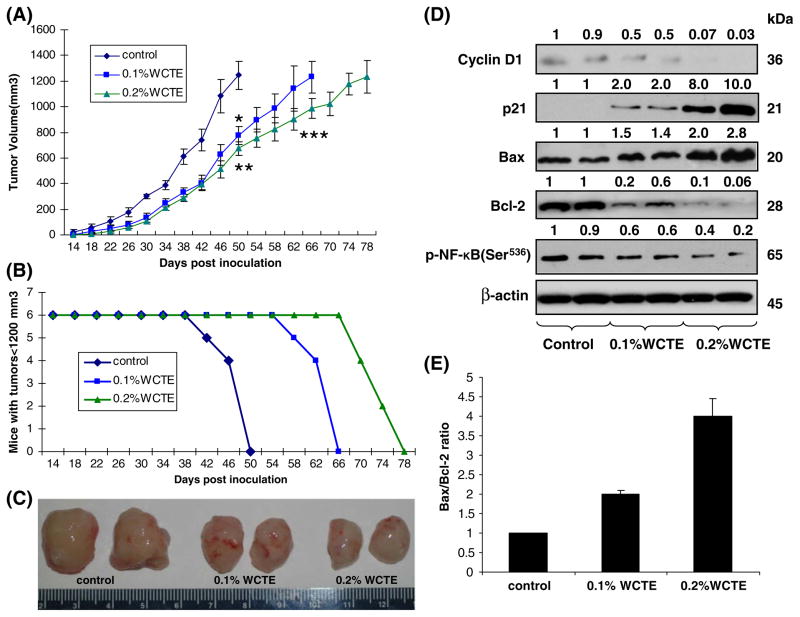
The treatment of nude mice with WCTE given as the sole source of drinking fluid resulted in inhibition of PC-3 tumor xenograft growth (Fig. 4C). The appearance of small solid tumor was observed in animals receiving water 14 days after cell inoculation. This latency period was prolonged to 18 days in animals receiving WCTE as drinking fluid. WCTE (0.1% and 0.2%) was administered orally ad libitum to these animals day 1 after tumor cell implantation. As shown in Fig. 4A, tumor growth, as inferred by computed tumor volume, was significantly inhibited in mice receiving both 0.1 and 0.2% WCTE with higher inhibitory effects observed in animals receiving 0.2% WCTE than in those receiving 0.1% WCTE. In this protocol, we sacrificed the animals when the tumors reached volume of 1,200 mm3. In the control group, the average tumor volume of 1,200 mm3 was reached in 50 days after tumor cells inoculation. At this time point, the average tumor volumes of 0.1 and 0.2% WCTE-fed groups were 775 and 674 mm3, respectively. The average tumor volume of 1,200 mm3 was achieved in 66 days after tumor cell inoculation in 0.1% WCTE-fed group. The 0.2% WCTE-fed group showed the most effective tumor growth inhibitory response with the targeted average tumor volume of 1,200 mm3 reached at 78 days after tumor cell inoculation. Tumor data was analyzed for survival probability by Kaplan–Meier analysis, which indicated that continuous WCTE infusion to athymic nude mice resulted in increased survival (P<0.0001, log-rank test), with a median survival of 66 and 78 days, (0.1 and 0.2% WCTE, respectively), compared with 50 days in water-fed mice (P<0.0001, log-rank test) (Fig. 4B).
Fig. 4.
Effect of oral administration of WCTE on PC-3 tumor growth in athymic nude mice. Approximately 1 million PC-3 cells were s.c. injected in each flank of the mouse to initiate tumor growth. 24 h after cell implantation, the control group of animals continued to receive drinking water whereas animals of group 2 and 3 received 0.1 and 0.2% WCTE, respectively, in the same drinking water ad libitum. Water bottles were changed every other day. Once tumors stated to grow, their sizes were measured twice weekly and the tumor volume was calculated. (A) Average tumor volume of water-fed, 0.1 and 0.2% WCTE-fed mice plotted over days after tumor cell inoculation. (B) Number of mice remaining with tumor volumes ≤1,200 mm3 after they consumed 0.1 and 0.2% WCTE for the indicated days. Values represent mean ± SE of six animals. *p<0.01, **p<0.001 versus the water-fed group of mice; ***p<0.01 versus the 0.1% WCTE-fed group of mice. Details are described in Materials and Methods. (C) photographs of excised tumors form each group. (D) Protein levels of Bax, Bcl-2, cyclin D1, p21 and phospho-NF-κB/p65 as determined by western blotting analysis in pooled tumors excised from mice treated with WCTE. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin antibody. Western blotting analysis was conducted in all animals of each group, and only representative blots of two animals from each group are shown. (E) Bax to Bcl-2 ratio.
Inhibition of Cyclin D1, Bcl-2 and p-NF-κB/p65 and Induction of p21 and Bax by WCTE in Tumor Tissue of Athymic Nude Mice
As WCTE treatment was observed to modulate the expression levels of p21, Bax and Bcl-2 under in vitro conditions, we determined the effect of WCTE administration on the expression levels of p21, Bax and Bcl-2 in tumors excised from three groups of animals. As shown in Fig. 4D, WCTE administration was found to decrease the protein expressions of Bcl-2 protein. Inversely, a significant increase in the expression levels of p21 and Bax was observed in tumor tissues of animals treated with WCTE. Further, Western blotting analysis also showed a significant decrease in the protein expression of cyclin D1 and p-NF-κB/p65.
DISCUSSION
Prostate cancer is the second leading cause of cancer-related deaths in American men; therefore, it is necessary to intensify our efforts to identify novel agents that could delay or prevent the development of PCa. Cocoa tea (Camellia ptilophylla) is a naturally decaffeinated tea plant, growing in southern China. Here, we demonstrated that exposure of PCa PC-3 cells to white cocoa tea extract for 72 h significantly inhibited cellular proliferation (Fig. 1A), caused cell apoptosis (Fig. 1B) and was associated with the G2/M phase arrest (Fig. 1C).
It has been reported that G2 abrogation prevents cancer cells from repairing DNA damage, forcing them into M phase. Thus, the G2 checkpoint has emerged as an attractive therapeutic target for cancer therapy (21). Our study indicated that white cocoa tea exerts strong growth inhibitory effects on human PCa PC-3 cells by arresting cells in G2/M phase. It is known that cell cycle is primarily regulated by complexes containing cdks and cyclins, which are critical for the progression of cell cycle and whose inactivation leads to cell cycle arrest (22,23). The observed inhibitory effects of WCTE on cyclin D1, D2 and E, cdk2, 4 and 6 in PCa PC-3 cells suggested its interference in cell cycle. Cdk activity is additionally regulated by cdk inhibitors such as the WAF1/p21 and KIP1/p27 families of proteins. Our data showed that WCTE arrested PCa PC-3 cells in G2/M phase via modulation of cell cycle regulatory molecules (Fig. 2).
Bcl-2 is an upstream effector molecule in the apoptotic pathway and has been identified as a potent suppressor of apoptosis (24), and most cancers, including PCa, generally overexpress Bcl2, thereby escaping apoptosis and undermining therapy (25,26). Bcl-2 forms a heterodimer with the apoptotic protein Bax and thereby neutralizes its apoptotic effects. Therefore, alteration in the ratio of Bax/Bcl2 is a decisive factor that plays an important role to determine whether cells will undergo apoptosis. We observed that WCTE significantly down-regulated Bcl2 protein and up-regulated levels of Bax protein in PCa PC-3 cells as well as in tumors from PC-3 xenografts, suggesting the involvement of an intrinsic apoptotic pathway by which WCTE induces apoptosis in PCa PC-3 cells and inhibits tumor growth in athymic nude mice (Figs. 3AB, 4DE).
Caspases are part of a growing family of cysteine proteases that have been shown to be involved in many forms of apoptosis (27–29). Activation of caspase proteases was previously shown to be required for the induction of apoptosis in different cell types (30,31). Caspases can be grouped into initiator caspases and effector caspases. Once the initiator caspases, such as caspases 8, 9, and 10, are activated through intrinsic or extrinsic pathway, they proteolytically cleave and thus activate the effector caspases, including caspases 3, 6, and 7, whose functions are known to be responsible for the cleavage of the intracellular substrates that leads to cell death. Caspase 3 is one of the key executioners of apoptosis. Upon activation, caspase 3 can cleave 5 substrates, including other effector caspases and fodrin, which forms a cytoskeletal network. In the present study, we have shown that procaspase-3 and -8 were decreased during WCTE-mediated apoptosis (Fig. 3C).
The NF-κB is shown to stimulate cell survival and promote cell proliferation, and its increased activity is positively associated with many cancer types, including prostate cancer (18). Inhibition of tumorigenesis often involves modulation of signal transduction pathways, leading to cell cycle arrest and, consequently, apoptosis. NF-κB is sequestered in the cytoplasm in an inactive form through interaction with IκB. Phosphorylation of IκB by IκB kinase (IKK) causes ubiquitination and degradation of IκB, thus releasing NF-κB, which then translocates to the nucleus, where it binds to specific B binding sites in the promoter regions of several genes (32). In the present study, we investigated the effect of WCTE on the pattern of NF-κB activation. Our results showed that treatment of WCTE in PC-3 cells significantly inhibited IKKα and phosphorylation and degradation of IκBα protein. This study suggested that the effects of WCTE on NF-κB/p65 are through inhibition of phosphorylation and subsequent proteolysis of IκBα (Fig. 3D, E).
To establish the relevance of these in vitro findings to in vivo situation, athymic nude mice were implanted with human PCa PC-3 cells. We found that the oral administration of WCTE significantly slowed the progression of PC-3 tumor growth in athymic nude mice. These in vivo growth inhibitory effects of WCTE could be correlated well with the induction of apoptosis and inhibition of known cell proliferative biomarkers. A significant increase in p21, Bax/Bcl2 protein ratio, and the inhibition of cyclin D1, p-NF-κB/p65 protein levels in WCTE-treated tumors suggested the involvement of similar molecular events as those observed in the in vitro system (Fig. 4). Our findings are significant because the xenograft mouse model is extremely useful for preclinical studies of anticancer agents (17,33).
The present study is the first report showing the effect of white cocoa tea in inhibiting human PCa cell growth in in vitro model as well as in in vivo preclinical setting. In summary, based on the present findings, it is suggested that white cocoa tea could be developed as a potential anticancer agent against human PCa.
Acknowledgments
This work is supported by Chinese Government Scholarship to Li Peng to conduct research work in the laboratory of Prof. Hasan Mukhtar at University of Wisconsin-Madison and by United States Public Health Service Grants RO1 CA 78809, RO1 CA 101039, RO1 CA 120451 and P50 DK065303.
ABBREVIATIONS
- cdk
cyclin-dependent kinase
- EGCG
Epigallocatechin gallate
- GCG
Gallocatechin gallate
- MTT
Thiazolyl Blue Tetrazolium Bromide
- NF-κB
Nuclear factor kappa B
- PCa
Prostate cancer
- WCTE
White cocoa tea extract
Contributor Information
Li Peng, School of Life Science, Sun Yat-sen University, Guangzhou 510275, China. Department of Dermatology, University of Wisconsin Madison, Wisconsin 53706, USA.
Naghma Khan, Department of Dermatology, University of Wisconsin Madison, Wisconsin 53706, USA.
Farrukh Afaq, Department of Dermatology, University of Wisconsin Madison, Wisconsin 53706, USA.
Chuangxing Ye, School of Life Science, Sun Yat-sen University, Guangzhou 510275, China.
Hasan Mukhtar, Email: hmukhtar@wisc.edu, Department of Dermatology, University of Wisconsin Madison, Wisconsin 53706, USA.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Pu YS, Chiang HS, Lin CC, Huang CY, Huang KH, et al. Changing trends of prostate cancer in Asia. Aging Male. 2004;7:120–32. doi: 10.1080/13685530412331284687. [DOI] [PubMed] [Google Scholar]
- 3.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui IA, Saleem M, Adhami VM, Asim M, Mukhtar H. Tea beverage in chemoprevention and chemotherapy of prostate cancer. Acta Pharmacologica Sinica. 2007;28:1392–408. doi: 10.1111/j.1745-7254.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea consitituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 6.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 7.Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin Cancer Res. 2009;15:1947–53. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–82. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- 9.Xie BF, Liu ZC, Pan QC. The anticancer effect and anti-DNA topoisomerase II effect of extracts of camellia ptilophylla chang and camellia sinensis. Chin J Cancer. 1992;11:424–8. [Google Scholar]
- 10.Xian LJ, Liu ZC, Pan QC, Li HX. The inhibitory effect of extract of camellia sinensis and extract of camellia ptilophylla chang of DNA polymerase of ehrlich ascite carcinoma cells. Chin J Cancer. 1997;16:334–7. [Google Scholar]
- 11.Kurihara H, Shibata H, Fukui Y, Kiso Y, Xu JK, et al. Evaluation of the hypolipemic property of Camellia sinensis Var. ptilophylla on postprandial hypertriglyceridemia. J Agric Food Chem. 2006;54:4977–81. doi: 10.1021/jf0603681. [DOI] [PubMed] [Google Scholar]
- 12.He RR, Xie G, Yao XS, Kurihara H. Effect of cocoa tea (Camellia ptilophylla) co-administrated with green tea on ambulatory behaviors. Biosci Biotechnol Biochem. 2009;73:957–60. doi: 10.1271/bbb.80815. [DOI] [PubMed] [Google Scholar]
- 13.Yang XR, Ye CX, Xu JK, Jiang YM. Simultaneous analysis of purine alkaloids and catechins in Camellia sinensis, Camellia ptilophylla and Camellia assamica var. kucha by HPLC. Food Chem. 2007;100:1132–6. [Google Scholar]
- 14.Peng L, Song XH, Shi XG, Li JX, Ye CX. An improved HPLC method for simultaneous determination of phenolic compounds, purine alkaloids and theanine in Camellia species. J Food Compost Anal. 2008;21:559–63. [Google Scholar]
- 15.Wang ZY, Huang MT, Ferraro T, Wong CQ, Lou YR, et al. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-o-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52:1162–70. [PubMed] [Google Scholar]
- 16.Gupta S, Hastak K, Ahmad N, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 18.Hastak K, Gupta S, Ahmad N, Agarwal AK, Agarwal ML, et al. Role of p53 and NF- B in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4853–9. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 19.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–8. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen K, Berneman ZN, Van Bockstaele DR. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:165–75. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98:523–8. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devault A, Cavadore JC, Fesquet D, et al. Concerted roles of cyclin A, cdc25+ mitotic inducer, and type 2A phosphatase inactivating the cyclin B/cdc2 protein kinase at the G2/M phase transition. Cold Spring Harb Symp Quant Biol. 1991;56:503–13. doi: 10.1101/sqb.1991.056.01.057. [DOI] [PubMed] [Google Scholar]
- 23.van den Heuvel S, Harlow E. Distinct roles for cyclin dependent kinases in cell cycle control. Science. 1993;262:2050–4. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 24.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 25.Reed JC. Regulation of apoptosis by Bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–6. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Revelos K, Petraki C, Gregoakis A, Scorilas A, Papanastasiou P, et al. Immunohistochemical expression of Bcl-2 is an independent predictor of time-to-biochemical failure in patients with clinically localized prostate cancer following radical prostatectomy. Anti-cancer Res. 2005;25:3123–33. [PubMed] [Google Scholar]
- 27.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 28.Grutter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000;10:649–55. doi: 10.1016/s0959-440x(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 30.Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, et al. Biochem Biophys Res Commun. 2000;270:793–7. doi: 10.1006/bbrc.2000.2536. [DOI] [PubMed] [Google Scholar]
- 31.Hayakawa S, Saeki K, Sazuka M, Suzuki Y, Shoji Y, et al. Biochem Biophys Res Commun. 2001;285:1102–6. doi: 10.1006/bbrc.2001.5293. [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23:2507–22. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui IA, Zaman N, Aziz MH, et al. Inhibition of CWR22Rv1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–9. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]