Abstract
Objective
To assess the long-term effect of omega-3 polyunsaturated fatty acids (n-3 PUFA) intake during gestation on visual development.
Study design
We examined the long-term effects in 136 school-age Inuit children exposed to high levels of n-3 PUFAs during gestation using visual evoked potentials (VEPs). VEP protocols using color and motion stimuli were used to assess parvo- and magnocellular responses. Concentrations of the two major n-3 PUFAs (DHA and EPA) were measured in umbilical cord and child plasma phospholipids, reflecting pre- and postnatal exposure, respectively.
Results
After adjustment for confounders, cord plasma DHA was associated with shorter latencies of the N1 and P1 components of the color VEPs. No effects were found for current n-3 PUFA body burden or motion-onset VEPs.
Conclusion
This study demonstrates beneficial effects of DHA intake during gestation on visual system function at school age. DHA is particularly important for the early development and long-term function of the visual parvocellular pathway.
Keywords: Infant nutrition; Polyunsaturated fatty acids; Vision; Development; Neurotoxicity; Nunavik, Fish
Lipids influence neuronal function by modifying characteristics of the membrane, gene expression and eicosanoïd synthesis, all of which play a critical role in metabolism, growth, and differentiation of cells 1. Considerable accumulation of long-chain polyunsaturated fatty acids occurs in neural and retinal membranes during the third trimester of gestation and continues throughout the first postnatal year. Adequate intake of essential fatty acids during the pre- and postnatal periods is particularly important for optimal fœtal and neonatal brain development 2.
Eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) are two of the most important omega-3 polyunsaturated fatty acids (n-3 PUFAs). Although they can be synthesized in the liver from their precursor, the α-linolenic acid (ALA, 18:3n-3), only small amounts are produced in humans. Thus, intake of large quantities from dietary sources is needed, particularly from fish, seafood, and sea mammals. DHA is the principal n-3 PUFA found in the brain and is also highly concentrated in the photoreceptor outer segment membranes of the retina3. DHA plays an important role in sensory, perceptual, cognitive and motor function 4. In animals, inadequate DHA intake during early visual development decreases DHA concentrations in the brain and retina, which results in impairment in neurogenesis, neurotransmitter metabolism and visual function 5. Inadequate intake of EPA was associated with poorer motor function and mood disorders, but its importance for visual and brain development is less well understood 4.
Previous studies on the effects of dietary n-3 PUFAs on the visual system have focused on postnatal supplementation in term and preterm infants. Beneficial effects on vision have most clearly been demonstrated for preterm infants receiving supplementation during the first months of life 6. Furthermore, only visual acuity, measured either behaviorally or, more often, electrophysiologically using visual evoked potentials (VEPs), has been tested in these studies. Because VEPs reflect the maturation and the functional integrity of the visual system, damage along visual pathways leads to abnormal VEP latency and/or amplitude. The visual system is comprised of the parvocellular and the magnocellular pathways, which carry different types of information 7. The magnocellular pathway is optimally sensitive to low-to-medium spatial frequencies, low achromatic contrasts and high temporal frequencies. The parvocellular pathway, which mediates visual acuity, is optimally sensitive to medium-to-high spatial frequencies, high contrast and low temporal frequency. As a consequence, the magnocellular pathway is more sensitive to motion analysis; whereas the parvocellular pathway plays a major role in processing stimulus detail and chromatic analysis 8.
We assessed the benefits associated with prenatal intake of n-3 PUFAs in Inuit children living in Nunavik (Northern Québec, Canada). Because fish and marine mammals represent an important part of the Inuit diet, n-3 PUFA intake is substantially greater than in Southern Canada 9. However, this population is also exposed to high levels of several environmental contaminants 10, including methylmercury, polychlorinated biphenyls and lead, which were controlled statistically in this study in order to isolate the beneficial effects of DHA and EPA. Using two different VEP paradigms, we measured parvo- and magnocellular brain responses in school-age children to test the hypothesis that the beneficial effects of perinatal n-3 PUFA intake reported during infancy in this population 11 would continue to be evident in childhood. More specifically, we hypothesized significant moderate associations between n-3 PUFAs and the parvocellular responses considering that this system mediates visual acuity function, which was improved by increased perinatal n-3 PUFA intake.
Methods
The sample for this VEP study consisted of 171 Inuit children (mean age = 11.3 years old) and their mothers who had previously participated (n = 483) in the Nunavik Cord Blood Monitoring Program. This Canadian project was initiated in the early 1990s to detect environmental contaminants in the food chain 12 and measure several toxicants and nutrients in umbilical cord blood samples of Inuit newborns from Nunavik. Demographic background, smoking, alcohol and drug use during pregnancy, and other maternal characteristics was obtained by maternal interview at time of testing. The following inclusion criteria were used: children aged between 10 and 13 years, no known ophthalmic, neurological or developmental disorder, no medication, birth weight ≥ 2500g and gestation duration ≥ 37 weeks. Although the gestation duration was slightly lower than 37 weeks for four children (two children were born between 36 and 37 weeks and two between 35 and 36 weeks), their VEP responses did not differ significantly from the others (all Ps > 0.10).
Visual screening assessments for color and acuity were performed using the Ishihara Test for Color Blindness® and the Snellen E-chart. Visual acuity was considered normal for scores of 20/20 to 20/30. Visual function was also assessed using the Functional Acuity Contrast Test (F.A.C.T.®), which provides a fine measurement of near visual contrast sensitivity. This test assesses orientation discrimination of high quality sine-gratings (1.7 degrees of visual angle) at five spatial frequencies (1.5, 3, 6, 12, and 18 cycles/degree) and nine contrast levels distributed in five rows of increasing contrast. The contrast step between each grating is 0.15 log units. In each row the contrast diminishes from left to right and the child is required to indicate whether the gratings are upright, to the left, or to the right.
Adequate electrophysiological data were obtained for 136 of the 171 tested children. Inadequate data were due to technical/computer problems (n=1), lack of cooperation to assess visual acuity (n=6), insufficient signal-to-noise ratio (n=5) and abnormal visual acuity (≤ 20/40) (in one or both eyes (n=23)). Of the 136 children with adequate electrophysiological data, 134 were tested for color and 70 for motion-onset VEP. The research procedures were approved by the Sainte-Justine Hospital, Laval University, and Wayne State University ethics committees. A written informed consent was obtained from a parent of each participant and an oral assent from each child.
Visual evoked potentials
Visual stimuli were generated with Presentation software (Neurobehavioral system Inc.). Children viewed the stimuli binocularly from a distance of 57 cm in a dimly lit room (24° × 24° of visual field). They were instructed to fixate a small red dot located in the center of the screen (VP171b LCD ViewSonic monitor). Whenever the reflection of the stimulus was not centered over the pupil, the examiner interrupted the electrophysiological recordings to readjust the child’s head. Data were recorded with an INSTEP system®. The electro-oculogram (EOG) was recorded from the outer canthus of each eye (horizontal EOG) and above and below the right eye (vertical EOG). VEPs were recorded from Oz, T5, and T6 derivations according to the international 10–20 system using Ag–AgCl electrodes. The reference and the ground electrodes were located on the linked ear lobes and the forehead, respectively. Impedance was kept below 5 kΩ. The EEG signal was amplified and band-pass filtered at 0.1–100 Hz (sampling rate, 1000 Hz). One hundred trials were recorded for each condition (see below). VEPs were time-locked to the stimulus and averaged. Trials in which the response was greater than 75 μV at any recording site were rejected before averaging in order to eliminate ocular and muscular artifacts.
Parvocellular-related responses were recorded using an equiluminant color VEP protocol. Chromatic-contrast gratings (95% contrast, 1 cycle/degree) were generated by superimposing in a counter-phase manner red-black and green-black gratings of identical luminance. At the beginning of the study, a psychophysical experiment was conducted on six children to assess equiluminance for the color VEP protocol using heterochromatic flicker photometry. This method is based on the observation that, although the chromatic system is generally too slow to follow fast temporal changes, the luminance system can detect fast changing luminance differences between red and green. Therefore, if the perception of the flicker is minimized, the luminance difference is minimized as well. Mean values obtained in this experiment were used to generate the color stimuli that were used for the entire sample. The equiluminant gratings were presented in a reversal mode (2 reversals/sec) that typically produces a dominant negative component around 100 ms after stimulus onset followed by a positive component 13.
Magnocellular-related VEP responses were recorded using a motion-onset paradigm that is optimal for eliciting robust VEP response with low inter-subject variability 14. Achromatic motion-onset detection was elicited by the presentation of initial stationary concentric gratings for a period of 1120 ms followed by an abrupt onset (160 ms) of radial motion alternating at random intervals between expanding and contraction motion. In adults, such an onset/offset duty-cycle (13%, i.e., 160/160+1120) produces a typical P100/N180 complex where the N180 is the dominant motion-related component 15. In children, however, the negative component is manifest only after 200 ms 16. Spatial frequency was decreased (1–0.2 cycle/degree), and motion velocity was increased (5–25 deg/s) towards the periphery to account for different motion sensitivities in the centre versus the periphery of the visual field. The temporal frequency (5 cycles/sec) was thus kept constant over the whole stimulus field. Sinusoidal modulation of luminance and low contrast stimuli (10%) were used to eliminate high spatial frequencies and selectively target the magnocellular processing 17.
Biological samples
Umbilical cord and child blood samples were analysed for concentrations of n-3 PUFAs (DHA and EPA), as well as total mercury (Hg), polychlorinated biphenyls (PCBs), lead (Pb) and selenium (Se). A blood sample (30 mL) obtained from the umbilical cord was used as an indicator of prenatal exposure; a venous blood sample (20 mL) obtained from each participant was used to document body burden at time of testing. Contaminant analyses were performed at the Centre de Toxicologie du Québec, which is accredited by the Canadian Association for Environmental Analytical Laboratories. Fatty acid compositions of plasma phospholipids were performed at the University of Guelph Lipid Analytical Laboratory (B.J. Holub) using capillary gas-liquid chromatography. A 200-μL aliquot of plasma was extracted after the addition of chloroform:methanol (2:1, vol/vol), in the presence of a known amount of internal standard (diheptadecanoyl phospholipid). Total phospholipids were isolated from the lipid extract with thin-layer chromatography using heptane:isopropyl ether:acetic acid (60:40:3, by vol) as the developing solvent. After transmethylation with BF3/methanol, the fatty acid profile was determined with capillary gas-liquid chromatography. Concentrations of DHA and EPA were expressed as percentages of the total area of all fatty acid peaks from C14:0 to C24:1 (percent weight). Concentrations of the 14 most prevalent PCB congeners (IUPAC nos. 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187) were measured in purified plasma extracts using high-resolution gas chromatography (Hewlett-Packard HP5890 Seres II Plus) equipped with a 30-m DB-5 (J&W Scientific) and HP 5890B mass spectrometer (Agilent) according to the method described by Dallaire et al 18. Compounds are automatically extracted from the aqueous matrix using solid phase extraction. The detection limits were less than 0.05 μg/L for all PCB congeners except for PCB-52 (LOD = 0.15 μg/L). Total Hg, Pb and Se concentrations were determined in children whole blood samples by ICP-MS (Perkin Elmer Sciex Elan 6000 ICP-MS instrument for Pb and Se; PE DRC II instrument for Hg). Limits of detection were 0.002 μg/dL for Pb, 0.10 μg/L for Hg and 0.09 μmol/L for Se. DHA was measured in plasma phospholipids with the same procedure as in cord blood. For the present study, PCB congener 153, expressed on a lipid basis, was used as a marker of total PCB exposure since it is highly correlated with other PCB congeners and is considered an adequate marker of exposure to environmental PCB mixtures in the Arctic 19.
Statistical analyses
Because PCB 153, mercury, Pb and Se concentrations followed log normal distributions, all analyses were conducted with natural log-transformed values for these variables. Hierarchical multiple regression analyses were conducted to assess the relation between each DHA and EPA variable and each VEP measure when controlling for confounders. The control variables assessed in this study are listed in Table I. Selection of confounders was determined using a hybrid strategy combining significance test and change-in-estimate procedures: 1) among the set of control variables, each variable related to the VEP measure in question at p < .20 was selected; 2) the n-3 PUFA variable was entered in the first step of the regression analysis, each potential confounder meeting the p < .20 criterion was then entered hierarchically starting with the one showing the highest correlation with the outcome, continuing with the one showing the next highest correlation with the outcome, etc.; 3) a confounder was retained in the model if its inclusion changed the association (standardized β coefficient) between the n-3 PUFA variable and the outcome at step of entry by at least 10%. The 0.20 alpha level and 10% change value criteria were based on the work of Greenland and Maldonado 20, 21. All statistical analyses were performed using SPSS 16.0.
Table 1.
Descriptive statistics of color and motion VEPs, n-3 PUFAs, and potential confounding variables
| Color VEPs | N | Mean | SD | IQR |
|---|---|---|---|---|
| N1 latency (ms) | 134 | 104.2 | 9.0 | 80 - 154 |
| P1 latency (ms) | 134 | 138.9 | 19.4 | 101.6 - 212.9 |
| N1-P1 amplitude (μv) | 134 | 10.3 | 6.8 | 0.3 - 36.4 |
|
| ||||
| Motion VEPs | ||||
|
| ||||
| N2 latency (ms) | 70 | 237.5 | 25.3 | 179.7 - 296.9 |
| N2 amplitude (μv) | 70 | −7.0 | 3.5 | −18.0 - 0.4 |
|
| ||||
| N-3 PUFAs a | ||||
|
| ||||
| DHA – cord (% phospholipids) | 131 | 3.71 | 1.29 | 1.12-7.73 |
| DHA – child (% phospholipids) | 132 | 2.30 | 0.93 | 0.60-5.51 |
| EPA – cord (% phospholipids) | 131 | 0.45 | 0.43 | 0.00-2.89 |
| EPA – child (% phospholipids) | 132 | 0.60 | 0.50 | 0.04-2.96 |
|
| ||||
| Potential confounding variables | ||||
|
| ||||
| Contaminants a | ||||
| Mercury – cord (nmol/L) | 133 | 106.68 | 81.44 | 9-442 |
| Mercury – child (nmol/L) | 133 | 21.51 | 25.32 | 0.2-170.0 |
| PCB 153 – cord (μg/kg of lipids) | 132 | 128.18 | 97.55 | 21.61-653.60 |
| PCB 153 – child (μg/kg of lipids) | 132 | 79.08 | 94.61 | 6.82-809.52 |
| Lead – cord (μg/dL) | 133 | 4.77 | 3.32 | 0.83-19.48 |
| Lead – child (μg/dL) | 133 | 5.39 | 4.97 | 0.83-26.52 |
| Nutrients a | ||||
| Selenium – cord (μmol/L) | 123 | 4.56 | 2.54 | 1.93-20 |
| Selenium – child (μmol/L) | 133 | 2.46 | 1.22 | 0.86-12 |
| Others | ||||
| Age | 117 | 11.3 | 0.64 | 9.80-12.95 |
| Parity | 136 | 2.07 | 1.83 | 0-8 |
| Socioeconomic statusb | 136 | 29.69 | 12.75 | 8-66 |
| Breastfeeding (months) c | 55 | 13.3 | 15.95 | 1-60 |
| Sex (% females) | 136 | 52 | ||
| Marijuana use during pregnancy (% yes) | 117 | 21.4 | ||
| Smoking during pregnancy (% > 10 cigarettes/day)d | 122 | 44.3 | ||
| Binge drinking during pregnancy (% ≥ 5 standard drinks of alcohol per occasion) |
119 | 29.4 | ||
| Testing time (% AM) | 136 | 58.8 | ||
| Plane transportation during testing day (%yes) | 136 | 44.1 | ||
DHA, EPA, environmental contaminants (PCB, mercury, lead) and selenium concentrations are measured in umbilical cord and child blood samples. DHA and EPA concentrations were expressed in percentage according to plasma phospholipids. ms = milliseconds, μv = microvolts, SD = standard deviation, IQR = interquartile range, DHA = docosahexaenoic acid (22:6 n-3), EPA = eicosapentaenoic acid (20:5 n-3), PCB = polychlorinated biphenyls congener 153.
Child blood concentrations were measured at 11 years of age.
Hollingshead index 22 for the mother and her partner or, if she was not self-supporting, for her primary source of support.
40% of the children were breastfed.
82.8% of the mothers smoked during pregnancy.
Results
Descriptive data for the n-3 PUFAs, color and motion-onset VEPs and control variables are shown in Table I. The following potential confounding variables were examined: child’s sex, age at time of testing, time of day when testing occurred (AM or PM), parity, socioeconomic status (Hollingshead index) 22, mother’s education, transportation by airplane from a more remote village for testing (yes/no), breastfeeding duration, and exposure during gestation to maternal alcohol, marijuana use, or cigarette smoking. Maternal smoking is about 4 to 5 times higher in this community in comparison with the U.S. and southern Canada 23 (Table I). When the 134 children with valid VEP data were compared with the 37 whose data were discarded, no differences were seen in terms of the n-3 PUFAs and contaminants data collected at birth and at time of testing, and for any of the other control variables assessed (all Ps > .10).
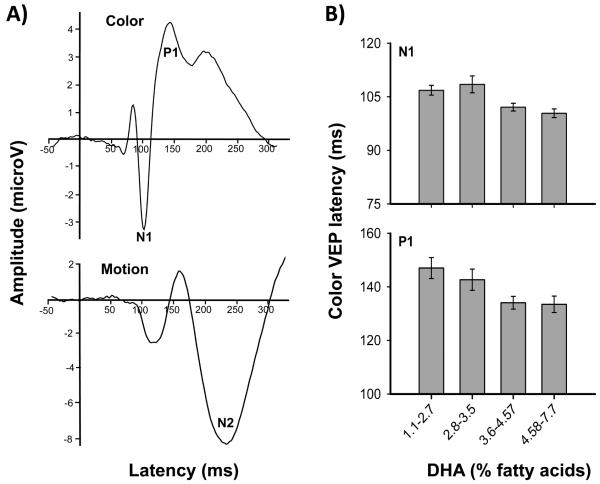
The color VEPs recorded from Oz elicited a major negative wave at approximately 104 ms (N1), followed by a major positive wave at approximately 139 ms (P1) (Table I). The amplitude of the N1-to-P1 averaged 10.3 μv, which is consistent with a report by Crognale 13. For motion VEPs, the major component recorded at electrode sites T5 and T6 was the N2. Mean latency was 237 ms with amplitude of −7.0 μv. Although these latter values are slightly different than those found in adults, for whom latency is typically shorter and amplitude lower, similar results have been observed in children 16 suggesting that motion-onset VEP is not yet mature at school age. Grand average waveforms for motion and color VEPs are presented in the Figure.
Intercorrelation and multiple regression analyses
Pearson correlations were conducted to examine the relationships among DHA, EPA and the environmental contaminants. Cord DHA was strongly associated with cord EPA, moderately associated with child DHA, weakly associated with child EPA and Hg as well as with PCB 153 (cord and child). No significant correlation (P > .05) was found between cord DHA and Pb, either for cord or child concentration. On the other hand, cord EPA was moderately associated with cord and child Hg, child PCB 153 and weakly associated with cord PCB 153 and child Pb. Cord EPA was not significantly correlated to child EPA and cord Pb (Table II).
Table 2.
Intercorrelations among DHA, EPA, mercury (log), PCB 153 (log) and lead (log) concentrations sampled from cord and child blooda
| DHA | EPA | Mercury | PCB 153 | Lead | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cord | Child | Cord | Child | Cord | Child | Cord | Child | Cord | Child | |
| DHA | ||||||||||
| Cord | 1 | 0.38*** | 0.61*** | 0.20* | 0.20* | 0.17* | 0.19* | 0.20* | 0.16 | 0.14 |
| Child | 1 | 0.30*** | 0.68*** | 0.00 | 0.34*** | 0.11 | 0.21* | −0.05 | 0.02 | |
| EPA | ||||||||||
| Cord | 1 | 0.15 | 0.33*** | 0.33*** | 0.21* | 0.31*** | 0.16 | 0.19* | ||
| Child | 1 | 0.07 | 0.30*** | 0.18* | 0.18* | −0.02 | 0.09 | |||
| Mercury | ||||||||||
| Cord | 1 | 0.51*** | 0.44*** | 0.40*** | 0.31*** | 0.14 | ||||
| Child | 1 | 0.35*** | 0.56*** | 0.19* | 0.23** | |||||
| PCB 153 | ||||||||||
| Cord | 1 | 0.48*** | 0.27** | 0.10 | ||||||
| Child | 1 | 0.26** | 0.29** | |||||||
| Lead | ||||||||||
| Cord | 1 | 0.17 | ||||||||
| Child | 1 | |||||||||
DHA = docosahexaenoic acid (22:6 n-3), EPA = eicosapentaenoic acid (20:5 n-3), PCB = polychlorinated biphenyl congener IUPAC 153.
Child blood concentrations were measured at 11 years of age.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
Table III shows Pearson correlations and regression coefficients relating the n-3 PUFAs to the VEPs. Raw regression coefficients are presented before and after adjustment for covariates; standardised regression coefficients, after adjustment. After adjustment for potential confounders, no significant relation was found between n-3 PUFAs, either cord or child and motion-onset VEPs. However, as predicted, cord DHA was associated with shorter latencies of the early N1 (β = −.28, P < .01) and P1 (β= −.22, P < .05) components of the color VEPs. No potential confounders were associated with either of these effects.
Table 3.
Relations between DHA and EPA at birth and in childhooda and VEP outcomes
| b ± (SE)b |
|||||||
|---|---|---|---|---|---|---|---|
| Variablesc | N | Confoundersd | Before | After | r | ß e | |
| Color VEPs | |||||||
| N1 latency | |||||||
| Cord DHA | 96 | None | −1.5 (0.5) | −1.5 (0.5) | −0.28** | −0.28** | |
| Child DHA | 96 | Cord DHA, cord Se, cord EPA | −1.2 (0.8) | −0.8 (0.8) | −0.15† | −0.1 | |
| Cord EPA | 96 | Cord DHA | −1.0 (1.5) | 2.6 (1.9) | −0.06 | 0.18 | |
| Child EPA | 96 | Cord DHA, child DHA, cord Se, cord EPA |
−0.9 (1.3) | 0.2 (1.8) | −0.07 | 0.02 | |
| P1 latency | |||||||
| Cord DHA | 97 | None | −3.2 (1.4) | −3.2 (1.4) | −0.22* | −0.22* | |
| Child DHA | 97 | Cord DHA, cord Se | −0.5 (2.1) | 0.5 (2.0) | −0.02 | 0.03 | |
| Cord EPA | 97 | Cord DHA, cord Se | −4.4 (4.0) | −0.9 (4.7) | −0.11 | −0.02 | |
| Child EPA | 97 | Cord DHA, cord Se | 5.4 (3.6) | 5.6 (3.3) | 0.16† | 0.16† | |
| N1-P1 amplitude | |||||||
| Cord DHA | 81 | None | −0.8 (0.7) | −0.8 (0.7) | −0.14 | −0.14 | |
| Child DHA | 81 | Child Se, cigarette | 1.4 (0.9) | 1.0 (0.9) | 0.17† | 0.12 | |
| Cord EPA | 81 | Child Se, cigarette, cord Se | 0.9 (1.8) | 1.1 (1.8) | 0.05 | 0.07 | |
| Child EPA | 81 | Child Se, cigarette | 1.8 (1.5) | 1.1 (1.4) | 0.14* | 0.08 | |
|
| |||||||
| Motion VEPs | |||||||
|
| |||||||
| N2 latency | |||||||
| Cord DHA | 45 | Cigarette, cord Hg, child DHA, child EPA |
0.7 (3.3) | −0.8 (3.7) | 0.03 | −0.04 | |
| Child DHA | 47 | Cigarette | 7.6 (4.0) | 5.9 (4.0) | 0.28* | 0.21 | |
| Cord EPA | 45 | Cigarette, cord Hg, child DHA, child EPA |
−3.0 (8.3) | −3.9 (8.6) | −0.55 | −0.07 | |
| Child EPA | 47 | Cigarette | 19.8(9.7) | 16.6 (9.6) | 0.29* | 0.24† | |
| N2 amplitude | |||||||
| Cord DHA | 57 | Gender, cord lead | 0.1 (0.4) | −0.1 (0.4) | 0.04 | −0.37 | |
| Child DHA | 57 | Gender, cord lead, child lead, cord Se |
−0.4 (0.6) | 0.0 (0.6) | −0.09 | 0.00 | |
| Cord EPA | 57 | Gender, cord lead | −0.0 (1.0) | −0.7 (1.1) | −0.00 | −0.09 | |
| Child EPA | 57 | Gender | −0.6 (1.5) | −0.3 (1.4) | −0.06 | −0.02 | |
Raw regression coefficients were measured before and after adjustment for confounders; standardised regression coefficients, after adjustment for confounders. The following control variables were considered for inclusion in the regression models : child’s gender, age, hemoglobin concentrations at time of testing, time of day when testing occurred (AM or PM), parity, socioeconomic status, mother’s education, plane transportation (if travel by plane was required), breastfeeding duration, mother’s cigarette smoking, alcohol consumption and marijuana use during pregnancy; the following contaminants and nutrients measured in cord and child blood: PCB = polychlorinated biphenyl congener IUPAC 153, Hg = mercury, lead, Se = selenium, EPA = eicosapentaenoic acid (20:5 n-3) and DHA = docosahexaenoic acid (22:6 n-3).
Measured at 11 years of age.
Raw regression coefficient ± (standard error)
Each row presents the findings from one multiple regression analysis. First line shows the dependent variable; subsequent lines (indented) show the n-3 PUFA predictor that was entered in the first step of the regression analysis.
Listed in order of entry into the model
Standardized regression coefficient.
p ≤ 0.10
p ≤ 0.05
p ≤ 0.01.
To further study these effects, cord DHA concentration was divided into quartiles. Dose-response relations were examined in analyses of covariance (ANCOVA), in which each VEP component found to be associated with prenatal DHA intake in the regression analyses was analyzed in relation to DHA group, after adjustment for the potential confounders used in the regression analyses (Fig. 1B). As expected, a main effect of DHA quartiles was found for both N1 latency (F(3, 1160) = 5.17, P = .002) and P1 latency (F(3, 3474) = 3.23, P = .025). Post hoc analyses for the N1 ANCOVA revealed a threshold effect; there was a significant (P < .05) shortening of latency between the two lowest quartiles and the two highest quartiles (Fig. 1B), suggesting beneficial effects in children whose cord DHA concentrations exceeded at least 3.6% of plasma phospholipids but no significant differences between the first two quartiles or between the two last quartiles (both P’s > .1). By contrast, the relation of cord DHA to P1 latency was dose dependent with no significant differences between the adjacent groups (P’s > .05). The difference in latency between the lowest and highest quartile was 7 ms for N1 latency (P = .014) and 14 ms for P1 latency (P = .011), both of which were equivalent to 0.7 standard deviations.
Figure.
A) Color (Oz site) and motion (T5-T6 sites) VEP grand mean average from valid subjects for 134 and 70 children, respectively. B) Color VEP N1 and P1 latency as a function of cord plasma phospholipid DHA concentration.
Snellen visual acuity score and contrast sensitivity scores, as assessed by the F.A.C.T., were also analysed to explore the hypothesis that beneficial effect of DHA on VEPs could be detected with behavioral testing. Prenatal n-3 PUFAs indicators (DHA and EPA) were not correlated with visual acuity and any of the five F.A.C.T. scores (Ps > .10). The absence of associations was corroborated in multiple regression analysis controlling for potential confounders.
Discussion
The timing and shape of the VEP responses in our cohort were similar to those found in the general population13, 16. We assessed the impact of prenatal exposure to n-3 PUFAs on VEPs in school-age children. In a previous study of infants from this same population, we reported that cord DHA phospholipids were associated with better visual acuity measured behaviorally using the Teller Acuity Card test11. The results of the present study confirm and extend these findings by showing that the beneficial fetal DHA intake on visual development persist onto late childhood and suggest that this effect is specific to parvocellular function, which is known to mediate visual acuity and color processing. Although this effect appears subtle and subclinical — because it was not significantly related to our behavioral measurements of visual acuity — this finding is of scientific and public health importance in that it demonstrates a beneficial role of n-3 PUFA exposure during pregnancy on visual processing through late childhood. The DHA-related improvement in latency between the lowest and highest cord DHA quartiles averaged 0.7 standard deviations for both latency measures. Again these differences are difficult to interpret in terms of clinical significance but they are clearly not negligible in terms of optimal visual function. Although DHA concentrations in the cord phospholipids plasma in Arctic Quebec are about three times higher than in Southern Quebec 9, it is of interest to note that cord DHA levels in Nunavik are similar to those reported in several other Western countries, notably Europe 24 25 and Massachusetts in the US 26. Because of the heterogeneity of laboratory analysis and quantification methods, direct cross-national comparisons are difficult. The beneficial effects can be seen with relatively modest increases of DHA; that is at or above 3.6% of fatty acids (Figure, 1B).
The effects on color VEPs were specific to DHA and not related to Hg, Pb, or PCBs, which often co-occur with exposure to PUFAs. Prenatal exposure to these contaminants was measured in umbilical cord blood; postnatal exposure at the time of testing. Although it would have been preferable to obtain assessments of postnatal exposure at more than one time point, PCBs, which have a long half-life in biological tissue 19 are likely to reflect exposure during an extended period, 11-year blood Hg concentration will represent long-term exposure if the child’s fish intake is relatively stable, and 11-year Pb body burden is likely to reflect postnatal exposure from the toddler period, when most Pb exposure occurs 27.
It has been suggested in other studies in fish eating populations, such as in the Seychelles Islands that the benefits from increased intake of nutrients from fish can outweigh the risks of the increased Hg exposure 28. Similarly, data from questionnaire reports from the Avon Longitudinal Study of Parents and Children (ALSPAC) suggest that the risks of a deficiency from maternal seafood nutrients during pregnancy are greater on the child’s neurodevelopment than the risks of harm from contaminants 29. Our results do not indicate that PUFAs can protect against adverse effects of Hg or other contaminants. However, it is clear that increased prenatal DHA intake is beneficial for childhood visual processing even in presence of contaminants, at least for the endpoints investigated here. By contrast, other endpoints that were examined in this cohort were vulnerable to contaminant toxicity even after controlling for the beneficial effects of DHA e.g. 30, 31.
No significant association was found between cord DHA and motion-onset VEPs. This finding needs to be interpreted with caution, as the sample for the motion onset protocol was smaller. However, given that the magnitude of the association between cord DHA and the motion onset N2 was close to zero, it seems unlikely that a significant effect would have emerged for this outcome with a larger sample. Our results add to the growing body of evidence that increased levels of DHA during fetal development and early life are associated with more optimal perceptual and cognitive function, particularly vision 32. Most of the studies in this field have focused on postnatal n-3 PUFAs supplementation during the first months of life, providing evidence that such supplementation can enhance visual acuity during the first months of life 33. However, these results have been observed primarily in preterm infants, and the picture is much less clear in healthy term infants 34. Of particular interest is a recent study which showed that beneficial effects on visual acuity continue to be evident through 4 years of age in breast-fed or DHA-supplemented healthy term children, compared with children receiving n-3 PUFAs-free formula 35.
The finding that the beneficial effects on parvocellular function were seen in relation to cord but not child plasma DHA suggests that DHA intake during the prenatal period plays a critical role in the early development of the visual system, whose beneficial effects continue to be detectable well into school age. The maternal-to-fetal transfer of DHA during gestation is heavily influenced by maternal circulating and dietary intake of DHA 36. Because placental fatty acid transfer involves diffusion as well as membrane and systolic fatty acid binding proteins, genetic factors presumably also affect fetal supply. However, maternal intake of essential fatty acids during pregnancy is clearly a critical factor. We found a strong correlation between the DHA concentrations in maternal and cord plasma phospholipids of Inuit newborns11. Mean cord plasma DHA concentration was significantly higher than maternal plasma DHA concentration, indicating that the mother transmits a significant proportion of her DHA to the fetus.
Maternal DHA supplementation from fish oil during pregnancy affected the offspring visual development. In a study by Judge et al 37, infants who were supplemented during gestation performed significantly better on visual acuity at 4 months postpartum as measured with the Teller Acuity Card test. In an earlier study, the effects of maternal DHA supplementation on healthy term infants were assessed using VEPs 38. One hundred women were supplemented with either fish oil capsules rich in DHA or placebo from Week 15 of pregnancy until delivery. There were no significant differences in any of the VEP measures between supplementation groups, possibly due to the low levels of DHA in the blended fish oil supplements, which were about seven times lower than those used by Judge et al 37. Nonetheless, it is of particular interest that, regardless of the supplementation, DHA status of the infant at birth was associated with shorter P100 peak latencies as tested with pattern-reversal VEPs at 12.5 and 16.5 months post-conceptional age 38. In conclusion, this study provides new evidence for the importance of in utero DHA for development of the visual system, in particular for parvocellular function.
Acknowledgments
Acknowledgments available at www.jpeds.com.
Supported by the National Institute of Environmental Health and Sciences/U.S. National Institutes of Health (R01 ES07902 to J.J.), Indian and Northern Affairs Canada--Northern Contaminants Program (G.M.), the Joseph Young, Sr., grant from the State of Michigan (S.J.), the Nunavik Regional Board of Health and Social Services, and the Canadian Institutes of Health Research (D.S-A.).
LIST OF ABBREVIATIONS
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FACT
Functional Acuity Contrast Test
- Hg
Mercury
- N-3
Omega-3
- Pb
Lead
- PCBs
Polychlorinated biphenyls
- PUFAs
Polyunsaturated fatty acids
- Se
Selenium
- VEPs
Visual evoked potentials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. 1999;19:63–90. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]
- 2.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4-5):329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22(2):79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 4.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12(3):207–227. [PubMed] [Google Scholar]
- 5.Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal N-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res. 2005;58(5):865–872. doi: 10.1203/01.pdr.0000182188.31596.5a. [DOI] [PubMed] [Google Scholar]
- 6.SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105(6):1292–1298. doi: 10.1542/peds.105.6.1292. [DOI] [PubMed] [Google Scholar]
- 7.Shapley R. Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- 8.Livingstone, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas M, Dewailly E, Muckle G, Ayotte P, Bruneau S, Gingras S, et al. Gestational age and birth weight in relation to n-3 fatty acids among Inuit (Canada) Lipids. 2004;39(7):617–626. doi: 10.1007/s11745-004-1274-7. [DOI] [PubMed] [Google Scholar]
- 10.Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Determinants of polychlorinated biphenyls and methylmercury exposure in inuit women of childbearing age. Environ Health Perspect. 2001;109(9):957–963. doi: 10.1289/ehp.01109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the inuit of arctic Quebec. J Pediatr. 2008;152(3):356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Muckle G, Dewailly E, Ayotte P. Prenatal exposure of Canadian children to polychlorinated biphenyls and mercury. Can J Public Health. 1998;89(Suppl 1):S20–25. 22–27. [PubMed] [Google Scholar]
- 13.Crognale MA. Development, maturation, and aging of chromatic visual pathways: VEP results. J Vis. 2002;2(6):438–450. doi: 10.1167/2.6.2. [DOI] [PubMed] [Google Scholar]
- 14.Kuba M. Motion-onset visual evoked potentials and their diagnostic applications. RNDr. Frantisek Skopec, CSc. - Nucleus HK; 2006. [Google Scholar]
- 15.Bach M, Ullrich D. Motion adaptation governs the shape of motion-evoked cortical potentials. Vision Res. 1994;34(12):1541–1547. doi: 10.1016/0042-6989(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 16.Langrova J, Kuba M, Kremlacek J, Kubova Z, Vit F. Motion-onset VEPs reflect long maturation and early aging of visual motion-processing system. Vision Res. 2006;46(4):536–544. doi: 10.1016/j.visres.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci U S A. 1986;83(8):2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect. 2009;117(9):1380–1386. doi: 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E, Inuit Cohort S. Assessment of pre- and postnatal exposure to polychlorinated biphenyls: lessons from the Inuit Cohort Study. Environ Health Perspect. 2003;111(9):1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland S, Rothman KJ. Introduction to stratified analysis. In: Rothman KJGS, editor. Modern Epidemiology. Lippincot, Williams, & Wilkins; Philadelphia: 1998. pp. 253–279. [Google Scholar]
- 21.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 22.Hollingshead A. Four factor index of social status. Yale University Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- 23.Andres RL, MC D. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- 24.Rump P, Mensink RP, Kester AD, Hornstra G. Essential fatty acid composition of plasma phospholipids and birth weight: a study in term neonates. Am J Clin Nutr. 2001;73(4):797–806. doi: 10.1093/ajcn/73.4.797. [DOI] [PubMed] [Google Scholar]
- 25.Krauss-Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jimenez M, et al. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr. 2007;85(5):1392–1400. doi: 10.1093/ajcn/85.5.1392. [DOI] [PubMed] [Google Scholar]
- 26.Donahue SM, Rifas-Shiman SL, Olsen SF, Gold DR, Gillman MW, Oken E. Associations of maternal prenatal dietary intake of n-3 and n-6 fatty acids with maternal and umbilical cord blood levels. Prostaglandins Leukot Essent Fatty Acids. 2009;80(5-6):289–296. doi: 10.1016/j.plefa.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiodo L, Jacobson S, Jacobson J. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26(3):359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Myers G, Davidson P, Strain J. Nutrient and methyl mercury exposure from consuming fish. J Nutr. 2007;137(12):2805–2808. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- 29.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369(9561):578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 30.Plusquellec P, Muckle G, Dewailly E, Ayotte P, Begin G, Desrosiers C, et al. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31(1):17–25. doi: 10.1016/j.neuro.2009.10.008. Epub 2009 Oct 2023. [DOI] [PubMed] [Google Scholar]
- 31.Boucher O, Bastien C, Saint-Amour D, Dewailly E, Ayotte P, Jacobson J, et al. Prenatal exposure to methylmercury and PCBs affects distinct stages of information processing: An event-related potential study with Inuit children. Neurotoxicology. 2010;18:18. doi: 10.1016/j.neuro.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22(5-6):474–480. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 33.Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Invest Ophthalmol Vis Sci. 1992;33(11):3242–3253. [PubMed] [Google Scholar]
- 34.Gibson RA, Makrides M. Polyunsaturated fatty acids and infant visual development: a critical appraisal of randomized clinical trials. Lipids. 1999;34(2):179–184. doi: 10.1007/s11745-999-0352-1. [DOI] [PubMed] [Google Scholar]
- 35.Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev. 2007;83(5):279–284. doi: 10.1016/j.earlhumdev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Judge MP, Harel O, Lammi-Keefe CJ. A docosahexaenoic acid-functional food during pregnancy benefits infant visual acuity at four but not six months of age. Lipids. 2007;42(2):117–122. doi: 10.1007/s11745-006-3007-3. [DOI] [PubMed] [Google Scholar]
- 38.Malcolm CA, McCulloch DL, Montgomery C, Shepherd A, Weaver LT. Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F383–390. doi: 10.1136/fn.88.5.F383. [DOI] [PMC free article] [PubMed] [Google Scholar]