INTRODUCTION
HIV infection has been associated with pro-atherogenic lipid profiles, including elevated triglycerides (TG) and decreased high density lipoprotein cholesterol (HDL-C), but also with decreased low density lipoprotein cholesterol (LDL-C) in both men and women [1-3]. These alterations may contribute to the increased risk of cardiovascular disease (CVD) reported in HIV-infected persons [4].
Assessment of the concentrations of lipoprotein particles (which transport cholesterol and TG) may provide information regarding CVD risk beyond the standard lipid panel [5, 6], since individuals with the same LDL-C or HDL-C level may have different concentrations of LDL particles (LDL-p) or HDL particles (HDL-p). Greater small LDL-p has been associated with increased CVD risk in some studies from the general population [7, 8], while lower HDL-p (especially small HDL-p) has been associated with CVD risk [9, 10].
Few studies have examined whether HIV and HAART use are associated with lipoprotein particle concentrations after adjusting for standard lipids. A small study from the pre-HAART era found that the association of HIV with small LDL-p was attenuated after adjustment for TG [11]. The few studies from the HAART era that have found an association between HIV and lipoprotein particle concentrations did not adjust for standard lipids [12, 13]; this adjustment is desirable as elevations in TG are common after HAART initiation [2].
Using a large cohort of HIV-infected and uninfected women, we quantified the association HIV infection and current HAART use had with LDL-p and HDL-p, and examined whether any relationships found persisted after adjustment for standard lipids. To quantify particle concentrations, we employed the same nuclear magnetic resonance (NMR) spectroscopy technique used by published HIV studies in the HAART era so that our findings could be compared to other studies.
METHODS
Study Population
The Women's Interagency HIV Study (WIHS) is a multicenter prospective cohort study that was established in 1994 to investigate the progression of HIV in women with and at risk for HIV. A total of 3,766 women (2,791 HIV-infected and 975 HIV-uninfected) was enrolled in either 1994-95 (n=2,623) or 2001-02 (n=1,143) from six United States locations (Bronx, Brooklyn, Chicago, Los Angeles, San Francisco and Washington DC). Baseline socio-demographic characteristics and HIV risk factors were similar between HIV-infected and uninfected women [14, 15]. An institutional review board approved study protocols and consent forms, and each study participant gave written informed consent.
At each semiannual visit, participants undergo a comprehensive physical examination, provide biological specimens for CD4 cell count and HIV RNA determination, and complete an interviewer-administered questionnaire, which collects information on demographics, disease characteristics, and specific antiretroviral therapy use. Standard lipid and lipoproteins are measured annually from fasting samples using the Roche Modular System (Roche Diagnostics Corporation, Indianapolis, IN) at a centralized laboratory (Quest Diagnostics, Baltimore, MD).
From April 2004 to March 2007, NMR data, which included high volume assessment of lipoprotein size, concentration, and subclass concentration, were collected at a single study visit (referred to subsequently as the index visit) on a total of 1410 participants enrolled in WIHS cardiovascular disease or metabolic substudies [16, 17]. Of these 1410 women, 401 (28%) were HIV-uninfected, 146 (10%) were HIV-infected and HAART-naïve up through and including the index visit, and 863 (61%) were HIV-infected and reported initiating HAART at or prior to their index visit. One-hundred and seventy-five of the 863 women who initiated HAART did not report using HAART in the six months prior to the index visit and were excluded from analyses. Our final study population was comprised of the 1077 women (361 [90% of 401] HIV-uninfected, 128 [88% of 146] HIV-infected/HAART naïve, and 588 [85% of 688 = 863-175] HIV-infected/on HAART) who had complete data on all covariates of interest (described below).
Primary Outcomes
Lipoprotein particle concentration and subclass concentration were estimated from freshly thawed plasma samples using an automated proton NMR spectroscopic assay (LipoScience, Raleigh, NC), as previously described [18]. Particle concentrations of total LDL (nmol/L), small LDL (nmol/L), total HDL (μmol/L), and small HDL (μmol/L) were analyzed.
Primary Exposures
The definition of HAART was guided by the DHHS/Kaiser Panel [DHHS/Kaiser 2008] guidelines and is defined as the reported use of three or more antiretroviral medications, one of which has to be a protease inhibitor (PI), a non-nucleoside reverse transcriptase inhibitor (NNRTI), one of the nucleoside reverse transcriptase inhibitors (NRTIs) abacavir or tenofovir, an integrase inhibitor (e.g., raltegravir), or an entry inhibitor (e.g., maraviroc or enfuvirtide). All non-HAART regimens were considered combination therapy or monotherapy.
Study participants were stratified into three groups for primary analyses: HIV-uninfected, HIV-infected/HAART naïve, and HIV-infected/on HAART. At the index visit, the 588 HIV-infected women on HAART reported HAART use at a median of nine visits corresponding to approximately 4.5 years of exposure. Participants were classified as HAART-naïve if they did not report HAART at any visit up through and including the index visit. One-hundred and twenty-three (96%) of the 128 women who were HAART naïve reported using no antiretroviral medications in the six months prior to the index visit.
Covariates
All analyses were adjusted for current age at the index visit, race/ethnicity, current menopause status, number of years participant reported smoking cigarettes prior to the index visit, hepatitis C infection status (HCV RNA positive versus anti-HCV negative or anti-HCV positive and HCV RNA negative), any report of lipid-lowering therapy usage in the six months prior to the index visit, current waist size (cm), current homeostatic model assessment-insulin resistance (HOMA =insulin [μU/mL]× glucose [mg/dL]) / 405), and diabetes status (participant was classified as having diabetes mellitus [DM] if DM was reported, fasting glucose ≥ 126 mg/dL, or anti-diabetic medication was reported at any visit at or prior to the index visit) [19]. For analyses of small LDL-p (small HDL-p), we further adjusted for TG and HDL-C (LDL-C), which were entered into the model separately and then jointly. In ancillary analyses which included only the women on HAART, we examined the association of NNRTI use and PI use at the index visit with small HDL-p concentrations, with further adjustment for CD4 cell count, log10 HIV RNA, and clinical AIDS status. If data on a specific covariate were missing at the index visit then data from the visit closest to and prior to the index visit (up to two visits prior to the index visit) were used.
Statistical Analyses
Because the distributions of the outcome variables were typically somewhat skewed, we chose to base statistical comparisons on medians and other percentiles rather than means. The distribution of each of the primary outcomes (total LDL-p, small LDL-p, total HDL-p, and small HDL-p) by primary exposure group were summarized using enhanced box plots that depict not only the median, inter-quartile range, and outliers, but also the 5th, 10th, 90th, and 95th percentiles [20]. Quantile regression models [21] were used to compare the differences in the 10th, 25th, 50th, 75th, and 90th percentiles of each of the primary outcomes between the three primary exposure groups after adjusting for the covariates listed above. In quantile regression models, values of individual percentiles are predicted by the model, rather than the mean response. Quantile regression models are appropriate when the effect of an exposure on different parts of the outcome distribution is of interest, not simply a central value (e.g., the median), or when the normality or constant variance assumptions required by standard least squares regression are not satisfied [22]. One of the reasons for the observed skewness of outcome distributions is effects in the tails, which can be studied by estimation of percentiles such as the 75th or 90th.
The quantile regression models were parameterized so that regression coefficients provided estimates of the difference in a given percentile of an outcome comparing either HIV-infected/HAART naïve women or HIV-infected women on HAART to HIV-uninfected women. All continuous covariates were centered at median values when included in regression models so that the models' intercept represents the expected percentile of a particular particle concentration for the group of women with the constellation of continuous covariates at the centering values and reference values of categorical covariates.
RESULTS
Table 1 shows the characteristics at the index visit of the 1077 participants included in the analysis by HIV infection and HAART status. Compared to the HIV-uninfected participants, HIV-infected women were older, more likely to be menopausal, and HCV-infected. More than half of the women were African-American. HIV-uninfected and HAART naïve women had similar waist size and HOMA values. Duration of smoking and HOMA values were greatest in women currently on HAART. Triglycerides were higher in HIV-infected women than uninfected women; the highest levels were observed among those on HAART. HDL-C was lowest in HAART naïve women and highest in HIV-uninfected women. Only five percent of the study population reported using lipid-lowering medications. HIV-infected women reported using lipid-lowering medications more than HIV-uninfected women. Among HIV-infected women, being HAART naïve was associated with a higher median CD4 count and a higher HIV viral load. A history of clinical AIDS was more common in those on HAART. Among those on HAART, 38% reported using a NNRTI and 59% reported using a PI.
Table 1.
Characteristics of 1077 participants of the Women's Interagency HIV Study at the index visita by HIV infection and HAART status
| Variable | HIV-uninfected N=361 |
HIV-infected/HAART-naïve N=128 |
HIV-infected/on HAART N=588 |
|---|---|---|---|
| Median (IQR) age, years | 37.7 (30.2, 45.9) | 40.5 (36.2, 47.6) | 41.9 (36.5, 48.3) |
| Race, No. (%) | |||
| African American | 231 (64%) | 90 (70%) | 337 (57%) |
| Hispanic | 94 (26%) | 26 (20%) | 186 (32%) |
| Caucasianb | 36 (10%) | 12 (9%) | 65 (11%) |
| Menopausal, No. (%) | 58 (16%) | 29 (23%) | 157 (27%) |
| Median (IQR) years smoked cigarettes | 10.8 (0.0, 23.0) | 11.0 (0.0, 22.5) | 14.0 (0.0, 24.4) |
| HCV RNA positive, No. (%) | 45 (12%) | 31 (24%) | 122 (21%) |
| Use lipid-lowering medicationc, No. (%) | 11 (3%) | 6 (5%) | 41 (7%) |
| Median (IQR) waist size, cm | 90.1 (78.9, 103.9) | 90.1 (79.1, 101.5) | 89.1 (80.7, 99.9) |
| Median (IQR) HOMA, μU/mL | 2.2 (1.2, 3.7) | 2.1 (1.3, 3.8) | 2.6 (1.5, 4.3) |
| Median (IQR) LDL-C, mg/dl | 95 (74, 121) | 99 (81, 124) | 100 (78, 124) |
| Median (IQR) HDL-C, mg/dl | 51 (44, 63) | 41 (33, 52) | 47 (38, 58) |
| Median (IQR) Triglycerides, mg/dl | 88 (61, 120) | 94 (70, 128) | 118 (82, 166) |
| Diabetes Mellitusd, No. (%) | 65 (18%) | 18 (14%) | 109 (19%) |
| Median (IQR) log10 HIV RNA, copies/ml | NA | 3.30 (1.90, 4.23) | 1.90 (1.90, 3.06) |
| Median (IQR) CD4 count, cells/mm3 | 1006 (786, 1255) | 468 (341, 668) | 436 (265, 658) |
| Clinical AIDS, No. (%) | NA | 33 (26%) | 253 (43%) |
HAART, highly active antiretroviral therapy; HDL-C, high-density lipoprotein; IQR, inter-quartile range; NA, not applicable
if data were missing at index visit then data from visit closest to and prior to index visit (up to 2 visits prior to index visit) were used
includes 3% (n=11), 2% (n=3), 2% (n=14) Asian, Pacific Islander, Native American, Alaskan, other among HIV-uninfected, HIV-infected/HAART naïve, and HIV-infected/HAART experience women, respectively
reported taking medication to lower cholesterol and/or triglyceride
A participant was considered to have diabetes if any of the following occurred at or prior to index: fasting glucose ≥ 126 mg/dL, anti-diabetic medication use was reported, or diabetes was self-reported
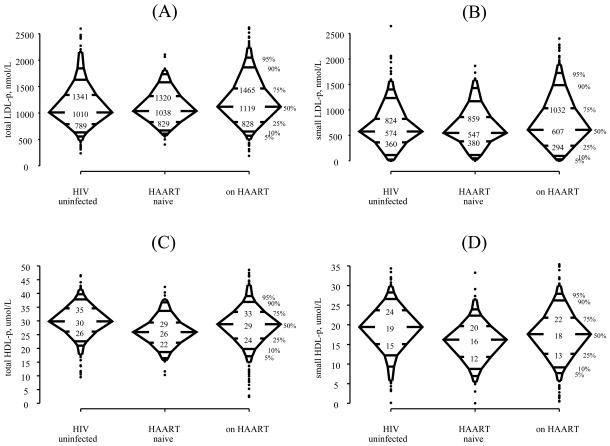
Figure 1 shows the distribution of total LDL-p (panel A), small LDL-p (panel B), total HDL-p (panel C), and small HDL-p (panel D) by HIV and HAART status. There was little difference in the three groups with respect to the 5th, 10th, 25th, and 50th percentiles of total and small LDL-p, but the 75th, 90th, and 95th percentiles in women on HAART were higher than the corresponding percentiles of the two other groups. Thus, entire distributions are not simply being shifted, as assumed by methods based on the comparisons of means with normally distributed errors, but rather stretched, i.e., the differences are in the upper tails of the distributions. HIV-uninfected women had higher total and small HDL-p than HIV-infected women at each of the percentiles shown.
Figure 1.
Distribution of total LDL particle concentration (panel A), small LDL particle concentration (panel B), total HDL particle concentration (panel C), and small HDL particle concentration (panel D) by HIV and HAART status. Three-hundred and sixty-one HIV-uninfected women, one-hundred and twenty-eight HIV-infected/HAART naïve, and five-hundred and eighty-eight HIV-infected women on HAART were included for each outcome. The numerical values of the 25th, 50th, and 75th percentiles are given. The 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of the distribution are denoted. Values <2.5th percentile or >97.5th percentile are denoted by closed circles.
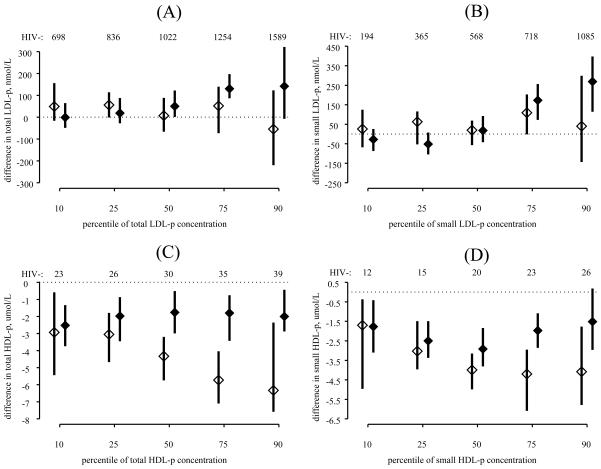
After adjustment for demographic and metabolic factors, there remained little difference in total LDL-p (Figure 2, panel A) and small LDL-p (Figure 2, panel B) between HAART naïve and HIV-uninfected women. Compared to HIV-uninfected women, those on HAART had greater 50th percentiles (50.0 nmol/L greater; 95% confidence interval [CI] 1.3 to 122.3) and 75th percentiles (130.3 nmol/L greater; 95% CI 86.7 to 197.3) of total LDL-p, as well as greater 75th percentiles (173.1 nmol/L greater; 95% CI 72.4 to 256.1) and 90th percentiles (268.7 nmol/L greater; 95% CI 114.1 to 397.3) of small LDL-p. Furthermore, the differences in total LDL-p and small LDL-p between women on HAART and HIV-uninfected women increased as the quantile of each outcome increased, e.g. the difference in the 75th percentiles was greater than the differences in the 50th percentiles, and the difference in the 90th percentiles was greater than the differences at the 75th percentile.
Figure 2.
Differences in the distributions of total LDL particle concentration (panel A), small LDL particle concentration (panel B), total HDL particle concentration (panel C), and small HDL particle concentration (panel D) between HIV-infected, HAART naïve women (open diamond) and HIV-infected women on HAART (closed diamond) compared with HIV-uninfected controls. The estimated 10th, 25th, 50th, 75th, and 90th percentiles for HIV-uninfected women who were 40 years of age, African-American, not menopausal, reported smoking for 15 years, HCV RNA negative, did not report using lipid-lowering medications, had a waist size of 90 centimeters, a HOMA value of 3.5 μU/mL, and did not have diabetes are shown along the top of each panel as a reference. Point estimates and 95% confidence intervals for differences in the 10th, 25th, 50th, 75th, and 90th percentiles are plotted. All estimates were adjusted for age, race/ethnicity, menopause status, smoking history, hepatitis C infection status, lipid lowering therapy, waist size, HOMA, and diabetes status.
Compared to HIV-uninfected women, being HAART naïve or on HAART was associated with lower 10th, 25th, 50th, 75th, and 90th percentiles of total HDL-p (Figure 2, panel C) and lower 10th, 25th, 50th, and 75th percentiles of small HDL-p (Figure 2, panel D). The differences between those on HAART and those who were HIV-uninfected were constant across percentiles of total HDL-p, while the differences between those who were HAART naïve and HIV-uninfected increased as total HDL-p and small HDL-p increased. When we examined the association of HIV/HAART status with large HDL-p, we found similar but weaker relationships (data not shown).
Because prior studies have shown a strong association of higher TG and lower HDL-C with greater small LDL-p [11], we also adjusted for TG and/or HDL-C in our multivariate models (Table 2). After adjustment for TG, the association of HAART with greater small LDL-p was attenuated and no longer significant at the median or 75th percentile. After adjustment for HDL-C, there was little change in the association of HAART with small LDL-p as shown in Figure 2. When both TG and HDL-C were included, HIV-infected women, regardless of HAART status, had lower values of small LDL-p compared to those HIV-uninfected: 10th and 25th percentiles were significantly lower for those on HAART and 25th and 50th percentiles were significantly lower for those who were HAART naïve.
Table 2.
The association between HIV-infection, HAART use, and small LDL particle concentration after adjustment for triglycerides and/or HDL-C.
| Differencesa in Indicated Percentiles of small LDL particle concentration (nmol/L) | |||||
|---|---|---|---|---|---|
| 10th percentile | 25th percentile | 50th percentile | 75th percentile | 90th percentile | |
| Adjustment for triglyceridesb | |||||
| on HAART (vs HIV-uninfected) | −66 (−132, 0) | −59 (−122, −26) | −58 (−126, 15) | 14 (−65, 74) | 50 (−61, 157) |
| HAART naïve (vs HIV-uninfected) | 11 (−58, 81) | −8 (−88, 88) | −16 (−102, 56) | −43 (−109, 98) | 19 (−122, 156) |
| Adjustment for HDL-Cb | |||||
| on HAART (vs HIV-uninfected) | −82 (−143, −7) | −58 (−108, −18) | −13 (−65, 58) | 81 (1, 180) | 138 (38, 252) |
| HAART naïve (vs HIV-uninfected) | −47 (−154, 22) | −72 (−150, −18) | −101 (−163, −13) | −75 (−201, 31) | −141 (−209, 25) |
| Adjustment for triglycerides and HDL-Cb | |||||
| on HAART (vs HIV-uninfected) | −86 (−163, −32) | −89 (−131, −38) | −57 (−124,34) | −1 (−81, 76) | −5 (−120,127) |
| HAART naïve (vs HIV-uninfected) | −42 (−172, 18) | −86 (−157, −11) | −82 (−169, −6) | −64 (−197, 23) | −115 (−229, 58) |
Each table entry represents the difference in indicated percentile of small LDL particle concentration comparing either HIV-infected women on HAART or HIV-infected women who were HAART naïve to those who were HIV-uninfected along with the 95% confidence interval of the difference
The quantile regression models were also adjusted for index visit values of age, race/ethnicity, menopause status, number of years of smoking cigarettes, hepatitis C infection status, lipid lowering therapy, waist size, HOMA, and diabetes status. If data were missing at index visit then data from visit closest to and prior to index visit (up to 2 visits prior to index visit) were used.
TG levels were strongly associated with greater small LDL-p (10th percentile: +61.8 nmol/L per 100 unit TG increase, 95% CI, 48.5 - 128.6; 25th percentile: +146.3, 95% CI, 79.9 - 189.4; 50th percentile: +213.6, 95% CI, 161.0 – 327.4; 75th percentile, +310.7, 95% CI, 227.2 – 423.2; 90th percentile, +405.8, 95% CI, 267.5 - 512.6). Similarly, a strong negative association of HDL-C with lower small LDL-p was observed (10th percentile: −21.3 μmol/L per 5 unit HDL-C increase, 95% CI, −30.7 to −17.1; 25th percentile: −28.7, 95% CI: −35.9 to −22.3; 50th percentile: −39.3, 95% CI −45.8 to −23.6; 75th percentile: −40.6, 95% CI −55.4 to −33.9; 90th percentile: −55.4, 95% CI −71.3 to −48.4).
When we adjusted for TG and LDL-C in a multivariate model with small HDL-p as the outcome (Table 3), HIV infection regardless of HAART status was associated with lower 25th, 50th, 75th, and 90th percentile values of small HDL-p. Specifically, those who were HAART naïve or on HAART had small HDL-p that was on average 3.6 μmol/L or 2.4 μmol/L lower, respectively, than the corresponding percentile of HIV-uninfected women.
Table 3.
The association between HIV-infection, HAART use, and small HDL particle concentration after adjustment for triglycerides and/or LDL-C.
| Differencesa in Indicated Percentiles of small HDL particle concentration (μmol/L) | |||||
|---|---|---|---|---|---|
| 10th percentile | 25th percentile | 50th percentile | 75th percentile | 90th percentile | |
| Adjustment for triglyceridesb | |||||
| on HAART (vs HIV-uninfected) | −1.8 (−3.1, −0.5) | −2.5 (−3.4, −1.6) | −3.2 (−4.0, −2.1) | −2.2 (−3.4, −1.4) | −2.0 (−3.2, −0.3) |
| HAART naïve (vs HIV-uninfected) | −1.7 (−5.0, −0.1) | −3.1 (−3.9, −1.3) | −4.3 (−5.4, −2.9) | −4.4 (−6.0, −3.0) | −4.0 (−5.8, −2.2) |
| Adjustment for LDL-Cb | |||||
| on HAART (vs HIV-uninfected) | −1.7 (−3.6, −0.6) | −2.7 (−3.6, −1.5) | −2.8 (−3.7, −1.5) | −2.0 (−3.0, −1.3) | −1.4 (−2.8, 0.0) |
| HAART naïve (vs HIV-uninfected) | −2.6 (−4.6, −0.2) | −3.1 (−4.4, −2.0) | −3.7 (−5.5, −2.2) | −4.3 (−6.1, −3.0) | −3.9 (−5.6, −2.4) |
| Adjustment for triglycerides and LDL-Cb | |||||
| on HAART (vs HIV-uninfected) | −1.8 (−3.7, −0.7) | −2.7 (−3.7, −1.8) | −3.1 (−3.8, −1.9) | −2.3 (−3.3, −1.3) | −2.2 (−3.4, −0.7) |
| HAART naïve (vs HIV-uninfected) | −2.7 (−4.5, 0.1) | −3.1 (−4.5, −2.0) | −3.9 (−5.3, −2.3) | −4.5 (−6.0, −3.0) | −4.0 (−6.0, −2.7) |
Each table entry represents the difference in indicated percentile of small HDL particle concentration comparing either HIV-infected women on HAART or HIV-infected women who were HAART naïve to those who were HIV-uninfected along with the 95% confidence interval of the difference
The quantile regression models were also adjusted for index visit values of age, race/ethnicity, menopause status, number of years of smoking cigarettes, hepatitis C infection status, lipid lowering therapy, waist size, HOMA, and diabetes status. If data were missing at index visit then data from visit closest to and prior to index visit (up to 2 visits prior to index visit) were used.
Because HAART use (in particular regimens containing a NNRTI) has been shown to increase HDL [23], we examined whether being on an NNRTI regimen was associated with greater small HDL-p in the 588 HIV-infected women on HAART. After adjustment for TG and LDL-C, we found that women on an NNRTI containing regimen had a median small HDL-p that was 1.3 μmol/L greater (95% CI 0.2 to 2.5) than women not on an NNRTI containing regimen. When we examined the association of PI containing regimens with HDL-p, we found that after adjustment for TG and LDL-C, women on a PI containing regimen had a median small HDL-p that was 1.3 μmol/L lower (95% CI −2.5 to −0.6) compared to women not on a PI containing regimen.
DISCUSSION
In our study of 1077 women with and at risk for HIV infection, we found that HIV infection was associated with greater 75th and 90th percentiles of small LDL-p in women on HAART and lower 10th, 25th, 50th, and 75th percentiles of small HDL-p, regardless of HAART status. However, after further adjustment for standard lipids (TG and HDL-C), the association of HAART with greater small LDL-p was no longer significant. In contrast, HIV infection remained associated with lower small HDL-p after further adjustment for standard lipids (TG and LDL-C). Unlike our findings with small HDL-p, our data suggests that small LDL-p concentrations likely confer little additional information regarding CVD risk beyond that of standard lipids in HIV-infected women
We found that the association of HAART with greater small LDL-p was strongly mediated by TG alone (which are known to increase as a result of HAART use). Furthermore, the estimates quantifying the association of TG with small LDL-p were much larger than the estimates quantifying the association between HIV infection and small LDL-p. In the setting of alterations in TG metabolism, cholesterol ester transfer protein activity is thought to increase leading to: 1) the transfer of cholesterol ester from HDL-p to apoB-containing lipoproteins and thus low HDL-C levels; and 2) cholesterol depletion and TG enrichment of LDL-p that facilitates the formation of small LDL-p [6, 24]. Our findings are consistent with a study from the pre-HAART era that showed that disturbances in TG metabolism, which have been reported to occur in the setting of advanced HIV disease, were associated with greater small LDL-p [11]. The lack of an association of being HAART naïve with greater small LDL-p in our study is likely because the majority of HAART naïve women did not have advanced HIV disease (median CD4 of 468 cells/mm3) and thus had TG levels (median=94 mg/dL) more similar to HIV-uninfected women (median=88 mg/dL) than those on HAART (median=118 mg/dL).
In contrast, our observation of an association of HIV infection (regardless of HAART use) with lower small HDL-p, even after further adjustment for standard lipids, suggests an effect of HIV on HDL-p that is not mediated through alterations in standard lipids. A recent small study of HIV-infected participants with relatively preserved immune function (median CD4 of 382 cells/mm3) who were not receiving ART hypothesized that the inflammation resulting from HIV infection might alter HDL-p, making HDL-p less atheroprotective [12]. When they studied correlations between lipid levels and biomarkers in a subset of 32 HIV-infected participants, they found a strong association of lower small HDL-p with greater levels of IL-6 (a marker of inflammation), sICAM-1 (a marker of endothelial activation), and D-dimer (a thrombotic factor). In our study, the decrease in small HDL-p was less in those on HAART compared to those who were HAART naïve. These findings suggest that viral suppression and the concomitant decrease in inflammation is associated with smaller decreases in small HDL-p. Further investigation of the relationship of HIV infection, inflammation, and HDL-p is needed in a larger study.
Our finding that HAART use was no longer associated with small LDL-p after adjustment for standard lipids is clinically important, because it provides insight into the relationship of small LDL-p and standard lipids with CVD risk in the setting of HIV infection. Data from some large prospective studies of healthy men and women from the general population [25-27] show that after adjustment for TG and HDL-C or cholesterol to HDL-C ratio, the independent association of LDL-p with CVD events was no longer significant. These studies concluded that LDL-p was comparable to standard lipid and lipoprotein assessments for CVD risk. Similarly, a recent study in mainly HIV-infected men from the SMART trial found that total, large and small HDL-p were significantly and inversely associated with CVD, in contrast to VLDL-p and LDL-p, where no association was found [9]. There was little change in the association of HDL-p with CVD risk after adjustment for LDL-C and triglycerides. On the other hand, additional adjustment for inflammatory markers (IL-6, C-reactive protein, and D-dimer) mitigated the association.
A limitation of our study was its cross-sectional design, which did not allow us to fully address the causal associations of HIV infection and HAART use with alterations in lipoprotein particle concentrations. While some consider density gradient ultracentrifugation [28] as the gold standard method to identify lipoprotein subclasses, we chose NMR spectroscopy to measure lipoprotein particle concentrations in order to be able to compare our findings with those of other HIV studies, which have mainly used the NMR technique. Because our study was performed in a cohort of women, our findings may not be generalizable to men, although our findings prior to further adjustment for standard lipids are consistent with the findings of the Multicenter AIDS Cohort Study [13]. Finally, as with all observational studies, our findings are subject to possible unmeasured confounding.
We conclude that the association of HAART use with greater small LDL-p concentration is strongly mediated by triglycerides, which are elevated in the setting of HAART, and to a lesser extent, by HDL-C. Our findings suggest that routine testing of LDL-p concentrations will not confer additional information beyond TG and HDL-C levels when assessing CVD risk. In contrast, adjusting for standard lipids did not alter the association of HIV infection with HDL-p concentrations, suggesting a direct effect of either HIV or possibly HIV-associated chronic inflammation. Further investigation will examine the relationship of HIV infection, inflammation, and small HDL-p, as well as the role of HDL-p in the link between HIV infection and CVD in women.
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co- funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). Drs. Tien and Glesby are supported by the National Institute of Allergy and Infectious Diseases through K23 AI 66943-04 and K24 AI078884, respectively. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. All authors played a role in editing the manuscript and approved the text as submitted to AIDS. Phyllis C. Tien designed the study and wrote the manuscript. Michael F. Schneider and Christopher Cox performed the data analysis and assisted in the interpretation of statistical data. Mardge Cohen, Roksana Karim, Mary Young, Jason Lazar, and Marshall J. Glesby reviewed and edited the manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Currier J, Scherzer R, Bacchetti P, et al. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008;48:35–43. doi: 10.1097/QAI.0b013e318164227f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. Jama. 2003;289:2978–82. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 3.Wohl D, Scherzer R, Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 5.Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6:381–7. doi: 10.1007/s11883-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 6.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90:22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 7.Kuller L, Arnold A, Tracy R, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–80. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 8.Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Duprez DA, Kuller LH, Tracy R, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–9. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 11.Feingold KR, Krauss RM, Pang M, Doerrler W, Jensen P, Grunfeld C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab. 1993;76:1423–7. doi: 10.1210/jcem.76.6.8501146. [DOI] [PubMed] [Google Scholar]
- 12.Baker J, Ayenew W, Quick H, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 201:285–92. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddler SA, Li X, Otvos J, et al. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–8. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 14.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 16.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids. 2008;22:1615–24. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin MT, Lu D, Cremers S, et al. Short-Term Bone Loss in HIV-Infected Premenopausal Women. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181bf6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992;38:1632–8. [PubMed] [Google Scholar]
- 19.Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. Aids. 2007;21:1739–45. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 20.Esty W, Banfield J. The Box-Percentile Plot. J Stat Softw. 2003;8:1–14. [Google Scholar]
- 21.Koenker R, K. H. Quantile regression. J Econ Perspect. 2001;51:143–56. [Google Scholar]
- 22.Terry MB, Wei Y, Esserman D. Maternal, birth, and early-life influences on adult body size in women. Am J Epidemiol. 2007;166:5–13. doi: 10.1093/aje/kwm094. [DOI] [PubMed] [Google Scholar]
- 23.van der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. Aids. 2001;15:2407–14. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 24.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 25.El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–53. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–9. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–7. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 28.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]